Abstract
Associations between efficient processing of brief, rapidly presented, successive stimuli and language learning impairments (LLI) in older children and adults have been well documented. In this paper we examine the role that impaired rapid auditory processing (RAP) might play during early language acquisition. Using behavioral measures we have demonstrated that RAP abilities in infancy are critically linked to later language abilities for both non-speech and speech stimuli. Variance in infant RAP thresholds reliably predict language outcome at 3 years-of-age for infants at risk for LLI and control infants. We present data here describing patterns of electrocortical (EEG/ERP) activation at 6 month-of-age to the same non-verbal stimuli used in our behavioral studies. Well-defined differences were seen between infants from families with a history of LLI (FH+) and FH− controls in the amplitude of the mismatch response (MMR) as well as the latency of the N250 component in the 70 ms ISI condition only. Smaller mismatch responses and delayed onsets of the N250 component were seen in the FH+ group. The latency differences in the N250 component, but not the MMR amplitude variation, were significantly related to 24-month language outcome. Such converging tasks provide the opportunity to examine early precursors of LLI and allow the opportunity for earlier identification and intervention.
Keywords: Auditory perception, Development, Rapid auditory processing, Language acquisition, Speech, EEG
1. Introduction
During the first year, the critical foundations of phonemic perception and later language are established and can be observed well before spoken language emerges. Such precursors of language are evident in the infant’s well-documented ability to process auditory, speech, and language information. In particular, the ability to perceive and categorize auditory signals occurring within tens of milliseconds is one of the skills essential to mounting language.
Psychophysical studies of infants strongly suggest that these acoustic abilities and the necessary underlying cortical substrates are in place from a very early age (Aslin, 1989; Eilers, Morse, Gavin, & Oller, 1981; Irwin, Ball, Kay, Stillman, & Rosser, 1985; Morrongiello & Trehub, 1987; see Fitch, Read, & Benasich, 2001 for review). While not at adult levels, the acoustic capabilities of the human infant are impressive and include keen sensitivity to auditory sweeps, gaps and changes in frequency (Aslin, 1989; Aslin & Hunt, 2001; Jusczyk, Pisoni, Walley, & Murray, 1980; Trehub, Schneider, & Henderson, 1995; Werner & Rubel, 1992). This ability to perform fine-grained acoustic analyses in the tens of millisecond range appears to be critical to the decoding of the speech stream and the subsequent establishment of phonemic maps (Aslin, 1989; Aslin, Pisoni, & Juczyk, 1983; Eimas, 1975; Eimas, Siqueland, Jusyzk, & Vigorito, 1971; Kuhl, 2004). Importantly, all the components that are necessary for children to process, discriminate, and acquire language are already in place at this early, preverbal stage of development.
A burgeoning body of research, conducted across laboratories, has shown that one of the fundamental skills underlying the development of language is the ability to efficiently and accurately process sequential rapidly presented, brief, auditory stimuli (reviewed in Benasich & Leevers, 2002; Fitch et al., 2001; see also Farmer & Klein, 1995; Hari & Kiesla, 1996; Kraus et al., 1996; Leonard, 1998; McAnally & Stein, 1996, 1997; McCrosky & Kidder, 1980; Neville, Coffey, Holcomb, & Tallal, 1993; Robin, Tomblin, & Kearney, 1989; Witton et al., 1998; Wright et al., 1997). The neural substrates that subserve efficient processing of brief, rapidly presented, successive auditory stimuli, or rapid auditory processing (RAP), are critical for accurately analyzing and segmenting the speech stream. Evidence suggesting that sensory processing in the time window of tens of milliseconds is relevant to language comprehension and achievement is drawn from studies of RAP in both children and adults (see Farmer and Klein, 1995; Leonard, 1998; Tallal, 2004; Wright et al., 1997 for reviews). Individuals with RAP deficits and an associated language learning impairment (LLI)1 hear normally and can sequence sounds. However, they need orders of magnitude more processing time than unimpaired children. Although most 5- to 10-year-olds can process stimuli in sequence that are only tens of milliseconds apart, children with LLI need a hundred or more milliseconds between the same stimuli to process their features accurately. These difficulties occur whether the incoming signals are language, for example, consonant–vowel (CV) syllables such as \da\ or \ga\, or non-language signals such as tone pairs containing brief within-pair interstimulus intervals (ISIs). It has also been well documented that LLI runs in families and that children born to families with affected parents or siblings are at an elevated risk for the disorder with about a threefold increase in incidence as compared to control children (Bishop, North, & Donlan, 1995; Bishop & Edmundson, 1986; Choudhury & Benasich, 2003; Lahey & Edwards, 1995; Neils & Aram, 1986; Tallal, Ross, & Curtiss, 1989; Tallal et al., 2001; Tomblin, 1989; Tomblin & Buckwalter, 1998; van der Lely & Stollwerck, 1996).
However, the earliest steps of language acquisition are still not clearly understood despite many years of concentrated research. Thus sorting out the etiology of LLI has been difficult. Children with LLI have extreme difficulty in acquiring language, while other cognitive abilities appear to remain relatively intact. Theories proposed to account for LLI range from arguments for the impairment of innate brain modules specialized for processing grammar (Gopnik & Crago, 1991), to limitations in processing capacity or working memory (Gathercole & Baddeley, 1990; Schul, Stiles, Wulfeck, & Townsend, 2004). Delays in learning the semantic and syntactic rules critical to development of language have most often been proposed as a primary determinant of LLI (Clahsen, 1992; Rice & Wexler, 1996; see Leonard, 1998 for a review). Another fundamental and consistent deficit implicated, and thought to be causal, in children with language or reading deficits is poor phonological processing (Bird, Bishop, & Freeman, 1995; Elliott & Hammer, 1988; Elliott, Hammer, & Scholl, 1989; Gathercole & Baddeley, 1990; Liberman, 1996; Scarborough, 1990; Stark & Tallal, 1988; Sussman, 1993; Wagner & Torgesen, 1987; Whitehurst & Fischel, 1994). However, the precise nature and origin of these deficits remains the focus of intense study and theoretical debate. A controversial issue is the question of whether phonological deficits are ‘speech-specific’, or whether lower level sensory-processing mechanisms play a crucial role in setting up the phonological building blocks of human language (see Fitch & Tallal, 2003). Further, considerable research has recently been directed towards the contribution of basic mechanisms such as attention, perception, and memory to this process (e.g. Farmer and Klein, 1995; Habib, 2000; Hari & Renvall, 2001; Hoffman & Gillam, 2004; Webster & Shevell, 2004; see Fitch & Tallal, 2003; Tallal, 2004 for a review). Much evidence suggests that the developing brain exhibits both ‘hard-wired’ characteristics (such as statistical learning; e.g. Maye, Werker, & Gerken, 2002; Saffran, Aslin, & Newport, 1996) as well as significant environmental modifiability of emergent language. However, critical areas of knowledge remain elusive.
Infants whose initial acoustic analysis of the speech stream diverges from the norm (i.e. possibly fairly subtle differences in segmenting, pattern analysis or “chunking” of information over time; see Tallal, 2004) might well show substantial variation in developmental trajectories for language acquisition. If early differences in acoustic processing endure, the result may be difficulty in identifying and mapping phonemes within the native language and subsequent delays in mounting age-appropriate language. Whether such early delays result in LLI is still under study, but arguably, the predictive value of these early responses to prelinguistic stimuli may reflect the earliest manifestations of individual differences in language acquisition. Findings in normal control infants using operant-conditioned and habituation/recognition-memory tasks, and both speech and non-speech stimuli, support this contention (Benasich & Tallal, 2002; Benasich, Choudhury, & Friedman, 2003), as do the retrospective studies of Trehub and Henderson (1996). The cited studies reported robust correlations between infant (6–7.5 months) acoustic processing thresholds and preschool expressive and receptive language outcome. Kuhl and colleagues have also recently reported good predictability of language outcome in normally developing children at 24 months of age from their discrimination scores at 6 months of native and non-native contrasts (Kuhl, Tsao, & Liu, 2003; Tsao, Liu, & Kuhl, 2004).
Early neurophysiological (EEG/ERP) responses have also been utilized to examine auditory processing as it relates to language development. Although ERPs undergo marked developmental changes from infancy to adulthood (Ceponiené, Rinne, & Näätänen, 2002; Courchesne, 1990; Kurtzberg, 1982; Kurtzberg, Hilpert, Kreuzer, & Vaughan, 1984; Kushnerenko et al., 2002a; Kushnerenko, Ceponiené, Balan, Fellman, & Näätänen, 2002b; Morr, Shafer, Kreuzer, & Kurtzberg, 2002; Novak, Kurtzberg, Kreuzer, & Vaughan, 1989; Shafer, Morr, Kreuzer, & Kurtzberg, 2000; Thomas & Crow, 1994; Weitzman & Graziani, 1968), waveform components that have been widely studied in relation to auditory processing (e.g. MMN) have been described in young infants and children. For example, a mismatch-like response2 (MMR) dependent on the immediate sound context can be observed in both preterm and full-term infants. This MMR can be elicited with speech stimuli (Cheour et al., 1997, 2002a, 2002c; Cheour-Luhtanen et al., 1995, 1996; Dehaene-Lambertz, 2000; Friedrich, Weber, & Friederici, 2004; Kushnerenko et al., 2002a, 2002b, 2001; Leppänen, Pihko, Eklund, & Lyytinen, 1999; Martynova, Kirjavainen, & Cheour 2003; Weber, Hahne, Friedrich, & Friederici, 2003) and also with tone stimuli differing in parameters such as frequency, intensity, location and duration (Ceponiené et al., 2000, 2002; Cheour et al., 2002a; Cheour, Kushnerenko, Ceponiené, Fellman, & Näätänen, 2002b; Dehaene-Lambertz, 2000; Kurtzberg, Vaughan, Kreuzer, & Fliegler, 1995; Leppänen, Eklund, & Lyytinen, 1997; Morr et al., 2002; see Cheour, Leppänen, & Kraus, 2000; Leppänen et al., 2004b for reviews).
