Abstract
Learning is an adaptive change in behavior in response to environmental stimuli. In mammals, there is a distinct female bias to learn skills that is still unprecedented in other animal taxa. Here we have investigated the biological determinants of performance in an associative learning paradigm in the nematode Caenorhabditis elegans. Using an assay of chemotactic reactions associated with food deprivation, wild-type male worms show inferior learning ability relative to hermaphrodites. Sex-based learning difference is therefore an ancient evolutionary feature appearing even in relatively simple animals. C. elegans mutants with reduced insulin/IGF-1 signaling also exhibit a greatly reduced learning ability in this assay. In addition, hyperactivation of insulin/IGF-1 signaling through loss-of-function mutations in the PTEN phosphatase daf-18, a negative regulator of insulin/IGF-1 signaling, enhances learning ability beyond that of wild type. According to our epistasis analysis, the effect of DAF-2 on learning acts via phosphatidylinositol 3,4,5-trisphosphate (PIP3) production, but not the DAF-16 FOXO transcription factor. This implies that the signaling pathway from DAF-2 affecting this learning paradigm branches between PIP3 production and DAF-16. However, learning capacity of nematodes is lowered by loss-of-function mutations in daf-16, suggesting involvement of noninsulin/IGF-1 signaling-dependent DAF-16 activation in learning. Potentially, sex and insulin/IGF-1 signaling affect performance in this learning assay via effects on the neurobiology of learning.
ASSOCIATIVE learning occurs when an animal learns to link a stimulus or behavior with a second temporally associated stimulus (Jorgensen and Rankin 1997). Progress in discovering conserved signaling pathways involved in learning is greatly facilitated by studying animals with a relatively simple and tractable nervous system (Hirsch and Boudreau 1958; Hotta and Benzer 1970; Kelleher et al. 2004). The free-living nematode Caenorhabditis elegans with 302 neurons in adult hermaphrodites is an excellent model organism to analyze neuronal function (White et al. 1986; Bargmann and Mori 1997; Kenyon 1997).
In C. elegans, a male-specific behavior, mate searching, is influenced by a conserved neuroendocrine system, the insulin/insulin-like growth factor (IGF) signaling pathway (Lipton et al. 2004). Aging and reproductive growth are also regulated hormonally by insulin/IGF-1 signaling in this organism (Kenyon 1997; Riddle and Albert 1997; Guarente and Kenyon 2000), and it is possible that this regulation is sexually dimorphic. For example, wild-type males are more predisposed than hermaphrodites to form diapausal dauer larvae in response to starvation and high population density (Ailion and Thomas 2000). (The self-fertilizing C. elegans hermaphrodite is essentially a female that generates sperm for a brief period before oogenesis.) Given that reduction in insulin/IGF-1 signaling or signaling via the daf-7 TGF-β pathway increases dauer formation (Riddle and Albert 1997), this suggests that flux through either pathway might be constitutively reduced in males. In addition, wild-type males live ∼20% longer than hermaphrodites (Gems and Riddle 2000). This male longevity advantage requires the insulin/IGF-1 signaling-regulated transcription factor DAF-16; however, in daf-2(m577) mutants a male longevity advantage was seen similar to that in wild type, suggesting that while DAF-16 levels are higher in males, the insulin/IGF-1 signaling level is not lower (Gems and Riddle 2000). In spite of these observations, the nature of sexual dimorphism in endocrine function in C. elegans remains almost unexplored. In addition, the tissues at which the insulin/IGF-1 signaling pathway controls lifespan appear to include the nervous system (Wolkow et al. 2000; Libina et al. 2003).
In this study, we investigate the role of insulin/IGF-1 signaling in the learning process in C. elegans and whether this regulation operates in a different way in males and hermaphrodites. We show that male nematodes perform less than hermaphrodites in a learning paradigm in which paired presentation of salt and starvation induces a change in behavior. We also demonstrate a strong influence of the insulin/IGF-1 signaling pathway on performance in this simple learning assay. Mutant worms with reduced insulin/IGF-1 signaling are defective in learning, while mutational hyperactivation of the insulin/IGF-1 signaling cascade results in enhancement of learning ability beyond that of wild type.
MATERIALS AND METHODS
C. elegans strains:
Strains were maintained on nematode growth medium (NGM) as described (Brenner 1974). The strain var. Bristol N2 was used as wild type. Single- and double-mutant strains used in this study were: DR466 him-5(e1490)V, CB1489 him-8(e1489)IV, CB1370 daf-2(e1370)III, DR1564 daf-2(m41)III, DR1572 daf-2(e1368)III, TJ1052 age-1(hx546)II, JT709 pdk-1(sa709)X, GR1307 daf-16(mgDf50)I, CF1038 daf-16(mu86)I, NS3227 daf-18(nr2037)IV, CB1375 daf-18(e1375)IV, GR1308 daf-2(e1370); daf-16(mgDf54), GR1309 daf-2(e1370); daf-16(mgDf47), BU050 daf-2(e1370); him-5(e1490), BU051 daf-2(e1368); him-8(e1489), BU052 daf-18(nr2037) him-8(e1489), BU053 daf-18(e1375) him-8(e1489), CB2590 tra-1(e1099)/+ dpy-18(e1096)/+III, and CB2810 tra-1(e1575)/+III; unc-42(e270)him-5(e1490)dpy-21(e428)V.
