Abstract
Sympathetic nerves arising from the superior cervical ganglion (SCG) protect the cerebrovasculature during periods of acute hypertension and may play a role in homeostasis of target organs. The functions of these nerves depend on calcium release triggered by activation of ryanodine receptor (RyR) channels. The function of RyR channels is in part dependent on genetic expression and regulation by numerous proteins modulators such as neuronal nitric oxide synthase (nNOS) neurons also found in the SCG. We have shown that release of calcium in SCG cells is altered during late maturation and advancing age. However, the underlying molecular mechanisms that may in part account for these data are elusive. Therefore we used molecular techniques to test the hypothesis that advancing age alters the pattern of genetic expression and/or protein levels of RyRs and their modulation by nNOS in the SCG in F-344 rats aged 6, 12 and 24 months. Surprisingly, ryr1 expression was undetectable in all age groups and ryr2 and ryr3 are the predominantly transcribed isoforms in the adult rat SCG. mRNA and protein levels for RyR2 isoform did not change with advancing age. However, ryr3 mRNA levels increased from 6 to 12 months and declined from 12 to 24 months. Similarly, RyR3 receptor protein levels also increased from 6 to 12 months and declined from 12 to 24 months. Because nNOS and the phosphorylation of the RyRs have been shown to modulate the function of RyRs, total phosphorylation and nNOS protein levels were analyzed in all age groups. Phosphorylation levels of the RyRs were similar in all age groups. However, nNOS protein levels increased from 6 months to 12 months followed by a decline from 12 months to 24 months. These data suggest that advancing age selectively impacts the genetic expression and protein levels of RyR3 as well as modulatory nNOS protein levels. In addition, these data may part provide some insight into the possible changes in the function of RyRs that may occur with the normal aging process.
INTRODUCTION
Clinical studies show that risk of stroke increases with age, and the single most important factor is rising systolic blood pressure (1, 14, 32, 69). Thus, a more comprehensive understanding of aging and modulation of the cerebrovasculature is necessary. Systolic blood pressure rises in both F344-Rats and in humans suggesting that the cerebrovasculature is subjected to increased pressure and risk of hyperemia and stroke (14, 68). The SCG provides sympathetic innervations to the cerebrovasculature, and dampen increased cerebral blood flow in response to hypertension or increased intracranial pressures (19). Therefore, sympathetic nerves arising from the SCG reduce the risk of blood brain barrier disruption and are becoming an increasingly important area in biomedical research (6, 14, 25, 55).
In addition to the importance of the SCG in the cerebrovasculature, the SCG innervates and impacts the function of many organs including the heart (29, 41, 61, 65, 66), the eye (23, 53, 62, 64), and secretory glands, such as the pineal gland (30, 31) thyroid/parathyroid and salivary glands (7, 9, 10, 26, 49). It has also been suggested that the SCG and other sympathetic neurons function as a peripheral neuroendocrine center (8, 9) serving as the communication bridge between the central nervous system and the endocrine system (9, 52). The SCG has also been shown to play an important role in the immune response (36, 48, 63).
The function of peripheral sympathetic and sensory neurons has been shown to be dependent on release of calcium from intracellular stores in response to stimulation-evoked increases in intracellular calcium ([Ca2+]i) (34, 44, 56–58). This process is known as calcium induced calcium release (CICR) and occurs through the activation of ryanodine receptor channels. The function of RyR channels depends in part on density and their regulation. The regulation of the function of RyRs is complex and overall this regulation serves to modulate the sensitivity of RyR’s to cellular Ca2+ levels. These modulators include FKBP proteins, which serve to activate or inhibit channel state depending on its binding status, and activators such as phosphorylation and intracellular molecules including cADP-ribose (cADPr) (24, 35, 45). In the case of cADPr these levels are modulated by nitric oxide (NO) released from nNOS containing neurons. Indeed our studies and others have shown that the SCG and the cerebrovasculature is innervated by nNOS neurons, which function to modulate stimulation-evoked norepinephrine release (12, 33, 37, 38).
To maintain the CICR process the [Ca2+]i stores must be refilled. Refilling depends on the amplification of [Ca2+]i, which occurs through such channels as voltage gated calcium channels, and store operated calcium channels, and by pumping Ca2+ back into the stores by smooth endoplasmic reticulum calcium ATP-ases (SERCA). We have previously demonstrated that there is an age related decline in SERCA-mediated Ca2+ uptake in the SCG (47). This decline in SERCA function influences caffeine-evoked release of Ca2+ and both rapid depolarization-induced and spontaneous refilling of SER Ca2+ stores after depletion (60). These data suggested that advancing age alters the capacity of SCG cells to release Ca2+ from SER stores and that the levels of the SER Ca2+ may also decline with age, which may possibly alter the function of the SCG.
The three isoforms of the ryanodine receptor family are RyR1 (found in skeletal muscle), RyR2 (found in cardiac muscle), and RyR3 (found in neurons, and other tissue types) have been shown to be responsible for CICR (45). As discussed above, these receptors are important in the function of sympathetic neurons and sympathetic neurons arising from the SCG may serve to protect the CNS from rupture of blood vessels at higher systemic pressures. As maturation and aging are normal and inevitable processes it is necessary to study underlying mechanisms that may account for how animals develop and age. Thus, molecular characterization of the RyRs and/or selective modulators may provide information on calcium release during the aging process. The expression, overall phosphorylation, and modulation of RyR’s by nNOS with late maturation or advancing age has not previously been studied in the rat SCG model. As the function of multiple subtypes of RyR’s are important to the overall function of numerous excitable cells the genetic expression and protein levels of RyR channels in models that have not been previously studied are warranted. Using molecular techniques of RT-PCR and ELISA analysis we tested the hypothesis that the genetic and protein expression of the predominant RyR isoform(s) in adult rat SCG, along with selective modulators, are altered during late maturation and advancing age in F-344 rats aged 6, 12 and 24 months.
MATERIALS AND METHODS
Experimental animals
Male Fischer 344 (F-344) rats, aged 6 mo (young adult), 12 mo (mature adult), and 24 mo (senescent), were obtained from National Institutes of Health-National Institute on Aging breeding colony (Harlan Sprague-Dawley, Indianapolis, IN). The age range designation comes from other studies showing the median life span in F-344 rats is ~24 months. The animals were allowed to eat and drink at will and were maintained on a 12:12-h light-dark cycle under controlled temperature (72–77°F). All procedures used in this study were approved by the Institutional Animal Care and Use Committee at Loma Linda University, and the approved guidelines were adhered to throughout the study.
SCG isolation and sample preparation
Rats were anesthetized with CO2 (45 s) followed by decapitation. Both carotid arteries were dissected from each male F-344 rat. SCG were dissected from the carotid artery bifurcation as previously described (60). Tissue was pulverized in liquid nitrogen into a fine powder using a metal mortar and pestle, then placed in cold lysis buffer (8.77g NaCl, 7.88g Tris HCL, 3.29g EDTA, 5ml 0.5% Tween-20, and broad spectrum protease inhibitors and sonicated until homogenous.
Isolation of SCG total RNA
Total RNA from 6, 12 and 24 month old animals were isolated using the Ambion RiboPure RNA isolation kit (Ambion, Austin, TX) according to the manufacturer’s recommendations. Briefly, the rat SCG was dissected out of the 6, 12 or 24 month old animals and pulverized using liquid nitrogen. The pulverized tissue was then re-suspended in RNAwiz and the RNA was separated from SCG proteins by the addition of chloroform and centrifugation. The RNA was precipitated and treated with DNAse 1 to eliminate genomic DNA. The integrity of the RNA was determined by assessing the 260/280 ratio. RNA samples with ratios of 1.8–2.0 indicate high purity of RNA and samples yielding these values were used for all RT-PCR reactions.