ERPs have been found to relate to later language outcome (see Leppänen, Choudhury, Benasich, & Lyytinen, 2004a for a review). For example, Molfese and Molfese (1985, 1997) identified specific brain response patterns in healthy newborns that later discriminated between groups of children with high and low language skills at the ages of 3 and 5 years. These responses reflected group differences in consonant discrimination in the left hemisphere (at 70–320 ms) and in both hemispheres (at around 660 ms). The authors argue that the ERPs reflect a mechanism underlying the perception of phonemic boundaries, possibly inherent within the mammalian auditory system (Kuhl, 2004).
In a prospective, longitudinal study of a population of infants at risk for familial dyslexia, Guttorm, Leppänen and colleagues reported that group differences at birth in ERP-components were significantly associated with later language development (Guttorm, Leppänen, Poikkeus, Eklund, Lyytinen, & Lyytinen, 2005; Guttorm, Leppänen, Richardson, & Lyytinen, 2001; Guttorm, Leppänen, Tolvanen, & Lyytinen, 2003). The “at risk” response pattern for consonant–vowel (CV) syllables (i.e., larger and prolonged responses to/ga/at 540–630 ms) in the right hemisphere was related to significantly poorer receptive language skills at 2.5 years and a similar ERP pattern in the left hemisphere was associated with poorer verbal memory skills at 5 years across children with and without familial risk for dyslexia. Similarly Friedrich et al. (2004) used CV-syllables and an EEG/ERP paradigm to assess auditory processing abilities in 2-months old German infants at risk for specific language impairment and their normal controls. The authors report significant group differences in the latency of a mismatch-like change-detection response (mismatch response, MMR), with infants at risk for LLI showing a significantly slower MMR to a deviant stimulus than normal control infants. Thus it appears that both behavioral and EEG/ERP paradigms that assess acoustic processing during the first year of life provide important information concerning later language attainment.
The research reviewed thus far suggests that questions about the ontogeny of language can be explored by studying factors, across development, thought to contribute to mature language ability and attending carefully to the time course of acquisition. Thus, prospective longitudinal studies beginning in early infancy allow the interactive processes essential to language development (both normative and atypical) to be investigated and clarified. In the study presented here, we further examine the role that impaired rapid auditory processing (RAP) might play in early language acquisition by using converging paradigms and a developmental framework that is both prespeech and non-language specific. Thus, one may address the question of whether RAP deficits simply co-occur with the difficulties in phonological and syntactic decoding seen in children with LLI, or whether they precede and predict those impairments. Such an approach also allows more general questions to be asked, specifically those relating to general prelinguistic mechanisms that might perturb emerging language in normally developing children.
In our current prospective longitudinal studies that start at 6 months-of-age, we are collecting both behavioral and electrocortical (dense array EEG/ERP) data. The use of such converging paradigms permits careful examination of recurring relational patterns that may be linked to poor speech and language skills concurrently and/or predictively (Benasich, Thomas, Choudhury, & Leppänen, 2002). We have begun to uncover important precursor abilities (such as rapid auditory processing) that allow efficient mounting of native language (Benasich & Leevers, 2002).
Infants at high risk for LLI by virtue of having a family history of specific language disorder (FH+) and infants with no such history (FH−) were studied (Benasich & Tallal, 1996, 2002; Benasich et al., 2002; Jing, Choudhury, Thomas-Friedman, & Benasich, 2003; see Choudhury & Benasich, 2003 for a review). Across studies, our results demonstrated that RAP efficiency evaluated with behavioral measures (operant-conditioned head-turn and habituation/recognition paradigms) differs as a function of family history and is predictive of later language outcome at 16, 24 and 36 months in both FH+ infants and their FH− controls (Benasich & Tallal, 2002; Benasich et al., 2002; Benasich & Leevers, 2002; Choudhury & Benasich, 2003; Jing et al., 2003; Leppänen, Choudhury, Thomas, Jing, & Benasich, 2002a).
More recently we have begun to collect dense array EEG/ERP data in order to examine developmental changes and maturation of infant brain responses to rapidly changing auditory cues and their relation to behavioral performance (Benasich et al., 2002; Jing et al., 2003; Leppänen et al., 2002a). Below, we present our initial analyses of the 6-month EEG/ERP data, describe these early brain responses and explore their relations to 24-months cognitive and language measures.
2. Main experiment: converging EEG/ERP results in a new sample
2.1. Methods
2.1.1. Subjects
Two groups of infants are included in this study: normal control infants and infants born into families with a history of language-based learning disorders. Infants are tested at 6, 12, 24 and 36 months of age using a battery of assessments (ERPs, habituation and recognition memory, operantly conditioned head-turn, and standardized cognitive and language measures as well as mother–infant interaction) (Fig. 1) Families were recruited from urban and suburban communities in New Jersey and were assigned to one of the two groups based on parental report of family history of LLI, the family history positive group (FH+) and the family history negative group (FH−).
Fig. 1.

Photograph of a 6-month-old child seated on his mother’s lap during an ERP testing session using a dense array Geodesic Sensor Net system (Electric Geodesic, Inc., Eugene, Oregon, USA).
The FH+ group consists of 13 full-term normal birth weight healthy infants (six males and seven females). Infants from FH+ families were recruited from local newspaper birth announcements and pediatric clinics. In order to be classified as FH+, families provided clinical reports of expressive and receptive language scores and a general cognitive score for at least one affected immediate family member (the “proband”); 75% were siblings and the remainder were parents (see Choudhury & Benasich, 2003 for more detail). If the language scores for the proband were at least one standard deviation below the age-appropriate mean, and performance on standardized tests of general cognitive ability was within the normal range, the family was recruited into the FH+ group. Families with children who received a primary diagnosis of attention deficit disorder, or families with children who had language impairments because of hearing loss, neurological disorders, oral motor impairment, a diagnosis of pervasive developmental disorder or autism were not included in this sample. The FH− group consists of 30 infants (14 males and 16 females) with no reported family history of specific language impairments or of dyslexia, learning disability, attention deficit disorder, pervasive developmental disorder, or autism in the nuclear or in the extended family (grandparents, aunts and uncles). Informed consent was obtained from all parents prior to their child’s inclusion in these studies.
2.1.2. Stimuli
The stimuli are complex tones with fundamental frequencies of 100 or 300 Hz with 15 harmonics (6 dB roll-off per octave). The duration of each tone is 70 ms (5 ms rise time and 5 ms fall time) and the intensity is 75 dB SPL. The tones are presented as pairs with varying interstimulus intervals (ISI’s) of 300 and 70 ms. The standard tone pair is a low–low pair (100–100 Hz) and the deviant tone pair is a low–high pair (100–300 Hz). These acoustic stimuli are presented in a passive oddball paradigm using a blocked design; the 70 ms ISI stimuli are presented first followed by a second block of 300 ms ISI stimuli (Fig. 2). In both blocks the low–low tone pair comprises 85% of the stimuli (708 standard tone pairs) and the low-high tone pair the remaining 15% (120 deviant tone pairs) of the total stimuli (828 tone pairs). At least three and no more than 12 standard tone pairs are presented before each deviant pair. The onset-to-onset inter-trial interval (ITI) is 915 and 1140 ms, and the offset-to-onset ITI is 705 and 700 ms, for 70 and 300 ISI ms conditions, respectively. All acoustic stimuli are presented free field via speakers located on either side of the subject.
Fig. 2.
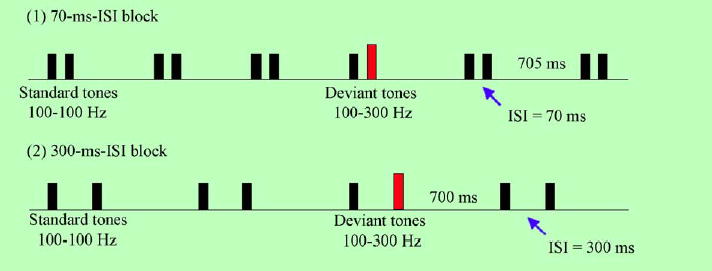
The acoustic stimuli were presented in a passive oddball paradigm using a blocked design. Complex tone pairs had either a 300 or 70 ms within-pair interstimulus interval (ISI). Tones had a fundamental frequency of 100 or 300 Hz with 15 harmonics (6 dB roll-off per octave). In both blocks the 100–100 Hz (low–low) tone pair comprised 85% of the stimuli and the 100–300 Hz (low–high) tone pair comprised the remaining 15%. The 70 ms ISI stimuli were presented first followed by a second block of 300 ms ISI stimuli. The onset-to-onset inter-trial interval (ITI) was 915 and 1140 ms, and the offset-to-onset ITI was 705 and 700 ms for the 70 and 300 ISI ms conditions, respectively.
2.1.3. Auditory event-related potential recording
The EEG signals were recorded from the subjects while they were seated comfortably on their parent’s lap or in a chair in a sound attenuated and electrically shielded room. Silent movies or cartoons were played on a monitor in front of the children to engage them and minimize their movement. An experimenter engaged the children’s attention with a puppet show or other toys if they lost interest in the video.
ERPs were recorded from 62 scalp sites using EGI recording system with a geodesic sensor net (Electric Geodesic, Inc., Eugene, Oregon, USA). The vertex was used as the online reference electrode, the signal was sampled at 250 Hz, and bandpass filtered online at 0.1–100 Hz. The signals were rereferenced off-line to an average (whole head) reference and bandpass filtered at 0.5–25 Hz. The continuous EEG was segmented into epochs according to the stimulus type (predeviant standard or deviant), with the segment length being the same as each onset-to-onset ITI. In addition, a 50 ms prestimulus segment was included for baseline correction and zero was taken as the time of onset of the first tone in the pair. Eye movements were estimated from EEG at the electrodes slightly above and lateral to both eyes. EEG segments containing signals greater than ±200 μV from the baseline at any EEG channel were excluded. A minimum of 90 artifact-free predeviant standard and 90 artifact-free deviant EEG segments was used in each block for averaging ERPs (300 ms ISI condition: average = 122 segments, ranges; 92–150 and 96–150 segments for standards and deviants segments, respectively; 70 ms ISI condition: average = 127 segments, ranges; 92–160 and 96–162 segments for standards and deviants segments, respectively;).3
2.1.4. Analyses of ERP data
Latencies and amplitudes were calculated from frontal (F3 and F4), fronto-central (Fc3 and Fc4), central (C3 and C4), temporal (T3 and T4), parietal (P3 and P4), and occipital (O1 and O2) areas from each of the hemispheres (see Luu & Feree, 2000 for equivalencies between the Geodesic sensor net electrode positions and their 10–10 international equivalents). The latencies reported in this paper are measured from the onset of the first tone in the pair (i.e. absolute values from point zero). Relative latencies, if reported, are presented in parentheses and are measured from the onset of the second tone (after 370 ms in the 300 ms ISI condition and after 140 ms in the 70 ms ISI condition). Amplitudes reported are deflections from baseline (0 μV) in either the positive or negative direction.