RNA interference:
To generate a daf-18/PTEN RNA interference (RNAi) construct, a reverse-transcriptase (RT)–PCR experiment was performed to amplify a daf-18 cDNA fragment that was cloned into the vector pPD129.36 (kindly provided by A. Fire). Forward and reverse primers were used as follows: 5-CAT GCC ATG GCA TGT TCC ATC ACA ACG ACG CTA C-3 and 5′-CAT GCC ATG GCA TGC CGA ACA CTT CGC TCT TTT C-3′. RNAi experiments were performed as described (Kamath et al. 2000).
Behavioral assays:
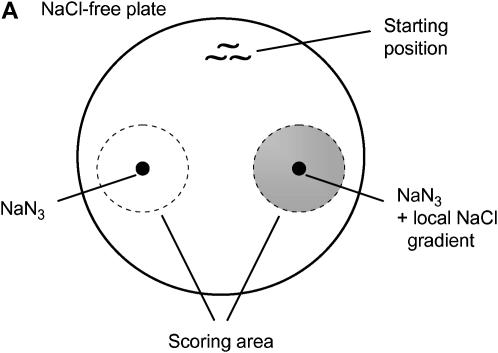
Chemotaxis toward NaCl was assayed as described (Saeki et al. 2001), with some modifications. For preparing the assay plate, an agar plug from a 100-mm NaCl-containing NGM plate was placed on a NaCl-free NGM plate for 12 hr. The plug was then removed, and 1 μl of 0.5 m sodium azide (NaN3) was added onto the same position to anesthetize the worms. As a control, 1 μl NaN3 was also spotted at 4 cm away from the center of the NaCl gradient. For assaying changes in chemotaxis, well-fed animals were washed off from NGM plates and transferred either directly onto the assay plates (naive) or onto food-free NGM plates (conditioned). Bacterium-free NGM plates without NaCl were used as controls. After 4 hr of conditioning, ∼100 young adults were transferred individually by platinum wire, equidistant from the two NaN3 spots, onto an assay plate. Worms were left there to move freely for 30 min, and the number of animals within 1.5 cm of each spot was counted. The chemotaxis index (CI) was calculated as CI = NN − NC/NT (NN, the number of animals around the NaCl gradient; NC, the number of animals around the control spot; NT, total number of animals). Assays were performed in triplicate, and thus in each single experiment N represents ∼300 animals.
Dauer formation analysis:
The progeny of mated hermaphrodites (50/50% male/hermaphrodite ratio) were grown at a temperature (for wild type, 25°; for daf-2 mutants, 22.5°) that gives a mix of dauers and nondauers in the population (Ailion and Thomas 2000). Dauer formation of wild-type worms was induced by applying partially purified exogenous dauer pheromone. Dauers were then recovered under permissive conditions, and the sex of nondauers and recovered dauers was scored.
RESULTS
Effect of sex on the capacity of C. elegans to associate NaCl with starvation:
Grown under well-fed conditions, C. elegans shows chemoattraction to 0.1–200 mm NaCl (Ward 1973). However, migration of worms toward salt falls dramatically following starvation on agar plates with NaCl (Saeki et al. 2001). Since this altered response to salt is induced by paired presentation of NaCl and food deprivation, it involves experience and thus represents a form of associative learning. Chemotaxis toward Na+ and Cl− ions in C. elegans requires a sensory amphid neuron pair called ASE (Bargman and Horvitz 1991), but little is known about the mechanisms underlying such behavioral plasticity (Saeki et al. 2001; Ishihara et al. 2002). This study is an exploration of several aspects of these mechanisms.
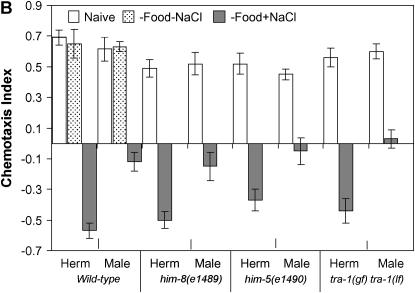
First, we compared learning ability in wild-type C. elegans males and hermaphrodites. To quantify changes in chemotactic behavior, we deprived young adult worms of food for 4 hr and then transferred them individually from conditioning plates to assay plates (Figure 1A). Interestingly, wild-type males conditioned with paired presence of starvation and 100 mm NaCl (salt) were less repelled by salt than hermaphrodites. Whereas 44% of wild-type males continued to move toward salt after conditioning, only 23.5% of hermaphrodites behaved in the same way under identical conditions (Figure 1B). This weaker behavioral change of males was also apparent in mutant strains that segregate males with high frequency (Hodgkin et al. 1979). Both him-8(e1489) and him-5(e1490) mutant males were able to respond to conditioning, and they were less likely to avoid the salt than the corresponding hermaphrodites (Figure 1B). The fact that both sexes showed an equal degree of attraction to NaCl rules out the explanation that different response to paired NaCl and starvation is due to the slight hyperactivity of males.
