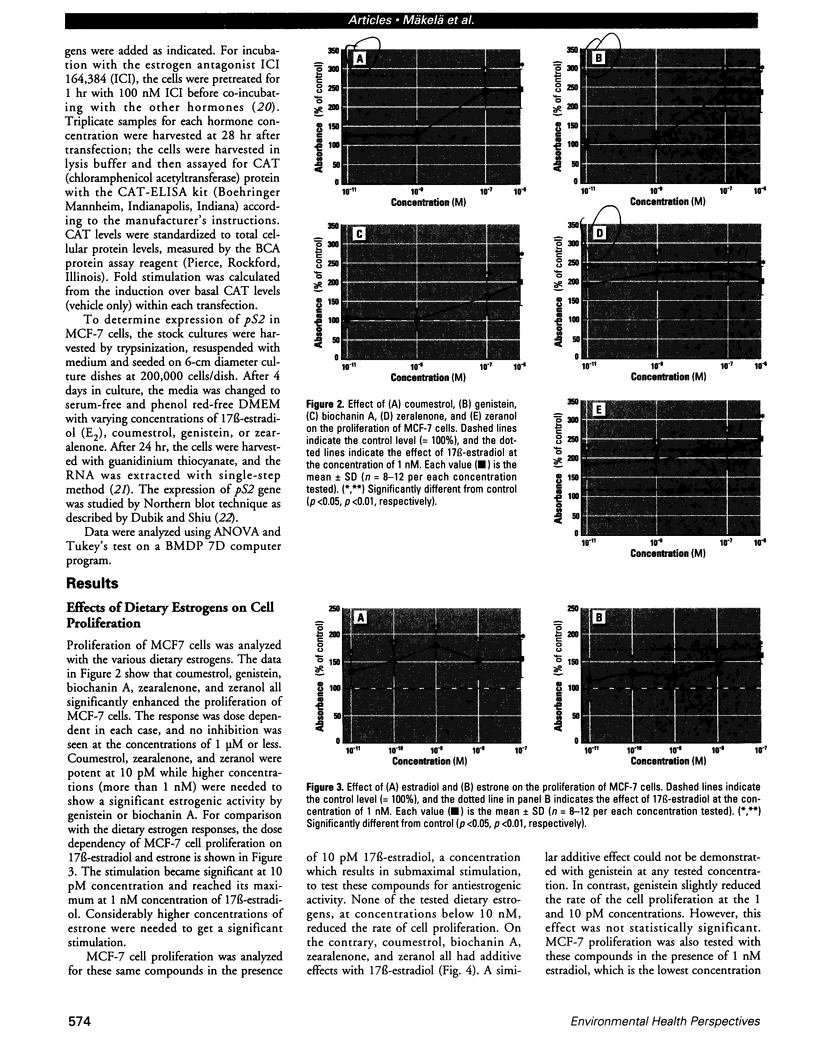
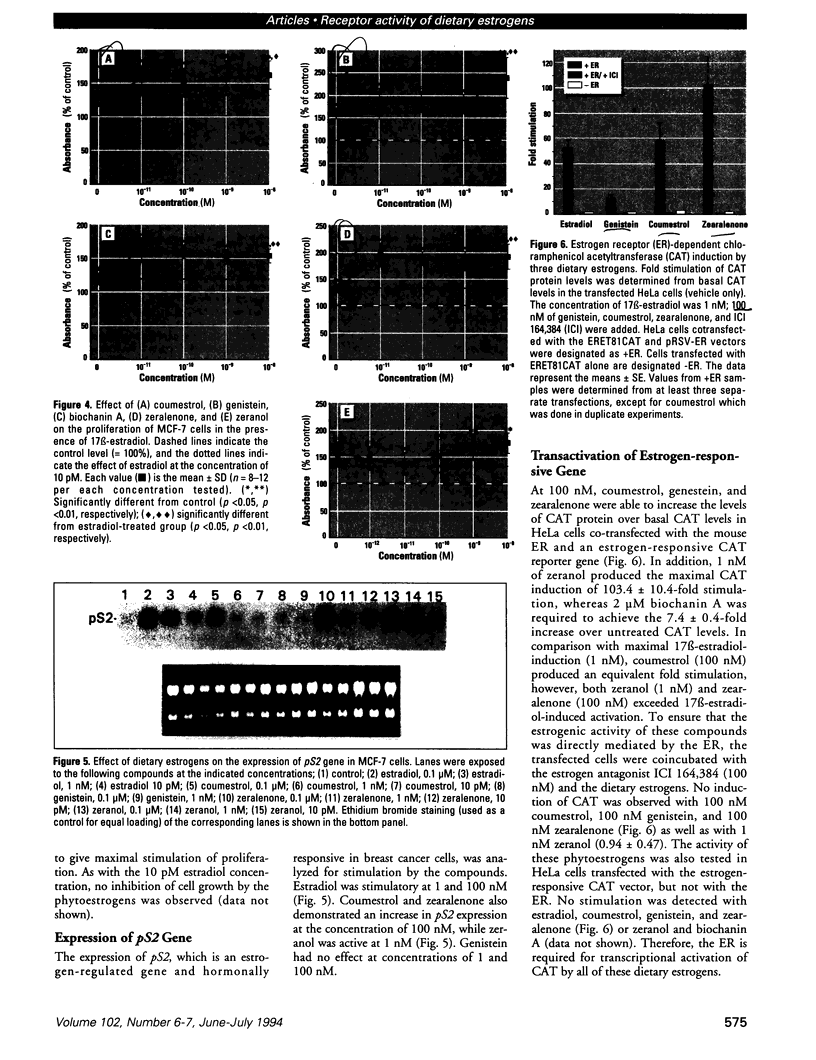
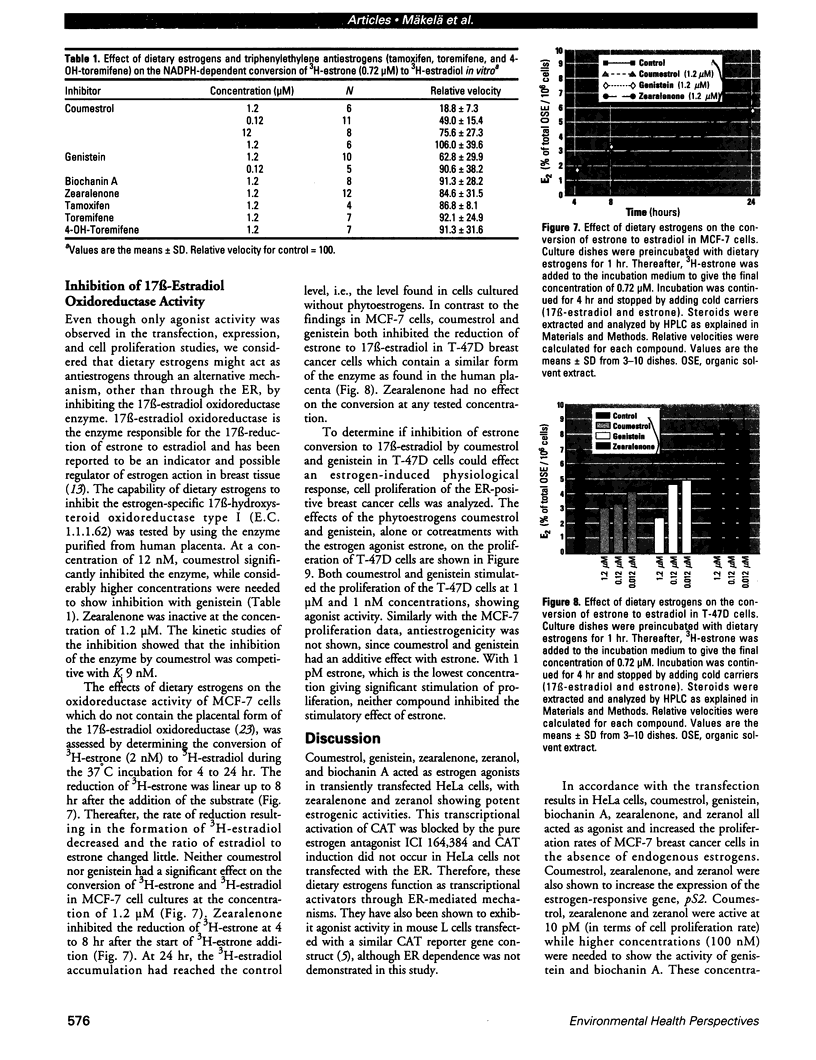
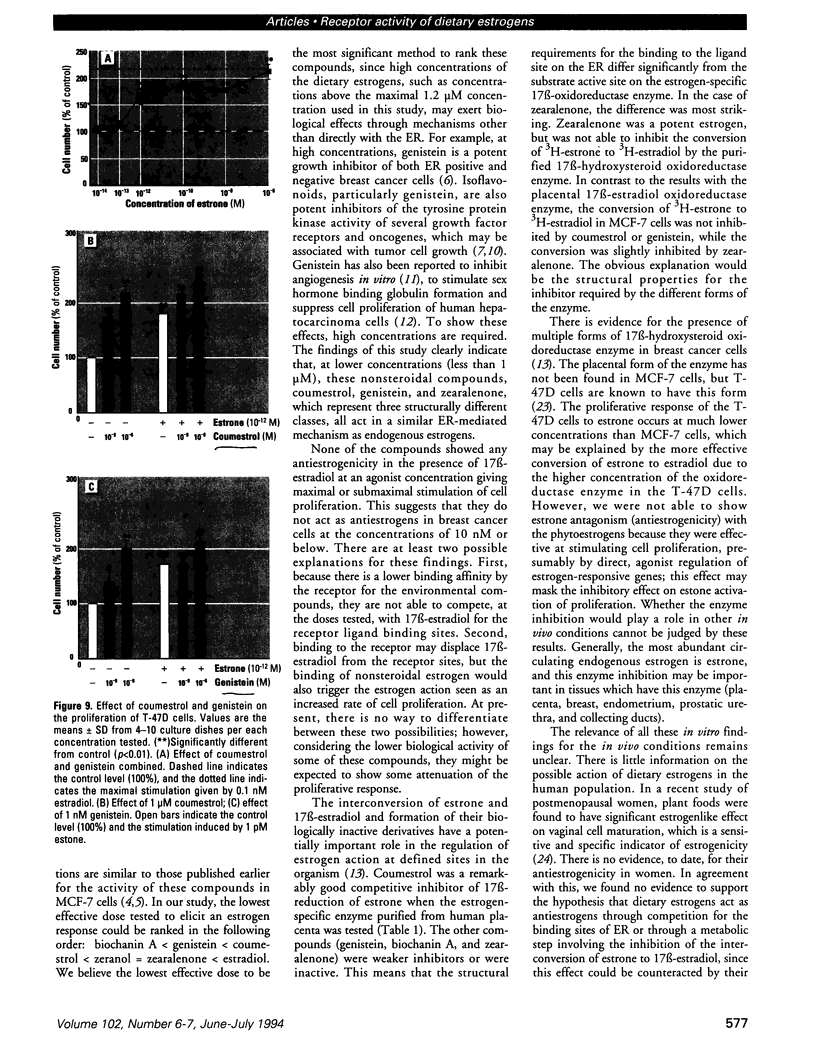
Abstract
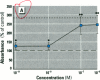
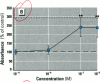
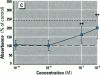
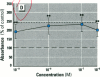
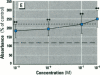
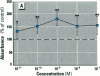
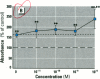
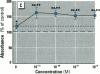
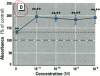
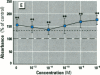
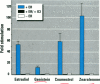
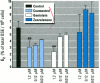
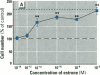
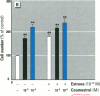
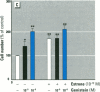
Dietary estrogens are believed to exert their estrogenic or antiestrogenic (chemopreventive) action in estrogen responsive cells by interacting with the estrogen receptor (ER). The present study was undertaken to evaluate a direct role of ER in estrogenic or antiestrogenic activities of three dietary estrogens (coumestrol, genistein and zearalenone). HeLa cells were transiently co-transfected with an expression vector for ER and an estrogen-responsive reporter gene construct. Coumestrol, genistein, and zearalenone all increased the activity of the reporter gene, only in the presence of the ER, and the activation was blocked with the ER antagonist ICI 164,384, demonstrating an ER-specific, agonist response. In addition, in MCF-7 cells, coumestrol and zearalenone increased the expression of the estrogen-responsive pS2 gene. Coumestrol and genistein inhibited the purified estrogen-specific 