Abstract
Bridges (1916) observed that X chromosome nondisjunction was much more frequent in XXY females than it was in genetically normal XX females. In addition, virtually all cases of X nondisjunction in XXY females were due to XX ↔ Y segregational events in oocytes in which the two X chromosomes had failed to undergo crossing over. He referred to these XX ↔ Y segregation events as “secondary nondisjunction.” Cooper (1948) proposed that secondary nondisjunction results from the formation of an X-Y-X trivalent, such that the Y chromosome directs the segregation of two achiasmate X chromosomes to opposite poles on the first meiotic spindle. Using in situ hybridization to X and YL chromosomal satellite sequences, we demonstrate that XX ↔ Y segregations are indeed presaged by physical associations of the X and Y chromosomal heterochromatin. The physical colocalization of the three sex chromosomes is observed in virtually all oocytes in early prophase and maintained at high frequency until midprophase in all genotypes examined. Although these XXY associations are usually dissolved by late prophase in oocytes that undergo X chromosomal crossing over, they are maintained throughout prophase in oocytes with nonexchange X chromosomes. The persistence of such XXY associations in the absence of exchange presumably facilitates the segregation of the two X chromosomes and the Y chromosome to opposite poles on the developing meiotic spindle. Moreover, the observation that XXY pairings are dissolved at the end of pachytene in oocytes that do undergo X chromosomal crossing over demonstrates that exchanges can alter heterochromatic (and thus presumably centromeric) associations during meiotic prophase.
IN the article that began this journal in 1916, Calvin Bridges observed that X chromosome nondisjunction was much more frequent in XXY females than it was in genetically normal XX females (Bridges 1916). He further observed that nearly all cases of nondisjunction in XXY females involved achiasmate X chromosomes and that nondisjunction was caused by the segregation of the two achiasmate X chromosomes from the Y chromosome (XX ↔ Y segregations). Realizing that this nondisjunctional process was mechanistically different from whatever nondisjunctional processes occurred in genetically normal females, he dubbed the nondisjunction observed in XXY females “secondary nondisjunction.”
Bridges' initial observation that the vast majority (97%) of secondary nondisjunction events involved nonexchange (E0) X chromosomal bivalents has been further supported by two lines of evidence. First, a thorough analysis of exchange and nondisjunction in XXY females performed by both Sturtevant and Beadle (1936) and by O'Tousa (1982) confirmed Bridges' original finding that 90–96% of secondary nondisjunctional events involved E0 tetrads. [However, Carpenter (1973) obtained a somewhat lower frequency of E0 tetrads (75%) among the secondary nondisjunctional events observed in her study]. Similarly, the limited cytological evidence available suggests that the X chromosomes that undergo nondisjunction in XXY females are usually, although not invariably, achiasmate (Puro and Nokkala 1981). The cases of secondary nondisjunction that do involve exchange X chromosomes predominantly involve X chromosomal bivalents that carry a single very distal exchange (Carpenter 1973; Lüning 1982; O'Tousa 1982).
Second, the frequency of secondary nondisjunction is greatly elevated in females in which X chromosomal exchange has been reduced, either as a consequence of the presence of recombination-defective meiotic mutants (Carpenter and Sandler 1974) or as a consequence of heterozygosity for paracentric inversions (Sturtevant and Beadle 1936; Cooper 1948). However, the presence of structural heterology alone does not appear to influence the probability that an oocyte with two achiasmate X chromosomes and a Y chromosome will undergo nondisjunction; the proportion of E0 tetrads that undergo nondisjunction in FM7/X/Y females (70–80%; Cooper 1948) is roughly similar to the fraction of E0 tetrads that nondisjoin in XXY females bearing two normal-sequence X chromosomes (66–83%; O'Tousa 1982). Thus, the ability of the Y chromosome to induce two X chromosomes to segregate to the opposite pole at anaphase I depends on the exchange status of the X chromosome bivalent, and not on structural heterozygosity. And the probability that two nonexchange X chromosomes will undergo secondary nondisjunction in an XXY female is not affected by sequence divergence. The frequency of secondary nondisjunction in otherwise genetically normal XXY females carrying isogenic X chromosomes is similar to that observed in females that carry different normal-sequence X chromosomes (Rutherford and Carpenter 1988).