Figure 1.—
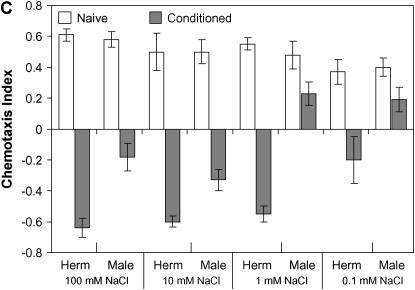
Sex differences in the ability of young adult worms to modulate behavior. (A) Schematic of an assay plate. The shaded area indicates a local NaCl-containing region. Young adults were picked up individually from naive or conditioning plates, placed on the starting position of the assay plate, and left to move freely. The number of worms around each NaN3 spot was counted. (B) Reduced capacity in starvation-induced suppression of chemotaxis of wild-type (N2) males as well as of him-8(e1489), him-5(e1490), and tra-1(e1099) mutant males. P < 0.001, except for him-5(e1490) males, where P < 0.01 (unpaired t-test). For the chemotaxis index (CI = NN − NC/NT, where NN is the number of animals around the NaCl gradient, NC is the number of animals around the control spot, and NT is the total number of animals), positive values demonstrate attraction to NaCl and negative values repulsion from NaCl. Worms were assayed for chemotaxis directly (naive) or conditioned by starving on a NGM plate either without (−) or with (+) NaCl for 4 hr. No mutants exhibited significant changes in behavioral response from the corresponding naives when they were conditioned on NaCl-free plates (data not shown). tra-1(e1575) gain-of-function mutants with XO karyotype are hermaphrodites whereas tra-1(e1099) loss-of-function mutant animals with XX karyotype are fertile males. (C) Chemotaxis of wild-type nematodes toward various concentrations of NaCl. At 0.1 mm NaCl, where only a fraction of nematodes responds to salt, males are still less repelled by salt than hermaphrodites (P < 0.01, unpaired t-test). Herm, hermaphrodite. Error bars indicate mean ± standard error.
The primary determinant of sex in C. elegans is karyotype: animals with one sex chromosome (XO) are normally males, and animals with two sex chromosomes (XX) are hermaphrodites. To test whether the sex difference in learning ability is a direct consequence of karyotype or of the resultant state of differentiation, we monitored behavioral plasticity in mutant nematode populations where the relationship between genotypic and phenotypic sex was reversed. tra-1(e1099) loss-of-function mutant XX animals form fertile males (pseudomales), while tra-1(e1575) gain-of-function mutant XO animals are fertile females (Hodgkin 1987). In this learning assay, tra-1(e1099) XX males behaved like wild-type XO males (i.e., they learned less well), and tra-1(e1575) XO females behaved like wild-type XX hermaphrodites (i.e., they showed superior learning ability) (Figure 1B). Thus, the sex difference in learning is the result of phenotypic rather than genotypic sex.
One possibility is that the sex difference in learning is the result of a sex difference in the capacity to sense salt. To test this possibility we determined the male and hermaphrodite threshold responses in this associative learning paradigm. We first compared chemoattraction to salt of hermaphrodites and males over a range of salt concentrations. Over a 1- to 100-mm concentration range, in both sexes, a similar degree of attraction was seen (Figure 1C). However, both sexes showed a significant reduced attraction to 0.1 mm NaCl (P = 0.001, unpaired t-test). Importantly, there was no significant sex difference in chemoattraction, even at 0.1 mm NaCl, implying sexual equality in the ability to sense salt (P = 0.65, unpaired t-test).
We also compared associative learning in hermaphrodites and males over this range of salt concentrations (Figure 1C). The threshold salt concentration at which animals showed full associative learning proved to be lower in hermaphrodites (0.1–1 mm) than in males (1–10 mm). Furthermore, at all NaCl concentrations tested, males learned less well than hermaphrodites (Figure 1C). Because males are no less able to sense salt than hermaphrodites (as judged by their chemoattractive responses), this difference in threshold salt concentration for full associative learning is likely to reflect a sex difference in learning rather than a capacity to sense salt.
Our results imply the existence of a sex difference in learning ability in C. elegans. However, it remains possible that this difference is specific to the assay used here. For example, it could reflect a sex difference in the biology of nutrition. Potentially, males may be less sensitive to the nutritional environment, in terms of either chemosensation of food or internal sensing of starvation or satiety. This could explain why they show less avoidance of salt after conditioning.
