Abstract
Mutations in >30 genes that regulate different pathways and developmental processes are reported to cause a melanotic phenotype in larvae. The observed melanotic masses were generally linked to the hemocyte-mediated immune response. To investigate whether all black masses are associated with the cellular immune response, we characterized melanotic masses from mutants in 14 genes. We found that the melanotic masses can be subdivided into melanotic nodules engaging the hemocyte-mediated encapsulation and into melanizations that are not encapsulated by hemocytes. With rare exception, the encapsulation is carried out by lamellocytes. Encapsulated nodules are found in the hemocoel or in association with the lymph gland, while melanizations are located in the gut, salivary gland, and tracheae. In cactus mutants we found an additional kind of melanized mass containing various tissues. The development of these tissue agglomerates is dependent on the function of the dorsal gene. Our results show that the phenotype of each mutant not only reflects its connection to a particular genetic pathway but also points to the tissue-specific role of the individual gene.
HALF a century ago melanotic tumors in Drosophila larvae and adults were viewed as the equivalent of cancer and as events of controlled histological differentiation that could be manipulated genetically. The participation of blood cells in the formation of some melanotic tumors was reported at about the same time (Oftedal 1953; Barigozzi 1958; Rizki 1960). Black melanotic spots are found in a number of different mutants and have been called, interchangeably, melanotic tumors or pseudotumors. These “tumors” are usually not invasive and involve tumorous overgrowth only in some instances. Therefore we use the term “melanotic masses” to describe the phenotype generally and “melanotic nodules” and “melanizations” to describe more specific phenotypes.
Well-documented studies of larval melanotic masses have shown the involvement of the immune response mediated by hemocytes (Rizki and Rizki 1983; Harrison et al. 1995; Rodriguez et al. 1996; Qiu et al. 1998; Nappi et al. 2005). This cellular immune response includes cell aggregation, phagocytosis, encapsulation of foreign material, and induction of the melanization cascade (for reviews see De Gregorio et al. 2002a; Fossett et al. 2003; Meister and Lagueux 2003; Nappi and Christensen 2005). Phagocytosis is carried out by the most abundant class of hemocytes, the plasmatocytes. Lamellocytes, functioning in encapsulation, are rare in healthy larvae and increase substantially during metamorphosis and after infection. Melanization/melanogenesis is facilitated by a distinct type of blood cells, the crystal cells. Crystal cells express the enzyme phenoloxidase (Pro-phenoloxidase A1), responsible for the initiation of the melanogenesis cascade, at rate-limiting levels (Lebestky et al. 2000; Duvic et al. 2002; Sugumaran 2002; Nappi and Christensen 2005). The activation of Pro-phenoloxidase is partially controlled by the serine protease inhibitor serpin 27A (Spn27A). Spn27A mutant larvae show a melanotic phenotype and excessive melanization in response to immune challenge (Nappi et al. 2005). This phenotype is linked to the activation of the Toll pathway (Tingvall et al. 2001; De Gregorio et al. 2002b; Ligoxygakis et al. 2002a,b; Nappi et al. 2005).
The Toll pathway is responsible for the formation of melanotic masses in Drosophila (Lemaitre et al. 1995). The pathway is named after the transmembrane receptor Toll that, when activated, controls the nuclear targeting of the Drosophila NF-κB/Rel proteins Dorsal and Dif. The pathway is a major effector of innate immunity and is also involved in hematopoiesis. Constitutive activation of the pathway in Toll gain-of-function or cactus loss-of-function mutants leads to overproliferation of hemocytes, in particular lamellocytes, resulting in the formation of melanotic nodules (Lemaitre et al. 1995; Qiu et al. 1998; Lavine and Strand 2002). Jak/STAT is another signaling pathway functioning in innate immunity and hematopoiesis. Constitutive activation of this pathway in HopTum mutants leads to phenotypes similar to those observed in constitutive activation of Toll, including overproliferation of hemocytes and melanotic nodules (Harrison et al. 1995; Lagueux et al. 2000; Luo et al. 2002; Evans et al. 2003; Meister 2004).
The Drosophila immune deficiency (IMD)/Relish pathway also functions in the immune response and has a connection to melanotic nodule formation. After immune challenge of larvae with constitutive expression of the peptidoglycan-recognition protein-LE, functioning upstream of the IMD pathway, melanotic masses were observed in the cuticle and hemolymph (Takehana et al. 2002).
The activation of other pathways like the Ras/MAPK in hemocytes by the expression of transgenes leads to hemocyte proliferation and formation of melanotic masses (Asha et al. 2003; Zettervall et al. 2004). There are also several loss-of-function mutations leading to similar phenotypes. These include mutations in yantar, functioning in hemocyte differentiation (Sinenko et al. 2004; Sinenko and Mathey-Prevot 2004), mutations in the ribosomal gene rpS6 that lead to hemocyte malignancies and aberrant immune system behavior (Watson et al. 1992; Stewart and Denell 1993), black-pearl (blp), a distant homolog of DnaJ (Becker et al. 2001), and the malignant blood neoplasm gene l(3)mbn (Konrad et al. 1994). Additional mutants resulting in melanotic phenotypes were identified in genetic screens and many were linked to immunity (Watson et al. 1991, 1994; Rodriguez et al. 1996; Zettervall et al. 2004).
A large number of “tumorogenic” mutants were identified before the era of whole-genome sequencing and modern methods of gene mapping. The observed phenotypes lead to gene names like tumorous (tu-s), malignant blood neoplasm (mbn-s), and aberrant immune response (air-s; reviewed in Gateff 1977; Watson et al. 1991, 1994; Lindsley and Zimm 1992). More than 30 genome annotated genes have been reported to cause melanotic phenotypes in larvae. The tumors in ∼20 of these genes were not histologically characterized and the involvement of blood cells was not established. Rather, it is commonly assumed that all melanotic masses are caused by abnormalities in the immune response.
Here we report a morphological and immuno-histochemical analysis of melanotic nodules produced by mutations in 14 genes, representing an array of pathways and cellular functions and characterized to various degrees. Our experiments are aimed at understanding if all melanotic phenotypes are linked to the cellular immune response conventionally associated with encapsulation by hemocytes. We found that nodules in half of the mutants are surrounded by blood cells, in particular, lamellocytes. Most of these mutations are in genes previously reported to function in hematopoiesis and in the immune response. Three genes, zfrp8, Su(var)205, and spag, have melanotic nodules surrounded by hemocytes, but the function of these genes in hematopoiesis or immunity is not established.
Interestingly, more than half of the mutants produce melanizations in the gut, trachea, cuticle, or salivary glands, and no hemocytes were detected surrounding these nodules. This suggests that the mechanism for their formation does not involve most aspects of the cellular immune response, except possibly melanization.