Peak latencies and amplitudes of the first positive and negative components (P150 and N250) were extracted from waveforms to the predeviant standard and deviant tone pairs in both the 300 and 70 ms ISI blocks. The components were defined according to the averaged peak latencies of each component (Shafer et al., 2000). The peak latencies were measured in the range of 100–300 ms for the P150component and 130–300 ms for the N250 component. Amplitudes were measured as the largest positive or negative peak amplitudes relative to the corrected baseline. In the 300 ms ISI condition an additional ERP component was extracted. This was the first negative component following the second tone in the pair (N1*)4 and it peaked at about 480 ms (range 460–580 ms).
Latencies and amplitudes of a mismatch-like response (MMR) were also measured from the difference wave (deviant–predeviant standard) within the window of 300–540 ms in the 70 ms ISI condition and 530–770 ms in the 300 ms ISI condition (i.e., 160–400 ms from the onset of the second tone in the pair in both 70 and 300 ms. conditions). Amplitudes were measured as the largest positive or negative peaks relative to the corrected baseline within the aforementioned time windows. The results were visually verified to ensure accuracy.
2.1.5. Standardized language and cognitive measures
The Preschool Language Scale-3 (PLS-3) (Zimmerman, Steiner, & Pond, 1992) provides age-normed language scores for children from birth to 7 years of age and was administered in the laboratory to participants at 24 months. The PLS-3 assesses receptive (Auditory Comprehension) and expressive (Expressive Communication) language skills and yields standard scores (Mean = 100, S.D. = 15), percentile ranks, and age scores for the subscales as well as a total language score. In these analyses standard scores for the auditory comprehension and expressive communication subscales were used.
The Bayley Scales of Infant Development, Mental Development Index (MDI) (Bayley, 1993) was assessed at 24-months. The MDI consists of a range of items, administered according to age, assessing various cognitive abilities such as search, social interaction, imitation, vocalization, and puzzle completion. The scale provides a standardized score with a mean of 100 and a standard deviation of 16 points.
2.1.6. Statistical analyses
As this longitudinal study is still in progress, we present here the initial EEG/ERP findings for the 6-month FH+ group only as compared to their same age FH− controls. We also present a subset of the behavioral results from this sample, in order to examine prospective associations between ERP waveform components and language and cognitive outcomes at 24 months of age.
Analysis of variance (ANOVA) models were used to assess differences in ERP amplitude and latency by group (FH+ versus FH−), rate (70 versus 300 ms ISI), stimulus type (standard versus deviant), hemisphere (left versus right), and brain area (anterior–posterior)5. In all models the between subjects factor was group and within-subjects factors were amplitude or latency of the component of interest from the predeviant standard, deviant (P150, N250, and N1*) and difference waves (MMR). Differences were deemed significant when p < 0.05. In addition, ANOVA’s (gender by group) were used to assess differences in ERP components at 6 months and language and cognitive abilities at 24 months, and Pearson’s product moment correlations were conducted to assess associations between ERP components and 24-month language and cognitive abilities. Preliminary analyses were conducted to assess the role of socio-economic status (SES) and gender on infant ERP responses. Results indicated no association between SES and ERP components, and no differences in the amplitude or latency of ERP waveforms based on gender.
2.2. Results
2.2.1. Morphology and analyses of the P150, N250, and N1* of the standard and deviant wave
2.2.1.1. Morphology of the 300 ms ISI ERP waveform
In both the FH+ and FH− groups the 300 ms ISI stimuli elicited a biphasic responses to the first tone in the tone pair identified as the P150 and the N250 (see Fig. 3). Infants in both groups showed a clear positive component followed by a negative component. The positive peak (P150) appeared between 140 and 200 ms and the negative peak (N250) at about 230–280 ms in all electrode sites examined. The onset of the second tone in the pair elicited a negative response (N1*) in the standard and the deviant waves in both groups. This component peaked at about 470–490 ms in the deviant (i.e. 100–120 ms after the onset of the deviant tone) and standard waves in frontal, central and temporal regions, and at about 520–570 in parietal and occipital regions. In the deviant wave both groups had a large positivity (approximately 6.1–7.8 μV in both groups) occurring at about 650 ms (i.e. 280 ms after the onset of the second tone) in frontal, central and temporal channels. This positivity was inverted in the posterior regions (parietal and occipital electrodes), considerably smaller in magnitude over parietal regions (approximately −3.0 to −3.8 μV) and occurred at about 700 ms.
Fig. 3.
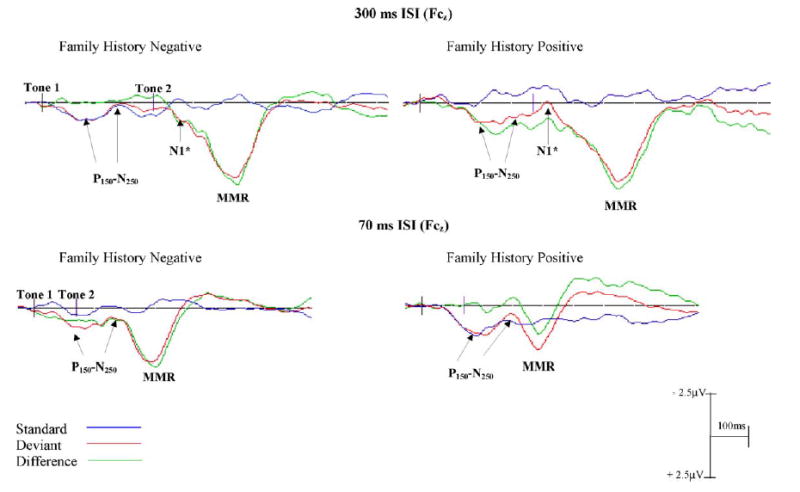
Grand averaged ERP waveforms for FH− and FH+ infants to 300 ms ISI (top) and 70 ms ISI (bottom) conditions. Standard (blue line) deviant (red) and difference (green) waveforms are presented for Fcz (electrode 4). The onsets of the tones are shown by the black vertical lines on the baseline (tones 1–2 represent a single tone pair). Negativities are plotted up, positivities down. The P150–N250 components are visible in the standard and deviant waveforms in both 300 and 70 ms ISI conditions for both groups. The absence of the N1* waveform in the 70 ms ISI condition from the standard and the deviant waves suggests a merged response for these more rapidly presented stimuli.
2.2.1.2. Statistical analysis of the 300 ms ISI ERP waveform
Analyses of variance revealed no group differences on any of the components of interest (P150, N250, N1* and the large positivity) on either the standard or the deviant wave.
2.2.1.3. Morphology of the 70 ms ISI ERP waveform
Waveforms of the ERP’s to the 70 ms ISI stimuli were significantly different from those to 300 ms ISI. These stimuli elicited a more merged response. In both FH+ and FH− groups there were clear positive peaks (P150) at about 155–208 ms6 followed by negative peaks (N250). In the FH+ group the N250 appeared 280–310 ms (i.e., approximately 155 ms after the onset of the second tone) in both the standard and the deviant waves in all electrode regions examined. However, in the control group the N250 appeared at approximately 270–290 ms (135 ms after the onset of the second tone) in the standard wave and at approximately 260–280 ms in the deviant wave. A robust positive peak, (FH−: 4.2–5.4 μV and FH+: 2.4–3.9 μV) at 411 ms (i.e., 271 ms after the onset of the second tone), was observed in the deviant wave following the N250 component.
2.2.1.4. Analysis of the 70 ms ISI ERP waveform
A 2 × 2 (group by stimulus type) ANOVA revealed significant group differences for latency of the ERP response for frontal, fronto-central and central channels for the N250 (see Table 1). Overall, infants in the control (FH−) group had significantly faster N250 responses as compared to FH+ infants. In addition, a significant interaction effect was observed for the frontal and central channels: the N250 response for the deviant wave of FH+ infants was appeared significantly later than the response for the standard wave for FH+ infants only. A second 2 × 2 (group by hemisphere) ANOVA revealed significant laterality effect for frontal channels only (see Table 2): right hemispheric N250 responses for FH+ infants appeared significantly later in time compared to the response in the left hemispheric. No such laterality effect was observed for FH− infants (Fig. 4). Latencies and amplitudes for posterior channels were comparable in both groups with the N250 peaking at about 290–340 ms in standard and deviant waves. ANOVA revealed no group differences in the latency or the amplitude of any other component.
Table 1.
Mean (S.D. error) differences in tlie latency (ms) of the N250 response iii the standaid and deviant waves for family history positive (FH+) and control (FH−) infants in the left and right hemispheres for 70 ms I SI condition
| FH+
|
FH−
|
|||||
|---|---|---|---|---|---|---|
| Stimuli type | Standard | Deviant | Standard | Deviant | F | p |
| L frontal | 284 (8) | 307 (11) | 282 (7) | 266 (6) | 6.05 | 0.02 |
| R frontal | 289 (8) | 305 (6) | 289 (7) | 264 (7) | 7.47 | 0.01 |
| L fronto-central | 289 (2) | 288 (10) | 299 (7) | 269 (7) | 2.93 | 0.09 |
| R fronto-central | 294 (11) | 277 (10) | 286 (7) | 262 (6) | 6.14 | 0.02 |
| L central | 294 (10) | 279 (12) | 298 (8) | 269 (7) | 6.06 | 0.02 |
| R central | 285 (12) | 274 (10) | 285 (6) | 273 (6) | 1.02 | 0 80 |
| L temporal | 297 (12) | 267 (12) | 299 (S) | 298 (8) | 2.69 | 0.12 |
| R temporal | 306 (12) | 278 (12) | 298 (8) | 292 (8) | 1.25 | 0.27 |
| L parietal | 290 (13) | 309 (10) | 273 (9) | 324 (7) | 2.25 | 0.14 |
| R parietal | 286 (13) | 315 (10) | 277 (9) | 321 (7) | 0.69 | 0.41 |
| L occipital | 287 (11) | 288 (14) | 287 (9) | 331(7) | 3.84 | 0.06 |
| R occipital | 298 (12) | 287 (14) | 290 (9) | 328 (8) | 4.45 | 0.04 |
Table 2.