We probed this possibility by examining the effect of food on locomotion in males. Nutritionally replete males moved more slowly in the presence of food (22 ± 0.7 bends in the anterior body region of moving animals during a 30-sec interval) than in the absence of food (28 ± 1.5 bends). This is similar to the basal locomotion response of wild-type hermaphrodites to food (Sawin et al. 2000). In addition, males moved much more slowly on food after starvation (enhanced slowing response: 14 ± 1.9 bends on plates with bacteria vs. 29 ± 0.9 bends on plates without bacteria). Thus, the gross behavioral responses to food are similar in the two sexes. This would seem to suggest that the sex difference in learning reported here reflects sex differences in associative learning rather than in response to the nutritional environment.
Effects of insulin/IGF-1 signaling on the capacity of nematodes to associate NaCl with starvation:
We next investigated the possible genetic basis of this sex-dependent behavioral response. In C. elegans, reproductive growth, life span, and several aspects of behavior are regulated hormonally by a conserved neuroendocrine system, the insulin/IGF-1 signaling pathway (Figure 2A). Several findings are consistent with sex differences in neuroendocrine function in C. elegans (see Introduction). We therefore asked whether insulin/IGF-1 signaling influences associative learning in C. elegans.
Figure 2.—
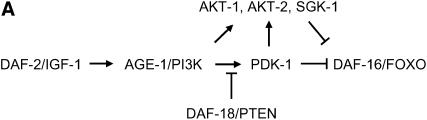
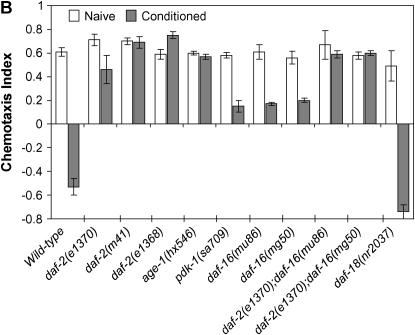
Mutant worms with decreased insulin/IGF-1 signaling are defective in associative learning. (A) Regulatory hierarchy among the components of the insulin/IGF-1 genetic cascade in C. elegans. Arrows indicate activations, bars indicate negative regulatory interactions. DAF-2, the worm insulin/IGF-1 receptor; AGE-1, phosphatidylinositol-3-OH kinase (PI3K); DAF-18, PTEN phosphatase—the only known negative regulator of insulin/IGF-1 signaling; PDK-1, AKT-1, AKT-2, and SGK-1, phosphoinositide-dependent serine/threonine kinases; DAF-16, FOXO forkhead transcription factor. (B) Wild-type and insulin/IGF signaling-defective mutant hermaphrodites maintained at 20° were shifted to 25° and assayed for chemotaxis directly (naive) or conditioned on food-free NGM plates with NaCl. None of these strains displayed significant changes in behavior from the corresponding naives when they were conditioned on NaCl-free plates (data not shown). A genetic null mutation in daf-18, nr2037, improves the ability of hermaphrodites to change behavior. Error bars indicate mean ± standard error.
As shown in Figure 2B, reduction-of-function mutations in the gene daf-2, which encodes the worm insulin/IGF-1 receptor (Kimura et al. 1997), blocked associative learning in hermaphrodites at 25°, where daf-2 mutant traits are highly temperature sensitive. In the assay used these daf-2 mutant animals continued to migrate toward NaCl instead of avoiding it. We also tested two downstream components of this signal transduction pathway, age-1 and pdk-1 (Figure 2A), which encode a phosphatidylinositol-3-OH kinase (PI 3-kinase) catalytic subunit and a phosphoinositide-dependent kinase, respectively (Morris et al. 1996; Paradis et al. 1999). age-1(hx546) and pdk-1(sa709) mutants were defective in associative learning, although only moderately in the latter (Figure 2B).
The poor performance in this learning assay of mutants with reduced insulin/IGF-1 signaling could reflect a role of the insulin/IGF-1 signaling pathway in learning; alternatively, it could reflect simply general defects in neuronal or other functions in insulin/IGF-1 signaling mutants. Only if the former were true would an engineered increase in insulin/IGF-1 signaling be predicted to result in an enhancement of learning ability. We therefore tested whether increased insulin/IGF-1 signaling results in nematodes with enhanced capacity to associate salt with starvation, employing nr2037, a null mutation in daf-18. This gene encodes an ortholog of the human tumor suppressor protein PTEN, a negative regulator of the insulin/IGF-1 signaling pathway (Ogg and Ruvkun 1998; Gil et al. 1999; Mihaylova et al. 1999; Rouault et al. 1999). daf-18(0) elevates insulin/IGF-1 signaling activity by increasing the level of phosphatidylinositol 3,4,5-trisphosphate (PIP3), the lipid secondary signal produced by the AGE-1/AAP-1 PI 3-kinase. Significantly, daf-18(0) resulted in associative learning that was enhanced relative to wild type (Figure 2B).