Reverse-transcriptase PCR
The primers used for reverse transcription-PCR (RT-PCR) analysis were specific for the ryr1, ryr2, ryr3 and GAPDH determined by using the NCBI blast database for Rattus norvegicus (Table 1). RT-PCR reactions (50 μl) using RT-PCR reaction mix (Promega, Madison, WI) were performed with samples containing RNA ranging from 10 ng to 1 μg for optimization. To test for DNA contamination we performed negative controls, which consisted of the same RT-PCR reaction mix contained in the biological samples minus reverse transcriptase.
Table 1.
Primers used in this study
| Primer Sequences | |
| RyR1 FWD | 5′CCA GGG GAG GAT GAC ATA GAA GG 3′ |
| RyR1 REV | 5′GAG GGT CAG GTT TGC GCT CAT TG 3′ |
| RyR2 FWD | 5′ACA CCC TCC GCC TCT ACT CT 3′ |
| RyR2 REV | 5′GGG TGA TGC TCT TTG TCT CC 3′ |
| RyR3 FWD | 5′GAA CCG AGA TGT TGC TGT GTG 3′ |
| RyR3 REV | 5′GTC CTG CCC AGC AAA AAT G 3′ |
| GAPDH FWD | 5′ACC ACA GTC CAT GCC ATC ACT 3′ |
| GAPDH REV | 5′AGT TGG GAT AGG GCC TCT CTT 3′ |
| Amplicon Size | |
| RrR1 = 520bp | |
| RyR2 = 245bp | |
| RyR3 = 392bp | |
| GAPDH= 540bp | |
SDS-PAGE and Immunoblot analysis
This study utilized an ELISA assay along with selective antibodies to determine relative RyR2, RyR3 levels and nNOS levels in SCG. Therefore, we first validated the selectivity of our antibodies for these respective proteins using sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) electrophoresis and Western blot analysis. We have shown in our previous studies that the antibodies for nNOS are highly selective and yield single bands (37).
Similarly for RyR2 and RyR3 levels we determined the selectivity of polyclonal rabbit anti-rat antibodies for the respective RyR’s using a modified method [Figure 4, (21)]. Briefly, SDS-PAGE was performed using a 4 to 12% Bis-Tris polyacrylamide gel (NuPAGE Novex gels, Invitrogen, Carlsbad CA) in MOPS (morpholinepropanesulfonic acid)-SDS running buffer according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA.). Samples were prepared [(65% sample containing 25 μg of total protein), (25% 4x NuPAGE LDS sample buffer), (10% NuPAGE reducing agent)], heated at 72°C for 10 min, and then electrophoresed at 200 V for 120 minutes in the XCell SureLock Mini-Cell system using the Hi-Mark high molecular weight standards (Invitrogen, Carlsbad, CA.). The separated proteins were then transferred to PVDF membrane (Millipore, Bedford, MA). The blots were probed with ryanodine-specific antibodies (Chemicon, Temecula CA). Immunoreactive proteins were detected by the procedure described in the Western Lightning chemiluminescence reagent plus kit (Perkin-Elmer Life Sciences. Boston, MA.). Selectivity of the RyR2 and RyR3 are further validated by Chemicon using RyR2 and RyR3 positive and negative tissues and assurance of these controls are provided by Chemicon. With this information from Chemicon and our independent analysis together, suggest that the RyR2 and RyR3 selective antibodies are reliable for quantification of these isoforms using ELISA assay.
Figure 4. Ryanodine receptor expression levels with advancing age.
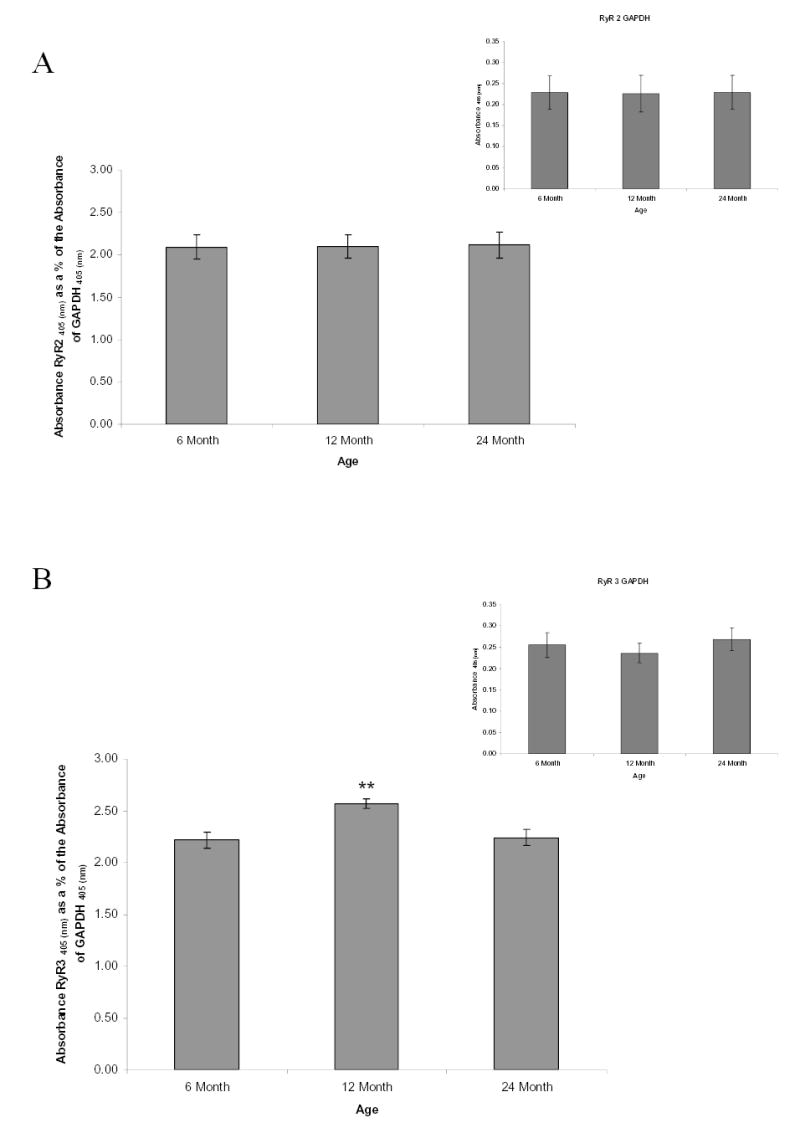
High binding ELISA plates were coated with 500ng of rat SCG proteins from all age groups. The RyR2, RyR3 and GAPDH (normalization controls, inset) proteins were probed with rabbit RyR2, RyR3 or GAPDH-selective antibodies. H2O2 and ABTS serves as substrates for the conjugated HRP secondary antibody to RyR2, RyR3 or GAPDH antibody complex. ABTS is oxidized to yield a green chromophore and absorbance was measured at 405 nm. (A): Results demonstrate that RyR2 protein expression does not significantly change with age. (B): RyR3 protein expression levels change with age. Bars represent the mean ± S.E. and were analyzed by ANOVA and Fischer-PLSD test. ** = significantly different from two other age groups, P < 0.03. n=3 experiments using 6 pooled ganglia per experiment.