Mean (S.D. error) differences in the latency (ms) of the N250 response in the deviant wave between family history positive (FH+) and control (FH−) infants in 70 ms ISI condition
| FH+
|
FH−
|
|||||
|---|---|---|---|---|---|---|
| Brain regions | Left | Right | Left | Right | F | p |
| Frontal* | 284(8) | 305(8) | 266 (6) | 263 (5) | 11.03 | 0.01 |
| Fronto-central | 282 (10) | 287(9) | 265(7) | 270 (6) | 8.25 | 0.01 |
| Central | 294 (11) | 275 (10) | 279 (7) | 272(7) | 0.74 | 0.40 |
| Temporal | 267 (12) | 278 (13) | 292 (8) | 292 (9) | 3.45 | 0.07 |
| Parietal | 309 (10) | 315 (10) | 324 (7) | 321 (7) | 1.07 | 0.31 |
| Occipital | 288 (10) | 287 (12) | 331 (7) | 328 (8) | 11.99 | 0.01 |
Significant laterality effects were also observed for frontal channels F(1, 40) = 5.78, p < .01, in FH+ infants only.
Fig. 4.
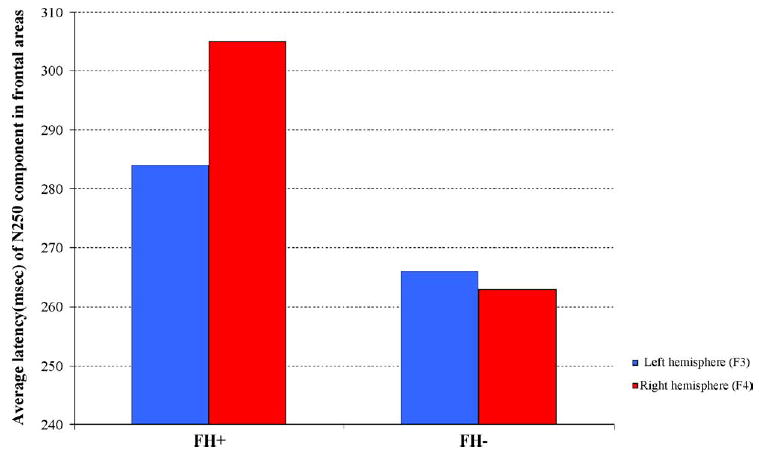
Bar graphs representing the averaged latency of the N250 ERP waveforms for the left (blue) and right (red) hemispheres for FH+ and FH− infants to the 70 ms ISI stimuli are presented here. A significant latency by group effect was observed. Overall FH+ infants had longer N250 latencies and in this group the latency for the right hemisphere was significantly longer than that of the left side. No such laterality difference was observed in the FH− group.
2.2.2. Mismatch response
2.2.2.1. 300 ms ISI
The MMR was obtained from the difference wave (deviant–predeviant standard). In the 300 ms ISI block the MMR appeared at about 615–700 ms (i.e. 245–330 ms after the onset of the second tone) in both groups. An ANOVA revealed no group differences in either the latency or the amplitude of the MMR response.
2.2.2.2. 70 ms ISI
The amplitude of the MMR for the 70 ms ISI block significantly differed between the FH+ and FH− groups at frontal, fronto-central and central channels (Table 3). In addition a significant group by hemisphere interaction was found in frontal channels, F(1, 40) = 6.47, p < .01; FH− infants demonstrated significantly more robust responses compared to FH+ infants (7 μV versus 3 μV) in the left hemisphere, while responses on the right side were comparable in both groups (8 μV versus 6 μV) (see Fig. 5). Amplitudes in the more posterior regions were smaller than those observed in the anterior regions and were comparable in both groups. There were no group differences in the latency of this component (occurring at about 400–430 ms all channels in both groups).
Table 3.
Mean (S.D. error) differences in the amplitude (μV) of the mismatch response (MMR) between family history positive (FH+) and control (FH−) infants in 70 ms ISI condition
| FH+
|
FH−
|
|||||
|---|---|---|---|---|---|---|
| Brain regions | Left | Right | Left | Right | F | p |
| Frontal* | 3.3 (0.8) | 6.4 (1.3) | 7.1 (0.6) | 8.1 (0.9) | 6.58 | 0.01 |
| Fronto-central | 2.5 (1.2) | 3.0 (1.3) | 6.9 (0.8) | 5.9 (0.9) | 8.62 | 0.01 |
| Central | 0.6 (0.9) | 0.1 (1.0) | 3.1 (0.6) | 2.4 (0.6) | 5.33 | 0.03 |
| Temporal | 6.8 (1 7) | 10.3 (1.2) | 7.0(1.2) | 7.4 (0.8) | 1.09 | 0.30 |
| Parietal | 0.4 (l.l) | 0.3 (0.9) | 1.1(0.7) | 0.6 (0.6) | 0.11 | 0.74 |
| Occipital | 3.1(1.2) | 5.2(1.8) | 0.5 (0 8) | 2.1(1.1) | 3.18 | 0.08 |
Significant, laterality effects were also observed for frontal channels F(1, 40) = 6.47, p < .01.
Fig. 5.
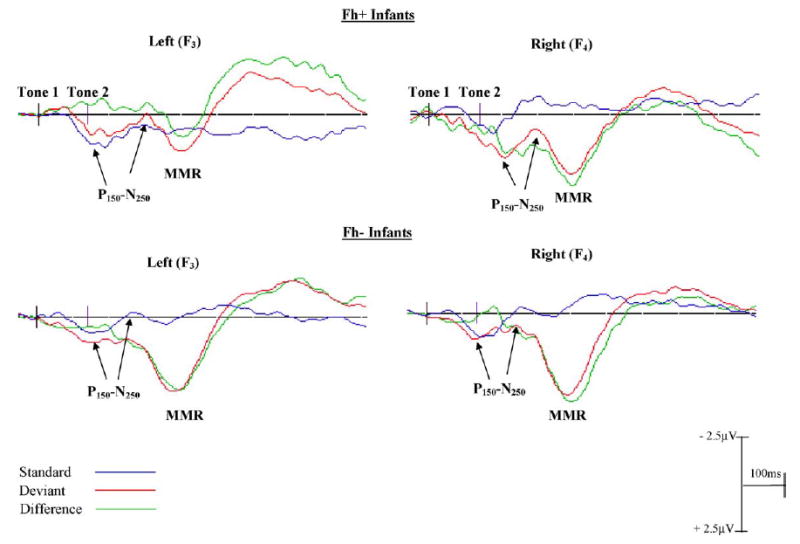
Grand averaged ERP waveforms for FH+ (top) and FH− (bottom) infants to the 70 ms ISI stimuli are presented here. The standard (blue line), deviant (red) and difference (green) waveforms for F3 (electrode13; left) and F4 (electrode 62; right) frontal channels are shown. The onsets of the stimuli are shown by the black vertical lines on the baseline (tone 1-tone 2 represent a single tone pair separated by 70 ms ISI). Negativities are plotted up, positivities down. A significant amplitude difference in the MMR was observed both between and within groups: FH+ infants had a smaller MMR overall as compared to FH−infants, and in the frontal areas only a significant laterality effect for FH+ infants was evident (left < right). No such laterality difference was observed in the FH− group.
Topographic maps representing amplitude of the MMR over the surface of the head are shown in Fig. 6. Peak MMR latency for 300 ms ISI (top) and 70 ms ISI (bottom) are shown for FH+ and FH− infants. Latency was 659 ms for the 300 ms ISI stimulus and 414 for the 70 ms ISI stimulus. Note that there are no significant differences in the 300 ms ISI, but for the 70 ms ISI condition, FH+ infants show reduced positivity at frontal, frontocentral and central channels as well as a significantly smaller MMR (reduced positivity) in the left hemisphere as compared to the FH− infants. There was no significant group by hemisphere interaction for the right hemisphere.
Fig. 6.
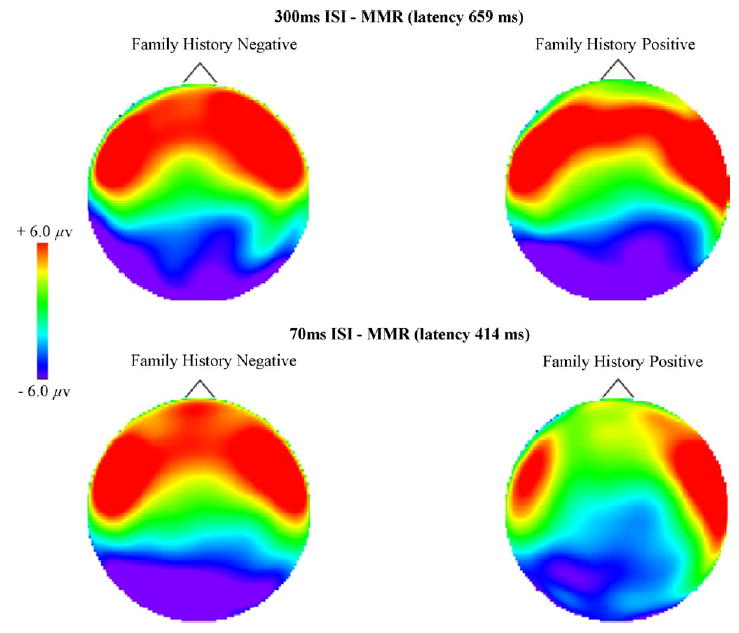
Topographic maps representing amplitude of the mismatch response (MMR) over the entire surface of the head for 300 ms ISI (top) and 70 ms ISI (bottom) are shown at peak MMR latency (659 and 414 ms, respectively) for FH+ and FH− infants. Anterior to posterior represented top to bottom (nose at top). There are no significant group differences in the 300 ms ISI, but for the 70 ms ISI condition the FH+ infants show reduced positivity (MMR) at frontal, frontocentral and central channels as compared to the FH− infants. Additionally, FH+ infants demonstrated a significantly smaller MMR (reduced positivity) in the left hemisphere as compared to FH− infants. There was no significant group by hemisphere interaction for the right hemisphere.
