Abstract
Purpose
To investigate basement membrane (BM) formation during ex vivo expansion of limbal corneal epithelial cells on intact amniotic membrane (iAM) and epithelially denuded (d)AM.
Methods
Human limbal explants were cultured on iAM and dAM. Expression of BM components, including laminin-5, type IV collagen, type VII collagen, perlecan, integrin α6, and epithelial cell differentiation markers such as p63, cytokeratin 3 (K3), and cytokeratin 12 (K12), were investigated by immunostaining. Levels of matrix metalloproteinase (MMP)-2 and MMP-9 and tissue inhibitor of matrix metalloproteinase (TIMP)-1 in the conditioned media were determined by ELISA and gelatin zymography.
Results
All four BM components were preserved in both iAM and dAM before culturing, but dissolved 1 week afterward when MMP-2 was increased. Epithelial outgrowth correlated with increased expression of MMP-2 and -9 for both cultures. Resynthesis of BM began with laminin-5 followed by other components. This process took place at 1 week on iAM but at 2 weeks on dAM after culturing. At 4 weeks, BM was more maturely deposited as a linear band from the explant toward the leading edge on iAM and temporally correlated with a sharp decline of MMP-9 levels. In contrast, such BM deposition began at the leading edge on dAM only when TIMP-1 levels were increased. Epithelial cell outgrowth on iAM expressed more p63 but less K3 and K12 than did that on dAM.
Conclusions
After dissolution of original amniotic BM, new BM formed by ex vivo expanded human limbal corneal epithelial cells on iAM deposits much faster and is more mature, resulting in regeneration of a limbal epithelial phenotype. In contrast, BM deposition is delayed and remains immature on dAM, resembling wound healing by a corneal epithelial phenotype. Thus, BM resynthesis may be used as another objective readout for assessing the success of ex vivo expansion of limbal epithelial progenitor cells on AM.
Limbal stem cell deficiency is a severe corneal abnormality caused by Stevens-Johnson syndrome, ocular cicatricial pemphigoid, aniridia, and chemical and thermal burns. Transplantation of limbal corneal epithelial cells expanded ex vivo on amniotic membrane (AM) is a new surgical strategy for restoring a normal corneal epithelial surface on these limbus-deficient corneas.1–5 To achieve this objective, different experimental protocols have been attempted that vary in using cells derived from limbal tissue explants6–8 or limbal epithelial cell suspension, with9 or without10 prior expansion on a 3T3 fibroblast feeder layer and in coculturing limbal explants or cell suspension, with10,11 or without7,8 3T3 fibroblast feeder layers. Furthermore, AM can be prepared either as intact (i)AM—that is, with devitalized amniotic epithelial cells7,8,12,13—or as denuded (d)AM, without devitalized amniotic epithelial cells.9–13 Such ex vivo–expanded tissue composites have been successfully transplanted for reconstructing the corneal surface afflicted by total limbal stem cell deficiency.1–4,14 Despite these variations, it is generally assumed that retention of the basement membrane (BM) in cryopreserved AM is essential for achieving ex vivo expansion of corneal limbal epithelial cells.
Epithelial BM is composed of an intricate network of extracellular matrix proteins that interact at the epithelial stromal interface. The major constituents of the BM include type IV and VII collagens, several members of the laminin family (types 1, 5, 6, and 7), the heparan sulfate proteoglycan perlecan, and nidogen (for review, see Ref. 15). Interactions between epithelial cells and these BM components modulate epithelial cell adhesion, migration, proliferation, and differentiation.16 AM, the innermost layer of the fetal membrane, consists of a simple epithelium, a BM, and a subjacent avascular stroma. The amniotic BM contains type IV collagen,17–19 type VII collagen,18 and laminin-5.17,20
Up to now, there has been no study demonstrating how BM is formed during ex vivo expansion of limbal explants, although transmission electron microscopy from several studies has shown no complete BM formation underneath the epithelial outgrowth derived from rabbit or human limbal explants cultured on either iAM or dAM for 3 to 4 weeks.1,6,7,12 In the study described herein, we were surprised to note that BM components of type IV collagen, type VII collagen, laminin-5, and perlecan of the original amniotic BM actually dissolved before epithelial outgrowth took place from the limbal explant onto either iAM or dAM. Such early dissolution of BM components correlates with high expression of matrix metalloproteinase (MMP)-2, but not MMP-9. The epithelial outgrowth coincided with high expression of MMP-9 and -2 activities. However, deposition of new BM components at the interface underneath the expanded epithelium occurred earlier and was more mature when cultured on iAM than when cultured on dAM. Such an early assembly of the new BM was correlated with a sharp downregulation of MMP-9, but not MMP-2. The significance of these dynamic changes of BM dissolution and reformation and their correlation with MMPs and tissue inhibitor of matrix metalloproteinase (TIMP)-1 expression are further discussed in the context of improving the future success of ex vivo expansion of limbal epithelial progenitor cells.
Materials and Methods
Dulbecco’s modified Eagle’s medium (DMEM), Ham’s/F12 medium, HEPES buffer, amphotericin B, gentamicin, fetal bovine serum (FBS), and mouse epidermal growth factor (EGF) were purchased from Invitrogen (Carlsbad, CA). Dispase II was obtained from Roche (Indianapolis, IN). Hydrocortisone, dimethyl sulfoxide, cholera toxin, insulin-transferrin-sodium selenite medium supplement, propidium iodide, Triton X-100, bovine serum albumin (BSA), mouse anti-type IV collagen and type VII collagen monoclonal antibodies, FITC conjugated anti-mouse, goat, and rat IgGs were from Sigma (St. Louis, MO). Mouse anti-laminin-5 monoclonal antibody, rat anti-perlecan antibody, and mouse anti-cytokeratin 3 (K3) monoclonal antibody were from Chemicon (Temecula, CA). Mouse anti-human integrin α6 and p63 monoclonal antibodies were from DakoCytomation (Carpinteria, CA), goat anti-cytokeratin 12 (K12) polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), and ABC-AP kit (mouse IgG) was from Vector Laboratories (Burlingame, CA). A cell-viability assay (Live/Dead) was obtained from Invitrogen (Eugene, OR). MMP-2 and -9 and TIMP-1 ELISA kits were from R&D Systems (Minneapolis, MN). Zymogram development buffer was from Bio-Rad Laboratories (Hercules, CA).