ELISA
There are two issues with regards to Western analysis of RyRs. First, RyRs are greater than 500 kDa and due to its high molecular weight may not transfer well. Secondly, incomplete transfer will affect the accuracy of the quantitation of RyR protein levels. Thus, ELISA assays were developed to measure their levels eliminating the need for transfer of proteins from separation gels to blotting membranes (21). We used a modified ELISA assay to measure RyR2 and RyR3 protein levels relative to GAPDH levels. Similarly, we used the ELISA to measure nNOS levels relative to GAPDH. We first performed a saturation curve to determine the optimal amount of protein to use in quantification of RyR’s and nNOS in rat SCG (Figure 4). The serial dilutions of total proteins, ranging from 15.6ng-2μg, were incubated in high binding 96-well plates (Corning, Corning NY) for 16–24 hours at 4°C. The unbounded proteins were washed away using PBS-T (138 mM NaCl, 2.7mM KCl, pH 7.4, 0.1% Tween-20) and blocked using 1% BSA for 1 hour at room temperature. The antigen-coated wells were washed twice with PBS-T and then incubated with the RyR2, RyR3, GAPDH or nNOS antibodies for 16 hours at 4°C. The antigen-coated plates were then washed three times with PBS-T and incubated with the HRP-conjugated antibodies for 1 hour at 37°C. The unbound antibodies were washed away using PBS-T and then incubated with hydrogen peroxide and 2,2′-Azino-di-(3-ethylbenz-thiazoline) Sulfonic Acid (ABTS) (Zymed, Invitrogen, Carslbad, CA) for 15–20 minutes ABTS is oxidized to yield a green chromophore and absorbance was measured at 405 nm using a microplate reader. The optimal concentrations of protein to be used for experiments were assessed and experiments were followed as described above. Negative controls were performed in the absence of primary antibodies. GAPDH was also determined in each age group to serve as a loading control. In addition, all colorimetric intensity values obtained for RyR’s were normalized to GAPDH.
Phosphorylation analysis of the RyRs
The relative total RyRs present in adult rat SCG were analyzed for levels of phosphorylation according to manufacturer’s protocol (Molecular Probes, Invitrogen, Carslbad, CA). Briefly, 25 μg determined by Bradford assay (BioRad) of total SCG protein from 6, 12 and 24 month old animals were subjected to SDS-PAGE for separation. After 1 hour of electrophoresis, the PeppermintStick phosphoprotein molecular weight standard (Molecular Probes, Invitrogen, Carlsbad CA) was then loaded on the gel and electrophoresed for an additional 1 hours. This standard served as a positive phosphoprotein control. The gel was fixed, washed, stained with the Pro-Q Diamond phosphoprotein stain and destained as described by manufacturer’s protocol. The phosphoproteins were imaged using transillumination at 540 nm. The images were documented and the proteins were then stained using SYPRO Ruby protein gel stain. The total proteins were then documented and ratio of phosphoprotein stain signal to total protein stain signal of the total RyRs were assessed. To ensure that the proper protein band was being assessed, immunoblot analysis was performed using mouse anti-RyR (clone 34C,from ascites fluid) antibodies, which detect all three isoforms (Sigma, St. Louis MO). As an additional internal control to insure that the method accurately detected phosphorylated forms of the ryanodine receptors, we prepared gels using two different amounts of total protein (12.5 μg and 25 μg). There was an increase in intensity of phorsporylated ryanodine receptor for the higher total protein, however, the ratio of total ryanodine protein to phosphorylated ryanodine did not change.
RESULTS
Saturation curve for RT-PCR analysis and validation of primer specificity
Reverse-transcriptase PCR is a powerful tool and has commonly been used to semi-quantitate mRNA levels. However, because PCR is an exponential process, if not optimized correctly, PCR type detection can be problematic. To ensure that saturation of the amplified products do not occur, primers for GAPDH, ryr2, and ryr3 were used to establish a curve to determine the optimal concentration of total RNA concentrations to use. Total RNA concentrations ranging from 10ng–120ng were used and amplified for 50 cycles. 60–75ng of total RNA showed to be ideal to use for quantitative purposes (data not shown). The desired molecular weights were determined by electrophoresis of the amplicons (Table 1). To ensure that the amplified products were the desired products and not non-specific priming of the oligonucleotides, the amplified products were sequenced by the UC Davis sequencing core facility and were shown to be ryr2, ryr3 or GAPDH.
Identification of the major ryanodine receptor isoforms in rat and impact of age on their expression
The predominant isoforms of the ryr in rat SCG has not previously been demonstrated. To identify what ryr isoform transcript(s) are predominantly present in rat SCG, RT-PCR performed on SCG DNase treated total RNA isolated from 6 month, 12 month and 24 month animals. Concentrations RNA up to 1 μg, did not yield any amplified fragment consistent with the ryr1 (Fig. 1). Since ryr1 was shown to be present in the brain, we utilized RNA isolated from the cerebral cortex of a 6 month old rat as a positive control to ensure that primer conditions were appropriate and primers were specific. The predicted size fragment was amplified from brain RNA, but not from the SCG, confirming that proper primer conditions were used. ryr2 and ryr3 were transcribed in rat SCG demonstrating that these two isoforms are the predominant ryr transcripts in rat SCG cells (Figs 2A, C). To ensure no contaminating DNA was present, the reaction was also performed in the absence of reverse transcriptase (data not shown). No amplified fragment was observed.
Figure 1.

The ryr1 transcript is not the predominant ryr isoform in adult SCG. 75 ng of DNase treated total RNA isolated from rat SCG ganglia or rat brain was subjected to reverse transcriptase-PCR (RT-PCR) using ryr1-specific oligonucleotides. RT-PCR products were then subjected to gel electrophoresis and stained with ethidium bromide and visualized by UV-excitation. No detectable amplified product consistent with the predicted ryr1 transcript was detected from rat SCG RNA. To ensure primer conditions were optimized and specific, RNA from rat brain was used as a control.
Figure 2. Transcriptional profiling of ryr2 and ryr3 with advancing age.
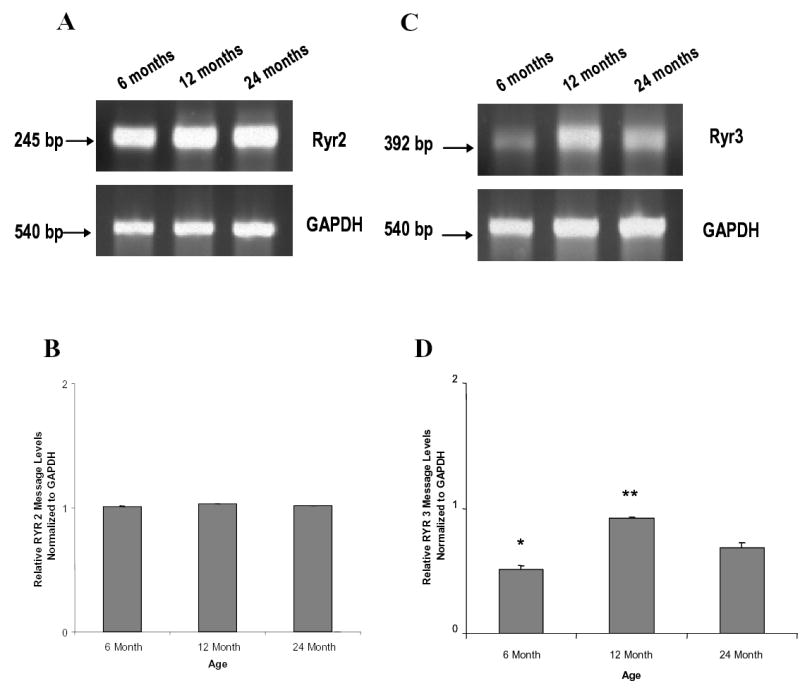
(A, C) 60 ng of DNase treated total RNA isolated from rat SCG was subjected to RT-PCR using ryr2 or ryr3 specific oligonucleotides shown in Table 1. RT-PCR products were amplified then subjected to gel electrophoresis and stained with ethidium bromide and visualized by UV-excitation and optical density was measured. (B, D) Density analysis of the ryr messages were normalized to the GAPDH transcript; (A, C). (A, B): There is no detectable age-related alteration of ryr2 transcript in adult rat SCG. (C, D): The ryr3 message expression changes with age in rat SCG. Data represent the mean ± S.E. n = 3 replicates from 12 pooled ganglia from each age group, performed in two independent experiments. Data were analyzed by ANOVA and Fischer-PLSD test. * = significantly different from one other age group, P <0.02. ** = significantly different from two other age groups, P<0.02.