How does insulin/IGF-1 signaling influence behavior in this learning paradigm? There are many possibilities, given that insulin/IGF-1 signaling affects various aspects of the biology of C. elegans, including development, morphology, behavior, metabolism, and aging (Riddle and Albert 1997; Gems et al. 1998; Lipton et al. 2004). One possibility is that it directly controls the neurobiological processes involved in associative learning. In mammals, insulin and IGF-1 signaling mediate effects of food on metabolism and growth. Thus, mutational inhibition of the insulin/IGF-1 signaling pathway may result in a lack of response to the nutritional environment, due to an internal perception of starvation. To probe this latter possibility, we monitored basal and enhanced slowing responses in insulin/IGF-1 signaling-deficient worms (Sawin et al. 2000). Both well-fed and starved daf-2(e1370) mutant nematodes showed a significant response, in terms of locomotion, to food and to starvation (data not shown). This is consistent with at least some perception of food in daf-2 mutants. Moreover, mutations in daf-2 and age-1 were shown recently to influence behavioral plasticity of temperature-induced responses (Murakami et al. 2005), supporting the role of insulin/IGF-1 signaling in learning per se.
Associative learning is partially dependent on insulin/IGF-1 signaling-independent action of the DAF-16 FOXO transcription factor:
A range of mutant phenotypes of insulin/IGF-1 signaling-deficient worms requires the activity of the forkhead transcription factor DAF-16, for example, constitutive dauer formation (Riddle and Albert 1997), increased life span (Kenyon et al. 1993; Ogg et al. 1997), and reduced fecundity and movement (Gems et al. 1998). Interestingly, mutational inactivation of daf-16 only partially suppressed the learning-defective phenotype of daf-2(e1370) hermaphrodites (Figure 2B). Thus, DAF-2 acts independently of DAF-16 in its effects on this learning paradigm. In fact, in contrast to daf-18, mutation of daf-16 in an otherwise wild-type background markedly reduced hermaphrodite learning capacity (Figure 2B).
Possible link between sex bias in associative learning and insulin/IGF-1 signaling:
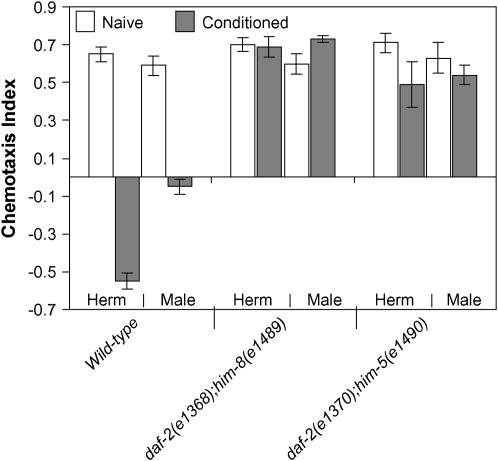
Maleness and lowered insulin/IGF-1 signaling in C. elegans lead to inferior ability to associate salt with starvation. Is it possible that a constitutive reduction in insulin/IGF-1 signaling in wild-type male nematodes accounts for their inferior performance in this assay? We probed further the relationship between sex and DAF-2 activity. Wild-type males exposed to dauer pheromone form more dauer larvae than hermaphrodites (Ailion and Thomas 2000). This male bias to dauer larva formation was not seen when dauers were produced constitutively as the result of reduction-of-function mutations in daf-2 (Table 1). We also compared associative learning in daf-2 mutant males and hermaphrodites and observed the same failure to learn in each (Figure 3).
TABLE 1.
Suppression of the male bias to form dauers by mutations in daf-2
| Genotype | Temp | % males forming dauers (±SE) | % herms forming dauers (±SE) | M:H dauer ratio | N | P |
|---|---|---|---|---|---|---|
| Wild type, pheromone | 25° | 35.8 ± 8.9 | 29.9 ± 5.0 | 1.20 | 369 | >0.1 |
| daf-2(e1370) | 22.5° | 31.9 ± 5.2 | 40.0 ± 5.4 | 0.80 | 766 | >0.1 |
Progeny of mated hermaphrodites were raised at a temperature that gave a mix of dauers and nondauers. The sex of recovered dauers was scored and the overall ratio of male:hermaphrodite (M:H) dauer formation was determined. Herms, hermaphrodites.
Figure 3.—
The sex bias in associative learning in C. elegans is suppressed by mutations in daf-2. P < 0.001 (unpaired t-test). Error bars indicate mean ± standard error.