One could imagine two causes for elevated X chromosomal nondisjunction in XXY females that do not involve X and Y chromosomal pairing. First, the Y might reduce the amount or distribution of X chromosomal exchange. Such a reduction in exchange would then lead to an increase in nondisjunction, as is commonly observed for recombination-defective mutants. However, Y chromosomes do not reduce the frequency of exchange in XXY females bearing normal-sequence X chromosomes (cf. Bridges 1916; Sturtevant and Beadle 1936; O'Tousa 1982; Ashburner et al. 2005). Indeed, the presence of the Y chromosome usually slightly increases X exchange, as measured among regular gametes, in XXY females with normal-sequence X chromosomes. Similarly, although the Y chromosome can induce small increases and decreases in the residual exchange observed in inversion heterozygotes and some inversion homozygotes (Grell 1962; Merriam 1967), these changes are too small to account for the high levels of nondisjunction observed in XXY females heterozygous for these inversions. Second, the kinds of exceptional offspring recovered from XXY females demonstrate that the ability of the Y chromosome to induce secondary nondisjunction is not simply a negative effect of the presence of a Y chromosome in the female germline on the fidelity of X chromosome disjunction. If this were true, then the segregation of two X chromosomes to the same pole should be concomitant with the random segregation of the Y chromosome, and XXY oocytes and oocytes carrying no sex chromosomes would be as common as XX- or Y-bearing oocytes. Such XXY- or 0-bearing oocytes are only rarely observed (see below).
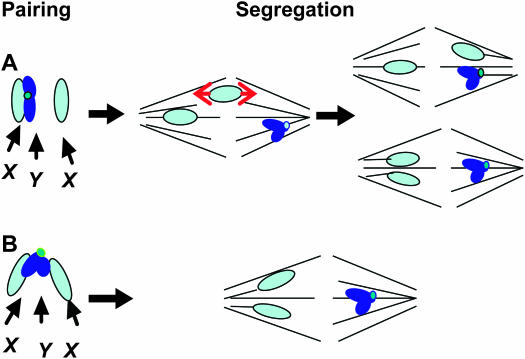
To explain this phenomenon, Bridges (1916) initially suggested that XX ↔ Y segregational events occurred as a consequence of competitive pairing and synapsis of the Y chromosome with one of the two X chromosomes. He proposed that one X paired with and segregated from the Y chromosome, while the remaining X chromosome segregated at random (see Figure 1A). To quote Bridges (1916, p. 116), “That synapsis in an XXY female does not involve all three chromosomes at once, but is between two of them with the third chromosome left unsynapsed, is proved by the fact that the chromosomes in XX eggs are never crossovers, while the Xs of the X and XY eggs are crossovers in about the usual percent. A difference in the paths followed by these two chromosomes originated before the stage at which crossingover became possible.” As shown in Figure 1, a crucial prediction of this model is that the frequency of secondary nondisjunction cannot exceed 50% of the frequency of E0 bivalents because the unsynapsed X chromosome will cosegregate only with the X chromosome in 50% of the cases. Thus, the maximum observed frequency of nondisjunction will be 33% of the E0 frequency because of the inviability of XXX and 0Y embryos.
Figure 1.—
Models of secondary nondisjunction in XXY females. (A) Bridges' (1916) model of secondary nondisjunction in which the Y chromosome pairs with, and segregates from, only one of the two X chromosomes. Because the unpaired X is free to segregate at random, it will segregate only from the Y in 50% of the cases. Thus, the maximum frequency of secondary nondisjunction cannot exceed half the frequency of nonexchange X chromosomes. (B) Cooper's (1948) model of secondary nondisjunction in which each of the two arms of the Y chromosome pairs with the heterochromatin of one of the two X chromosomes, creating an X-Y-X trivalent. The Y chromosome then directs the segregation of the two X chromosomes to opposite poles. This model allows the frequency of secondary nondisjunction to be substantially higher than half the frequency of nonexchange X chromosomes.