Semi-quantitative RT-PCR analysis of ryr2 normalized to GAPDH from the 6, 12 and 24 month showed no significant alteration in the ryr2 transcript with advancing age (Fig 2A, B.). In contrast to ryr2, ryr3 normalized to GAPDH message significantly increased in the 12 month in comparison to the 6 month, followed by a significant decline in 24 month-old animals (Fig 2C, D).
Saturation curve analysis for ELISA, and validation of antibody specificity
Since RyRs are very high molecular weight proteins, quantification by standard western blotting procedure can be very difficult due to incomplete transfer. Because of these factors, ELISA assay was used to measure RyR levels as it is commonly used for protein detection and quantification. To determine that the antibodies used in these ELISA are specific for RyR2 and RyR3, SCG proteins were transferred onto a PVDF membrane, blocked and then probed using RyR2 and RyR3 specific antibodies. Immunoreactive bands larger than 500 kDa, consistent with the size of the ryanodine receptors were detected (Fig. 3A). These data demonstrate that the antibodies are selective for the ryanodine receptors 2 and 3, which were detected in the SCG samples. To ensure saturation does not occur with the ELISA procedure, a serial dilution of the SCG sample ranging from 15.6 ng-2ug of total protein was used, in addition various antibody dilutions ranging from 1:500–1:2000 were also used. These data demonstrate 500 ng of total SCG protein/well and a 1:500 dilution of both RyR2 and RyR3 were optimal (Fig. 3B).
Figure 3.
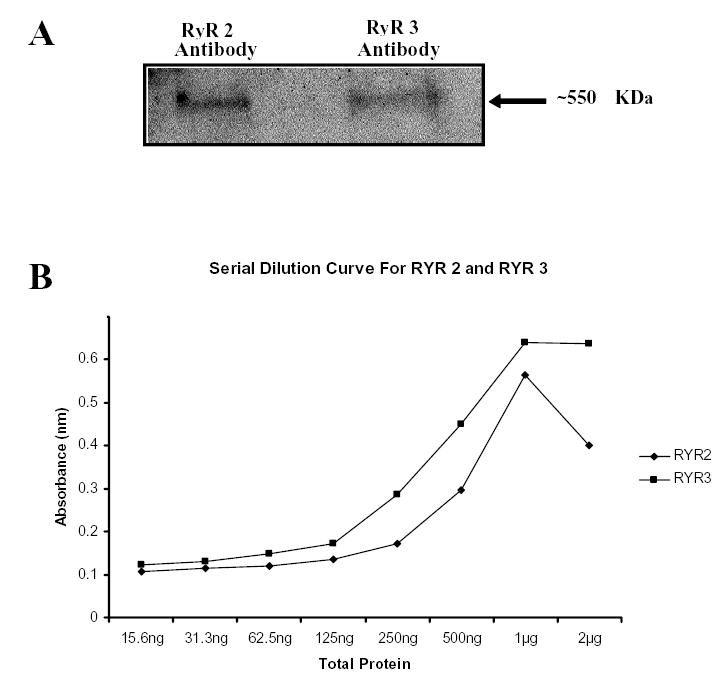
Validation of RyR2 and RyR3 antibody specificity, and saturation curve analysis for RyR2 and RyR3 ELISA. (A) 25 μg of total rat SCG protein were subjected to SDS-PAGE, transferred to PVDF membrane and probed with RyR2 or RyR3 antibodies. The ryanodine receptor antibodies immunoreacted with high molecular weight bands approximately 550 kDa, demonstrating the selectivity of the antibodies for RyR2 and RyR3. (B) SCG were isolated from 12 month old rats. ELISA plates were coated with serially diluted SCG protein, concentrations ranging from 23.5 ng-2μg total protein. Saturation curves determined that 500 ng of total SCG protein/well and a 1:500 dilution of both RyR2 and RyR3 were optimal.
Protein expression analysis of RyR2, RyR3 and nNOS by ELISA assay and phosphorylation of total RyR’s with advancing age
ELISA quantification showed that RyR2 levels normalized to GAPDH showed no significant differences with advancing age (Fig. 4A). In these studies GAPDH served as a normalization control since there is no change in its levels over the age range 6, 12 and 24 months (Fig. 4A inset). In contrast to RyR2, levels of RyR3 normalized to GAPDH, there was a significant increase in RyR3 levels in the 12 month old SCG in comparison to the SCG of the 6 month old animals followed by a significant decrease in the RyR3 levels at 24 months (Fig. 4B with inset GAPDH). In addition to changing RyR expression levels, phosphorylation level of the total RyR was assessed. Ratio of phosphostaining to total protein staining showed no significant alterations in the levels of RyR phosphorylation with advancing age (Fig. 5). As an added control to insure detection of phosphorylated RyR’s we used a phosphoprotein standard kit containing ovalbumin phosphoprotein, which was positively phosphostained. In contrast the non-phosphophorylated protein controls bovine serum albumin and beta-glactosidase did not stain with the phosphostain (data not shown).
Figure 5.
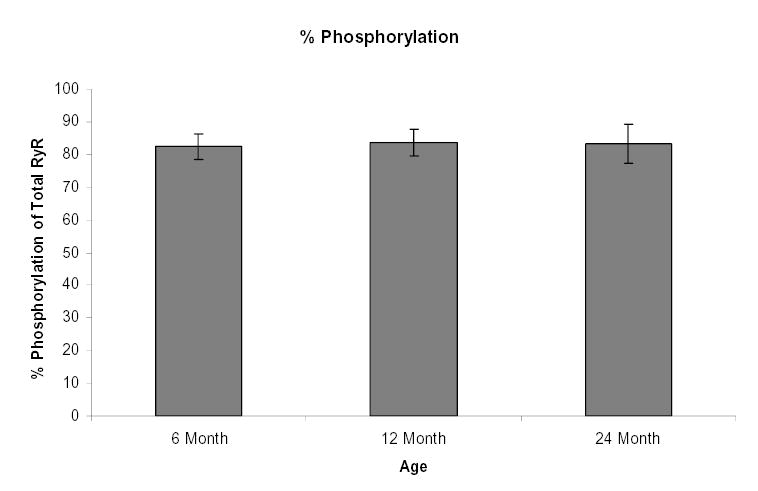
Phosphorylation of total ryanodine receptors do not change with age. 25 μg of total SCG protein from 6, 12 and 24 month old animals were separated and the RyRs were analyzed for phosphate groups stained with the Pro-Q Diamond phosphoprotein stain or stained using SYPRO Ruby protein for total protein. The phosphoproteins and total proteins were imaged using transillumination at 540 nm. The ratio of phosphoprotein stain signal to total protein stain signal of the total RyRs were assessed and plotted. Bars represent the mean ± S.E. and were analyzed by ANOVA and Fischer PLSD test. n= 3 experiments with 6 pooled ganglia per experiment.