17ß-hydroxysteroid oxidoreductase enzyme and the conversion of estrone to 17ß-estradiol in T-47D cells, which contain this enzyme. However, they did not inhibit the estrone-induced proliferation of T-47D cells. In conclusion, coumestrol, genistein, and zearalenone are all potent estrogens in vitro, and they act through ER mediated mechanism. Our findings give no evidence to support the idea that these compounds act as antiestrogens through competition for the binding sites of ER or by inhibition of the conversion of estrone to 17ß-estradiol in breast cancer cells, since this effect was nullified by their agonist action on cell proliferation. Therefore, their suggested chemopreventive action in estrogen-related cancers must be mediated through other mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B. Enzymatic regulation of estradiol-17 beta concentrations in human breast cancer cells. Breast Cancer Res Treat. 1992 Mar;20(3):145–154. doi: 10.1007/BF01834620. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Honjo H., Higashi A., Fotsis T., Hämäläinen E., Hasegawa T., Okada H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991 Dec;54(6):1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dean N. M., Kanemitsu M., Boynton A. L. Effects of the tyrosine-kinase inhibitor genistein on DNA synthesis and phospholipid-derived second messenger generation in mouse 10T1/2 fibroblasts and rat liver T51B cells. Biochem Biophys Res Commun. 1989 Dec 15;165(2):795–801. doi: 10.1016/s0006-291x(89)80036-1. [DOI] [PubMed] [Google Scholar]
- Dubik D., Shiu R. P. Transcriptional regulation of c-myc oncogene expression by estrogen in hormone-responsive human breast cancer cells. J Biol Chem. 1988 Sep 5;263(25):12705–12708. [PubMed] [Google Scholar]