The studies of secondary nondisjunction in inversion heterozygotes by both Sturtevant and Beadle (1936) and Cooper (1948) necessitated a modification of Bridges' model. These studies obtained observed frequencies of secondary nondisjunction of ∼60% in females in which the X chromosomes were heterozygous for one or more inversions that strongly suppressed the actual occurrence of exchange. When these measurements are corrected for the inviability of XXX and 0Y embryos, they yield estimates of the true frequency of secondary nondisjunction in the range of 70%. Both Sturtevant and Beadle (1936) and Cooper (1948) realized that these frequencies of nondisjunction suggested that the vast majority (80–90%) of E0 bivalents underwent secondary nondisjunction in XXY females.
Similar observations of high frequencies of secondary nondisjunction in inversion and balancer heterozygotes have been made by others (cf. Gershenson 1935; Zitron and Hawley 1989; Zhang and Hawley 1990). These observations clearly contradict Bridges' original “competitive” pairing model, which predicts a maximum frequency of secondary nondisjunction of 50% (when corrected for the inviability of XXX and 0Y embryos), even in a population of oocytes in which the E0 frequency was 100%. Cooper proposed instead that secondary nondisjunction reflects the formation of an X-Y-X trivalent such that each arm of the entirely heterochromatic Y chromosome pairs with and thus co-orients the heterochromatic regions of one of the two X chromosomes (see Figure 1B). This trivalent then directs the XX ↔ Y segregations that are observed as secondary nondisjunction.
Cooper (1948) proposed that structural dissimilarities within the euchromatin caused by inversion heterozygosity created a situation where the strongest X-X pairings would involve the heterochromatin, a region with substantial homology to the Y chromosome. Thus, in the absence of proper euchromatic synapsis and subsequent crossing over, pairing would be determined primarily by the heterochromatic and Y homologous regions of the X chromosome. To quote Cooper (1948, p. 183), “a moment's reflection will bring conviction that were the euchromatic lengths of the two X chromosomes to be made wholly dissimilar, but the so-called inert or chromocentral regions to remain essentially unaltered, then conjunction between the two Xs would become a primarily heterochromatic affair–a process occurring almost exclusively between the chromocentral regions … those regions of the X wherein pairing with the Y normally occurs.” Indeed, Cooper proposed, that “both arms of the Y chromosome share homology with the X and thus may conjoin with an X.”
Thus, Cooper's model differs from Bridges' explanation in two crucial ways. First, Bridges proposes that the Y actually competes with the second X chromosome to pair with the first, and in doing so prevents X chromosome synapsis and pairing, while Cooper proposes that the X's associate with the Y chromosome only when euchromatic synapsis and crossing over fails or is prevented by structural heterozygosity. Second, while Bridges proposes that one X segregates from the Y and the remaining X segregates at random, Cooper proposes that the three sex chromosomes form a simple trivalent that usually segregates the two X's to one pole and the Y to the other (see Figure 2 of Cooper 1948).
Figure 2.—
Sites of hybridization for the heterochromatic probes used in this study. The 1.686 satellite sequences on the X chromosome and the AATAC repeats on the Y chromosome were chosen as probes for in situ hybridization (Dernburg 2000). The 1.686 satellite is located in the pericentric heterochromatin on both the normal-sequence X chromosome and the X chromosome carrying the euchromatic inversion In(1)dl-49. The 1.686 satellite sequence is separated into two parts on the FM7 balancer chromosome; the larger block of satellite sequences is near the distal tip of the chromosome and the smaller region of 1.686 satellite sequences is located near the centromere. Thus, as shown in Figure 3, in prometaphase oocytes the FM7 and X chromosomes can be easily differentiated because the normal-sequence X chromosome has one large block of 1.686 signal near its centromere, while FM7 has a small block of 1.686 signal near the centromere and a larger block of 1.686 signal near its distal tip. The AATAC repeats are located medially on the long arm of the Y chromosome. The y+Y chromosome also carries the tip of the In(1)sc8 chromosome appended to the distal tip of YL. This fragment of the X chromosome contains a large block of 1.686 satellite sequence, resulting in both X and Y signals being observed for the y+Y chromosome.