Human Tissue Preparation
Human tissue was handled according to the Declaration of Helsinki. Corneoscleral tissues from human donor eyes were obtained from the Florida Lions Eye Bank (Miami, FL) immediately after the central corneal button had been used for corneal transplantation. The tissue was rinsed three times with DMEM containing 50 μg/mL gentamicin and 1.25 μg/mL amphotericin B. After careful removal of excessive sclera, conjunctiva, iris, and corneal endothelium, the remaining tissue was placed in a culture dish and exposed to dispase II (1.2 U/mL in complete culture medium) at 37°C in humidified 5% CO2 for 10 minutes. After three rinses with complete culture medium for 10 minutes each, the scleral rim was trimmed to obtain limbal tissue cubes of 1 clock hour of ~1 × 1.5 × 2.5-mm size.
Human Limbal Explant Cultures on AM
Preserved human AM was kindly provided by Bio-Tissue (Miami, FL). Immediately before use, AM was thawed, washed three times with sterile PBS, and cut into pieces approximately 2.5 cm2 in size. For preparation of dAM, membranes were deprived of their devitalized amniotic epithelial cells by incubation with 0.02% EDTA at 37°C for 1 hour, to loosen the cellular adhesion, followed by gentle scraping with a cell scraper. After that, AM with the epithelium or the BM side facing up was fastened to a culture insert, as previously reported.21 On the center of the AM insert, a human limbal explant was placed and cultured in supplemented hormonal epithelial medium (SHEM) made of an equal volume of HEPES-buffered DMEM containing bicarbonate and Ham’s/F12. The medium was supplemented with 5% FBS, 0.5% dimethyl sulfoxide, 2 ng/mL mouse EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 0.5 μg/mL hydrocortisone, 1 nM cholera toxin, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. Cultures were incubated at 37°C under 5% CO2 and 95% air, and the medium was changed every 48 hours, conditioned medium was centrifuged (3000g for 15 minutes), and the supernatant was stored at −80°C. All explants were kept submerged in the culture medium (2 mL) throughout the culture duration. Freeze-thawed, devitalized explants and iAM and dAM without explants were used as the control. Parallel experiments were killed at the end of each week until 4 weeks, and AM together with the explant was removed and embedded in optimal cutting temperature (OCT) compound for cryosectioning.
Histology and Immunostaining
Cryostat sections (4-μm) of the limbal explants as well as outgrowth on AM were fixed in acetone for 10 minutes at −20°C. The sections used for immunostaining were rehydrated in PBS and then incubated in 0.2% Triton X-100 for 10 minutes. After three rinses with PBS for 5 minutes each and preincubation with 2% BSA to block nonspecific staining, the sections were incubated with primary antibodies (antitype IV collagen at 1:400, others at 1:100) for 1 hour. After three washes with PBS for 15 minutes, they were incubated with an FITC-conjugated secondary antibody (goat anti-rabbit or anti-mouse IgG at 1:100) for 45 minutes. After three additional PBS washes for 15 minutes, they were counterstained with PI (1:2000) for 1 minute, mounted in mounting medium, and analyzed with a fluorescence microscope. For immunohistochemical staining of p63, endogenous peroxidase activity was blocked by 0.6% hydrogen peroxide for 10 minutes. Nonspecific staining was blocked by 1% normal goat serum for 30 minutes. The sections were then incubated with p63 antibody (1:50) for 1 hour. After three washes with PBS for 15 minutes, sections were incubated with biotinylated rabbit anti-mouse IgG (1:100) for 30 minutes, followed by incubation with ABC reagent for 30 minutes. The reaction product was developed with 3,3′-diaminobenzidine (DAB) for 5 minutes, followed by dehydration, and examined under a light microscope.
ELISA of MMP-2, MMP-9, and TIMP-1
Quantitative ELISA assays for human MMP-2, MMP-9, and TIMP-1 were performed according to the manufacturer’s protocol. In brief, 100 μL of assay buffer and 50 μL of standard dilutions of recombinant human MMP-2, MMP-9, TIMP-1, and supernatants of the conditioned media were dispensed into a 96-well polystyrene microplate coated with a mouse monoclonal antibody against MMP-2, MMP-9, or TIMP-1. The plate was sealed, incubated for 2 hours at room temperature (RT). After three washes, 200 μL horseradish peroxidase–conjugated secondary antibody was added to each well and incubated for 1 hour at RT. After three washes, 200 μL of the substrate solution tetramethyl-benzidine (TMB) was applied for 30 minutes, to develop a blue color, and the reaction was stopped by adding 50 μL of 2 N sulfuric acid. Absorbance was read at 450 nm by an automatic plate reader with a reference wavelength of 570 nm.
Gelatin Zymography
To determine the relative concentration and activity of gelatinases in the conditioned medium from limbal explant cultures on iAM or dAM, SDS-PAGE gelatin zymography was performed by using a previously reported method.22 Briefly, 10 μL of each conditioned medium was treated with SDS-PAGE sample buffer without boiling and reduction. Samples were fractionated in a 10% polyacrylamide gel by electrophoresis at 100 V for 90 minutes at 4°C. The gels were soaked in 0.25% Triton X-100 for 30 minutes at RT, to remove the SDS, and incubated in a development buffer (50 mM Tris-HCl [pH 7.5] 200 mM NaCl, 5 mM CaCl2, and 0.02% Brij-35) containing 5 mM phenylmethylsulfonyl fluoride, a serine protease inhibitor, at 37°C overnight, to allow proteinase digestion of the substrate, and then stained with 0.25% Coomassie blue R-250 in 40% isopropanol for 2 hours and destained with 7% acetic acid. Gelatinolytic activities appeared as clear bands of digested gelatin against a dark blue background of stained gelatin.
Statistical Analysis
All experiments were repeated three times, each in triplicate. For MMP-2, MMP-9, and TIMP-1 detection, each group had six samples, and each sample was assayed in duplicate. Group means were compared using the appropriate version of Student’s unpaired t-test. Test results were reported as two-tailed, where P < 0.05 was considered statistically significant. Summary data are reported as the mean ± SD.
