Abstract
In this article we show that hypomorphic loss-of-function alleles of the JIL-1 histone H3S10 kinase are strong suppressors of position effect variegation (PEV) of the wm4 allele and that lack of JIL-1 activity can counteract the effect of the dominant enhancer E(var)2-1 on PEV.
HIGHER-ORDER chromatin structure is important for epigenetic regulation and control of gene activation and silencing. In Drosophila euchromatic genes can be transcriptionally silenced as a result of their placement in or near heterochromatin, a phenomenon known as position effect variegation (PEV) (reviewed by Wallrath 1998; Henikoff 2000; Schotta et al. 2003). Repression typically occurs in only a subset of cells and can be heritable, leading to mosaic patterns of gene expression (Schotta et al. 2003; Delattre et al. 2004). PEV in Drosophila has served as a major paradigm for the identification and genetic analysis of evolutionarily conserved determinants of epigenetic regulation of chromatin structure through the isolation of mutations that act as suppressors [Su(var)] or enhancers [E(var)] of variegation (Schotta et al. 2003). Some of the strongest suppressors of PEV described, Su(var)3-1 mutations, were recently identified to be alleles of the JIL-1 locus that generate proteins with COOH-terminal deletions (Ebert et al. 2004). JIL-1 is a tandem kinase that localizes specifically to euchromatic interband regions of polytene chromosomes (Jin et al. 1999). Analysis of JIL-1 null and hypomorphic alleles has shown that JIL-1 is essential for viability and that reduced levels of JIL-1 protein lead to a global disruption of chromosome structure (Jin et al. 2000; Wang et al. 2001; Zhang et al. 2003; Deng et al. 2005). These defects are correlated with severely decreased levels of histone H3S10 phosphorylation demonstrating that JIL-1 is the predominant kinase regulating the phosphorylation state of this residue at interphase (Wang et al. 2001). Ebert et al. (2004) provided evidence that the Su(var)3-1 alleles of JIL-1 consist of dominant gain-of-function mutations that may antagonize the expansion of heterochromatin formation; however, these experiments did not directly address JIL-1's normal function.
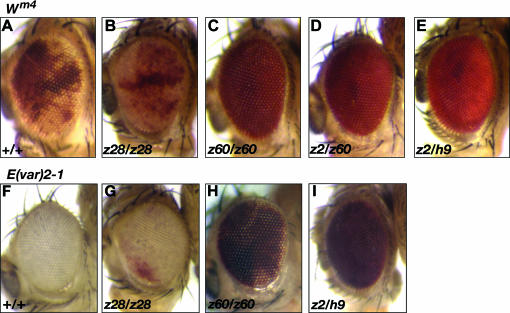
Thus, to examine the role that JIL-1 plays in higher-order chromatin structure and gene expression, we examined the effect of an allelic series of hypomorphic JIL-1 alleles on PEV of the wm4 allele. The In(1)wm4 X chromosome contains an inversion that juxtaposes the euchromatic white gene and heterochromatic sequences adjacent to the centromere (Muller 1930; Schultz 1936). The resulting somatic variegation of wm4 expression occurs in clonal patches in the eye reflecting heterochromatic spreading from the inversion breakpoint that silences wm4 expression in the white patches and euchromatic packaging of the w gene in those patches that appear red (reviewed in Grewal and Elgin 2002). Studies of this effect suggest that the degree of spreading may depend on the amount of heterochromatic factors at the breakpoint (reviewed in Weiler and Wakimoto 1995). In these experiments the In(1)wm4 chromosome was crossed into different JIL-1 mutant backgrounds that combined hypomorphic and null JIL-1 alleles (JIL-1z28, JIL-1z60, and JIL-1z2) to generate progeny expressing different amounts of wild-type JIL-1 protein. The JIL-1z28 allele is a weak hypomorph producing 45% of the normal level of wild-type JIL-1 protein; the JIL-1z60 allele is a strong hypomorph producing only 0.3% of wild-type JIL-1 protein levels, whereas the JIL-1z2 allele is a true null and homozygous animals do not survive to adulthood (Wang et al. 2001; Zhang et al. 2003). The JIL-1h9 allele expresses a truncated JIL-1 protein that lacks part of the second kinase domain and the entire COOH-terminal domain (Zhang et al. 2003). The JIL-1z2/JIL-1z60 heteroallelic combination is semilethal and only a few eclosed animals from large-scale crosses could be analyzed. Flies with the different genotypes were scored for the percentage of the eye that was red and variegated wm4; +/+ flies containing wild-type levels of JIL-1 protein were used as controls (Figure 1, A–E and Table 1). As JIL-1 protein levels were reduced, an increasing percentage of flies showed fully pigmented eyes, with 100% of the JIL-1z2/JIL-1z60 and JIL-1z2/JIL-1h9 animals showing completely red eyes (Figure 1, D and E and Table 1). This is in contrast to the control crosses in which none of the flies exhibited completely red eyes (Figure 1A and Table 1). These results strongly indicate that loss of JIL-1 protein results in suppression of PEV of the wm4 allele.