Consistent with this hypothesis of an X-Y-X trivalent, Cooper (1948) demonstrated that a one-armed Y chromosome derivative (YS) shows a much reduced ability to induce secondary nondisjunction when compared to a structurally normal metacentric Y chromosome [i.e., the frequency of secondary nondisjunction in In(1)dl-49,BM1/+/YS females is 36% compared to 78% in In(1)dl-49,BM1/+/Y females]. Moreover, Y chromosomes differ in their effects on the frequency of secondary nondisjunction (Neuhaus 1941; Cooper 1948). These differences appear to be related to the size of the Y. Deleted Y chromosomes [i.e., R(YL), scV1.YS] have a much weaker effect than normal-size Y chromosomes (Chadov 1981). This observation supports the interpretation that the cause of secondary nondisjunction is a physical interaction between the two X chromosomes and both arms of the Y chromosome.
Cooper's trivalent model was given credence by the finding of similar heterochromatic sequences, most notably the AATAT and AAGAG satellites as well as the rDNA, on both the X and Y chromosomes (Lohe et al. 1993). More important, the suggestion that such homologies might facilitate both stable heterochromatic pairings and achiasmate segregation is given real credence by the observations of Dernburg et al. (1996), who demonstrated that meiosis in Drosophila melanogaster females exhibits a modified diplotene-like stage in which heterochromatic associations are maintained until the end of prophase while euchromatic pairings are dissolved at the end of pachytene. These heterochromatic pairings can be maintained even during prometaphase (Dernburg et al. 1996 and below) and Hawley et al. (1993) and Karpen et al. (1996) demonstrated that these heterochromatic pairings were both necessary and sufficient to ensure the segregation of achiasmate homologs. Finally, the ability of a metacentric partner of a trivalent to direct the segregation of its two acrocentric homologs to the opposite poles is well documented by classic and modern cytogenetic studies (cf. MacKnight and Cooper 1944; Haaf et al. 1989). Thus, it seems fully reasonable to propose that the heterochromatic regions of X chromosomes, which surround the centromere, actually do pair with the entirely heterochromatic Y chromosome and that, in the absence of exchange, these pairings are sufficient to ensure segregation.
Finally, Grell (1962, 1976) has proposed a model of secondary nondisjunction that is similar to Cooper's in suggesting that the X chromosomes that failed to synapse and crossover were free to form a trivalent with a metacentric Y chromosome, or indeed with any metacentric chromosome, during a postulated second round of so-called distributive pairing that was presumed to be homology independent. It is, however, clear from numerous cytological studies that no such secondary round of pairing exists (Hawley and Theurkauf 1993; Dernburg et al. 1996). Moreover, it is equally clear that the homology-independent segregations on which Grell based her model for secondary nondisjunction (such as those involving compound autosomes) do not involve the physical interaction of segregating chromosomes (Dernburg et al. 1996).
For those reasons, Cooper's 1948 model for secondary nondisjunction has been the predominant explanation for this phenomenon and has been widely accepted and taught for the last six decades. However, until now it has never been directly tested. Here we use the technique of fluorescence in situ hybridization (FISH) to X and Y chromosomal satellite sequences developed by Dernburg et al. (1996) to study the association of X and YL heterochromatin in meiotic prophase in XXY oocytes in which exchange has or has not occurred. In these studies we suppress exchange by using either the highly rearranged balancer chromosome FM7 or an X chromosome bearing the euchromatic inversion In(1)dl-49, which suppresses X exchange without disrupting the homology of the pericentric X heterochromatin. These chromosomes are diagrammed in Figure 2.