Results
Epithelial Morphology
Limbal corneal epithelial cells started to migrate from the explant to iAM and dAM 4 to 5 days after culturing (Fig. 1A, 1E), which is consistent with our previous report.13 A prominent leading edge of outgrowth was visible on iAM cultures at 1 week (Fig. 1B), and spread out gradually, but did not reach confluence at 4 weeks of culturing (Fig. 1D). In contrast, the leading edge of outgrowth on dAM was not discernible by the naked eye, but could only be traced under the microscope (Fig. 1F, 1G). The outgrowth on dAM was subconfluent at 2 weeks and reached confluence at 4 weeks, indicating that the outgrowth rate on dAM was higher than that on iAM, which is also in agreement with our previous report.13 The epithelial morphology visualized by the cell viability assay showed that epithelial cells on iAM were uniformly small and had a cuboidal shape (Fig. 1I), whereas cells on dAM were large and had a migratory spindle shape (Fig. 1J). Hematoxylin and eosin (H&E) staining of the 4-week culture showed that the leading edge of the epithelial outgrowth on iAM was stratified whereas the remaining area had one to two cell layers (Fig. 2A). In contrast, the leading edge of the epithelial outgrowth on dAM was not stratified but was uniformly one to two cell layers throughout (Fig. 2B). Because the cell viability assay staining did not reveal any dead cells underneath the epithelial outgrowth on iAM (Fig. 1I), we concluded that there were no devitalized AM epithelial cells residing underneath the epithelial outgrowth. This interpretation was also supported by the finding that the devitalized amniotic epithelial cells were pushed away on iAM by expanding epithelial outgrowth (not shown).
Figure 1.
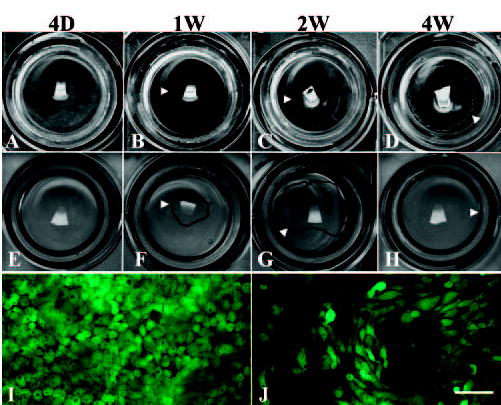
Morphologic difference between the outgrowth on iAM (A, B, C, D) and that on dAM (E, F, G, H) during ex vivo expansion. A prominent leading edge can be seen on iAM under the naked eye (B, C, D, arrowheads). In contrast, the leading edge is not visible, but can be traced under a microscope with a pen (F, G, H, arrowheads). Epithelial outgrowth did not reach confluence on iAM, even at 4 weeks of culturing (D), but already reached confluence by 4 week of culturing (between G and H). Cell viability assay staining showed a monolayer of uniformly small cuboidal cells on iAM (I), but large and spindle cells on dAM (J). Bar: (I, J) 100 μm.
Figure 2.
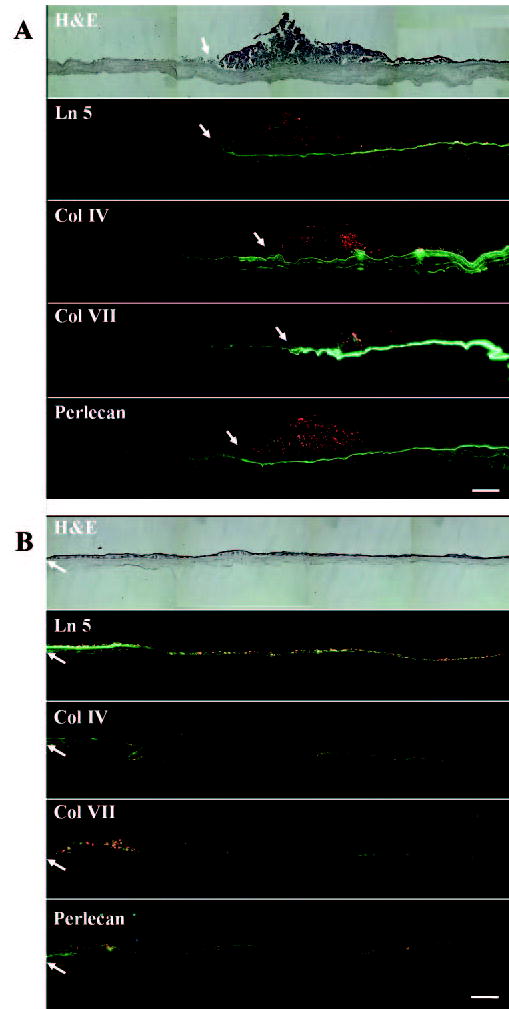
Overview of BM formation of limbal corneal explants cultured on iAM or dAM for 4 weeks. When an explant was cultured on iAM for 4 weeks (A), Ln-5, Col IV, Col VII, and perlecan were strongly expressed as a continuous band underneath the epithelial outgrowth, but decreased and became negative farther out from the outgrowth’s leading edge (arrow). In contrast, when an explant was cultured on dAM for 4 weeks (B), Ln-5, Col IV, Col VII, and perlecan started to deposit in a linear-segmented band close to the leading edge of the outgrowth (arrow) and showed a spotted and cytoplasmic staining in the major part of the outgrowth. Bar, 200 μm.
BM Components in iAM or dAM before Culturing
H&E staining showed that a monolayer of amniotic epithelial cells was well kept on iAM before culturing, whereas these cells were completely removed on dAM after EDTA treatment and mechanical scraping (Fig. 3). The BM components of laminin-5, type IV collagen, type VII collagen, and perlecan were preserved as a linear stain underneath the amniotic epithelium in iAM (Fig. 3). Similarly, these BM components were well preserved, as evidenced by the linear staining on dAM, confirming that all BM components were not removed by denudation of the amniotic epithelium (Fig. 3). Of note, type IV collagen was also present in the AM stroma as punctuate staining (Fig. 3).
Figure 3.