Our results show that the histological location of black masses is a reliable primary indication if encapsulation by hemocytes is involved. While most mutants in a given pathway showed similar phenotypes, we also observed tissue-specific effects particular to individual pathway components. This is seen, for instance, in hypomorphic alleles of cact, which produce melanotic aggregates not seen in Toll gain-of-function alleles. Our results show that cact may control the activity of Rel proteins in some tissues independently of Toll.
MATERIALS AND METHODS
Fly strains:
Flies were raised on standard cornmeal/molasses food at 21°. Fly stocks bearing the P-element insertions spagk12101, dcp-1k05606, dcp-102132, Arkk11502, dpldk08815a, skpAG0037, skpAG0389, and pr-set7P{hsneo}1, the mutations Toll3, cact1, l(3)mbnE1, and hopTum, corresponding deficiency alleles, and the armadillo (arm-GAL4) driver were obtained from the Bloomington Stock Center. The hemocyte–fat-body-specific driver collagen (cg-GAL4), dronc2, dronc51, Su(var)20502, and Su(var)20504 were kindly provided by C. Dearolf (Massachusetts General Hospital, Boston), A. Bergmann (University of Texas, Houston), J. Abrams (University of Texas Southwestern Medical Center, Dallas), and L. Wallrath (University of Iowa, Iowa City, IA), respectively. Flies carrying UAS-EGFP-dl, UAS-ECFP-Dif, and UAS-p-Relish were obtained from T. Ip (University of Massachusetts, Worcester, MA).
GFP-marked balancers were used to identify mutant larvae. Homozygous mutant larvae were collected on egg-laying plates and incubated with additional yeast on standard cornmeal/molasses food at 23° until the individuals reached the third instar larval stage and developed melanotic nodules.
Immunostaining:
For antibody staining, third instar larvae were dissected in phosphate-buffered saline (PBS) and tissues containing melanotic nodules were immediately transferred to a glass slide. Excess liquid was removed with tissue paper and the samples were air dried for 30 sec and then fixed at 80° for 1 min. The slides were kept at −70° for several days. For each mutant samples from 10 to 20 larvae were analyzed. The samples were additionally fixed for 40 min in 4% paraformaldehyde in PBST (PBS, 0.1% Tween 20) and washed several times in PBST. Antibody staining was performed essentially as described in Whalen and Steward (1993). Anti-Hemese monoclonal antibody (H2) and antibodies specific for lamellocytes (L1) were obtained from I. Ando (Biological Research Center, Szeged, Hungary) and used at 1:500 dilution. Secondary Cy-3-conjugated anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:500. F-actin was visualized by incubation with Alexa488 phalloidin (Molecular Probes, Eugene, OR) at 1:80 for 2 hr. DNA was stained using Hoechst 33258 (Molecular Probes). Samples were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and examined with a Zeiss Axioplan-2 microscope. Hematopoietic nodule images were captured using a Leica DM IRBE laser scanning confocal microscope. The images were analyzed with Image Pro Plus and Leica Microsystems software and further processed using Adobe PhotoShop.
RESULTS
Analysis of melanotic masses:
We decided to investigate if all melanotic masses indeed involve the cellular immune response, particularly the encapsulation of the mass by hemocytes. We also wanted to establish if mutations in genes functioning in the same process or in a given pathway result in the formation of melanotic nodules with similar characteristics. To this end, we analyzed whenever possible at least two mutant alleles in 14 genes, all reported to cause melanotic phenotypes. For this study we selected mutations in Toll and cact affecting the NF-κB pathway: dronc, Dcp-1, and Ark involved in apoptosis; Pr-Set7, Su(var)205, and skpA functioning in chromatin organization; hop activating the Jak/STAT pathway; and a number of genes whose functions have not been studied in detail, l(3)mbn, mxc, spag, zfrp8, and dpld (see Table 1).
TABLE 1.
Mutations causing different types of melanotic masses
| Type of melanotic phenotype
|
|||||
|---|---|---|---|---|---|
| Mutation | Gene name, function, references | Ha | Gutb | Otherc | Uniqued |
| skpAG0037 | skpA, chromatin condensation, cell cycle, Peter et al. (2002); Murphy (2003) | − | − | + | − |
| skpAG0389 | − | + | − | − | |
| pr-Set720 | pr-set7, histone H4 methylation, chromatin structure, cell cycle, Karachentsev et al. (2005) | + | − | + | − |
| pr-set7P{hsneo}1/Df(3R)red3l | − | −/+ | − | − | |
| Su(var)20502/Df(2L)TE29Aa-11 | Supressor of variegation, chromatin structure cell cycle, Bushey and Locke (2004); Norwood et al. (2004) | + | − | − | − |
| Su(var)20502/Su(var)20504 | + | − | − | − | |
| Su(var)20504/Df(2L)TE29Aa-11 | + | − | − | − | |
| dronc2/dronc51 | dronc or Nc, Nedd2-like caspase, programmed cell death, Laundrie et al. (2003); Chew et al. (2004); Xu et al. (2005) | + | + | + | − |
| dronc2/Df(3L)AC1 | − | + | + | ||
| Dcp-1k05606 | Death caspase-1, programmed cell death, also affect pita gene, Laundrie et al. (2003) | − | + | − | − |
| Dcp-102132 | − | + | − | − | |
| Dcp-1k05606/Df(2R)or-BR6 | − | + | − | − | |
| Arkk11502 | Apaf-1-related killer, caspase activator, programmed cell death, Rodriguez et al. (1999, 2002); Zhou et al. (1999); Zimmermann et al. (2002) | − | + | + | − |
| Arkk11502/Df(2R)BSC49/ | − | + | − | − | |
| Df(2R)P803-D1/Arkk11502 | − | + | − | − | |
| dpldk08815a | dappled, homolog of C. elegans lin-41, Rodriguez et al. (1996) | − | + | − | − |
| dpldk08815a/Df(2R)ST1 | − | + | − | − | |
| mxc22a-6/Y | multi sex comb, tumor suppressor, larval hematopoiesis, Santamaria and Randsholt (1995); Saget et al. (1998); Remillieux-Leschelle et al. (2002) | − | + | − | − |
| hopTum/+ | Hopscotch, Janus kinase, Jak-STAT signaling, Harrison et al. (1995); Luo et al. (2002) | + | − | − | − |
| l(3)mbnE1 | malignant blood neoplasm, Konrad et al. (1994) | + | − | − | − |
| l(3)mbnE1/+ | + | − | − | − | |
| cact A2 | cactus, IκB homolog, Rel pathway, Roth et al. (1991); Belvin et al. (1995); Qiu et al. (1998) | + | + | − | + |
| cactA2/cactE10R01 | + | + | − | + | |
| cactG8 | + | + | − | + | |
| cact1 | + | + | − | + | |
| Toll10B/+ | Toll, transmembrane receptor, Rel pathway, Gerttula et al. (1988) | + | − | − | − |
| Toll3/+ | + | − | − | − | |
| zfrp8M-1-1 | zinc finger RP8, homolog of mammalian PDCD2 (programmed cell death 2), Minakhina et al. (2003); S. Minakhina (unpublished results) | + | − | − | − |
| zfrp8M-1-1/Df(2R)SM183 | + | − | − | − | |
| spagk12101 | spaghetti, TPR-protein intaracting with Hsp90, Marhold (2000) | + | − | − | − |
Melanotic nodules found in the hemocoel or in association with Hemese-positive lymph glands.