- Fotsis T., Pepper M., Adlercreutz H., Fleischmann G., Hase T., Montesano R., Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Gompel A., Malet C., Spritzer P., Lalardrie J. P., Kuttenn F., Mauvais-Jarvis P. Progestin effect on cell proliferation and 17 beta-hydroxysteroid dehydrogenase activity in normal human breast cells in culture. J Clin Endocrinol Metab. 1986 Nov;63(5):1174–1180. doi: 10.1210/jcem-63-5-1174. [DOI] [PubMed] [Google Scholar]
- Markovits J., Linassier C., Fossé P., Couprie J., Pierre J., Jacquemin-Sablon A., Saucier J. M., Le Pecq J. B., Larsen A. K. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989 Sep 15;49(18):5111–5117. [PubMed] [Google Scholar]
- Martikainen P., Nyman K., Nevalainen T. J. Toxic effects of human pancreatic and snake and bee venom phospholipases A2 on MCF-7 cells in culture. Toxicon. 1993 Jul;31(7):835–843. doi: 10.1016/0041-0101(93)90218-8. [DOI] [PubMed] [Google Scholar]
- Martin P. M., Horwitz K. B., Ryan D. S., McGuire W. L. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978 Nov;103(5):1860–1867. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- Mayr U., Butsch A., Schneider S. Validation of two in vitro test systems for estrogenic activities with zearalenone, phytoestrogens and cereal extracts. Toxicology. 1992 Sep;74(2-3):135–149. doi: 10.1016/0300-483x(92)90134-z. [DOI] [PubMed] [Google Scholar]
- Migliaccio S., Davis V. L., Gibson M. K., Gray T. K., Korach K. S. Estrogens modulate the responsiveness of osteoblast-like cells (ROS 17/2.8) stably transfected with estrogen receptor. Endocrinology. 1992 May;130(5):2617–2624. doi: 10.1210/endo.130.5.1572285. [DOI] [PubMed] [Google Scholar]