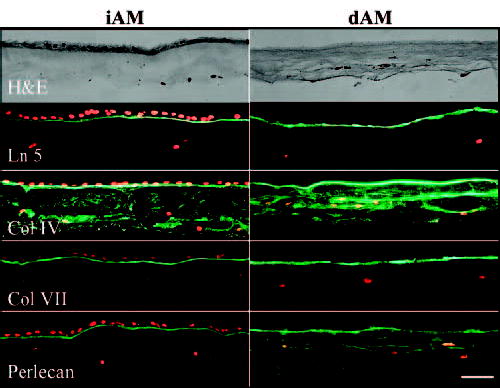
BM components in iAM and dAM before culturing. H&E staining showed that a monolayer of amniotic epithelial cells was present on iAM but absent on dAM. A linear staining of laminin-5 (Ln-5), type IV collagen (Col IV), type VII collagen (Col VII), and perlecan was noted in the BM zone of both iAM and dAM. Punctate staining of collagen IV was also found in the AM stroma of iAM and dAM, and punctuate staining of perlecan was found in the AM stroma of dAM. Bar, 50 μm.
Degradation of Amniotic BM Components and Reassembly of New BM Components during Epithelial Outgrowth
To investigate whether there was any change in the BM components of AM during expansion of epithelial outgrowth from the limbal explant, we performed immunostaining of each BM component at different times of iAM and dAM culture. We noted unexpectedly that all four BM components that preexist in both iAM and dAM (Fig. 3) disappeared at 1 week of culture, suggesting that they were degraded after 1 week (Fig. 4). To strengthen our interpretation further, we cultured freeze-thawed, devitalized limbal explants for 2 weeks on either iAM or dAM as a control, as well as iAM or dAM without explants as another control, and noted that all four BM components were well preserved (data not shown). These findings further indicate that degradation of BM components was dependent on live cells derived from the limbal explant.
Figure 4.
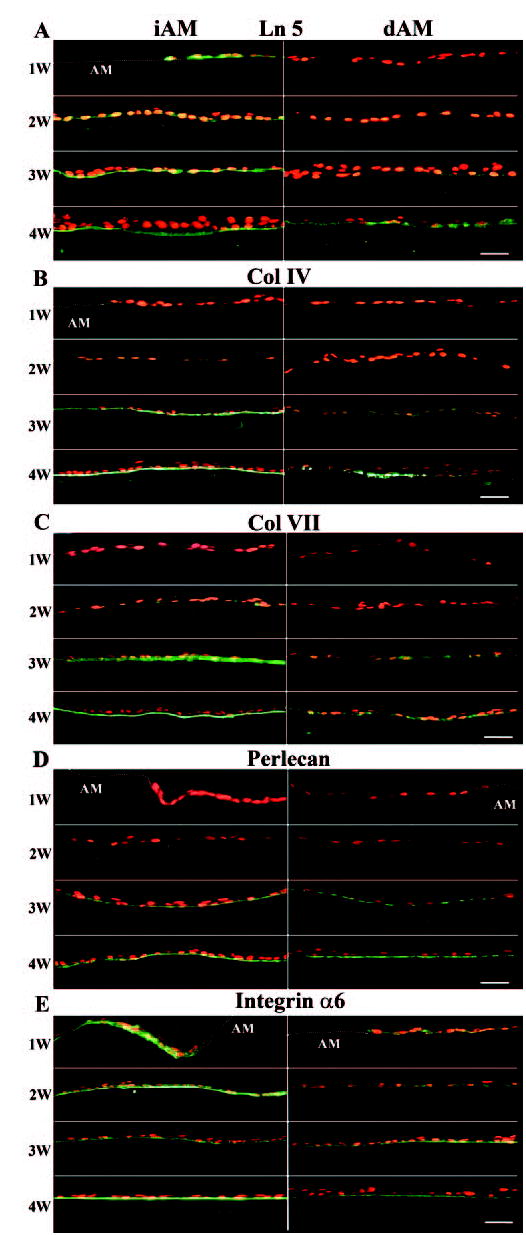
Immunostaining of BM components of laminin-5 (A), type IV collagen (B), type VII collagen (C), perlecan (D) and integrin α6 (E) during ex vivo expansion on iAM and dAM. At 1 week of culture, all four BM components disappeared in both iAM and dAM, indicating the degradation of preexisting BM components of AM. The reexpression of new BM components followed the order of intracellular punctuate staining, extracellular punctate deposition beneath the cells, and a continuous linear staining between the epithelial outgrowth and AM stromal matrix. The new synthesis of Ln-5 was first noted in the stromal matrix. The new synthesis of Ln-5 was first noted in the epithelial outgrowth at 1 week (1W), but that of Col IV, Col VII, and perlecan started at 2 weeks (2W) and finished as a linear band on iAM by 4 weeks (4W). In contrast, new synthesis of these four components was delayed 1 week in dAM and there was still no linear staining by 4W. Expression of integrin α6 by epithelial outgrowth was intracellular at 1W on both iAM and dAM, but became linear at 2W on iAM but at 3W on dAM. Bar, 50 μm.
Continuous observation revealed that there was new synthesis of BM components by outgrowth of epithelial cells on iAM, as shown by initial intracellular expression, followed by punctate deposition beneath the cells, and finally by a continuous band formed at the interface between outgrowth of epithelial cells and AM stromal matrix. When explant was cultured on iAM, the earliest BM component expressed by the epithelial outgrowth was laminin-5 at 1 week, which exhibited strong intracellular staining, followed by the same staining pattern of type IV collagen, type VII collagen, and perlecan, beginning at 2 weeks. Compared with the iAM culture, there was a 1-week delay in the expression of all BM components in dAM cultures. At the end of 4 weeks of culturing, all four BM components showed a continuous band on iAM, but not on dAM, where the staining was of lower intensity and remained fragmented and an intracellular pattern.
At 4 weeks of culturing, the outgrowth on iAM still did not reach confluence whereas outgrowth on dAM had already reached confluence (Fig. 1). Low-magnification composites showed that laminin-5, type IV collagen, type VII collagen, and perlecan were all strongly stained continuously underneath the outgrowth on iAM. Moreover, expression of type IV collagen and perlecan was also found in the AM stroma with a gradient coming from the explant. Staining of all four BM components was notably stronger and more prominent toward the explant, but became more attenuated toward the leading edge of outgrowth and was negative in iAM outside the leading edge of outgrowth. These results indicate that deposition of BM components was mature after 4 weeks of culturing on iAM and constituted a gradient starting from the explant to the leading edge of the outgrowth. In contrast, when the explant was cultured on dAM, the staining of all four BM components was most prominent at the leading edge of the outgrowth in contact with the plastic wall, became markedly attenuated toward the explant, and showed an intracellular punctuate pattern in nearly all of the outgrowth. These results indicate that the deposition of BM components remained immature after 4 weeks of culturing on dAM and adopted a gradient starting from the leading edge of the outgrowth toward the explant, the opposite the gradient of iAM cultures.