Gut melanization not accompanied by tissue overgrowth and hemocyte encapsulation.
Melanizations found in other tissues, such as the trachea and salivary glands.
Unique melanotic mass-type-containing variable tissues.
All melanotic masses were dissected from third instar mutant larvae. The nodules are often located in the hemocoel (body cavity) but in some mutants black masses are found in other tissues. To investigate whether all the melanotic masses were associated with blood cells, we dissected the melanized tissues and determined whether the hemocyte-specific marker Hemese was expressed in or around the nodules (Figure 1 and Figure 2; Kurucz et al. 2003). To reveal the cell shape and the size of nuclei, we also stained the tissues for filamentous actin and DNA.
Figure 1.—
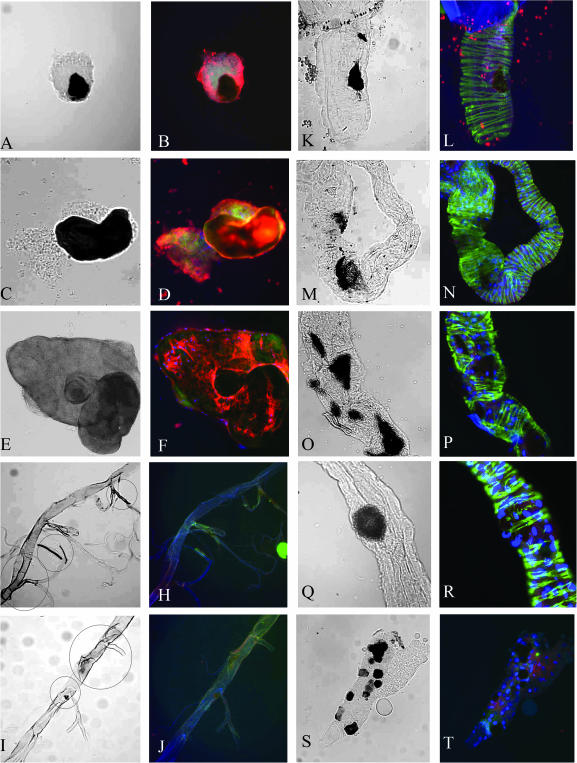
Melanizations and melanotic nodules. Melanotic nodules isolated from the hemocoel of dronc2/dronc51 (A and B), cact A2 (C and D), and spagk12101(E and F). Trachea isolated from Pr-Set720 (G and H; note melanization within the circles), Su(var)20502/Su(var)20504 (I and J; darkening tissue and melanized spot within the circles), nodules in the intestine observed in dronc2/dronc51 (K and L), mxc22a-6 (M and N), Arkk11502 (O and P), and dcp-1k05606 (Q and R). Melanization of salivary gland cells in Arkk11502 (S and T). In B, D, F, H, J, L, N, P, R, and T, tissues were stained with anti-Hemese (red), phalloidin for F-actin (green), and Hoechst33258 for DNA (blue). Bar, 40 μm.
Figure 2.—
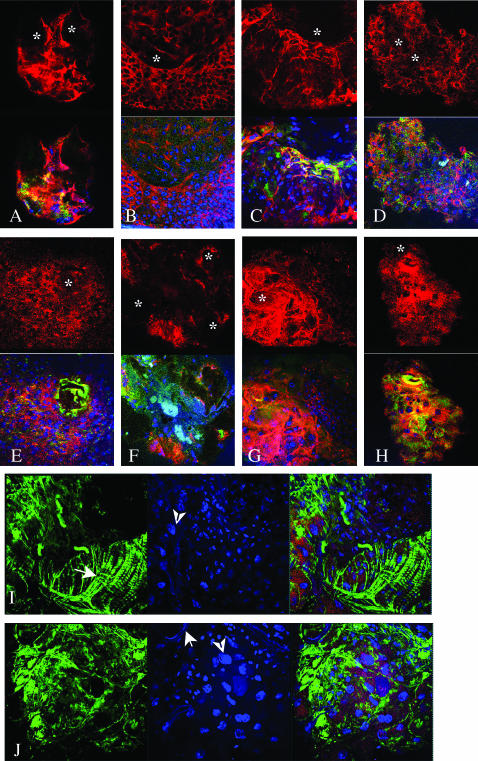
Melanotic nodules. Confocal crossections taken close to the surface of melanized nodules derived from (A) HopTum/+, (B) l(3)mbnE1/+, (C) Su(var)20502/Su(var)20504, (D) zfrp8M-1-1, (E) Toll10B/+, and (F, I, and J) cact A2. Muscle and tube-like structures are indicated with arrows and polyploid nuclei with arrowheads in I and J. (G and H) cact A2 and dl2. Tissues were stained with anti-Hemese (red), phalloidin for F-actin (green), and Hoechst33258 for DNA (blue). In A–H, Hemese staining alone is shown in the top panels. The melanized foci usually do not stain and are indicated by stars. Bar, 40 μm.
Surprisingly, only half of the mutants showed the classical melanotic phenotype: nodules that are located in the hemocoel and contain hemocytes, Hemese-positive cells (Figure 1, A–F, and Figure 2, Table 1). The other mutations, previously reported to have a melanotic phenotype, generally have melanizations found in nonhematopoietic tissues such as gut and salivary glands or melanization of trachea without involvement of hemocytes (see Figure 1, G–T; Table 1).
Melanizations in nonhematopoietic tissues:
Melanizations in different regions of the larval gut were observed in mxc, dpld, Dcp-1, and Ark and less frequently in dronc and cact larvae (see Table 1). The location and frequency of the melanizations varies in different mutants. Arkk11502 homozygous mutant larvae develop multiple black spots along the gut and sometimes in the salivary glands, while other allelic combinations of Ark show only gut melanizations. Most hemizygous and a large number of heterozygous mxc22a-6 larvae develop melanizations in the gut walls. This was also observed by Saget et al. (1998) who reported strong melanization associated with the hindgut ring in several mxc alleles. A total of 30–50% of dpld larvae show gut melanization, while the rest die at earlier stages without a detectable melanotic phenotype. Gut abnormalities as well as melanotic phenotypes were previously described for other alleles of dpld (Rodriguez et al. 1996). Several Dcp-1 mutant alleles also show consistent gut melanization. Gut melanizations are found in 20–30% of cact and dronc larvae along with the blood-associated melanotic nodules that we discuss below.