We assessed the protein levels of nNOS normalized to GAPDH (Fig 6 inset) in the SCG over the age range from 6–24 month-old animals. There was a significant increase in the proteins levels of nNOS 6 month to 12 month, followed by a significant decline of nNOS protein levels in 24 month old animals (Fig. 6).
Figure 6.
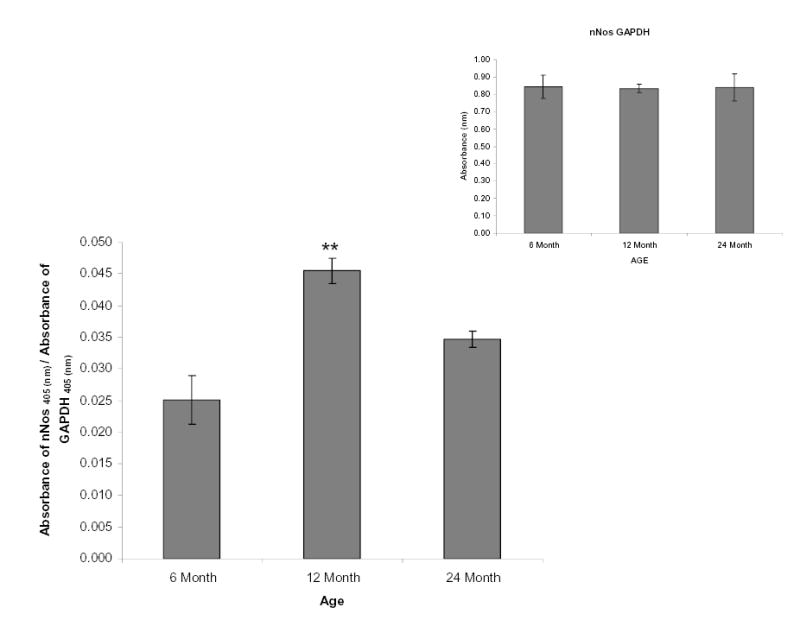
nNOS levels increase from 6 – 12 months and then decline from 12 – 24 months. High binding ELISA plates were coated with 500ng of rat SCG proteins from all age groups. The nNOS protein was probed with monoclonal nNOS-selective antibodies, and GAPDH (normalization control, inset) was probed with Rabbit GAPDH-selective antibodies. Relative levels of nNOS were normalized to GAPDH, and determined as stated for RyR2 and RyR3. Bars represent the mean ± S.E. and were analyzed by ANOVA and Fischer-PLSD test. ** = significantly different from two other age groups, P < 0.03. n= 3 experiments with 6 pooled ganglia per experiment.
Discussion
We have previously demonstrated a decline in caffeine-evoked release of Ca2+ and a decline in the ability of the superior cervical ganglia cells to sustain release of intracellular Ca2+ with age (60). In that study caffeine-evoked calcium transients increased from 6–12 months and then declined at 24 months. Thus, we chose these age groups for the current study as we anticipated the greatest changes in the genetic expression of ryr and RyR protein levels and modulatory nNOS levels to occur at these ages. In the present study we attempted to illuminate some of the possible molecular mechanisms underlying our previous functional studies. We have molecularly identified and characterized the ryanodine receptors with advancing age in this study. Surprisingly, we have found that the ryr1 message is not predominantly expressed in the rat SCG contrasting other studies demonstrating ryr1 is present in excitable cells including neurons (Reviews (17, 51). Thus, a novel conclusion from these studies is that independent of late maturation and advancing age RyR1 does not appear to play role in mediating the release of calcium in the SCG. However, RyR2 and RyR3 are the major receptor isoforms that regulate calcium release from RyR sensitive stores in the SCG in all age groups. In addition, late maturation and advancing age from adult to senescent animals only alters the ryr3 mRNA and RyR3 protein levels. Overall phosphorylation of RyR2 and RyR3 did not change in any of the age groups, suggesting that aging does not necessarily alter modulation of RyR’s via phosphorylation. However, nNOS has been shown to play a role in modulation of RyR sensitivity to changes in [Ca2+]i levels through modulation of cADPr levels (13, 27). Our data demonstrates the presence of nNOS in the SCG and late maturation and senescent aging significantly increase then decrease nNOS levels.
The nature of the biphasic alteration in RyR3 and nNOS levels is intriguing and suggest that there are important maturational changes in the function of RyR’s followed by a decline during senescent aging. In light of the current study and our published functional data (60) there appears to be an important increase in the function of RyR’s during late maturation which is then followed by a decline in function in the senescent animals. Overall the combination of age-related changes in RyR3 levels, altered modulation by nNOS and our previous data suggesting the SER calcium levels may decline with age (47, 60) may combine to significantly alter the function of RyR3 and possibly RyR2 in the SCG and alter the function of the calcium release mechanism.
Aging and genetic expression ryr1, 2 and 3
It has been demonstrated that the ryr1 is predominantly expressed in skeletal muscle, ryr2 is predominantly expressed in cardiac muscle and ryr3 is expressed in many tissue types, but predominantly in brain and neurons [Reviewed in (17, 18, 35, 39, 45, 50, 51)]. Numerous studies have shown differential genetic expression of ryr 1, 2 and 3 isoforms in various neuronal models (15, 28, 42, 43, 59). However, to our knowledge this is the first report of on the genetic expression of ryr1, 2 and 3 in the SCG model. In this study, transcriptional analysis of adult rat SCG demonstrated the transcription of both ryr2 and ryr3, however, to our surprise no transcription of ryr1 was detected in the SCG using total RNA concentrations up to 1 microgram, yet it was detectable in substantial quantity in our positive control, the cerebral cortex. This data does not necessarily suggest that RyR1 is not expressed in adult rat SCG, but suggests that it is not the major isoform of the adult rat SCG and may be detected using higher concentrations of RNA. Furthermore, these observations may vary among tissues of different developmental age. It has been shown that during the embryonic stage of the mouse brain, ryr1 expression was the highest in the rostral cortical plate, ryr3 expression was the highest in the caudal cortical plate, and low levels of ryr2 were expressed in the diencephalon and brain stem. However, after 7 days postnatal, ryr2 appeared to be the major isoform in many regions of the brain (42). Indeed this observation has also been shown during embryonic development in the mouse cerebral cortex where the expression of ryr2 progressively increases and becomes the predominant isoform, where the other isoforms remained lower (15). This may also possibly occur in the embryonic and postnatal development of the rat SCG and is a subject of ongoing investigation in our laboratory.
Transcriptional analysis of the ryanodine receptors in the SCG demonstrates that ryr2 and ryr3 are the major ryr transcripts present in SCG and their gene products may be responsible for calcium release from the SER stores. The ryr2 gene expression did not change with age suggesting that ryr2 levels remain stable during late maturation and senescent aging. However, mRNA coding for the ryr3 isoform increased from 6 to 12 months and then significantly declined from 12 to 24 months of age. In light of the studies mentioned above, demonstrating that there is differential expression of ryr isoforms in development. It is possible that the differential expression of ryr3 in the SCG observed in this study may play a role in a late developmental transition from 6 – 12 month and may possibly represent an increase in function of RyR3. To our knowledge this study is the first to show that there appears to be a late maturational increase in the ryr3 isoform with a subsequent decline with senescence. These results do not necessarily differentiate between changes in genetic expression or altered stability of the mRNA in the age groups. Thus, both possibilities may explain the results. Overall, the data suggest that the contribution of ryr3 to the RyR3 protein levels and ultimately calcium signaling in the SCG may change with age while the contribution provided by RyR2 levels remains stable.