Hemidesmosome Assembly of Epithelial Cell Outgrowths
Hemidesmosomes are specialized junctional complexes that mediate adhesion of epithelial cells to the underlying BM. Hemidesmosomal components include the α6β4 integrin, HD1 plectin, and the bullous pemphigoid antigens BP180 and BP230 (for review, see Ref. 23). We used integrin α6 as a marker to investigate hemidesmosome formation. The results showed that integrin α6 was present intracellularly along the outgrowth on both iAM and dAM from 1 week (Fig. 4E). The staining of integrin α6 became concentrated along the basal epithelial membrane on both iAM and dAM at 3 weeks and formed a continuous band at 4 weeks. At this time, the staining was much stronger on iAM than dAM, suggesting mature formation of hemidesmosomes and restoration of polarity of epithelial cell outgrowth.
Correlation of Degradation and Reassembly of BM Components with Levels of MMP-2, MMP-9, and TIMP-1 in Conditioned Media
To investigate the mechanism of degradation and reassembly of BM components in our culture system, we measured levels of MMP-2, MMP-9, and TIMP-1 proteins in the conditioned media with ELISA assays. MMP-2 and -9 activity was further analyzed by gelatin zymography. The results showed that MMP-2 concentration increased in the first week in iAM and dAM cultures. After that, MMP-2 decreased at the second week and maintained at a stable plateau at the third and fourth weeks in iAM cultures. However, a high level of MMP-2 was maintained from the second to fourth weeks in dAM cultures, and the concentration was higher in dAM than in iAM from days 10 to 26 (P < 0.05; Fig. 5A). In contrast, MMP-9 was kept at a negligible low level in the first week and increased dramatically in the second week on both iAM and dAM. A high level of MMP-9 was maintained from the second to fourth weeks on dAM, but decreased in the fourth week on iAM. MMP-9 concentration was higher in iAM than in dAM in the third week, but was lower in iAM in the fourth week (P < 0.05 at days 16, 22, 24, and 26; Fig. 5B). TIMP-1 also increased in the first week in both iAM and dAM cultures. After that, TIMP-1 decreased gradually and was kept at a low level on iAM from the second to the fourth weeks. However, TIMP-1 was kept at a high level on dAM and increased dramatically in the fourth week (P < 0.01 at day 10, 12, and days 18–26; Fig. 5C). The control with iAM or dAM alone without limbal explants showed negligible or undetectable levels of MMP-2, MMP-9, and TIMP-1 throughout the culture duration.
Figure 5.
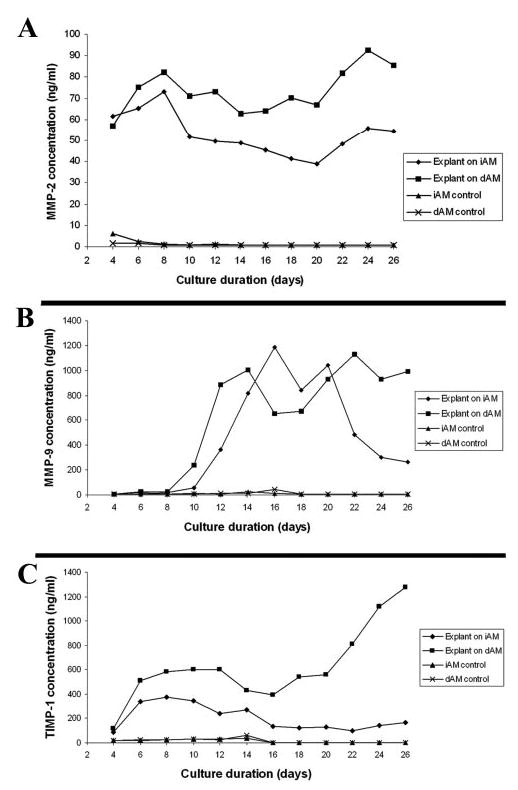
MMP-2, MMP-9, and TIMP-1 in conditioned medium of limbal explants cultured on iAM or dAM. MMP-2 concentration increased in the first week in iAM and dAM cultures. After that, MMP-2 decreased in the second week and kept stable in the third and fourth weeks in iAM cultures. High level MMP-2 sustained throughout the culture duration from the second to fourth weeks in dAM cultures (A). MMP-9 remained at a very low level in the first week and increased dramatically in the second week both on iAM and dAM. A high level of MMP-9 was sustained from the second to fourth week in dAM, but declined in the fourth week in iAM (B). TIMP-1 increased in the first week in both iAM and dAM cultures. After that, TIMP-1 decreased gradually and remained at a low level in iAM from the second to fourth weeks. However, TIMP-1 stayed at a high level in dAM and increased dramatically in the fourth week (C). The control without explants showed negligible or undetectable levels of MMP-2, MMP-9, and TIMP-1 throughout the culture duration.
Gelatin zymography assay results showed an active form of MMP-2 only in the explant-cultured conditioned medium (Figs. 6A, 6B), but not in the control culture with iAM (Fig. 6C) or dAM (Fig. 6D) alone. MMP-2 expression was relatively stable throughout the culture period and was higher on dAM (Fig. 6B) than on iAM (Fig. 6A), in agreement with ELISA assay results. MMP-9 expression was undetectable at 4 days when the explant was cultured on either iAM or dAM, but it quickly increased in the second week. Expression of MMP-9 remained high in the second and third weeks on both iAM and dAM, but decreased in the fourth week on iAM but not on dAM. This finding was also in agreement with ELISA assay results. MMP-9 activities were undetectable in the control of either iAM or dAM without explants.
Figure 6.