Melanization of the trachea was observed in two mutants, Pr-Set720, a null mutation in the histone H4-K20 mono-methytransferase, and in a strong, P-element-induced mutation in the skpA (skpAG0037) gene encoding a component of the SCF ubiquitin ligases involved in centrosome duplication (Murphy 2003; Karachentsev et al. 2005). These two genes show the largest variations of phenotypes of all the mutants studied here. Some homozygous Pr- Set720 larvae have melanotic nodules in the hemocoel and cuticle along with melanization of the trachea. In Pr-Set7P{hsneo}1/Df(3R)red3l, melanizations are rare and found only in the gut and cuticle. A similar phenotype is observed in skpAG0389 larvae, suggesting that the histological appearance of the melanotic phenotype in both Pr-Set7 and skpA probably depends on the strength of the allele.
Notably, all melanizations found in the gut wall were surrounded by relatively normal-looking gut tissue that does not express the Hemese marker. Moreover, Hemese is not detected in or around pigmented trachea and melanized salivary gland tissue (Figure 1, G–J, S, and T). Thus the melanization in these organs can be induced without the activation of the cellular immune response, or, more precisely, without the encapsulation response.
Melanotic nodules associated with hemocytes:
In about half of our mutants all melanotic masses were found in the hemocoel or in association with the lymph glands and they all show high levels of Hemese expression (Figure 1, B, D, and F; Figure 2). In this study we call this phenotype “melanotic nodules.”
Criteria for classifying melanotic nodules were suggested by Watson et al. (1991, 1994). On the basis of the dissection of nodules and the morphology of lymph glands, Watson and colleagues divided mutants with melanotic phenotypes into two classes. The class 1 mutants produce melanotic nodules as a “response of an apparently normal immune system to the presence of abnormal target tissues” such as brain or imaginal disc tissue. This process is often called an “autoimmune response” (Watson et al. 1991; Nappi et al. 2004). Class 2 represents the “true blood cell tumor” mutants showing overgrowth of hematopoietic organs, leading to blood cell aggregations that form melanotic nodules. In both classes, the cellular immune system is believed to be activated and to trigger encapsulation and melanization.
Our melanotic nodule group consists partly of mutants with well-defined tumor phenotypes—hop, l(3)mbn, cact, Toll—and mutants in which the melanotic phenotype has not been described in detail before—Su(var)205, spag, and zfrp8 (alleles and phenotypes are listed in Table 1).
P-induced lethal mutations in spag (spagk12101) cause multiple nodules surrounded by hemocytes (Figure 1B). The lymph glands in these mutant larvae are about two times larger than wild-type glands. The nodules are located symmetrically along the body wall and often replace imaginal discs, suggesting that the formation of melanotic nodules may be provoked by an immune response to the self-tissue. The enlargement of lymph glands in this case may happen as part of this autoimmune reaction. Therefore, this is the only mutant that we analyzed that could be included in the Watson class 1 genes. The other molecularly characterized class 1 gene, krz, was described by Roman et al. (2000). Krz is expressed in the nervous system and fat body and mutants have melanotic nodules in the fat bodies that are surrounded by hemocytes.
All other mutations with melanotic nodules tested in our study cause overproliferation of hemocytes and belong to class 2, following the Watson classification. RpS6/air8 and hop are original members of this class (Watson et al. 1991, 1992, 1994). How mutations in RpS6/air8 lead to aberrant immune response and to hemocyte overproliferation is not known. It is, however, known that gain-of-function mutations in hop (hopTum) and also expression of an activated hop transgene result in the constitutive activation of the Jak/STAT pathway, resulting in hemocyte proliferation, lamellocyte differentiation, and constitutive immune response (Luo et al. 2002; Asha et al. 2003; Agaisse and Perrimon 2004; Zettervall et al. 2004). Similar phenotypes are produced by overactivation of the Nf-κB/Rel pathway in the gain-of-function Toll10B mutant and the loss-of-function cactA2 mutant (Qiu et al. 1998). Our results confirm the hematopoietic origin of all nodules found in hopTum and Toll (Toll10B and Toll3) mutants, where Hemese-positive cells aggregate to form masses with one or multiple melanized foci. The shapes of cells in these masses are deformed and F-actin is increased (Figure 2, A and E).
The phenotype of melanotic masses found in cact larvae stands apart. About half of the melanotic masses are Hemese positive and are similar to the nodules seen in Toll mutants, while others have additional features that will be discussed below.
Melanotic nodules in l(3)mbn, encoding a plasma membrane protein, result from excessive proliferation of hemocytes and abnormal differentiation of these cells into giant plasmatocytes and lamellocytes. (Konrad et al. 1994; Kurucz et al. 2003). Our analysis shows that all l(3)mbnE1 melanotic nodules are found in the hemocoel and are covered by large, flat F-actin/Hemese-positive cells, stretched over the surface of the nodules (Figure 2B). Often hemocytes on the surface of the melanized nodules stain more strongly with anti-Hemese antibody than other hemocytes (Figure 2). Su(var)205 nodules look similar to those observed in l(3)mbn but they are associated with the lymph gland lobes. Homozygous or hemizygous mutants in zfrp8 (Minakhina et al. 2003; S. Minakhina, unpublished results), a homolog of mammalian PDCD2 (programmed cell death-2), also cause melanotic nodules in the lymph glands. Because nodules caused by mutations in these two genes are always found in association with the lymph gland, the genes may have an essential function in hematopoiesis.
Encapsulation of melanotic nodules:
All melanotic masses found in the hemocoel or in association with the lymph gland contain hemocytes. The structure of the nodules, size, shape, level of melanization, and the amount of surrounding tissue vary not only between different mutants, but also within one larva. To further characterize the tissue surrounding the melanotic nodule and to investigate whether all the nodules result from encapsulation by lamellocytes, we used, in addition to the hemocyte-specific antibody (Hemese; Figure 2), the L1 antibody, specific for lamellocytes (Asha et al. 2003; Kurucz et al. 2003). Crystal cells are difficult to detect, because they are unstable and are destroyed once they have caused the initiation of the melanization cascade by releasing pro-phenoloxydase (Lanot et al. 2001; Evans and Banerjee 2003).