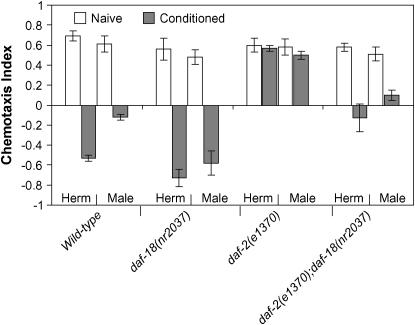
If the inferior learning capacity of wild-type male nematodes described here results from a constitutive reduction in insulin/IGF-1 signaling, then increasing insulin/IGF-1 signaling levels should increase associative learning in males to that of hermaphrodites. To this end we used the daf-18(0) mutation, as described above. Strikingly, daf-18(0) resulted in an elevated learning capacity that was not different from that in mutant hermaphrodites (Figure 4). nr2037 also weakly suppressed the learning-defective phenotype of daf-2(e1370) mutants in both sexes (Figure 4). The results for daf-2 or daf-18 could imply that the sex difference reflects constitutive reduction in insulin/IGF-1 signaling in males. Alternatively, daf-2 and daf-18 mutations could be epistatic to the sex bias, or the sex bias could arise from a parallel pathway.
Figure 4.—
Mutations in the PTEN phosphatase daf-18 increase the ability of nematodes to modulate behavior. The daf-18 null mutation nr2037 restores the ability of males to change behavior to levels comparable with those of mutant hermaphrodites. P < 0.22 (unpaired t-test). nr2037 partially suppresses the learning-defective phenotype of daf-2(e1370) males. P < 0.01 (unpaired t-test). Herm, hermaphrodite. Error bars indicate mean ± standard error.
DISCUSSION
Sex differences in learning ability have been observed in higher animal taxa, including primates, where males have an inferior learning ability (Blote and Van Gool 1989; Vederhus and Krekling 1996; Londsdorf et al. 2004). In this study we showed the existence of a distinct sex bias in associative learning in C. elegans (Figure 1, B and C). Male nematodes learn less well than hermaphrodites in a learning paradigm in which paired presentation of salt and starvation induces changes in chemotactic behavior (Saeki et al. 2001). Because males respond to the presence and absence of food, this learning bias is not a simple consequence of the male's incapability to sense food. However, it cannot be excluded that there might be differences in the efficiency of food sensation between males and hermaphrodites that could influence the learning process. So far there is no indication of this possibility.
We also provide evidence of an apparent regulatory role of insulin/IGF-1 signaling in the nematode rudimentary learning capacity (Figure 2B). daf-2 and age-1 mutant nematodes exhibit inferior learning ability relative to that of wild type (Figure 2B). Furthermore, mutations in the PTEN phosphatase daf-18, which increase insulin/IGF-1 signaling, result in an enhancement of learning ability. The role of the insulin/IGF-1 signaling cascade in nematode learning is further supported by a recent study of insulin/IGF-1 signaling pathway mutants, which demonstrated their influence on behavioral plasticity in response to changing temperature (Murakami et al. 2005).
Evidence is accumulating implicating insulin/IGF-1 signaling in cognitive functions in mammals. In mice, for example, the intestinal glucagon-like peptide (GLP)-1, which influences glucose metabolism by stimulating insulin synthesis and secretion, improves learning and memory (During et al. 2003). The presence of insulin receptor and IGF-1 receptor in discrete regions of the rat brain (Adamo et al. 1989; Unger et al. 1991; Zhao et al. 1999) suggests their functional involvement in brain cognition function. Lesions to the hippocampus produce severe loss of learning ability and memory that can be significantly reversed by insulin treatment (De Castro and Balagura 1976; Horel 1978; Huppert and Piercy 1979; Spiers et al. 2001). Both insulin receptor substrate-1 and phosphatidyinositol-3 phosphate kinase are abundantly expressed in the rat hippocampus (Folli et al. 1994). The PI3K inhibitor LY294002 blocks learning and memory in mice (Barros et al. 2001; Lin et al. 2001). Together, these data point to a role of insulin/IGF-1 signaling in cognitive functions in divergent animal phyla. The mechanisms by which insulin/IGF-1 signaling controls mammalian cognition are poorly understood (During et al. 2003; Zhao et al. 2004). This study shows that C. elegans represents a tractable genetic model organism for unraveling the details of how the insulin/IGF-1 signaling hormonal system affects learning and memory.
It is intriguing that the effect of mutation of daf-2 on C. elegans performance in the salt-starvation association assay involves PI3-kinase and PIP3 generation, but not the forkhead transcription factor DAF-16 FOXO (Figure 2B). This implies that the insulin/IGF-1 signaling pathway branches at PIP3 to control learning, via a distinct PIP3-sensitive pathway, whose identity it would be interesting to learn. Interestingly, our findings clearly show that daf-16 is partially required for learning in daf-2(+) animals. Together, these results suggest that daf-16 plays a role in learning in the assay used that is daf-2 independent. One possibility is that starvation results in DAF-16 activation, as part of the stress response, and that this response constitutes part of the experience of starvation that is associated with salt. In this context, DAF-16 could be responding to the stress-sensitive c-Jun N-terminal kinase (JNK) pathway, which activates DAF-16 (Oh et al. 2005). However, a positive role of DAF-16 in learning argues against that a constitutive decrease of insulin/IGF-1 signaling and increase in DAF-16 activity account for the poor learning ability of males. This working hypothesis was probed by testing the effect of mutations of daf-2 and daf-18 on sex differences in learning. In both cases, the sex difference was suppressed; however, this could imply (a) that the sex difference reflects constitutive reduction in insulin/IGF-1 signaling in males, (b) that daf-2 and daf-18 mutations are epistatic to the sex bias, or (c) that the sex bias arises from a parallel pathway.