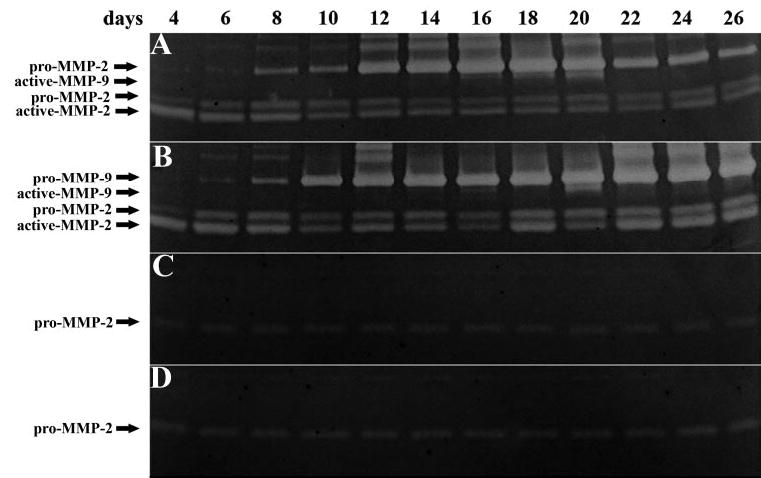
Gelatin zymography of MMP-2 and -9 in explant culture conditioned medium. The active form of MMP-2 was only present in explant culture on iAM (A) and dAM (B) conditioned medium, but not in the control with iAM (C) or dAM (D) alone without explants. MMP-2 activities were relatively stable throughout the culture period, but the activity was higher on dAM than on iAM. MMP-9 activities were undetectable at 4 days when the explant was cultured on both iAM (A) and dAM (B), but quickly increased in the second week. A high expression of MMP-9 was maintained in the second and third weeks on both iAM and dAM, but it decreased in the fourth week on iAM, but remained at a high level on dAM. MMP-9 activities were undetectable in the control without explants (C, D).
Correlation of Epithelial Differentiation and Reassembly of New BM Components
Deposition and assembly of the BM occurs concurrently with the normalization of epithelial growth, morphogenesis, and differentiation.24,25 To study the differentiation of corneal limbal epithelial cells expanded on iAM or dAM, we investigated the expression of p63, K3, and K12 in the outgrowth. The results showed that after 4 weeks of culture, there was more nuclear staining of p63 in the outgrowth on iAM. K3 was negative or weakly positive in the basal epithelial cells on iAM, but was strongly positive in all epithelial cell outgrowths on dAM. K12 was weakly positive on iAM, but almost all cells were strongly positive on dAM (Fig. 7). These results indicated that the epithelial cell outgrowths on iAM remained poorly differentiated, resembling limbal basal epithelial cells, whereas cells on dAM were more differentiated, similar to corneal epithelial cells.
Figure 7.
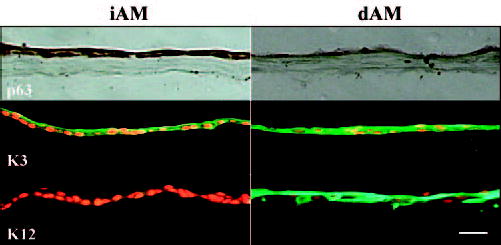
Differentiation markers expressed by outgrowth epithelial cells expanded on iAM or dAM for 4 weeks. There was more nuclear staining of p63 on iAM than dAM. K3 was negative or weakly positive in basal epithelial cells on iAM, but was strongly positive in all outgrowth epithelial cells on dAM. K12 was weakly positive on iAM, whereas almost all cells were strongly positive on dAM. Bar, 50 μm.
Discussion
BM formation is an important criterion for structural and functional regeneration of the epidermis when skin equivalents are engineered and transplanted.26 Only tissues with well-structured BM show optimal epithelial tissue architecture after transplantation.27 This study is the first attempt at determining whether BM formation can be used as another readout to assess the success of ex vivo expansion of limbal epithelial progenitor cells before transplantation.
Our study demonstrates dynamic changes of BM degradation and reassembly during ex vivo expansion of limbal corneal epithelial cells on AM, and provides four important findings. The first surprising finding was the degradation of preexisting BM components such as type IV collagen, type VII collagen, laminin-5, and perlecan on iAM and dAM before migration of epithelial outgrowth (Figs. 3, 4). In mouse corneas, epithelial BM is found partially disassembled after extensive epithelial debridement before complete epithelialization.28 If what we observed herein also had taken place in vivo, it would seriously challenge our prior belief that preexisting amniotic BM components serve as a scaffold to promote epithelialization after AM transplantation (for review, see Ref. 29). Because these BM components were still well preserved on both iAM and dAM without limbal explants or with devitalized limbal explants after 2 weeks of culture in the same medium (data not shown), we conclude that the degradation of preexisting BM is due to the release of gelatinases by cells in live limbal explants. Because MMP-2 levels were readily detectable and dramatically increased in both iAM and dAM cultures, whereas MMP-9 levels were negligible during the first week when epithelial migration just started, we strongly speculate that MMP-2, not MMP-9, is responsible for BM degradation. This speculation is supported by the finding that MMP-2 can degrade type IV collagen,30 type VII collagen,31 and laminin-5.32 Because selective action of MMP-2 on laminin-5 results in stimulation of mammary epithelial cell migration,32 we speculate that the removal of preexisting laminin-5 is an important stimulus to promote epithelial outgrowth migration onto either iAM and dAM. Because BM also acts as a reservoir for latent growth factors and cytokines, such as transforming growth factor-β and basic fibroblast growth factor,33–35 proteolysis of BM components by MMP-2 may help release and activate these factors-cytokines that may play an important role in triggering or supporting subsequent epithelial migration, proliferation, and differentiation. Because MMP-2 may be secreted by both epithelial cells and fibroblasts in the anterior segment of the eye36 (for review, see 37), future studies are needed to determine whether limbal epithelial cells, stromal mesenchymal cells, or both are responsible for the early release of MMP-2 into the conditioned medium and how such an early release is regulated.