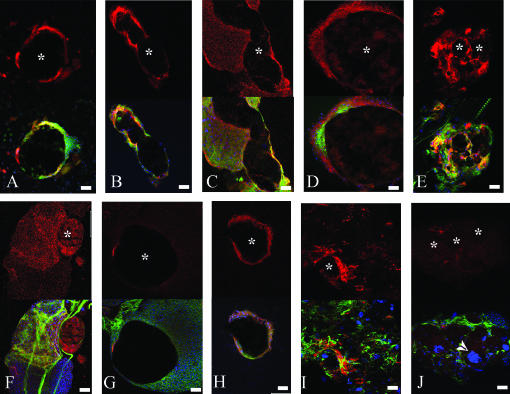
The cells in immediate contact with the melanized tissue in spag, mbn, Hop, Toll, and Su(var)205 mutant larvae are L1 positive, indicating that they are lamellocytes (Figure 3, A–E). This suggests that the encapsulation by lamellocytes and the proposed function of crystal cells are sufficient for the formation of melanotic nodules.
Figure 3.—
Participation of lamellocytes in melanotic nodule encapsulation. Confocal crossection through the central part of melanotic nodules derived from (A) l(3)mbnE1/+, (B) spagk12101, (C) hopTum/+, (D) Toll3/+, (E) Su(var)20502/Df(2L)TE29Aa-11, (F and G) zfrp8M-1-1/Df(2R)SM183, and (H, I, and J) cact1 and cactG8. Tissues were stained with L1-lamellocyte-specific antibodies (red), phalloidin for F-actin (green), and Hoechst33258 for DNA (blue). L1 staining alone is shown in the top panels; melanized tissues (indicated with stars) generally do not stain (A, B, E, G, and I), but sometimes accumulate the L1 marker (D and F) . Bar, 40 μm.
In cact and zfrp8 mutants, there are melanotic nodules that are surrounded by lamellocytes (Figure 3, F and I) as well as nodules that are surrounded by other cells (Figure 3, G and J). In cact mutants, three types of nodules are found (see below). The zfrp8 nodules are located in the lymph gland, a tissue that contains hemocytes at different stages of differentiation (Lanot et al. 2001; Jung et al. 2005). In this case, melanized tissues can be surrounded either by differentiated lamellocytes (Figure 3F) or by small, L1-negative prohemocytes (Figure 3G).
Melanotic masses in cactus:
The majority of the melanotic nodules in all cactus alleles are located in the hemocoel. Only half of the cactA2 melanotic nodules have the same composition as the nodules found in Toll gain-of-function mutants (Figure 3, D and H). This is surprising because Toll functions upstream from cact in the Rel pathway and in both Toll10B and cactA2 the NF-κB/Rel proteins Dorsal and Dif are constitutively nuclear.
The other half of the nodules consist of tissue agglomerates that contain one or more melanized foci and also tube-like structures, possibly tracheae, visceral muscles, and polyploid cells (arrows and arrowheads in Figure 2, I and J; Figure 3J). Only some of the agglomerates containing the melanotic foci are surrounded by lamellocytes (compare Figure 3, I and J). Similar masses are found at lower frequency in the homozygous cact G8 and cact 1 larvae.
The differentiation of cells within cact melanotic masses into various tissues may result from uncontrolled Rel-protein activation. To check if dorsal (dl) is responsible for the tissue transformation observed in cact mutants, we analyzed the nodules derived from cactA2, dl2 double mutants. Multiple cactA2, dl2 melanotic nodules show clear hematopoietic origin (Figure 2, G and H). They are located in the hemocoel and lymph glands and consist of large, flat, F-actin-rich lamellocytes or both lamellocyte and plasmatocyte-like cells. Melanotic agglomerates containing different tissue types are not observed. The fact that in the double mutant the melanotic nodule phenotype is still detected, but dl loss of function suppresses the formation of the complex agglomerates, may be due to the redundant function of Dorsal and Dif in hematopoiesis and immunity and is consistent with cact and dl having additional functions in development.
General overexpression of dorsal is lethal:
We reasoned that upregulating the three Rel proteins in different tissues and observing the phenotypes produced by this overexpression would help us understand the cact loss-of-function phenotype. To this end, we expressed the Drosophila Rel proteins coupled to fluorescent peptides, EGFP-Dorsal, ECFP-Dif, and EYFP-Relish (Bettencourt et al. 2004) under the control of the arm-GAL4 and cg-GAL4 driver. The fluorescence observed in larvae confirmed that arm-GAL4 induces virtually ubiquitous but low expression of transgenes, while cg-GAL4 drives a high level of expression specifically in hemocytes and fat body (Asha et al. 2003).
The expression of the three Rel transgenes did not cause melanotic nodule formation but had specific effects on larval viability. The expression of EYFP-Relish does not reduce larval and adult viability regardless of driver. ECFP-Dif larvae have reduced viability (∼25% viability) when driven by arm-GAL, but cg-GAL4; ECFP-Dif animals are viable. Activation of EGFP-dl expression in immune tissues (CG-GAL4) does not affect viability; however, EGFP-dl expression driven by arm-GAL4 causes 100% lethality at the first and second instar larval stages. This phenotype resembles the phenotype of cact null alleles, which are also lethal at the first and second instar larval stages (Roth et al. 1991; Qiu et al. 1998) and suggests that the essential function of Cactus during postembryonic development is to inhibit Dorsal activity in somatic tissues and to regulate Dorsal and Dif function in the immune response.
DISCUSSION
We could subdivide melanotic masses into two categories on the basis of staining with the hemocyte-specific marker Hemese: (1) the melanotic nodules in the hemocoel or associated with the lymph glands evidently surrounded by blood cells and (2) Hemese-negative melanizations located in the gut, salivary glands, and tracheae. Absence of tissue overgrowth and lack of hemocytes surrounding the melanized foci indicate that they may result from cell death, possibly necrosis. It is not clear if activation of the melanization of these masses involves crystal cells.
Mutants in genes functioning in apoptosis generally show only melanizations. Mutants in Ark, dcp-1, and dronc develop melanizations located in the gut (see Table1). In Ark and dcp-1 mutants, no evidence of additional hyperplasia of the lymph glands or overproliferation of blood cells was observed (see also Song et al. 1997), but lymph-gland-associated nodules are sometimes seen in dronc larvae, and in some Ark alleles melanized foci are detected in the salivary glands. These variations may reflect the tissue-specific functions of the apoptosis genes or may be due to genetic background.
Mutations in Pr- Set7, skpA, and Su(var)205 show the highest divergence of phenotype. While Su(var)205 has a clear hematopoietic phenotype, both Pr-Set7 and skpA larvae show melanizations in other tissues, and the location of the melanized masses varies in different alleles. This pleiotropy may be explained by the fact that all three genes are involved in chromatin and chromosome structure and therefore may interfere with basic mechanisms of cell maintenance (Murphy 2003; Bushey and Locke 2004; Karachentsev et al. 2005).