Figure 1.—
The effect of JIL-1 hypomorphic alleles on PEV. (A–E) Suppression of PEV in the eyes of ln(1)wm4 (wm4) flies with hypomorphic allelic combinations of the JIL-1 alleles JIL-1z28 (z28), JIL-1z60 (z60), JIL-1h9 (h9), and JIL-1z2 (z2). Strong suppression of PEV is indicated by a completely red eye phenotype. (F–I) Hypomorphic allelic combinations of the JIL-1 alleles JIL-1z28 (z28), JIL-1z60 (z60), JIL-1h9 (h9), and JIL-1z2 (z2) overcome the effects of E(var)2-1 on PEV in the eyes of ln(1)wm4 flies. Fly stocks were maintained according to standard protocols (Roberts 1998). Canton-S was used for wild-type preparations. The JIL-1z2, JIL-1z60, and JIL-1h9 alleles are described in Wang et al. (2001) and in Zhang et al. (2003). In(1)wm4; Pr Dr/TM3 Sb Ser and In(1)wm4; CyO E(var)2-1/Sco stocks were generously provided by G. Reuter. In(1)wm4 and Su(var)3-13/TM3 Sb Ser stocks were obtained from the Bloomington Stock Center. Balancer chromosomes and markers are described in Lindsley and Zimm (1992). Strains containing the In(1)wm4 X chromosome and a loss-of-function JIL-1 allele (JIL-1z2, JIL-1z60, JIL-128, or JIL-1h9) heterozygous with the TM6 Sb Tb e third chromosome balancer were produced by standard crossing. Subsequent crosses between these strains generated flies with different JIL-1 allelic combinations in a wm4 background. As a control, wm4 PEV was analyzed in flies homozygous for a Canton-S wild-type third chromosome. A CyO second chromosome containing the E(var)2-1 allele was introduced into the In(1)wm4; JIL-1/TM6 stock by standard crosses. As a control for PEV in these stocks, individuals that carried the E(var)2-1 CyO chromosome were compared with siblings that did not. To quantify the variegated phenotype newly eclosed adults were collected, aged for 4–5 days at 25°, and were then sorted into different classes on the basis of the percentage of the eye that was red. Eyes from representative individuals from these crosses were photographed using an Olympus stereo microscope and a Spot digital camera (Diagnostic Instruments).
TABLE 1.
JIL-1 alleles suppress PEV of wm4
| % of flies categorized by the proportion of red ommatidia
|
||||||
|---|---|---|---|---|---|---|
| Genotypea | n | 0% red | 0–25% red | 25–75% red | 75–99% red | 100% red |
| +/+ | 542 | 0.0 | 36.3 | 41.5 | 22.1 | 0.0 |
| z28/z28 | 160 | 0.0 | 48.1 | 24.4 | 25.6 | 1.9 |
| z60/z60 | 397 | 0.0 | 0.0 | 0.0 | 8.3 | 91.7 |
| z2/z60 | 48 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| z2/h9 | 57 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
Genotype of the third chromosome. In addition, all flies were homozygous (females) or hemizygous (males) for wm4 on the X chromosome.
To confirm that loss of the JIL-1 protein produces a bona fide Su(var) phenotype, we examined the effect of decreased levels of JIL-1 protein in a wm4 genetic background that also carries the dominant enhancer E(var)2-1. This enhancer results in nearly completely white-eyed flies (Figure 1F) and has proven useful in identifying and characterizing strong Su(var) mutations in genetic screens (Schotta et al. 2003). As levels of JIL-1 protein decreased in this background, a corresponding increase in pigmentation was observed (Figure 1, G–I and Table 2) with 51.2% of JIL-1z60/JIL-1z60 and 90.0% of JIL-1z2/JIL-1h9 flies showing completely red eyes compared to 0% in control flies. Thus, lack of JIL-1 activity strongly counteracts the effect of the dominant enhancer E(var)2-1 on PEV of the wm4 allele.