Aging and protein levels of RyR2, RyR3 and modulatory nNOS
Changes in genetic expression alone do not necessarily hold a complete explanation for altered RyR protein levels or regulation of the RyR’s. Thus, we measured RyR2 and RyR3 protein levels using an ELISA assay and normalized to the stable marker GAPDH. Following a similar pattern mRNA RyR2 protein levels were not changed in any age groups. The lack of a change in RyR2 protein levels in any age group suggests that this isoform may remain stable and functional throughout the adult life span. On the other hand the turnover rate of the RyR2 in neuronal cells was not evaluated, which may possibly affect protein function. Indeed protein turnover plays a role in recycling of amino acids and ensures the destruction of proteins that have been damaged by cellular processes or oxidation (54). In addition, numerous proteins turnover rates decline with age (16). For example in muscular tissue, the turnover rate of the RyR was decreased by 25% in the aged rats in comparison to the younger rats (16). RyR turnover has not been evaluated in SCG and may change with age. Any alterations in turnover rate may have an impact on the RyR2 protein function such as increased oxidation and protein damage in the SCG. In contrast to RyR2, RyR3 protein levels increased from 6 to 12 months and then declined at 24 months. However, the decline at 24 months was not significantly smaller than at 6 months. Thus, a straight-forward conclusion as to functional consequences of this age-related decline is difficult to make. We have shown that caffeine-evoked release of calcium declines with age (60). As caffeine-evoked calcium release offers a measurement of the functional capacity of the RyR sensitive stores, it is difficult to interpret a mechanism based on the alterations in RyR3 protein levels alone. However, the function of the RyR’s also depends on SER calcium filling levels as well as the modulation of the RyR’s. Indeed, we have shown that SERCA function declines in the SCG (47) and that basal levels of [Ca2+]i also decline with age (60). Thus, filling levels of the SER may also be compromised which may alter the functional capacity of the release mechanism. In addition, protein levels do not necessarily fully correlate with protein function as some may undergo post-translation modification and may require accessory proteins and modulators to regulate their function. It has been reported that RyR function can be influenced by several factors, including phosphorylation, binding proteins, calcium levels and nNOS which modulates cADPr levels and in turn modulates RyR’s [Reviewed in (3, 11, 13, 20, 40)].
To evaluate if there are changes in selected modulators of the channels, we measured the levels of total phosphorylation. This type of assay provides a general probe as to the relative phosphorylation levels of the RyR’s and was used to determine if further investigation of phosphorylated RyR’s was necessary. Total phosphorylation of RyR channels was not altered with age. These data suggest that changes in steady state phosphorylation and hence regulation by this mechanism is not necessarily changing with age. NO synthesized by nNOS has been shown to be implicated in modulating calcium release in neurons (13). It is involved in the production of nitric oxide and can be directly regulated by calmodulin, a calcium binding protein. It is part of the signal transduction pathway responsible for cADPr synthesis, which directly affects the activity of the ryanodine receptors and modulates release of calcium from SER stores (Fig. 8, 13). In addition, we have observed that the addition of nitric oxide donors increased caffeine-induced calcium release in SCG cells (unpublished data). Given the importance of nNOS in the function of RyR’s we measured nNOS protein levels in the SCG. Our data demonstrate that nNOS is indeed present in the SCG which is consistent with other studies demonstrating that the SCG contains nNOS neurons in addition to adrenergic neurons (12, 67). Our data demonstrated that with advancing age, nNOS protein expression increases from 6 to 12 months and significantly declines from 12 to 24 months. In addition, as nNOS activity modulates cADPr levels, which in turn modulate RyR activity it may reasonable to speculate that these data may possibly suggest that cADPr levels may also decline with age. As there appear to only be two RyR’s contributing to calcium release in the SCG, overall, the age-related decline in RyR3, coupled with a decline in nNOS levels may in part provide an explanation for the decrease in RyR mediated calcium release with age noted in our previous study (60). We hypothesize that an age-related reduction in RyR3 receptor levels and cADPr levels may account in part for a decline in the function of RyR’s. We are currently determining if advancing age alters cADPR levels in the SCG, which may shed light on activity of nNOS during the aging process and regulation of RyR’s.
As previously noted the regulation of the RyR’s is very complex and there is not necessarily a consensus on the mechanisms governing the regulation of RyR’s. In addition to the regulatory mechanisms discussed above, we offer some speculative discussion on other processes not evaluated in this study that may also affect the function of the ryanodine receptors, for example, modulation by thiol oxidation (2, 22, 46). It has been reported that oxidizing conditions can affect the calcium sensitivity of the RyR2 and the affinity of the RyR2 for calmodulin (22). The oxidation states and the ability of the RyRs to be regulated by oxidation in the SCG cells has not been evaluated and may be altered in aged SCG compared to younger SCG. In addition, we cannot rule out the possibility that accumulating mutations may arise with natural aging. These mutations may compromise the function of these receptors. It has been shown that the mitochondria play an important role in calcium regulation from buffering of calcium to production of ATP for promoting proper cellular processes to maintain homeostasis (4, 5). It has been reported that defects and mutations of the mitochondrial DNA and genes accumulates with the natural aging process and these mutations may influence calcium regulation in the ER [Reviewed in (4)]. In addition, there is an increase in reactive oxygen species produced by the mitochondria with advancing age [ Reviewed in (4)], which may result in increased DNA or protein damage in the SER. This increase in oxidative stress in the aging brain and neurons may be due to the reported decrease in antioxidant enzyme activities [Reviewed in (4)].
In this study, we have identified the major ryanodine isoforms of the adult rat SCG and molecularly characterized their expression levels and at least one protein modulator, nNOS with advancing age. ryr1 does not appear to be expressed in the adult SCG suggesting that RyR1 does not contribute to calcium release in the adult SCG. However, RyR2 and RyR3 appear to be the major contributors to calcium release function in the SCG. Advancing age only altered RyR3 levels but to a small extent. Thus, based on results of this study and our previous work, the combination of a decline in RyR3, reduced nNOS levels, reduced function of calcium pumps that fill SER calcium stores, may possibly act in concert to alter modulation of RyR’s in the SCG and in part may play a role in the age-related decline in intracellular calcium release in adult rat SCG. Overall these data may have implications for the protective function that the SCG provides to the cerebrovasculature in the face of age-related elevations in blood pressure.
Acknowledgments
This work was supported in part by grants from the American Heart Association, National Center (#0040021N), NIH P01 31226, RO1 on development (R01 HL69078-01) and NIH award 2R25 GM060507.