The true melanotic nodule phenotype manifested by encapsulation of melanotic foci by lamellocytes was present in mutants with established defects in hematopoiesis and immunity, such as hop and Toll. It was also observed in mutants with no reported phenotypes in blood development, such as Su(var)205 and zfrp8. The finding of melanotic nodules in association with the lymph glands suggests that zfrp8 and Su(var)205 have an important function in blood development. Interestingly, nodules found in the lymph gland are often surrounded by lamellocytes, of which there are only a few in wild-type glands. Unlike in Su(var)205, in zfrp8 larvae melanotic capsules also can be formed by L1-negative blood cells, possibly prohemocytes. This lamellocyte-type function of the prohemocytes may indicate that these cells have the potential to differentiate into lamellocytes.
We assigned one new mutation, spag, to class 1 of Watson et al. (1991, 1994), in which melanotic nodules are formed as an auto-immune response to abnormalities in the imaginal discs. But we found it generally difficult to distinguish between class 1 and class 2 “melanotic tumors” as described by Watson et al. All melanotic nodules are associated with overproliferation of hemocytes. This overgrowth and the ensuing clumps of cells may be recognized by the immune system as “abnormal” and provoke an auto-immune response. Most melanotic nodules are surrounded by lamellocytes, similarly to the encapsulation of foreign objects.
Mutations in genes in a given pathway do not necessarily result in identical phenotypes. This is clearly illustrated by the analysis of Toll and cact mutants. Cact encodes the Drosophila IκB protein, that controls the activity of the Rel/Nf-κB transcription factors Dorsal and Dif. Activation of the transmembrane receptor Toll causes degradation of the IκB protein Cactus and translocation of activated Rel proteins into nuclei. Hence loss of function of cact and the gain of function of Toll are expected to cause similar phenotypes resulting from increased nuclear Rel activity. Indeed, such mutants exhibit comparable defects in innate immunity and hematopoiesis, including overproduction of hemocytes and multiple melanotic nodules (Whalen and Steward 1993; Belvin et al. 1995; Belvin and Anderson 1996; Govind 1996; Qiu et al. 1998; this work).
In addition, the partial loss of function of cact leads to formation of melanotic masses of complex composition. They often include patches of cells reminiscent of somatic tissues, such as muscles, tracheae, and polyploid cells, typical for fat body and gut. The formation of these tissue aggregates is suppressed in cact, dl double-homozygous mutants, suggesting that the transformation results from uncontrolled activation of the NF-κB/Rel protein Dorsal.
Unlike other Rel proteins, Dorsal has functions in addition to the immune response. Maternally deposited Dorsal is the ventral morphogen and directly activates and represses a large set of genes, thereby controlling the formation of the different germ layers. Later in development Dorsal and its inhibitor Cactus function in the maintenance of innervation of somatic muscles (Cantera et al. 1999; Beramendi et al. 2005). It is likely that in late embryos and larvae, in the absence of Cactus, Dorsal protein is constitutively active and this leads to abnormal gene expression and tissue differentiation. It has been shown before that the ectopic activation of one of the Dorsal targets, twist, can activate the muscle-specific marker tinmann and cause tissue transformations, including muscle differentiation (Furlong et al. 2001; Furlong 2004).
Why does activation of the Rel pathway in Toll mutants not cause tissue aggregates, as seen in cact? The answer may lie in the expression patterns and in the different requirements of Toll and cact. It is known that the expression pattern of Toll does not completely overlap with that of dl and cact. Toll expression is reportedly absent in postembryonic muscles and lymph glands (Gerttula et al. 1988; Hashimoto et al. 1991; Nose et al. 1992; Halfon et al. 1995; Kambris et al. 2002; Wang et al. 2005). dl is expressed in immune tissues and at neuromuscular junctions, gut, salivary glands, and trachea (Cantera et al. 1999; Beramendi et al. 2005). It is likely that the function of Dorsal is regulated in all these tissues by its cytoplasmic inhibitor, Cactus, under the control of a different transmembrane receptor, possibly one of the Toll homologs.
Some of the larval tissues appear particularly sensitive to the uncontrolled activation of Dorsal. This is manifested by the cact tissue transformation phenotype and early larval lethality of cact null mutants (Roth et al. 1991). Lethality at the same period is also observed when a dl transgene is generally expressed.
In conclusion, we propose to subdivide melanotic phenotypes into three classes, depending on the detection of hemocytes. True melanotic nodules are generally found in hematopoietic and immunity mutants. These nodules usually present lamellocyte-mediated encapsulation. Mutations in several genes form melanizations only in nonhematopoietic tissues without encapsulation. Melanotic masses consisting of tissue agglomerates reminiscent of vertebrate tumors are seen in cact mutants and may be caused by the uncontrolled activity of the NF–κB protein Dorsal.
Acknowledgments
We thank Istvan Ando, Andreas Bergmann, John Abrams, Charles Dearolf, Lori Wallrath, and Tony Ip for antibodies, mutant, and transgenic flies. We are grateful to Cordelia Rauskolb for helpful discussion of the manuscript. We also thank Le Nguyen and Marina Druzhinina for technical help. This work was supported by a grant from the National Institutes of Health and by the W. Horace Goldsmith Foundation.