2.2.3. Group differences in language and cognitive abilities at 24 months
Analysis of variance (2 × 2) with gender and group entered as independent variables revealed significant main effects for group and gender in 24-month language abilities (see Table 4). While all infants were within the normal range, infants in the FH+ group scored significantly lower on the expressive language subscale of the PLS-3 compared to infants from the control group. While FH+ infants had lower scores on the receptive language subscale compared to FH− infants this difference did not reach significance. Gender differences were also found on 24-month expressive (F(1, 30) = 8.82, p < 0.01) and receptive (F(1, 30) = 6.62, p < 0.01) language abilities favoring girls. There was no gender by group interactions.
Table 4.
Means (S.D. error) for 24-month-old language and cognitive scores for FH+ (n = 11) and FH− infants (n = 23)
| FH+ | FH− | F | p | |
|---|---|---|---|---|
| Language abilities | ||||
| Expression | 96 (13) | 112 (13) | 8.41 | 0.01 |
| Comprehension | 101(12) | 110 (17) | 2.56 | 0.12 |
| Cognitive abilities | ||||
| Bayley, MDI | 103 (10) | 98 (12) | 1.14 | 0.24 |
2.2.4. Associations between ERPs and language and cognitive abilities at 24 months
Predictions from 6-month ERPs to performance on language and cognitive measures, across groups, were assessed using Pearson’s product moment correlations. Results revealed a series of statistically significant and systematic associations between the brain responses to the 70 ms ISI stimuli and language abilities at 24 months (see Table 5). Specifically, the latency and amplitude of the negative component of the deviant wave (the N250 of the P150–N250 complex) immediately preceding the large positivity was inversely associated with expressive and receptive language abilities in the frontal and central channels (latency r = −0.32 to −0.47, p’s < 0.05; amplitude r = −0.36 to −0.47; p < 0.05). This indicates that infants with faster and more negative N250 waveforms scored higher on standardized measures of both expressive and receptive language. No significant correlations to 24-month language were seen for the amplitude differences in the MMR.
Table 5.
Pearson product moment correlations (r) between 6-month-old infants latency of response to 70 ms ISI stimuli (N250) and expressive and receptive language abilities at 24 months (n = 34)
| Left hemisphere
|
Right hemisphere
|
|||
|---|---|---|---|---|
| Expression | Comprehension | Expression | Comprehension | |
| Brain regions | ||||
| Frontal | −0.50* | −0.40* | −0.40* | −0.31* |
| Fronto-central | −0.36* | −0.35* | −0.41* | −0.40* |
| Central | −0.47* | −0.44* | −0.02 | −0.05 |
| Temporal | −0.03 | −0.01 | −0.09 | −0.05 |
| Parietal | 0.22 | −0.03 | 0.16 | −0.05 |
| Occipital | 0.44* | 0.28* | 0.48* | 0.31* |
p < 0.05.
3. Discussion and conclusions
In the EEG/ERP study described here, we show that infants with a family history of LLI (FH+) and a FH− control group with no such risk factors also differ in patterns of brain activation to the same non-verbal stimuli used in our behavioral studies. Well-defined differences were seen between FH+ and FH− groups in the amplitude of the mismatch response (MMR) as well as the latency of the N250 component in the 70ms ISI condition, but not the 300 ms ISI condition. Infants from families with a history of LLI showed smaller MMRs and delayed onset of the N250 component as compared to infants without familial risk. Whether the clear MMRs we report here for the deviant as compared to the standard tones correspond to the automatic change detection response, suggested to be reflected in MMN-like responses reported in infants (Cheour et al., 2000), will be determined through further analyses as we continue to follow this sample of children. It is not as yet clear what these components represent and how they may be different or similar to the well-described adult components.
The group differences described in 6-month-olds’ brain activation to non-speech stimuli are significantly associated with differences in linguistic performance at 24 months-of-age. Both language comprehension and expression were correlated with the latency of the N250 component. We chose to first examine the 24-month data given the burst in language and cognitive abilities as well as the large amount of individual variation in language development at this age (Fenson et al., 1993; Fenson et al., 1994). Thus, we expected to also see associations between later language and the amplitude differences in the MMR. However, no associations were found between the group differences seen for 6-month MMR and performance on standardized tests at 24 months.
At least one previous study, examining infant response to CV syllables, found similar group differences in MMR between infants at risk for LLI and controls (Friedrich et al., 2004). However, later language has not yet been examined in that sample. Another study of infants at familial risk for dyslexia, that also used CV syllables rather than non-speech, found long-range prediction from infancy (newborms) to 5 years of age (Guttorm et al., 2001, 2003, 2005). Interestingly, the predictive waveforms in this study were not MMR but rather waveforms in the 540–630 ms range. Thus it is important to note that, despite common usage of the MMR as an index of infant response, the underlying processes represented by these components are not clear and there may not be a one-to-one correspondence between the adult waveforms and those that appear at about the same latency in infants (Leppänen et al., 2004a). It may be the case that the N250 is the beginning of the discrimination response in infants (i.e. it is within the right latency range and also seems to have the same polarity as the adult MMN). However, only replications will clarify these results. We are currently analyzing the infant information processing variables and additional EEG/ERP results and will soon be able to report on further findings.
An interesting pattern was also observed in the FH+ group’s standard waveform. In the left frontal and fronto-central areas, in the 70 ms ISI condition only, the standard wave appears to fall somewhat above the baseline for the FH+ group (i.e. the overall waveform is more positive; see Fig. 3). We posit that this may reflect the greater power density in lower versus higher frequency bands (seen in the raw EEG data collected during quiet play) for the FH+ as compared to the FH− control group (Benasich, Gou, Choudhury, Friedman, & Harris, 2005; Gou, Choudhury, Friedman, Harris, & Benasich, 2004). Such differences may index delayed maturational trajectories for a subset of the FH+ group.
Although studies of changes in distribution of power density functions across development are sparse, to date the results demonstrate that as children mature, activity in the lower frequency bands decreases and faster waveform activity increases (Clarke, Barr, McCarthy, Selikowitz, 2001; Gasser, Jennen-Steinmetz, Sroka, Verleger, & Mocks, 1988a; Gasser, Verleger, Bacher, & Sroka, 1988b; John et al., 1980; Katada, Ozaki, Suzuki, & Suhara, 1981; Matousek & Petersen, 1973; Matsuura et al., 1985; Matthis, Scheffner, Benninger, Lipinski, & Stolzis, 1980). We have found that FH+ infants (including a subset of the infants studied here) and matched FH− control children show significant differences in frontal power density in the broad gamma range during resting EEG with lower power seen in the FH+ group (Benasich et al., 2005; Gou et al., 2004). These differences are strongly and significantly associated with concurrent language and cognitive skills at 24 months of age. Alternatively, or perhaps additively, distribution of power density may vary as a function of the recruitment of differing brain areas in order to process the more rapidly presented (i.e. more difficult) stimulus condition. Further studies will help clarify what mechanisms are operating.
The hemispheric pattern of brain response also differed between groups, again only for the more rapidly presented stimuli (70 ms ISI condition). FH+ infants showed lower peak amplitudes in fronto-central areas of the left hemisphere as compared to the right, whereas the FH− controls showed no laterality differences (see left versus right MMR response in Fig. 4.
The differences we report in hemispheric activation, specifically decreased amplitude in left as compared to right brain areas only in FH+ infants, is intriguing in light of research from converging literatures and is evocative of findings in an EEG/ERP study by Leppänen and colleagues (Leppänen et al., 2002b). The authors found that 6-month-old infants at risk for familial dyslexia, as compared to control infants, had reduced response amplitude at the left central hemisphere for a change in consonant duration embedded within a pseudo word (/ata/versus/atta/). Interestingly, although the stimuli they used differed from the non-speech complex tones used in the present study, some similarities are obvious. Specifically, their speech stimulus consisted of a silent within-‘pair’ interval between the vowel and the following stop consonant (95 versus 255 ms). The same research group also found that newborn infants with a familial history of dyslexia (a subset of these infants were seen in the 6-month ERP study) differed in the laterality of their response to CV syllables (/da/, /ga/, /ba/) as compared to matched newborn control infants (Guttorm et al., 2001, 2003). For the group of control infants, the responses to the three CV syllables differed from each other predominately over the left hemisphere; however, infants born into families with a history of dyslexia showed a greater response over the right temporal and parietal areas. One of the interpretations offered was that very early on, even in the newborn period, a family history of LLI might predispose one to the recruitment of differing brain regions for analysis of the speech stream.
Using ERPs with older children Neville et al. (1993) also found decreased activation in frontal and parietal regions while doing a phonological task. Additional insight and potential support for this hypothesis that differing brain regions may be recruited in children with positive family histories of language disorder comes from a large body of research into individuals with LLI using neuroimaging techniques. MRI studies of LLI brains have shown volumetric and asymmetry differences in older children and adults, including a lack of the normal left greater than right pattern in the planum temporale (including Broca’s area), and aberrant asymmetry in the parietal and frontal regions (e.g. Cowell, Jernigan, Denenberg, & Tallal, 1995; Larsen, Hoien, Lund-berg, & Odegaard, 1990; Leonard, Voeller, Lomabardino, Morris, & Hynd, 1993; Jernigan, Hesselink, Sowell, & Tallal, 1991; for a review, see Leppänen et al., 2004a). Functional neuroimaging studies including PET and fMRI have also shown decreased physiological activation in similar regions to both phonological and non-linguistic transient auditory stimuli (e.g. Hagman et al., 1992; Paulesu et al., 1996; Rumsey et al., 1992, 1997; Shaywitz et al., 1998; Temple et al., 2000). Identification and exploration of the possible etiology and developmental trajectory of such atypical brain organization is of much interest and could be examined within a prospective longitudinal study of infants. As we are following these samples of children through the age of 5 years, this is another area that would benefit from further analysis as data are acquired.
Our previous behavioral studies have repeatedly shown that rapid auditory processing skills can be reliably measured across the first year, well before spoken language is in place (Benasich & Leevers, 2002; Benasich & Tallal, 1996; Benasich & Spitz, 1999) and that efficient processing of brief, rapidly presented, successive auditory stimuli is critically important to early language acquisition (Benasich, 1999; Benasich & Tallal, 2002; Benasich et al., 2002).