References
- 1.Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Popper JS, Ross GW, Petrovitch H. Age-related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol. 2003;56:479–486. doi: 10.1016/s0895-4356(02)00611-x. [DOI] [PubMed] [Google Scholar]
- 2.Anzai K, Ogawa K, Ozawa T, Yamamoto H. Oxidative modification of ion channel activity of ryanodine receptor. Antioxid Redox Signal. 2000;2:35–40. doi: 10.1089/ars.2000.2.1-35. [DOI] [PubMed] [Google Scholar]
- 3.Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J Membr Biol. 2002;185:1–8. doi: 10.1007/s00232-001-0111-4. [DOI] [PubMed] [Google Scholar]
- 4.Brewer GJ. Neuronal plasticity and stressor toxicity during aging. Exp Gerontol. 2000;35:1165–1183. doi: 10.1016/s0531-5565(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz J, Tsai H, Foucart S, Duckles SP. Advancing age alters intracellular calcium buffering in rat adrenergic nerves. Neurobiol Aging. 1996;17:885–892. doi: 10.1016/s0197-4580(96)00179-0. [DOI] [PubMed] [Google Scholar]
- 6.Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161–211. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- 7.Cardinali DP, Stern JE. Peripheral neuroendocrinology of the cervical autonomic nervous system. Braz J Med Biol Res. 1994;27:573–599. [PubMed] [Google Scholar]
- 8.Cardinali DP, Vacas MI, Gejman PV. The sympathetic superior cervical ganglia as peripheral neuroendocrine centers. J Neural Transm. 1981;52:1–21. doi: 10.1007/BF01253092. [DOI] [PubMed] [Google Scholar]
- 9.Cardinali DP, Vacas MI, Gejman PV, Pisarev MA, Barontini M, Boado RJ, Juvenal GJ. The sympathetic superior cervical ganglia as "little neuroendocrine brains". Acta Physiol Lat Am. 1983;33:205–221. [PubMed] [Google Scholar]
- 10.Chiba T, Tanaka K. A target specific pathway from nitric oxide synthase immunoreactive preganglionic sympathetic to superior cervical ganglion neurons innervating the submandibular salivary gland. J Auton Nerv Syst. 1998;71:139–147. doi: 10.1016/s0165-1838(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 11.Danila CI, Hamilton SL. Phosphorylation of ryanodine receptors. Biol Res. 2004;37:521–525. doi: 10.4067/s0716-97602004000400005. [DOI] [PubMed] [Google Scholar]
- 12.Dun NJ, Dun SL, Chiba T, Forstermann U. Nitric oxide synthase-immunoreactive vagal afferent fibers in rat superior cervical ganglia. Neuroscience. 1995;65:231–239. doi: 10.1016/0306-4522(94)00455-e. [DOI] [PubMed] [Google Scholar]
- 13.Eu JP, Xu L, Stamler JS, Meissner G. Regulation of ryanodine receptors by reactive nitrogen species. Biochem Pharmacol. 1999;57:1079–1084. doi: 10.1016/s0006-2952(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 14.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Faure AV, Grunwald D, Moutin MJ, Hilly M, Mauger JP, Marty I, De WM, Villaz M, Albrieux M. Developmental expression of the calcium release channels during early neurogenesis of the mouse cerebral cortex. Eur J Neurosci. 2001;14:1613–1622. doi: 10.1046/j.0953-816x.2001.01786.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrington DA, Krainev AG, Bigelow DJ. Altered turnover of calcium regulatory proteins of the sarcoplasmic reticulum in aged skeletal muscle. J Biol Chem. 1998;273:5885–5891. doi: 10.1074/jbc.273.10.5885. [DOI] [PubMed] [Google Scholar]
- 17.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 18.Fill M, Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988;11:453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- 19.Furuichi S, Endo S, Haji A, Takeda R, Nisijima M, Takaku A. Related changes in sympathetic activity, cerebral blood flow and intracranial pressure, and effect of an alpha-blocker in experimental subarachnoid haemorrhage. Acta Neurochir (Wien ) 1999;141:415–423. doi: 10.1007/s007010050318. [DOI] [PubMed] [Google Scholar]
- 20.Galione A. Cyclic ADP-ribose: a new way to control calcium. Science. 1993;259:325–326. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- 21.Grunwald R, Meissner G. Lumenal sites and C terminus accessibility of the skeletal muscle calcium release channel (ryanodine receptor) J Biol Chem. 1995;270:11338–11347. doi: 10.1074/jbc.270.19.11338. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton SL, Reid MB. RyR1 modulation by oxidation and calmodulin. Antioxid Redox Signal. 2000;2:41–45. doi: 10.1089/ars.2000.2.1-41. [DOI] [PubMed] [Google Scholar]
- 23.Hill CE, Vidovic M. The role of competition in the refinement of the projections of sympathetic neurons to the rat eye during development. Int J Dev Neurosci. 1989;7:539–551. doi: 10.1016/0736-5748(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 24.Hua SY, Tokimasa T, Takasawa S, Furuya Y, Nohmi M, Okamoto H, Kuba K. Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron. 1994;12:1073–1079. doi: 10.1016/0896-6273(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 25.Insel PA. Adrenergic receptors, G proteins, and cell regulation: implications for aging research. Exp Gerontol. 1993;28:341–348. doi: 10.1016/0531-5565(93)90061-h. [DOI] [PubMed] [Google Scholar]
- 26.Jallageas M, Mas N, Saboureau M, Roussel JP, Lacroix A. Effects of bilateral superior cervical ganglionectomy on thyroid and gonadal functions in the edible dormouse Glis glis. Comp Biochem Physiol Comp Physiol. 1993;104:299–304. doi: 10.1016/0300-9629(93)90321-t. [DOI] [PubMed] [Google Scholar]
- 27.Khan SA, Hare JM. The role of nitric oxide in the physiological regulation of Ca2+ cycling. Curr Opin Drug Discov Devel. 2003;6:658–666. [PubMed] [Google Scholar]
- 28.Kihira T, Utunomiya H, Kondo T. Expression of FKBP12 and ryanodine receptors (RyRs) in the spinal cord of MND patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:94–99. doi: 10.1080/14660820510034442. [DOI] [PubMed] [Google Scholar]
- 29.Korsching S, Thoenen H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev Biol. 1988;126:40–46. doi: 10.1016/0012-1606(88)90236-9. [DOI] [PubMed] [Google Scholar]
- 30.Larsen PJ. Tracing autonomic innervation of the rat pineal gland using viral transneuronal tracing. Microsc Res Tech. 1999;46:296–304. doi: 10.1002/(SICI)1097-0029(19990815/01)46:4/5<296::AID-JEMT6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Larsen PJ, Enquist LW, Card JP. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur J Neurosci. 1998;10:128–145. doi: 10.1046/j.1460-9568.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- 32.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:776–785. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- 33.Lee TJ, Zhang W, Sarwinski S. Presynaptic beta(2)-adrenoceptors mediate nicotine-induced NOergic neurogenic dilation in porcine basilar arteries. Am J Physiol Heart Circ Physiol. 2000;279:H808–H816. doi: 10.1152/ajpheart.2000.279.2.H808. [DOI] [PubMed] [Google Scholar]
- 34.Marrion NV, Adams PR. Release of intracellular calcium and modulation of membrane currents by caffeine in bull-frog sympathetic neurones. J Physiol. 1992;445:515–535. doi: 10.1113/jphysiol.1992.sp018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marx SO, Marks AR. Regulation of the ryanodine receptor in heart failure. Basic Res Cardiol. 2002;97 (Suppl 1):I49–I51. doi: 10.1007/s003950200029. [DOI] [PubMed] [Google Scholar]