References
- Agaisse, H., and N. Perrimon, 2004. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 198: 72–82. [DOI] [PubMed] [Google Scholar]
- Asha, H., I. Nagy, G. Kovacs, D. Stetson, I. Ando et al., 2003. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barigozzi, C., 1958. Melanotic tumors in Drosophila. J. Cell. Physiol. 52: 371–381. [DOI] [PubMed] [Google Scholar]
- Becker, S., A. Gehrsitz, P. Bork, S. Buchner and E. Buchner, 2001. The black-pearl gene of Drosophila defines a novel conserved protein family and is required for larval growth and survival. Gene 262: 15–22. [DOI] [PubMed] [Google Scholar]
- Belvin, M. P., and K. V. Anderson, 1996. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 12: 393–416. [DOI] [PubMed] [Google Scholar]
- Belvin, M. P., Y. Jin and K. V. Anderson, 1995. Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 9: 783–793. [DOI] [PubMed] [Google Scholar]
- Beramendi, A., S. Peron, A. Megighian, C. Reggiani and R. Cantera, 2005. The inhibitor kappaB-ortholog Cactus is necessary for normal neuromuscular function in Drosophila melanogaster. Neuroscience 134: 397–406. [DOI] [PubMed] [Google Scholar]
- Bettencourt, R., H. Asha, C. Dearolf and Y. T. Ip, 2004. Hemolymph-dependent and -independent responses in Drosophila immune tissue. J. Cell. Biochem. 92: 849–863. [DOI] [PubMed] [Google Scholar]
- Bushey, D., and J. Locke, 2004. Mutations in Su(var)205 and Su(var)3–7 suppress P-element-dependent silencing in Drosophila melanogaster. Genetics 168: 1395–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantera, R., T. Kozlova, C. Barillas-Mury and F. C. Kafatos, 1999. Muscle structure and innervation are affected by loss of Dorsal in the fruit fly, Drosophila melanogaster. Mol. Cell. Neurosci. 13: 131–141. [DOI] [PubMed] [Google Scholar]
- Chew, S. K., F. Akdemir, P. Chen, W. J. Lu, K. Mills et al., 2004. The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell 7: 897–907. [DOI] [PubMed] [Google Scholar]
- De Gregorio, E., S. J. Han, W. J. Lee, M. J. Baek, T. Osaki et al., 2002. a An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev. Cell 3: 581–592. [DOI] [PubMed] [Google Scholar]
- De Gregorio, E., P. T. Spellman, P. Tzou, G. M. Rubin and B. Lemaitre, 2002. b The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21: 2568–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic, B., J. A. Hoffmann, M. Meister and J. Royet, 2002. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr. Biol. 12: 1923–1927. [DOI] [PubMed] [Google Scholar]
- Evans, C. J., and U. Banerjee, 2003. Transcriptional regulation of hematopoiesis in Drosophila. Blood Cells Mol. Dis. 30: 223–228. [DOI] [PubMed] [Google Scholar]
- Evans, C. J., V. Hartenstein and U. Banerjee, 2003. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5: 673–690. [DOI] [PubMed] [Google Scholar]
- Fossett, N., K. Hyman, K. Gajewski, S. H. Orkin and R. A. Schulz, 2003. Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc. Natl. Acad. Sci. USA 100: 11451–11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong, E. E., 2004. Integrating transcriptional and signalling networks during muscle development. Curr. Opin. Genet. Dev. 14: 343–350. [DOI] [PubMed] [Google Scholar]
- Furlong, E. E., E. C. Andersen, B. Null, K. P. White and M. P. Scott, 2001. Patterns of gene expression during Drosophila mesoderm development. Science 293: 1629–1633. [DOI] [PubMed] [Google Scholar]
- Gateff, E., 1977. Malignant neoplasms of the hematopoietic system in three mutants of Drosophila melanogaster. Ann. Parasitol. Hum. Comp. 52: 81–83. [DOI] [PubMed] [Google Scholar]
- Gerttula, S., Y. S. Jin and K. V. Anderson, 1988. Zygotic expression and activity of the Drosophila Toll gene, a gene required maternally for embryonic dorsal-ventral pattern formation. Genetics 119: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind, S., 1996. Rel signalling pathway and the melanotic tumour phenotype of Drosophila. Biochem. Soc. Trans. 24: 39–44. [DOI] [PubMed] [Google Scholar]
- Halfon, M. S., C. Hashimoto and H. Keshishian, 1995. The Drosophila toll gene functions zygotically and is necessary for proper motoneuron and muscle development. Dev. Biol. 169: 151–167. [DOI] [PubMed] [Google Scholar]
- Harrison, D. A., R. Binari, T. S. Nahreini, M. Gilman and N. Perrimon, 1995. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14: 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, C., S. Gerttula and K. V. Anderson, 1991. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development 111: 1021–1028. [DOI] [PubMed] [Google Scholar]
- Jung, S. H., C. J. Evans, C. Uemura and U. Banerjee, 2005. The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132: 2521–2533. [DOI] [PubMed] [Google Scholar]
- Kambris, Z., J. A. Hoffmann, J. L. Imler and M. Capovilla, 2002. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr. Patterns 2: 311–317. [DOI] [PubMed] [Google Scholar]
- Karachentsev, D., K. Sarma, D. Reinberg and R. Steward, 2005. PR-Set7-dependent methylation of histone H4 Lys 20 functions in repression of gene expression and is essential for mitosis. Genes Dev. 19: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad, L., G. Becker, A. Schmidt, T. Klockner, G. Kaufer-Stillger et al., 1994. Cloning, structure, cellular localization, and possible function of the tumor suppressor gene lethal(3)malignant blood neoplasm-1 of Drosophila melanogaster. Dev. Biol. 163: 98–111. [DOI] [PubMed] [Google Scholar]
- Kurucz, E., C. J. Zettervall, R. Sinka, P. Vilmos, A. Pivarcsi et al., 2003. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. USA 100: 2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagueux, M., E. Perrodou, E. A. Levashina, M. Capovilla and J. A. Hoffmann, 2000. Constitutive expression of a complement-like protein in toll and JAK gain-of-function mutants of Drosophila. Proc. Natl. Acad. Sci. USA 97: 11427–11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanot, R., D. Zachary, F. Holder and M. Meister, 2001. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 230: 243–257. [DOI] [PubMed] [Google Scholar]
- Laundrie, B., J. S. Peterson, J. S. Baum, J. C. Chang, D. Fileppo et al., 2003. Germline cell death is inhibited by P-element insertions disrupting the dcp-1/pita nested gene pair in Drosophila. Genetics 165: 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine, M. D., and M. R. Strand, 2002. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 32: 1295–1309. [DOI] [PubMed] [Google Scholar]
- Lebestky, T., T. Chang, V. Hartenstein and U. Banerjee, 2000. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288: 146–149. [DOI] [PubMed] [Google Scholar]
- Lemaitre, B., M. Meister, S. Govind, P. Georgel, R. Steward et al., 1995. Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J. 14: 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis, P., N. Pelte, J. A. Hoffmann and J. M. Reichhart, 2002. a Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297: 114–116. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis, P., N. Pelte, C. Ji, V. Leclerc, B. Duvic et al., 2002. b A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 21: 6330–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Luo, H., P. E. Rose, T. M. Roberts and C. R. Dearolf, 2002. The Hopscotch Jak kinase requires the Raf pathway to promote blood cell activation and differentiation in Drosophila. Mol. Genet. Genomics 267: 57–63. [DOI] [PubMed] [Google Scholar]
- Marhold, J., I. Torok, I. Iliopoulos, A. Mishra, C. De Lorenzo et al., 2000. The TPR-containing Spaghetti protein interacts with Hsp90 and is required for cell survival and differentiation in imaginal discs. Annual Drosophila Research Conference, The Genetics Society of America, Pittsburgh.