TABLE 2.
JIL-1 alleles overcome the effects of E(var)2-1 on PEV of wm4
| % of flies categorized by the proportion of red ommatidia
|
||||||
|---|---|---|---|---|---|---|
| Genotypea | n | 0% red | 0–25% red | 25–75% red | 75–99% red | 100% red |
| +/+ | 1209 | 89.7 | 10.3 | 0.1 | 0.0 | 0.0 |
| z28/z28 | 283 | 58.3 | 40.3 | 1.5 | 0.0 | 0.0 |
| z60/z60 | 41 | 0.0 | 0.0 | 9.8 | 39.0 | 51.2 |
| z2/h9 | 10 | 0.0 | 0.0 | 0.0 | 10.0 | 90.0 |
Genotype of the third chromosome. In addition, all flies were homozygous (females) or hemizygous (males) for wm4 and were E(var)2-1CyO/+.
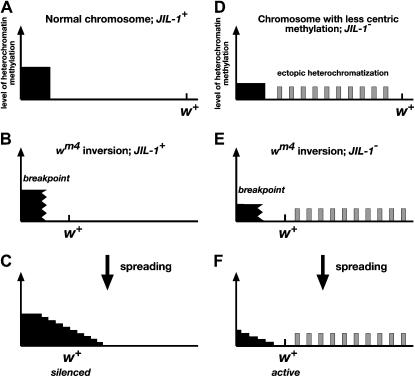
Zhang et al. (2006) recently demonstrated that a reduction in the levels of the JIL-1 histone H3S10 kinase results in a redistribution of the major heterochromatin markers H3K9me2 and HP1 to ectopic locations on the chromosome arms with the most pronounced increase on the X chromosomes. Interestingly, overall levels of heterochromatic factors remained unchanged, implying a concomitant reduction in the levels of pericentric heterochromatic factors (Zhang et al. 2006). On the basis of these findings a model was proposed wherein JIL-1 kinase activity functions to maintain euchromatic regions by antagonizing Su(var)3-9 mediated heterochromatization (Zhang et al. 2006). Thus, in the absence of JIL-1 function the dispersion of the H3K9me2 mark and HP1 to ectopic locations on the chromosomes would be expected to lead to heterochromatization and repression of gene expression at these sites, suggesting that loss of JIL-1 would result in an E(var) phenotype if the reporter locus were located at such a site. However, the results of Zhang et al. (2006) also showed that ectopic heterochromatization was not uniform and that not all active gene loci were repressed. This implies that certain chromatin sites are molecularly distinct and may be preferentially modified by Su(var)3-9 to recruit HP1 in the absence of JIL-1. Paradoxically, as a consequence of this combined with the redistribution of a fixed level of heterochromatic factors, a reduction in JIL-1 activity would be predicted to lead to suppression—not enhancement—of PEV at loci not affected by ectopic Su(var)3-9 activity but sensitive to the levels of heterochromatic factors at the pericentromeric chromatin, such as has previously been demonstrated for the wm4 allele (reviewed in Weiler and Wakimoto 1995). The results of this study support this hypothesis by demonstrating that JIL-1 hypomorphic loss-of-function alleles are strong suppressors of PEV of the wm4 allele and that lack of JIL-1 activity can counteract the effect of the dominant enhancer E(var)2-1 on PEV. We propose that the suppression of PEV of the wm4 allele in JIL-1 hypomorphic backgrounds is due to a reduction in the level of heterochromatic factors at the pericentromeric heterochromatin near the inversion breakpoint site that reduces its potential for heterochromatic spreading and silencing (Figure 2).
Figure 2.—
Model for suppression of PEV of the wm4 allele by JIL-1 null and hypomorphic alleles. (A–C) Spreading of heterochromatic factors (solid area) in a wild-type JIL-1 background. With normal levels of pericentric heterochromatic factors present the spreading across the inversion breakpoint can reach the w gene and silence gene expression. (D–F) Spreading of heterochromatic factors (solid area) in a JIL-1 null and hypomorphic allelic background. Shaded boxes indicate the redistribution to ectopic chromosome sites of heterochromatic markers occurring in JIL-1 hypomorphic mutants. Because of the reduced levels of pericentric heterochromatic factors in the JIL-1 mutant background the spreading is attenuated and does not extend far enough from the breakpoint to silence w expression.