Recently, it was shown that insulin/IGF-1 signaling interacts with the nutrient-sensing “target of rapamycin” (TOR) kinases to control life span, metabolism, and development (Saltiel and Kahn 2001; Vellai et al. 2003; Jia et al. 2004). The results presented here suggest a possible interrelationship between nutrition and learning through metabolism regulated by insulin/IGF-1 signaling. Potentially, the coordinated hormonal control of food intake, energy metabolism, and learning by the insulin/IGF-1 signaling cascade may improve the ability of an organism to utilize its limited food resources in such a way as to maximize fitness.
Acknowledgments
We thank T. Stiernagle and the Caenorhabditis Genetics Center founded by the National Institutes of Health for providing strains. We are also grateful to Fritz Müller for critical reading of the manuscript, István Aladzsity for generating bacteria expressing the daf-18 double-stranded RNA, Sára Simon for excellent technical help, and two anonymous referees for valuable comments on the manuscript. This work was supported by the grants EÜ Ministry 648/2003 and National Office for Research and Technology 1A/007/2004 (to T.V.) and Hungarian Scientific Research Fund T047241 (to A.L.K.) and by funds from the Biotechnology and Biological Sciences Research Council, United Kingdom (to D.M.), and the Royal Society and Wellcome Trust (to D.G.). T.V. is a grantee of the János Bolyai scholarship.
References
- Adamo, M., M. K. Raizada and D. LeRoith, 1989. Insulin and insulin-like growth factor receptors in the nervous system. Mol. Neurobiol. 3: 71–100. [DOI] [PubMed] [Google Scholar]
- Ailion, M., and J. M. Thomas, 2000. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics 156: 1047–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargman, C., and H. R. Horvitz, 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., and I. Mori, 1997. Chemotaxis and thermotaxis, pp. 717–738 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Barros, D. M., E. Mello, T. Souza, M. M. de Souza, H. Choi et al., 2001. LY294002, an inhibitor of phosphoinositide 3-kinase given into rat hippocampus impairs acquisition, consolidation and retrieval of memory for one-trial step-down inhibitory avoidance. Behav. Pharmacol. 12: 629–634. [DOI] [PubMed] [Google Scholar]
- Blote, A. W., and H. Van Gool, 1989. Writing behavior of children aged 4 to 5 and a half years. J. Hum. Mov. Stud. 17: 133–152. [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro, J. M., and S. Balagura, 1976. Insulin pretreatment facilitates recovery after dorsal hippocampal lesions. Physiol. Behav. 16: 517–520. [DOI] [PubMed] [Google Scholar]
- During, M. J., L. Cao, D. S. Zuzga, J. S. Francis, H. L. Fitzsimons et al., 2003. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 9: 1173–1179. [DOI] [PubMed] [Google Scholar]
- Folli, F., L. Bonfanti, E. Renard, C. R. Kahn and A. Merighi, 1994. Insulin receptors substrate-1 (IRS-1) distribution in the rat central nervous system. J. Neurosci. 14: 6412–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems, D., and D. L. Riddle, 2000. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics 154: 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil, E., E. Malone, L. Liu, C. Johnson and J. Lees, 1999. Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumour suppressor gene. Proc. Natl. Acad. Sci. USA 96: 2925–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente, L., and C. Kenyon, 2000. Genetic pathways that regulate ageing in model organisms. Nature 408: 255–262. [DOI] [PubMed] [Google Scholar]
- Hirsch, J., and J. C. Boudreau, 1958. Studies in experimental behaviour genetics. I. The heritability of phototaxis in a population of Drosophila melanogaster. J. Comp. Physiol. Psychol. 51: 647–651. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., 1987. A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes Dev. 1: 731–745. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., H. R. Horvitz and S. Brenner, 1979. Nondisjunction mutants of the nematode C. elegans. Genetics 91: 67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horel, J. A., 1978. The neuroanatomy of amnesia. A critic of the hippocampal memory hypothesis. Brain 101: 403–445. [DOI] [PubMed] [Google Scholar]
- Hotta, Y., and S. Benzer, 1970. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc. Natl. Acad. Sci. USA 67: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, F. A., and M. Piercy, 1979. Normal and abnormal forgetting in organic amnesia: effect of locus of lesion. Cortex 15: 385–390. [DOI] [PubMed] [Google Scholar]
- Ishihara, T., Y. Iino, A. Mohri, I. Mori, K. Gengyo-Ando et al., 2002. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109: 639–649. [DOI] [PubMed] [Google Scholar]
- Jia, K., D. Chen and D. L. Riddle, 2004. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131: 3897–3906. [DOI] [PubMed] [Google Scholar]