- 36.Mathison R, Carter L, Mowat C, Bissonnette E, Davison JS, Befus AD. Temporal analysis of the anti-inflammatory effects of decentralization of the rat superior cervical ganglia. Am J Physiol. 1994;266:R1537–R1543. doi: 10.1152/ajpregu.1994.266.5.R1537. [DOI] [PubMed] [Google Scholar]
- 37.Mbaku EM, Zhang L, Pearce WJ, Duckles SP, Buchholz J. Chronic hypoxia alters the function of NOS nerves in cerebral arteries of near-term fetal and adult sheep. J Appl Physiol. 2003;94:724–732. doi: 10.1152/japplphysiol.00771.2002. [DOI] [PubMed] [Google Scholar]
- 38.Mbaku EN, Zhang L, Duckles SP, Buchholz J. Nitric-oxide synthase-containing nerves facilitate adrenergic transmitter release in sheep middle cerebral arteries. J Pharmacol Exp Ther. 2000;293:397–402. [PubMed] [Google Scholar]
- 39.McPherson PS, Campbell KP. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993;268:13765–13768. [PubMed] [Google Scholar]
- 40.Meissner G. Regulation of mammalian ryanodine receptors. Front Biosci. 2002;7:d2072–d2080. doi: 10.2741/A899. [DOI] [PubMed] [Google Scholar]
- 41.Miura M, Okada J. Cardiac and non-cardiac preganglionic neurones of the thoracic vagus nerve: an HRP study in the cat. Jpn J Physiol. 1981;31:53–66. doi: 10.2170/jjphysiol.31.53. [DOI] [PubMed] [Google Scholar]
- 42.Mori F, Fukaya M, Abe H, Wakabayashi K, Watanabe M. Developmental changes in expression of the three ryanodine receptor mRNAs in the mouse brain. Neurosci Lett. 2000;285:57–60. doi: 10.1016/s0304-3940(00)01046-6. [DOI] [PubMed] [Google Scholar]
- 43.Mouton J, Marty I, Villaz M, Feltz A, Maulet Y. Molecular interaction of dihydropyridine receptors with type-1 ryanodine receptors in rat brain. Biochem J. 2001;354:597–603. doi: 10.1042/0264-6021:3540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa Y, Kurebayashi N, Murayama T. Putative roles of type 3 ryanodine receptor isoforms (RyR3) Trends Cardiovasc Med. 2000;10:65–70. doi: 10.1016/s1050-1738(00)00050-5. [DOI] [PubMed] [Google Scholar]
- 46.Pessah IN, Feng W. Functional role of hyperreactive sulfhydryl moieties within the ryanodine receptor complex. Antioxid Redox Signal. 2000;2:17–25. doi: 10.1089/ars.2000.2.1-17. [DOI] [PubMed] [Google Scholar]
- 47.Pottorf WJ, Duckles SP, Buchholz JN. SERCA function declines with age in adrenergic nerves from the superior cervical ganglion. J Auton Pharmacol. 2000;20:281–290. doi: 10.1046/j.1365-2680.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramaswamy K, Mathison R, Carter L, Kirk D, Green F, Davison JS, Befus D. Marked antiinflammatory effects of decentralization of the superior cervical ganglia. J Exp Med. 1990;172:1819–1830. doi: 10.1084/jem.172.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romeo HE, Gonzalez SC, Vacas MI, Rosenstein RE, Barontini M, Cardinali DP. Origins of the sympathetic projections to rat thyroid and parathyroid glands. J Auton Nerv Syst. 1986;17:63–70. doi: 10.1016/0165-1838(86)90044-5. [DOI] [PubMed] [Google Scholar]
- 50.Rossi D, Sorrentino V. Molecular genetics of ryanodine receptors Ca2+-release channels. Cell Calcium. 2002;32:307–319. doi: 10.1016/s0143416002001987. [DOI] [PubMed] [Google Scholar]
- 51.Sorrentino V. The ryanodine receptor family of intracellular calcium release channels. Adv Pharmacol. 1995;33:67–90. doi: 10.1016/s1054-3589(08)60666-3. [DOI] [PubMed] [Google Scholar]
- 52.Stern JE, Cardinali DP. Influence of the autonomic nervous system on calcium homeostasis in the rat. Biol Signals. 1994;3:15–25. doi: 10.1159/000109522. [DOI] [PubMed] [Google Scholar]
- 53.Stevens LM, Landis SC. Target influences on transmitter choice by sympathetic neurons developing in the anterior chamber of the eye. Dev Biol. 1990;137:109–124. doi: 10.1016/0012-1606(90)90012-8. [DOI] [PubMed] [Google Scholar]
- 54.Tavernarakis N, Driscoll M. Caloric restriction and lifespan: a role for protein turnover? Mech Ageing Dev. 2002;123:215–229. doi: 10.1016/s0047-6374(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 55.Toda N. Age-related changes in responses to nerve stimulation and catecholamines in isolated monkey cerebral arteries. Am J Physiol. 1991;260:H1443–H1448. doi: 10.1152/ajpheart.1991.260.5.H1443. [DOI] [PubMed] [Google Scholar]
- 56.Usachev YM, Thayer SA. All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J Neurosci. 1997;17:7404–7414. doi: 10.1523/JNEUROSCI.17-19-07404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Usachev YM, Thayer SA. Ca2+ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive Ca2+ stores. J Physiol. 1999;519(Pt 1):115–130. doi: 10.1111/j.1469-7793.1999.0115o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usachev YM, Thayer SA. Controlling the urge for a Ca(2+) surge: all-or-none Ca(2+) release in neurons. Bioessays. 1999;21:743–750. doi: 10.1002/(SICI)1521-1878(199909)21:9<743::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 59.van den BL, Verhoeven K, De SH, Wuytack F, Missiaen L, Robberecht W. Calcium handling proteins in isolated spinal motoneurons. Life Sci. 1999;65:1597–1606. doi: 10.1016/s0024-3205(99)00405-1. [DOI] [PubMed] [Google Scholar]
- 60.Vanterpool CK, Pearce WJ, Buchholz JN. Advancing age alters rapid and spontaneous refilling of caffeine-sensitive calcium stores in sympathetic superior cervical ganglion cells. J Appl Physiol. 2005;99:963–971. doi: 10.1152/japplphysiol.00343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verberne ME, Gittenberger-De Groot AC, Van IL, Poelmann RE. Contribution of the cervical sympathetic ganglia to the innervation of the pharyngeal arch arteries and the heart in the chick embryo. Anat Rec. 1999;255:407–419. doi: 10.1002/(SICI)1097-0185(19990801)255:4<407::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 62.Vidovic M, Hill CE. Withdrawal of collaterals of sympathetic axons to the rat eye during postnatal development: the role of function. J Auton Nerv Syst. 1988;22:57–65. doi: 10.1016/0165-1838(88)90154-3. [DOI] [PubMed] [Google Scholar]
- 63.Waddell SC, Davison JS, Befus AD, Mathison RD. Role for the cervical sympathetic trunk in regulating anaphylactic and endotoxic shock. J Manipulative Physiol Ther. 1992;15:10–15. [PubMed] [Google Scholar]
- 64.Wiley LA, Rupp GR, Steinle JJ. Sympathetic innervation regulates basement membrane thickening and pericyte number in rat retina. Invest Ophthalmol Vis Sci. 2005;46:744–748. doi: 10.1167/iovs.04-1023. [DOI] [PubMed] [Google Scholar]
- 65.Wingerd KL, Goodman NL, Leu ST, Clegg DO. Expression and function of integrin alpha4beta1 and vascular cell adhesion molecule-1 (VCAM-1) during sympathetic innervation of the heart. Dev Dyn. 2004;231:359–369. doi: 10.1002/dvdy.20120. [DOI] [PubMed] [Google Scholar]
- 66.Wingerd KL, Goodman NL, Tresser JW, Smail MM, Leu ST, Rohan SJ, Pring JL, Jackson DY, Clegg DO. Alpha 4 integrins and vascular cell adhesion molecule-1 play a role in sympathetic innervation of the heart. J Neurosci. 2002;22:10772–10780. doi: 10.1523/JNEUROSCI.22-24-10772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu SY, Dun NJ. Potentiation of IPSCs by nitric oxide in immature rat sympathetic preganglionic neurones in vitro. J Physiol. 1996;495 ( Pt 2):479–490. doi: 10.1113/jphysiol.1996.sp021608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, Fugleholm K, Day LB, Ye S, Weller RO, Day IN. Molecular pathogenesis of subarachnoid haemorrhage. Int J Biochem Cell Biol. 2003;35:1341–1360. doi: 10.1016/s1357-2725(03)00043-8. [DOI] [PubMed] [Google Scholar]