In a recently published study, infant RAP threshold and being male together predicted 39–41% of the variance in 36-month language outcome (Benasich & Tallal, 2002). Further, discriminant function analyses revealed that classification accuracy to “language-impaired” versus non-impaired groups at 36 months was 93.9% for the Stanford-Binet Verbal/Reasoning Vocabulary Subtest and 90.9% for the Stanford-Binet Verbal/Reasoning Comprehension Subtest. Differences in individual RAP thresholds in infancy were not only strongly related to later language development, but were found to be the single best predictor of expressive and receptive language outcome at all subsequent ages (Benasich & Tallal, 2002). We were intrigued by the strength of this effect in general, but also by the impact seen within the normally developing FH− control group.
Post hoc analysis of these 2002 data revealed a robust within group effect even though the sample sizes were fairly small. Mean RAP threshold at 7.5 months significantly predicted expressive and receptive language at 24 months of age in both groups. Specifically, within the FH− control group, RAP thresholds at the mean age of 7.5 months strongly predicted language comprehension (F(1, 31) = 47.0, p < .0001) and expression (F(1, 31) = 21.8, p < .0001) on the PLS-3 at 24 months. Even within the small FH+ group, infant RAP thresholds significantly predicted PLS-3 Language Comprehension (F(1, 10) = 9.8, p = .01) as well as Expression (F(1,10) = 7.8, p = .02). In the previous analyses reported in 2002, gender was significant across groups with males doing more poorly. However, no significant within-group contributions for gender were seen.
These additional data suggest that linguistic outcomes are as strongly related to early RAP in normally developing control infants as in infants at higher risk for LLI as a function of family history. Therefore, these findings further demonstrate the close link between RAP and emerging language and emphasize the importance of examining more general mechanisms that appear to be important for normal language development.
Research that uses the human infant as a prelinguistic, model holds considerable promise in resolving conflicting models of normal and atypical language development. It seems quite likely that early deficits in lower-level processing have their effect quite early on in development when acoustic and phonological maps are being constructed (Fitch et al., 2001). Such constraints (i.e. slower more effortful processing and difficulties in acoustic analysis) may contribute to an inability to extract the key acoustic features of language and to map the unique phonemic distribution of that language into auditory cortex. If these representations are poorly formed, expressive and/or receptive language delays may surface in early childhood, and reading, writing, and spelling deficits may follow given the importance of accurate phonographic (spoken) to orthographic (visual/written) mapping (Benasich & Read, 1998; Tallal, Merzenich Miller, & Jenkins, 1998; Tallal, 2004). It is also critical to examine more closely the contributions of general mechanisms such as attention, perception, and memory to variation in the establishment of early language.
Overall, our data suggest that ERPs, during infancy may in the not too distant future be a useful tool for early screening, which might be employed even at the individual level. Prelinguistic infants at high risk for LLI might be assessed and intervened for deficient RAP skills and perhaps the cascading effects of poor processing skills could be avoided (Benasich & Leevers, 2002). ERPs, when combined with behavioral measures, may also provide the predictive power necessary for detailed study of other populations at high risk for LLI (e.g. VLBW preterm infants). Use of converging electrophysiological and behavioral methodologies should allow better characterization of the nature and developmental origin of the deficits that contribute to a diagnosis of LLI. Finally, the research presented here illustrates the utility of examining early processing skills using converging methodologies to both validate and extend knowledge about the early precursors of LLI and provide the opportunity for earlier identification and intervention.
Acknowledgments
This research was supported by a grant to AAB from NICHD (RO1-HD29419) and a Rutgers University Board of Trustees Excellence in Research Award to AAB, with additional support from the Human Frontier Science Program Organization, the Don and Linda Carter Foundation and the Elizabeth H. Solomon Center for Neurodevelopmental Research. We thank Dr. Paavo Leppänen for his expertise and technical assistance with data collection and for input and critical discussions regarding the development of the ERP paradigm. In addition, we thank him as well as our referees for their many valuable comments on this manuscript.
Footnotes
For the sake of clarity, in this study the term language learning impairment (LLI) is used, instead of other terms that may have been used in the literature, to encompass language disorders that fit the general criteria of LLI.
The description of the MMR in the ERP literature is based on the well described adult mismatch negativity (MMN) component that is thought to reflect a pre-attentive auditory change detection process (for review see Näätänen, 1992; Näätänen, Gaillard, & Mäntysalo, 1978). Thus the MMN may provide a valuable index for investigating specific discrimination abilities such as fine-grained temporal processing in adults, children and preverbal infants. However, the underlying processes represented by these components are not clear and there may not be a one-to-one correspondence between adult waves and those that seem to be appear at the same latency in infants.
A subset of the files were adjusted for temporal offsets, see Jing, Heim, Chojnowskia, Thomas and Benasich (2004) for more detail. These files were also subjected to split-half within group comparison with unadjusted files. No differences in waveform amplitudes or latencies were seen in the FH− control group. More variability in amplitude and latency was seen in the FH+ group than in the control group. However, these differences in latency and amplitude (across groups) were highly correlated with individual behavioral performance, thus providing additional confirmation that group differences were strongly tied to risk status. Thus, those infants with faster and more negative N250 waveforms scored higher on standardized measures of both expressive and receptive language irrespective of group. A summary of these results can be seen in Tables 4 and 5.
The N1* is not equivalent to the adult N100. It is the first identifiable negative peak following the onset of the second tone observed only in the 300 ms ISI condition.
Activity in the anterior brain regions was collected from frontal, fronto-central and central electrodes (F3, F4, Fc3, Fc4, C3 and C4) while parietal and occipital electrodes were taken to represent activity over posterior regions (P3, P4, O1 and O2).
The second tone in the pair occurs at 140 ms. The P150-N250 in the 70 ms ISI stimuli block seems to be a response to both the tones in the pair (processed as a single event). For the sake of consistency and comparability these waveforms will also be referred to as the P150 and the N250.
References
- Aslin RN. Discrimination of frequency transitions by human infants. Journal of the Acoustical Society of America. 1989;86:582–590. doi: 10.1121/1.398237. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Hunt RH. Development, plasticity, and learning in the auditory system. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: The MIT Press; 2001. pp. 205–220. [Google Scholar]
- Aslin RN, Pisoni DB, Juczyk PW. Auditory development and speech perception in infancy. In: Haith MM, Campos JJ, Mussen PH, editors. Handbook of child psychology: Vol. 2. Infancy and developmental psychobiology. 4th ed. New York: Wiley; 1983. pp. 573–688. [Google Scholar]
- Bayley N. The Bayley scales of infant development. 2nd ed. New York: Psychological Corporation; 1993. [Google Scholar]
- Benasich AA. Temporal integration as an early predictor of speech and language development. In: von Euler C, Lundberg I, Llinás R, editors. Basic mechanisms in cognition and language, with special reference to phonological problems in dyslexia (Wenner-Gren International Series): Vol. 70. Oxford: Elsevier Science Ltd; 1999. pp. 123–142. [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT. Infant discrimination of brief sequential tone doublets is a better predictor of later language than discrimination of consonant–vowel syllables. Society for Neuroscience Abstracts. 2003;29 Program No. 288.3. [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, Friedman JT, Harris KD. EEG power spectral analysis of 24-month-old children with family history of specific language impairment. 2005 submitted for publication. [Google Scholar]
- Benasich AA, Leevers HJ. Processing of rapidly presented auditory cues in infancy: Implications for later language development. In: Fagan J, Haynes H, editors. Progress in infancy research. Vol. 3. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2002. pp. 245–288. [Peer-reviewed edited annual volume] [Google Scholar]
- Benasich AA, Read H. Representation: Picture or process? In: Sigel IE, editor. Theoretical perspectives in the development of representational (symbolic) thought. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1998. pp. 33–160. [Google Scholar]
- Benasich AA, Spitz RV. Insights from infants: Temporal processing abilities and genetics contribute to language development. In: Whitmore K, Hart H, Willems G, editors. A neurodevelopmental approach to specific learning disorders. London: Mac Keith Press; 1999. pp. 191–210. [Google Scholar]
- Benasich AA, Tallal P. Auditory temporal processing thresholds, habituation, and recognition memory over the first year. Infant Behavior and Development. 1996;19:339–357. [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behavioral Brain Research. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, Leppäne PHT. The importance of rapid auditory processing abilities to early language development: Evidence from converging methodologies. Developmental Psychobiology. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird D, Bishop D, Freeman N. Phonological awareness and literacy development in children with expressive phonological impairments. Journal of Speech and Hearing Research. 1995;38:446–462. doi: 10.1044/jshr.3802.446. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Edmundson A. Is otitis media a major cause of specific developmental language disorders? British Journal of Disorders of Communication. 1986;21:321–338. doi: 10.3109/13682828609019845. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, North T, Donlan C. Genetic basis of specific language impairment: Evidence from a twin study. Developmental Medicine and Child Neurology. 1995;37:56–71. doi: 10.1111/j.1469-8749.1995.tb11932.x. [DOI] [PubMed] [Google Scholar]
- Ceponiené R, Hukki J, Cheour M, Haapanen ML, Koskinen M, Alho K, et al. Dysfunction of the auditory cortex persists in infants with certain cleft types. Developmental Medicine and Child Neurology. 2000;42:258–265. doi: 10.1017/s001216220000044x. [DOI] [PubMed] [Google Scholar]