- Jorgensen, E. M., and C. Rankin, 1997. Neuronal plasticity, pp. 769–790 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser and J. Ahringer, 2000. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA In Caenorhabditis elegans. Genome Biol. 2: research0002.1–00002.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher, R. J., A. Govindarajan, H-Y. Jung, H. Kang and S. Tonegawa, 2004. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116: 467–479. [DOI] [PubMed] [Google Scholar]
- Kenyon, C., 1997. Environmental factors and gene activities that influence life span, pp. 791–814 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. A. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild-type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- Kimura, K. D., H. A. Tissenbaum, Y. Liu and G. Ruvkun, 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Libina, N., J. R. Berman and C. Kenyon, 2003. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115: 489–502. [DOI] [PubMed] [Google Scholar]
- Lin, C.-H., S.-H. Yeh, C.-H. Lin, K.-T. Lu, T.-H. Leu et al., 2001. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdale. Neuron 31: 841–851. [DOI] [PubMed] [Google Scholar]
- Lipton, J., G. Kleeman, R. Ghosh, R. Lints and S. W. Emmons, 2004. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J. Neurosci. 24: 7427–7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londsdorf, E. V., L. E. Eberly and A. E. Pusey, 2004. Sex differences in learning in chimpanzees. Nature 428: 715–716. [DOI] [PubMed] [Google Scholar]
- Mihaylova, V., C. Borland, L. Manjarrez, M. Stern and H. Sun, 1999. The PTEN tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin-like signalling pathway. Proc. Natl. Acad. Sci. USA 96: 7427–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. Z., H. A. Tissenbaum and G. Ruvkun, 1996. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382: 536–539. [DOI] [PubMed] [Google Scholar]
- Murakami, H., K. Bessinger, J. Hellmann and S. Murakami, 2005. Aging-dependent and -independent modulation of associative learning behavior by insulin/insulin-like growth factor-1 signal in Caenorhabditis elegans. J. Neurosci. 25: 10894–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg, S., and G. Ruvkun, 1998. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like signalling pathway. Mol. Cell 2: 887–893. [DOI] [PubMed] [Google Scholar]
- Ogg, S., S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee et al., 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. [DOI] [PubMed] [Google Scholar]
- Oh, S. W., A. Mukhopadhyay, N. Svrzikapa, F. Jiang, R. J. Davis et al., 2005. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 102: 4494–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, S., M. Ailion, A. Toker, J. H. Thomas and G. Ruvkun, 1999. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 13: 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle, D. L., and P. S. Albert, 1997. Genetic and environmental regulation of dauer larva development, pp. 739–765 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Rouault, J., P. E. Kuwabara, O. M. Sinilnikova, L. Duret, D. Thierry-Mieg et al., 1999. Regulation of dauer larva development in Caenorhabditis elegans by daf-18, a homologue of the tumour suppressor PTEN. Curr. Biol. 9: 329–332. [DOI] [PubMed] [Google Scholar]
- Saeki, S., M. Yamamoto and Y. Iino, 2001. Plasticity in chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 204: 1757–1764. [DOI] [PubMed] [Google Scholar]
- Saltiel, A. R., and C. R. Kahn, 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806. [DOI] [PubMed] [Google Scholar]
- Sawin, E. R., R. Ranganathan and H. R. Horvitz, 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. [DOI] [PubMed] [Google Scholar]
- Spiers, H. J., E. A. Maguire and N. Burgess, 2001. Hippocampal amnesia. Neurocase 7: 357–382. [DOI] [PubMed] [Google Scholar]
- Unger, J. W., J. V. Livingston and A. N. Moss, 1991. Insulin receptors in the central nervous system: localization, signaling mechanisms and functional aspects. Prog. Neurobiol. 36: 343–362. [DOI] [PubMed] [Google Scholar]
- Vederhus, L., and S. Krekling, 1996. Sex differences in visual spatial ability in 9-year-old children. Intelligence 23: 33–43. [Google Scholar]
- Vellai, T., K. Takacs-Vellai, Y. Zhang, A. L. Kovacs, L. Orosz et al., 2003. Influence of TOR kinase on lifespan in C. elegans. Nature 426: 620. [DOI] [PubMed] [Google Scholar]
- Ward, S., 1973. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc. Natl. Acad. Sci. USA 70: 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. N. Thomson and S. Brenner, 1986. The structure of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Wolkow, C. A., K. D. Kimura, M.-S. Lee and G. Ruvkun, 2000. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290: 147–150. [DOI] [PubMed] [Google Scholar]
- Zhao, W., H. Chen, H. Xu, E. Moore, N. Meiri et al., 1999. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J. Biol. Chem. 274: 34893–34902. [DOI] [PubMed] [Google Scholar]
- Zhao, W.-Q., H. Chen, M. J. Quon and D. L. Alkon, 2004. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 490: 71–81. [DOI] [PubMed] [Google Scholar]