- Meister, M., 2004. Blood cells of Drosophila: cell lineages and role in host defence. Curr. Opin. Immunol. 16: 10–15. [DOI] [PubMed] [Google Scholar]
- Meister, M., and M. Lagueux, 2003. Drosophila blood cells. Cell. Microbiol. 5: 573–580. [DOI] [PubMed] [Google Scholar]
- Minakhina, S., J. Yang and R. Steward, 2003. Tamo selectively modulates nuclear import in Drosophila. Genes Cells 8: 299–310. [DOI] [PubMed] [Google Scholar]
- Murphy, T. D., 2003. Drosophila skpA, a component of SCF ubiquitin ligases, regulates centrosome duplication independently of cyclin E accumulation. J. Cell Sci. 116: 2321–2332. [DOI] [PubMed] [Google Scholar]
- Nappi, A. J., E. Vass, D. Malagoli and Y. Carton, 2004. The effects of parasite-derived immune-suppressive factors on the cellular innate immune and autoimmune responses of Drosophila melanogaster. J. Parasitol. 90: 1139–1149. [DOI] [PubMed] [Google Scholar]
- Nappi, A. J., and B. M. Christensen, 2005. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol. 35: 443–459. [DOI] [PubMed] [Google Scholar]
- Nappi, A. J., F. Frey and Y. Carton, 2005. Drosophila serpin 27A is a likely target for immune suppression of the blood cell-mediated melanotic encapsulation response. J. Insect Physiol. 51: 197–205. [DOI] [PubMed] [Google Scholar]
- Norwood, L. E., S. K. Grade, D. E. Cryderman, K. A. Hines, N. Furiasse et al., 2004. Conserved properties of HP1(Hsalpha). Gene 336: 37–46. [DOI] [PubMed] [Google Scholar]
- Nose, A., V. B. Mahajan and C. S. Goodman, 1992. Connectin: a homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. Cell 70: 553–567. [DOI] [PubMed] [Google Scholar]
- Oftedal, P., 1953. The histogenesis of a new tumor in Drosophila melanogaster, and a comparison with tumors of five other stocks. Z. Indukt. Abstamm. Vererbungsl. 85: 408–422. [DOI] [PubMed] [Google Scholar]
- Peter, A., P. Schottler, M. Werner, N. Beinert, G. Dowe et al., 2002. Mapping and identification of essential gene functions on the X chromosome of Drosophila. EMBO Rep. 3: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, P., P. C. Pan and S. Govind, 1998. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 125: 1909–1920. [DOI] [PubMed] [Google Scholar]
- Remillieux-Leschelle, N., P. Santamaria and N. B. Randsholt, 2002. Regulation of larval hematopoiesis in Drosophila melanogaster: a role for the multi sex combs gene. Genetics 162: 1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki, M. T., 1960. Melanotic tumor formation in Drosophila. J. Morphol. 106: 147–157. [DOI] [PubMed] [Google Scholar]
- Rizki, T. M., and R. M. Rizki, 1983. Blood cell surface changes in Drosophila mutants with melanotic tumors. Science 220: 73–75. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A., Z. Zhou, M. L. Tang, S. Meller, J. Chen et al., 1996. Identification of immune system and response genes, and novel mutations causing melanotic tumor formation in Drosophila melanogaster. Genetics 143: 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, A., H. Oliver, H. Zou, P. Chen, X. Wang et al., 1999. Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat. Cell Biol. 1: 272–279. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A., P. Chen, H. Oliver and J. M. Abrams, 2002. Unrestrained caspase-dependent cell death caused by loss of Diap1 function requires the Drosophila Apaf-1 homolog, Dark. EMBO J. 21: 2189–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman, G., J. He and R. L. Davis, 2000. kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics 155: 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, S., Y. Hiromi, D. Godt and C. Nusslein-Volhard, 1991. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development 112: 371–388. [DOI] [PubMed] [Google Scholar]
- Saget, O., F. Forquignon, P. Santamaria and N. B. Randsholt, 1998. Needs and targets for the multi sex combs gene product in Drosophila melanogaster. Genetics 149: 1823–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria, P., and N. B. Randsholt, 1995. Characterization of a region of the X chromosome of Drosophila including multi sex combs (mxc), a Polycomb group gene which also functions as a tumour suppressor. Mol. Gen. Genet. 246: 282–290. [DOI] [PubMed] [Google Scholar]
- Sinenko, S. A., and B. Mathey-Prevot, 2004. Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene 23: 9120–9128. [DOI] [PubMed] [Google Scholar]
- Sinenko, S. A., E. K. Kim, R. Wynn, P. Manfruelli, I. Ando et al., 2004. Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev. Biol. 273: 48–62. [DOI] [PubMed] [Google Scholar]
- Song, Z., K. McCall and H. Steller, 1997. DCP-1, a Drosophila cell death protease essential for development. Science 275: 536–540. [DOI] [PubMed] [Google Scholar]
- Stewart, M. J., and R. Denell, 1993. The Drosophila ribosomal protein S6 gene includes a 3′ triplication that arose by unequal crossing-over. Mol. Biol. Evol. 10: 1041–1047. [DOI] [PubMed] [Google Scholar]
- Sugumaran, M., 2002. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 15: 2–9. [DOI] [PubMed] [Google Scholar]
- Takehana, A., T. Katsuyama, T. Yano, Y. Oshima, H. Takada et al., 2002. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc. Natl. Acad. Sci. USA 99: 13705–13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingvall, T. O., E. Roos and Y. Engstrom, 2001. The GATA factor Serpent is required for the onset of the humoral immune response in Drosophila embryos. Proc. Natl. Acad. Sci. USA 98: 3884–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Y. Tao, I. Reim, K. Gajewski, M. Frasch et al., 2005. Expression, regulation, and requirement of the toll transmembrane protein during dorsal vessel formation in Drosophila melanogaster. Mol. Cell. Biol. 25: 4200–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, K. L., T. K. Johnson and R. E. Denell, 1991. Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev. Genet. 12: 173–187. [DOI] [PubMed] [Google Scholar]
- Watson, K. L., K. D. Konrad, D. F. Woods and P. J. Bryant, 1992. Drosophila homolog of the human S6 ribosomal protein is required for tumor suppression in the hematopoietic system. Proc. Natl. Acad. Sci. USA 89: 11302–11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, K. L., R. W. Justice and P. J. Bryant, 1994. Drosophila in cancer research: the first fifty tumor suppressor genes. J. Cell Sci. 18 (Suppl.): 19–33. [DOI] [PubMed] [Google Scholar]
- Whalen, A. M., and R. Steward, 1993. Dissociation of the dorsal-cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J. Cell Biol. 123: 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D., Y. Li, M. Arcaro, M. Lackey and A. Bergmann, 2005. The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development 132: 2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall, C. J., I. Anderl, M. J. Williams, R. Palmer, E. Kurucz et al., 2004. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101: 14192–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., Z. Song, J. Tittel and H. Steller, 1999. HAC-1, a Drosophila homolog of APAF-1 and CED-4, functions in developmental and radiation-induced apoptosis. Mol. Cell 4: 745–755. [DOI] [PubMed] [Google Scholar]
- Zimmermann, K. C., J. E. Ricci, N. M. Droin and D. R. Green, 2002. The role of ARK in stress-induced apoptosis in Drosophila cells. J. Cell Biol. 156: 1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]