- Ceponiené R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clinical Neurophysiology. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Cheour M, Alho K, Sainio K, Reinikainen K, Runlund M, Aaltonen O, Eerola O, Näätänen R. The mismatch negativity to changes in speech sounds at the age of 3 months. Developmental Neuropsychology. 1997;13:167–174. [Google Scholar]
- Cheour M, Ceponiené R, Leppänen P, Alho K, Kujala T, Renlund M, Fellman V, Näätänen R. The auditory sensory memory trace decays rapidly in newborns. Scandinavian Journal of Psychology. 2002;43:33–39. doi: 10.1111/1467-9450.00266. [DOI] [PubMed] [Google Scholar]
- Cheour M, Kushnerenko E, Ceponiené R, Fellman V, Näätänen R. Electric brain responses obtained from newborn infants to changes in duration in complex harmonic tones. Developmental Neuropsychology. 2002;22:471–479. doi: 10.1207/S15326942DN2202_3. [DOI] [PubMed] [Google Scholar]
- Cheour M, Leppänen PHT, Kraus N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology. 2000;111:4–16. doi: 10.1016/s1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Cheour M, Martynova O, Näätänen R, Erkkola R, Sillanpää M, Kero P, et al. Speech sounds learned by sleeping newborns. Nature. 2002;415:599–600. doi: 10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- Cheour-Luhtanen M, Alho K, Kujala T, Sainio K, Reinikainen K, Renlund M, et al. Mismatch negativity indicates vowel discrimination in newborns. Hearing Research. 1995;82:53–58. doi: 10.1016/0378-5955(94)00164-l. [DOI] [PubMed] [Google Scholar]
- Cheour-Luhtanen M, Alho K, Sainio K, Rinne T, Reinikainen K, Pohjavuori M, et al. The ontogenetically earliest discriminative response of the human brain. Psychophysiology. 1996;33:478–481. doi: 10.1111/j.1469-8986.1996.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Benasich AA. The influence of family history and other risk factors on language development. Journal of Speech Language and Hearing Research. 2003;46:261–272. doi: 10.1044/1092-4388(2003/021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen H. The grammatical characterization of developmental dysphasia. Linguistics. 1992;27:897–920. [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Chronology of post-natal human brain development: Event-related potential, positron emission tomography, myelinogenesis, and synaptogenesis studies. In: Rohrbaugh JW, Parasuraman R, Johnson R Jr, editors. Event-related brain potentials: Basic issues and applications. New York: Oxford University Press; 1990. pp. 210–241. [Google Scholar]
- Cowell PE, Jernigan TL, Denenberg VH, Tallal P. Language and learning impairment and prenatal risk: An MRI study of the corpus callosum and cerebral volume. Journal of Medical Speech Language Pathology. 1995;3:1–13. [Google Scholar]
- Dehaene-Lambertz G. Cerebral specialization for speech and non-speech stimuli in infants. Journal of Cognitive Neuroscience. 2000;12:449–460. doi: 10.1162/089892900562264. [DOI] [PubMed] [Google Scholar]
- Eilers RE, Morse PA, Gavin WJ, Oller DK. Discrimination of voice onset time in infancy. Journal of the Acoustical Society of America. 1981;70:955–965. doi: 10.1121/1.387024. [DOI] [PubMed] [Google Scholar]
- Eimas PD. Auditory and phonetic coding of the cues for speech: Discrimination of the/r-l/distinction by young infants. Perception and Psychophysics. 1975;18:341–347. [Google Scholar]
- Eimas PD, Siqueland E, Jusczyk P, Vigorito J. Speech perception in infants. Science. 1971;171:303–306. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- Elliott LL, Hammer MA. Longitudinal changes in auditory discrimination in normal children and children with language-learning problems. Journal of Speech and Hearing Disorders. 1988;53:467–474. doi: 10.1044/jshd.5304.467. [DOI] [PubMed] [Google Scholar]
- Elliott LL, Hammer MA, Scholl ME. Fine-grained auditory discrimination in normal children and children with language-learning problems. Journal of Speech and Hearing Research. 1989;32:112–119. doi: 10.1044/jshr.3201.112. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Klein RM. The evidence for a temporal processing deficit linked to dyslexia: A review. Psychonomic Bulletin and Review. 1995;2(4):460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Fenson LS, Dale PS, Reznick JS, Bates E, Thal D, Pethick S. Variability in early communicative development. Monographs of the Society for Research in Child Development. 1994;59:1–173. [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. Technical manual for the MacArthur communicative development inventory. San Diego: Singular Press; 1993. [Google Scholar]
- Fitch RH, Read H, Benasich AA. Neurophysiology of speech perception in normal and impaired systems. In: Jahn A, Santos-Sacchi J, editors. Physiology of the ear. 2nd ed. San Diego: Singular Publishing Group, Inc; 2001. pp. 651–672. [Google Scholar]
- Fitch RH, Tallal P. Neural mechanisms of language-based learning impairments: Insights from human populations and animal models. Behavioral and Cognitive Neuroscience Reviews. 2003;2(3):155–178. doi: 10.1177/1534582303258736. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Weber C, Friederici AD. Electrophysiological evidence for delayed mismatch response in infants at risk for specific language impairment. Psychophysiology. 2004;41:772–782. doi: 10.1111/j.1469-8986.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Gasser T, Jennen-Steinmetz C, Sroka L, Verleger R, Mocks J. Development of the EEG of school-age children and adolescents. II. Topography. Electroencephalography and Clinical Neurophysiology. 1988;69:100–109. doi: 10.1016/0013-4694(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bacher P, Sroka L. I. Analysis of band power. Electroencephalography and Clinical Neurophysiology. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children: Is there a causal connection? Journal of Memory and Language. 1990;29:336–360. [Google Scholar]
- Gopnik M, Crago M. Familial aggregation of a developmental language disorder. Cognition. 1991;39:1–50. doi: 10.1016/0010-0277(91)90058-c. [DOI] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, Friedman JT, Harris KD, Benasich AA. EEG power spectral analysis of 24-month-old children with family history of specific language impairment. Society for Neuroscience Abstracts. 2004;30 Program No. 319.28. [Google Scholar]
- Guttorm TK, Leppänen PHT, Poikkeus AM, Eklund KM, Lyytinen P, Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41(3):291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Richardson U, Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. Journal of Learning Disabilities. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Tolvanen A, Lyytinen H. Event-related potential in newborns with and without familial risk for dyslexia: Principal component analysis reveals differences between the groups. Journal of Neural Transmission. 2003;110:1059–1074. doi: 10.1007/s00702-003-0014-x. [DOI] [PubMed] [Google Scholar]
- Habib M. The neurological basis of developmental dyslexia: An overview and working hypothesis. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- Hagman J, Wood F, Buchsbaum M, Flowers L, Katz W, Tallal P. Cerebral brain metabolism in adult dyslexics assessed with positron emission tomography during performance of an auditory task. Archives of Neurology. 1992;49:734–739. doi: 10.1001/archneur.1992.00530310082015. [DOI] [PubMed] [Google Scholar]
- Hari R, Kiesla P. Deficit of temporal auditory processing in dyslexic adults. Neuroscience Letters. 1996;205:138–140. doi: 10.1016/0304-3940(96)12393-4. [DOI] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Gillam RB. Verbal and spatial information processing constraints in children with specific language impairment. Journal of Speech Language and Hearing Research. 2004;47(1):114–125. doi: 10.1044/1092-4388(2004/011). [DOI] [PubMed] [Google Scholar]
- Irwin RJ, Ball A, Kay N, Stillman J, Roser J. The development of auditory temporal acuity in children. Child Development. 1985;56:614–620. [PubMed] [Google Scholar]
- Jernigan T, Hesselink JR, Sowell E, Tallal P. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Archives of Neurology. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Jing H, Choudhury N, Thomas-Friedman J, Benasich AA. Development of auditory event-related potentials and mismatch negativity across the first 2 years. Society for Neuroscience Abstracts. 2003;29 Program No. 148.15. [Google Scholar]
- Jing H, Heim S, Chojnowskia C, Thomas J, Benasich AA. Timing errors in auditory event-related potentials. Journal of Neuroscience Methods. 2004;138(1–2):1–6. doi: 10.1016/j.jneumeth.2004.03.009. [DOI] [PubMed] [Google Scholar]
- John ER, Ahn H, Pricep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Pisoni DB, Walley A, Murray J. Discrimination of relative onset time of two-component tones by infants. Journal of the Acoustical Society of America. 1980;67:262–270. doi: 10.1121/1.383735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada A, Ozaki H, Suzuki H, Suhara K. Developmental characteristics of normal and mentally retarded children’s EEGs. Electroencephalography and Clinical Neurophysiology. 1981;52:192–201. doi: 10.1016/0013-4694(81)90166-8. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: Cracking the speech code. Nature Reviews Neuroscience. 2004;5(11):831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences USA. 2003;100:9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D. Event-related potentials in the evaluation of high-risk infants. Annals of the New York Academy of Sciences. 1982;388:557–571. doi: 10.1111/j.1749-6632.1982.tb50816.x. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Hilpert PL, Kreuzer JA, Vaughan J. Differential maturation of cortical auditory evoked potentials to speech sounds in normal fullterm and very low-birthweight infants. Developmental Medicine and Child Neurology. 1984;26:466–475. doi: 10.1111/j.1469-8749.1984.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Vaughan HG, Jr, Kreuzer JA, Fliegler KZ. Developmental studies and clinical application of mismatch negativity: Problems and prospects. Ear and Hearing. 1995;16:105–117. doi: 10.1097/00003446-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Ceponiené R, Balan P, Fellman V, Huotilainen M, Näätänen R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport: For Rapid Communication of Neuroscience Research. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Ceponiené R, Balan P, Fellman V, Näätänen R. Maturation of the auditory change detection response in infants: A longitudinal ERP study. NeuroReport. 2002;13:1843–1848. doi: 10.1097/00001756-200210280-00002. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Cheour M, Ceponiené R, Fellman V, Renlund M, Soininen K, Alku P, Koskinen M, Sainio K, Näätänen R. Central auditory processing of durational changes in complex speech patterns by newborns: An event-related brain potential study. Developmental Neuropsychology. 2001;19:83–97. doi: 10.1207/S15326942DN1901_6. [DOI] [PubMed] [Google Scholar]
- Lahey M, Edwards J. Specific language impairment: Preliminary investigation of factors associated with family history and with patterns of language performance. Journal of Speech and Hearing Research. 1995;38:643–657. doi: 10.1044/jshr.3803.643. [DOI] [PubMed] [Google Scholar]