It has recently been demonstrated that the Su(var)3-1 alleles of JIL-1 consist of dominant gain-of-function alleles that also strongly suppress PEV (Ebert et al. 2004). However, JIL-1Su(var)3-1 alleles are characterized by deletions of the COOH-terminal domain that do not affect JIL-1 kinase activity or the spreading of heterochromatin markers (Ebert et al. 2004; Zhang et al. 2006). Furthermore, the results of Zhang et al. (2006) indicated that the COOH-terminal domain of JIL-1 is required for proper chromosomal localization and that JIL-1Su(var)3-1 proteins are mislocalized to ectopic chromosome sites. Thus, the dominant gain-of-function effect of the JIL-1Su(var)3-1 alleles may be attributable to JIL-1 kinase activity at ectopic locations possibly through phosphorylation of novel target proteins or by misregulated localization of the phosphorylated histone H3S10 mark (Ebert et al. 2004; Zhang et al. 2006). Consequently, the molecular mechanism of suppression of PEV of the wm4 allele is likely to be different for the dominant gain-of-function Su(var)3-1 alleles and for the hypomorphic loss-of-function JIL-1 alleles.
Acknowledgments
We thank members of the laboratory for discussion, advice, and critical reading of the manuscript. We also wish to acknowledge V. Lephart for maintenance of fly stocks and Laurence Woodruff for technical assistance. We especially thank G. Reuter for providing In(1)wm4 and E(var)2-1 stocks. This work was supported by National Institutes of Health grant GM-62916 (K.M.J.).
References
- Delattre, M., A. Spierer, Y. Jaquet and P. Spierer, 2004. Increased expression of Drosophila Su(var)3–7 triggers Su(var)3–9-dependent heterochromatin formation. J. Cell Sci. 117: 6239–6247. [DOI] [PubMed] [Google Scholar]
- Deng, H., W. Zhang, X. Bao, J. N. Martin, J. Girton et al., 2005. The JIL-1 kinase regulates the structure of Drosophila polytene chromosomes. Chromosoma 114: 173–182. [DOI] [PubMed] [Google Scholar]
- Ebert, A., G. Schotta, S. Lein, S. Kubicek, V. Krauss et al., 2004. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18: 2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal, S. I., and S. C. Elgin, 2002. Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12: 178–187. [DOI] [PubMed] [Google Scholar]
- Henikoff, S., 2000. Heterochromatin function in complex genomes. Biochim. Biophys. Acta 1470: 1–8. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Y. Wang, D. L. Walker, H. Dong, C. Conley et al., 1999. JIL-1: A novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol. Cell 4: 129–135. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Y. Wang, J. Johansen and K. M. Johansen, 2000. JIL-1, a chromosomal kinase implicated in regulation of chromatin structure, associates with the male specific lethal (MSL) dosage compensation complex. J. Cell Biol. 149: 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, New York.
- Muller, H. J., 1930. Types of visible variegations induced by X-rays in Drosophila. J. Genet. 22: 299–335. [Google Scholar]
- Roberts, D. B., 1998. Drosophila: A Practical Approach. IRL Press, Oxford.
- Schotta, G., A. Ebert, R. Dorn and G. Reuter, 2003. Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin. Cell Dev. Biol. 14: 67–75. [DOI] [PubMed] [Google Scholar]
- Schultz, J., 1936. Variegation in Drosophila and the inert chromosome regions. Proc. Natl. Acad. Sci. USA 22: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath, L. L., 1998. Unfolding the mysteries of heterochromatin. Curr. Opin. Genet. Dev. 8: 147–153. [DOI] [PubMed] [Google Scholar]
- Wang, Y., W. Zhang, Y. Jin, J. Johansen and K. M. Johansen, 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105: 433–443. [DOI] [PubMed] [Google Scholar]
- Weiler, K. S., and B. T. Wakimoto, 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29: 577–605. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Y. Jin, Y. Ji, J. Girton, J. Johansen et al., 2003. Genetic and phenotypic analysis of alleles of the Drosophila chromosomal JIL-1 kinase reveals a functional requirement at multiple developmental stages. Genetics 165: 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., H. Deng, X. Bao, S. Lerach, J. Girton et al., 2006. The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development 133: 229–235. [DOI] [PubMed] [Google Scholar]