- Mousavi Y., Adlercreutz H. Genistein is an effective stimulator of sex hormone-binding globulin production in hepatocarcinoma human liver cancer cells and suppresses proliferation of these cells in culture. Steroids. 1993 Jul;58(7):301–304. doi: 10.1016/0039-128x(93)90088-5. [DOI] [PubMed] [Google Scholar]
- Peterson G., Barnes S. Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem Biophys Res Commun. 1991 Aug 30;179(1):661–667. doi: 10.1016/0006-291x(91)91423-a. [DOI] [PubMed] [Google Scholar]
- Poutanen M., Moncharmont B., Vihko R. 17 beta-hydroxysteroid dehydrogenase gene expression in human breast cancer cells: regulation of expression by a progestin. Cancer Res. 1992 Jan 15;52(2):290–294. [PubMed] [Google Scholar]
- Pylkkänen L., Santti R., Mäentausta O., Vihko R. Distribution of estradiol-17 beta hydroxysteroid oxidoreductase in the urogenital tract of control and neonatally estrogenized male mice: immunohistochemical, enzymehistochemical, and biochemical study. Prostate. 1992;20(1):59–72. doi: 10.1002/pros.2990200108. [DOI] [PubMed] [Google Scholar]
- Trevillyan J. M., Lu Y. L., Atluru D., Phillips C. A., Bjorndahl J. M. Differential inhibition of T cell receptor signal transduction and early activation events by a selective inhibitor of protein-tyrosine kinase. J Immunol. 1990 Nov 15;145(10):3223–3230. [PubMed] [Google Scholar]
- Wilcox G., Wahlqvist M. L., Burger H. G., Medley G. Oestrogenic effects of plant foods in postmenopausal women. BMJ. 1990 Oct 20;301(6757):905–906. doi: 10.1136/bmj.301.6757.905-a. [DOI] [PMC free article] [PubMed] [Google Scholar]