- Larsen JP, Hoien T, Lundberg I, Odegaard H. MRI evaluation of the size and symmetry of the planum temporale in adolescents with developmental dyslexia. Brain and Language. 1990;39:289–301. doi: 10.1016/0093-934x(90)90015-9. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Voeller KKS, Lombardino LJ, Morris MK, Hynd GW. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Archives of Neurology. 1993;50:461–469. doi: 10.1001/archneur.1993.00540050013008. [DOI] [PubMed] [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Leppänen PHT, Choudhury N, Benasich AA, Lyytinen H. Classifications of developmental language disorders: Theoretical issues and clinical implications. Mahwah, NJ: Lawrence Elrbaum Press; 2004. Neuroimaging measures in the study of specific language impairment; pp. 99–137. [Google Scholar]
- Leppänen PHT, Choudhury N, Thomas J, Jing H, Benasich AA. Brain event-related potentials index rapid auditory processing in adults and 24-month-old children. Journal of Cognitive Neuroscience. 2002;14(Suppl):100B. [Google Scholar]
- Leppänen PHT, Eklund KM, Lyytinen H. Event-related brain potentials to change in rapidly presented acoustic stimuli in newborns. Developmental Neuropsychology. 1997;13:175–204. [Google Scholar]
- Leppänen PHT, Guttorm TK, Pihko E, Takkinen S, Eklund KM, Lyytinen H. Maturational effects on newborn ERPs measured in the mismatch negativity paradigm. Experimental Neurology. 2004;190:S91–S101. doi: 10.1016/j.expneurol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Leppänen PHT, Pihko E, Eklund M, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: II. Group effects. NeuroReport. 1999;10:969–973. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- Leppänen PHT, Richardson U, Pihko E, Eklund KM, Guttorm TK, Aro M, Lyytinen H. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Developmental Neuropsychology. 2002;22(1):407–422. doi: 10.1207/S15326942dn2201_4. [DOI] [PubMed] [Google Scholar]
- Liberman AM. Speech: A special code. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Luu P, Ferree T. Electrical Geodesics, Inc; Oregon, US: 2000. Determination of the Geodesic Sensor Nets average electrode positions and their 10–10 international equivalents., Technical note. [Google Scholar]
- Martynova O, Kirjavainen J, Cheour M. Mismatch negativity and late discriminative negativity in sleeping human newborns. Neuroscience Letters. 2003;340:75–78. doi: 10.1016/s0304-3940(02)01401-5. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Yamamoto K, Fukuzawa H, Okubo Y, Uesugi H, Moriwa M, Kojima T, Shimazono Y. Age development and sex differences of various EEG elements in healthy children and adults-quantification by a computerized wave form recognition method. Electroencephalography and Clinical Neurophysiology. 1985;60:394–406. doi: 10.1016/0013-4694(85)91013-2. [DOI] [PubMed] [Google Scholar]
- Matthis P, Scheffner D, Benninger C, Lipinski C, Stolzis L. Changes in the background activity of the electroencephalogram according to age. Electroencephalography and Clinical Neurophysiology. 1980;49:626–635. doi: 10.1016/0013-4694(80)90403-4. [DOI] [PubMed] [Google Scholar]
- Matusek M, Petersen L. Automatic evaluation of EEG background activity by way of age-dependent quotients. Electroencephalography and Clinical Neurophysiology. 1973;35:603–612. doi: 10.1016/0013-4694(73)90213-7. [DOI] [PubMed] [Google Scholar]
- Maye J, Werker JF, Gerken L. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 2002;82:B101–B111. doi: 10.1016/s0010-0277(01)00157-3. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Stein JF. Auditory temporal coding in dyslexia. Proceedings of the Royal Society of London. 1996;263:961–965. doi: 10.1098/rspb.1996.0142. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Stein JF. Scalp potentials evoked by amplitude modulated tones in dyslexia. Journal of Speech, Language, and Hearing Research. 1997;40:939–945. doi: 10.1044/jslhr.4004.939. [DOI] [PubMed] [Google Scholar]
- McCrosky R, Kidder H. Auditory fusion among learning disabled, reading disabled and normal children. Journal of Learning Disabilities. 1980;13:69–76. doi: 10.1177/002221948001300205. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Molfese VJ. Electrophysiological indices of auditory discrimination in newborn infants: The bases for predicting later language development? Infant Behavior and Development. 1985;8:197–211. [Google Scholar]
- Molfese DL, Molfese VJ. Discrimination of language skills at 5 years of age using event-related potentials recorded at birth. Developmental Neuropsychology. 1997;13:135–156. [Google Scholar]
- Morr ML, Shafer VL, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear and Hearing. 2002;23(2):118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Morrongiello BA, Trehub SE. Age-related changes in auditory temporal perception. Journal of Experimental Child Psychology. 1987;44:413–426. doi: 10.1016/0022-0965(87)90043-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Attention and brain function. Hillsdale, NJ: Lawrence Erlbaum; 1992. [Google Scholar]
- Näätänen R, Gaillard AWK, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Neils J, Aram DM. Family history of children with developmental language disorders. Perceptual and Motor Skills. 1986;63:655–658. doi: 10.2466/pms.1986.63.2.655. [DOI] [PubMed] [Google Scholar]
- Neville H, Coffey S, Holcomb P, Tallal P. The neurobiology of sensory and language processing in language impaired children. Journal of Cognitive Neuroscience. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- Novak GP, Kurtzberg D, Kreuzer JA, Vaughan HG., Jr Cortical responses to speech sounds and their formants in normal infants: Maturational sequence and spatiotemporal analysis. Electroencephalography and Clinical Neurophysiology. 1989;73:295–305. doi: 10.1016/0013-4694(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K. A phenotype of specific language impairments: Extended optional infinitives. In: Rice ML, editor. Towards a genetics of language. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 215–237. [Google Scholar]
- Robin D, Tomblin JB, Kearney A. Auditory temporal pattern learning in children with severe speech and language impairment. Brain and Language. 1989;36:604–613. doi: 10.1016/0093-934x(89)90089-8. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, Pikus A, Rapoport JL, Cohen RM. Failure to activate the left temporoparietal cortex in dyslexia. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donohue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives of Neurology. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Scarborough HS. Very early language deficits in dyslexic children. Child Development. 1990;61:1728–1743. [PubMed] [Google Scholar]
- Shafer VL, Morr ML, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in school-age children. Ear and Hearing. 2000;21:242–251. doi: 10.1097/00003446-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Science, USA. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schul R, Stiles J, Wulfeck B, Townsend J. How ‘generalized’ is the ‘slowed processing’ in SLI? The case of visuospatial attentional orienting. Neuropsychologia. 2004;42:661–671. doi: 10.1016/j.neuropsychologia.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Stark RE, Tallal P. San Diego: College-Hill; 1988. Language, speech and reading disorders in children: Neuropsychological studies. [Google Scholar]
- Sussman JE. Perception of formant transition cues to place of articulation in children with language impairments. Journal of Speech and Hearing Research. 1993;36:1286–1299. doi: 10.1044/jshr.3606.1286. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Crow CD. Development of evoked electrical brain activity in infancy. In: Dawson G, editor. Human behavior and the developing brain. New York: Guilford Publications; 1994. –207.pp. 231 [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nature Reviews Neuroscience. 2004;5(9):721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tallal P, Hirsch LS, Realpe-Bonilla T, Miller S, Brzustowicz LM, Bartlett C, Flax JF. Familial aggregation in specific language impairment. Journal of Speech Language and Hearing Research. 2001;44(5):1172–1182. doi: 10.1044/1092-4388(2001/091). [DOI] [PubMed] [Google Scholar]
- Tallal P, Merzenich MM, Miller S, Jenkins W. Language learning impairment: Integrating basic science technology and remediation. Experimental Brain Research. 1998;123:210–219. doi: 10.1007/s002210050563. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Prot opapas A, Nagarajan S, Salz T, Tallal P, Merzenich MM, Gabrieli JD. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from fMRI. Proceedings of the National Academy of Sciences. 2000;97(25):13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehub SE, Henderson JL. Temporal resolution in infancy and subsequent language development. Journal of Speech and Hearing Research. 1996;39(6):1315–1320. doi: 10.1044/jshr.3906.1315. [DOI] [PubMed] [Google Scholar]
- Tallal P, Ross R, Curtiss S. Familial aggregation in specific language impairment. Journal of Speech Language and Hearing Disorders. 1989;54(2):167–173. doi: 10.1044/jshd.5402.167. [DOI] [PubMed] [Google Scholar]
- Tomblin BJ. Familial concentration of developmental language impairment. Journal of Speech and Hearing Disorders. 1989;54(2):287–295. doi: 10.1044/jshd.5402.287. [DOI] [PubMed] [Google Scholar]
- Tomblin BJ, Buckwalter PR. Heritability of poor language achievement among twins. Journal of Speech, Language and Hearing Research. 1998;41:188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Henderson JL. Gap detection in infants, children, and adults. Journal of the Acoustical Society of America. 1995;98:2532–2541. doi: 10.1121/1.414396. [DOI] [PubMed] [Google Scholar]
- Tsao FM, Liu HM, Kuhl PK. Speech perception in infancy predicts language development in the 2nd year of life: A longitudinal study. Child Development. 2004;75(4):1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Stollwerck L. A grammatical specific language impairment in children: An autosomal dominant inheritance? Brain and Language. 1996;52:484–504. doi: 10.1006/brln.1996.0026. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and it’s causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Weber C, Hahne A, Friedrich M, Friederici AD. Discrimination of word stress in early infant perception: Electrophysiological evidence. Cognitive Brain Research. 2003;18:149–161. doi: 10.1016/j.cogbrainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Webster RI, Shevell MI. Neurobiology of specific language impairment. Journal of Child Neurology. 2004;19(7):471–481. doi: 10.1177/08830738040190070101. [DOI] [PubMed] [Google Scholar]
- Werner LA, Rubel EW. Washington, DC: American Psychological Association; 1992. Developmental Psychoacoustics. [Google Scholar]
- Weitzman ED, Graziani LJ. Maturation and topography of the auditory evoked response of the prematurely born infant. Developmental Psychobiology. 1968;1:79–89. [PubMed] [Google Scholar]
- Whitehurst GJ, Fischel JE. Practitioner review: Early developmental language delay: What, if anything, should the clinician do about it. Journal of Child Psychology and Psychiatry. 1994;35:613–648. doi: 10.1111/j.1469-7610.1994.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Witton C, Talcott J, Hansen P, Richardson A, Griffiths T, Rees A, et al. Sensitivity to dynamic auditory and visual stimuli predicts non-word reading ability in both dyslexic and normal readers. Current Biology. 1998;8:791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- Wright BA, Lombardino LJ, King WM, Puranik CS, Leonard CM, Merzenich MM. Deficits in auditory temporal and spectral resolution in language-impaired children. Nature. 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool language scale-3. New York: The Psychological Corporation; 1992. [Google Scholar]