The second finding was that active epithelial outgrowth from the explant correlated with a dramatic increase of MMP-9 in the conditioned medium of both iAM and dAM cultures (Fig. 5B). Recently, Sun et al.38 also reported elevation of MMP-9 in the conditioned medium during the outgrowth of limbal explants on AM. MMP-9 has been found expressed mostly in migrating epithelial cells in bronchial epithelial wounds39 and in migrating basal corneal epithelial cells in scrape40 or excimer laser–ablated36 wounds. The mechanism by which MMP-9 is upregulated in our system remains unclear. In epidermal keratinocytes, absence of laminin-5 triggers upregulation of phosphorylation of p38 MAPK,41 which has been linked to corneal epithelial migration42–44 and to upregulation of MMP-9 in other cell systems.45,46 Therefore, it is possible that early dissolution of BM components by MMP-2 triggers phosphorylation of p38 and as a result induces MMP-9 expression. At this moment, we do not know why MMP-9 decreased dramatically in iAM culture, but stayed at a high level in dAM during the fourth week.
The third finding revealed different patterns of BM formation on iAM and dAM. In several models of epidermal wound healing, BM deposition is a late event and starts at the leading edge after migrating epithelial cells fill in the wounded area, presumably due to “contact inhibition.”47–50 A similar phenomenon was observed in dAM cultures where linear BM deposition started at the leading edge when epithelial outgrowth reached the plastic wall at the end of 4 weeks and became markedly attenuated toward the explant (Fig. 4B). In contrast, BM linear deposition in iAM cultures started much earlier, before the leading edge reached the plastic wall, and formed a gradient starting from the explant toward the leading edge, a pattern exactly opposite to that of dAM cultures (Fig. 2A). This surprising finding prompted us to speculate that epithelial cell growth and migration on dAM resemble epithelial wound healing, whereas cells expanding on iAM resemble epithelial regeneration. The major difference between iAM and dAM is the presence of devitalized amniotic epithelial cells in the former. Besides the possibility that such devitalized amniotic epithelial cells serve as a physical barrier to slow down the outgrowth, which may trigger BM deposition, the other possibility is that they serve as a feeder layer to promote BM formation. This concept was suggested by a recent report showing the success of using devitalized amniotic epithelial cells as a feeder layer to support growth and maintain the undifferentiated status of primate embryonic stem cells.51 In epidermal organotypic cultures, BM assembly is facilitated when keratinocytes are cocultured with dermal fibroblasts.27 Future studies are needed to determine whether the protocol of coculturing limbal epithelial outgrowth with 3T3 fibroblast feeder layers may also promote BM assembly in dAM cultures, and if so whether such a mechanism lends support of this method in expanding limbal epithelial progenitor cells.10,11
Temporal and spatial correlation further indicated that deposition of BM components started from laminin-5 followed by type IV, VII collagen and perlecan, and that the pattern started from subepithelial punctuate staining to a linear band in both iAM and dAM cultures (Fig. 3). These results further support the pivotal role of laminin-5 in paving the way of BM deposition.52 Although at this moment, we do not know what factor(s) triggers laminin-5 deposition.
In epidermal organotypic cultures, addition of MMP inhibitors promotes BM formation,53 indicating that a decrease in MMP leads to BM deposition. If this interpretation is correct, we noted herein the fourth intriguing finding that a sharp decline of MMP-9 levels without a concomitant increase of TIMP-1 was temporally correlated with mature deposition of BM in iAM cultures. In contrast, an increase of TIMP-1 was correlated with BM deposition on dAM during the fourth week. These findings further suggest that different mechanisms are used for BM formation during limbal epithelial outgrowth on iAM and dAM. Future studies are needed to investigate how MMPs and TIMP-1 are differentially modulated in iAM and dAM culture.
We noted that early BM reassembly by iAM correlated with higher expression of p63 and lower expression of K3 and K12, compared with dAM (Fig. 7). Although studies from different groups showed discrepancies of p63 expression patterns in corneal and limbal epithelium, it was generally believed that limbal basal p63-positive cells include corneal epithelial stem cells, and that p63 positivity also represents cells in a proliferative state, such as transit amplifying cells (for review, see Ref. 54). Together with expression of K3 and K12, which had been used as differentiated corneal epithelial cell marker,55–57 we believe early BM formation correlates well with the preservation of limbal epithelial phenotype. These results were consistent with our previous report showing that ex vivo expansion on iAM preserves the limbal epithelial phenotype, whereas that on dAM promotes a corneal epithelial phenotype.13 We then propose that BM formation may be used as another objective sign for assessing the experimental variables used in different protocols for successful ex vivo expansion of limbal epithelial progenitor cells in the future.
Acknowledgments
The authors thank Szu-Yu Chen for technical assistance.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2005.
Disclosure: W. Li, Tissue Tek (F, E); H. He, Tissue Tek (F, E); C.-L. Kuo, Tissue Tek (F, E); Y. Gao, Tissue Tek (F, E); T. Kawakita, Tissue Tek (F, E); S.C.G. Tseng, Tissue Tek (F, I, E); Bio Tissue, Inc. (P)
Supported by National Eye Institute Grants R01 EY06819 and R01 EY015735 (SCGT).
References
- 1.Tsai RJF, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 2.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 4.Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290. doi: 10.1016/s0161-6420(02)01089-8. [DOI] [PubMed] [Google Scholar]
- 5.Ti SE, Grueterich M, Espana EM, et al. Correlation of long term phenotypic and clinical outcomes following limbal epithelial transplantation cultivated on amniotic membrane in rabbits. Br J Ophthalmol. 2004;88:422–427. doi: 10.1136/bjo.2003.026054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000;19:65–71. doi: 10.1097/00003226-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grueterich M, Tseng SCG. Human limbal progenitor cells expanded on intact amniotic membrane. Arch Ophthalmol. 2002;120:783–790. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 9.Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891–986. [PMC free article] [PubMed] [Google Scholar]
- 10.Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–2121. [PubMed] [Google Scholar]
- 11.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119:298–300. [PubMed] [Google Scholar]
- 12.Koizumi N, Fullwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–2513. [PubMed] [Google Scholar]
- 13.Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- 14.Grueterich M, Espana EM, Touhami A, et al. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 2002;109:1547–1552. doi: 10.1016/s0161-6420(02)01105-3. [DOI] [PubMed] [Google Scholar]
- 15.McMillan JR, Akiyama M, Shimizu H. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J Dermatol Sci. 2003;31:169–177. doi: 10.1016/s0923-1811(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 16.Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 17.Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Human corneal basement membrane heterogenicity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- 18.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 19.Endo K, Nakamura T, Kawasaki S, Kinoshita S. Human amniotic membrane, like corneal epithelial basement membrane, manifests the alpha5 chain of type IV collagen. Invest Ophthalmol Vis Sci. 2004;45:1771–1774. doi: 10.1167/iovs.03-0952. [DOI] [PubMed] [Google Scholar]
- 20.Kurpakus-Wheater M. Laminin-5 is a component of preserved amniotic membrane. Curr Eye Res. 2001;22:353–357. doi: 10.1076/ceyr.22.5.353.5494. [DOI] [PubMed] [Google Scholar]
- 21.Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- 22.Solomon A, Li D-Q, Lee S-B, Tseng SCG. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2000;41:2154–2163. [PubMed] [Google Scholar]
- 23.Nievers MG, Schaapveld RQ, Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999;18:5–17. doi: 10.1016/s0945-053x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 24.Bohnert A, Hornung J, MacKenzie IC, Fusenig NE. Epithelial-mesenchymal interactions control basement membrane production and differentiation in cultured and transplanted mouse keratinocytes. Cell Tissue Res. 1986;244:413–429. doi: 10.1007/BF00219217. [DOI] [PubMed] [Google Scholar]
- 25.Marinkovich MP, Keene DR, Rimberg CS, Burgeson RE. Cellular origin of the dermal-epidermal basement membrane. Dev Dyn. 1993;197:255–267. doi: 10.1002/aja.1001970404. [DOI] [PubMed] [Google Scholar]
- 26.Llames SG, Del RM, Larcher F, et al. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation. 2004;77:350–355. doi: 10.1097/01.TP.0000112381.80964.85. [DOI] [PubMed] [Google Scholar]
- 27.Andriani F, Margulis A, Lin N, et al. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J Invest Dermatol. 2003;120:923–931. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- 28.Sta Iglesia DD, Stepp MA. Disruption of the basement membrane after corneal debridement. Invest Ophthalmol Vis Sci. 2000;41:1045–1053. [PubMed] [Google Scholar]
- 29.Tseng SCG, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surface. 2004;2:177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 30.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 31.Seltzer JL, Eisen AZ, Bauer EA, et al. Cleavage of type VII collagen by interstitial collagenase and type IV collagenase (gelatinase) derived from human skin. J Biol Chem. 1989;264:3822–3826. [PubMed] [Google Scholar]
- 32.Giannelli G, Falk-Marzillier J, Schiraldi O, et al. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 33.Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 34.Dowd CJ, Cooney CL, Nugent MA. Heparan sulfate mediates bFGF transport through basement membrane by diffusion with rapid reversible binding. J Biol Chem. 1999;274:5236–5244. doi: 10.1074/jbc.274.8.5236. [DOI] [PubMed] [Google Scholar]
- 35.Vukicevic S, Kleinman HK, Luyten FP, et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 36.Ye HQ, Azar DT. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. 1998;39:913–921. [PubMed] [Google Scholar]
- 37.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 38.Sun CC, Cheng CY, Chien CS, et al. Role of matrix metalloproteinase-9 in ex vivo expansion of human limbal epithelial cells cultured on human amniotic membrane. Invest Ophthalmol Vis Sci. 2005;46:808–815. doi: 10.1167/iovs.04-0370. [DOI] [PubMed] [Google Scholar]
- 39.Legrand C, Gilles C, Zahm JM, et al. Airway epithelial cell migration dynamics: MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol. 1999;146:517–529. doi: 10.1083/jcb.146.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsubara M, Girard MT, Kublin CL, et al. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. 1991;147:425–439. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]
- 41.Harper EG, Alvares SM, Carter WG. Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J Cell Sci. 2005;118:3471–3485. doi: 10.1242/jcs.02475. [DOI] [PubMed] [Google Scholar]
- 42.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 43.Saika S, Okada Y, Miyamoto T, et al. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- 44.Hutcheon AE, Guo XQ, Stepp MA, et al. Effect of wound type on Smad 2 and 4 translocation. Invest Ophthalmol Vis Sci. 2005;46:2362–2368. doi: 10.1167/iovs.04-0759. [DOI] [PubMed] [Google Scholar]
- 45.Suarez-Cuervo C, Merrell MA, Watson L, et al. Breast cancer cells with inhibition of p38alpha have decreased MMP-9 activity and exhibit decreased bone metastasis in mice. Clin Exp Metastasis. 2004;21:525–533. doi: 10.1007/s10585-004-3503-x. [DOI] [PubMed] [Google Scholar]
- 46.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- 47.Larjava H, Salo T, Haapasalmi K, et al. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark RA, Lanigan JM, DellaPelle P, et al. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982;79:264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- 49.Olerud JE, Gown AM, Bickenbach J, et al. An assessment of human epidermal repair in elderly normal subjects using immunohistochemical methods. J Invest Dermatol. 1988;90:845–850. doi: 10.1111/1523-1747.ep12462083. [DOI] [PubMed] [Google Scholar]
- 50.El GA, Hensbergen P, Gibbs S, et al. Fibroblasts facilitate reepithelialization in wounded human skin equivalents. Lab Invest. 2004;84:102–112. doi: 10.1038/labinvest.3700014. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto K, Hayashi K, Suzuki T, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22:433–440. doi: 10.1634/stemcells.22-4-433. [DOI] [PubMed] [Google Scholar]
- 52.Fleischmajer R, Utani A, MacDonald ED, et al. Initiation of skin basement membrane formation at the epidermo-dermal interface involves assembly of laminins through binding to cell membrane receptors. J Cell Sci. 1998;111:1929–1940. doi: 10.1242/jcs.111.14.1929. [DOI] [PubMed] [Google Scholar]
- 53.Amano S, Akutsu N, Matsunaga Y, et al. Importance of balance between extracellular matrix synthesis and degradation in basement membrane formation. Exp Cell Res. 2001;271:249–262. doi: 10.1006/excr.2001.5387. [DOI] [PubMed] [Google Scholar]
- 54.Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C-Y, Zhu G, Converse R, et al. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt112. J Biol Chem. 1994;260:24627–24636. [PubMed] [Google Scholar]
- 57.Chen WYW, Mui M-M, Kao WW-Y, et al. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Curr Eye Res. 1994;13:765–778. doi: 10.3109/02713689409047012. [DOI] [PubMed] [Google Scholar]