We demonstrate that the XXY associations occur at high frequency in early prophase and persist through midmeiotic prophase for all genotypes studied. These XXY associations are usually lost by late prophase in oocytes that are free to undergo X chromosomal crossing over. However, XXY associations are maintained until late prophase in XXY oocytes in which X chromosomal exchange fails to occur. We presume that those XXY associations that persist until prometaphase create the trivalent proposed by Cooper and thus facilitate the segregation of the two X chromosomes and the Y chromosome to opposite poles on the developing meiotic spindle. Thus, the XX ↔ Y segregations observed in exchange-suppressed oocytes are indeed presaged by physical associations of the X and Y chromosomal heterochromatin that persist throughout meiotic prophase.
However, our data disagree with Cooper's view that XXY associations are formed following failure of the two X chromosomes to properly synapse and crossover. First, Gong et al. (2005) demonstrated that in FM7/X females the FM7 balancer and the normal-sequence X chromosome pair and synapse normally. Second, our data argue that XXY associations are visible from the beginning of meiotic prophase in FM7/X/Y females and are usually maintained until the end of prophase.
The fact that the early XXY associations observed in oocytes that undergo X chromosomal exchanges are dissolved following the end of the pachytene demonstrates that exchanges can alter heterochromatic (and thus presumably centromeric) associations long before nuclear envelope breakdown (NEB). These observations are consistent with those of Kemp et al. (2004) and Tsubouchi and Roeder (2005) in yeast, which demonstrated a role of exchange in controlling centromeric associations prior to NEB.
MATERIALS AND METHODS
Drosophila stocks:
In this study, the normal X, which carries the markers y and w, was obtained from a stock that also carries the y+Y chromosome. The FM7a,w chromosome (denoted as FM7), which is marked with y sc w v and B, is derived from a stock that is homozygous for FM7a and also carries y+Y. In(1)dl-49 is marked with y Hw m and g. The normal Y chromosome was obtained from our copy of the Oregon-R stock.
Crossing schemes:
To obtain FM7/FM7/y+Y females, rare females that were phenotypically non-yellow, white, and strong-Bar were selected from the FM7 stock. These FM7/FM7/y+Y exceptional females were then continuously crossed with FM7/y+Y males to create a stock. Similarly, rare exceptional yw/yw/ y+Y were obtained from our male yw/y+Y stock. These yw/yw/ y+Y females were then crossed with yw/y+Y males to continuously produce yw/yw/y+Y female offspring. These females are denoted by the designation “X/X/y+Y.” Both stocks were selected from every generation to maintain X/X/y+Y females. To obtain In(1)dl-49/X/y+Y females, FM7/yw/y+Y females were crossed with In(1)dl-49, y Hw m g/Y males and the yellow-plus, white-plus Hairy-wing non-Bar females with the genotype of In(1)dl-49, y/yw/y+Y [denoted In(1)dl-49/X/Y] were collected. To create XXY females bearing a normal-sequence Y chromosome, we crossed either y w/y w/y+Y or FM7/y w/y+Y females with w+/Y (Oregon-R) males and obtained the desired yellow y w/y w/Y (denoted simply as XXY) or FM7/y w/Y offspring (denoted FM7/X/Y).
Secondary nondisjunction assays:
To measure the frequency of secondary nondisjunction, females for each genotype were crossed individually to attached-XY, y+ v f B; C(4)RM, ci eyR males. For details of this cross and its analysis, see Hawley et al. (1993) and Harris et al. (2003). Although this cross does allow us to measure fourth chromosome nondisjunction as well as X chromosome nondisjunction, fourth chromosome exceptions were rare in all genotypes examined and are not considered here.
Probes for in situ hybridization:
The 1.686 satellite sequences (also known as the 359-bp repeats) on the X chromosome and AATAC repeats on the Y chromosome were chosen as probes for in situ hybridization (Dernburg et al. 1996; Dernburg 2000). The 1.686 satellite is located in the pericentromeric region on both normal X chromosomes and the euchromatic inversion X chromosome, In(1)dl-49 (Figure 2). The FM7 balancer chromosome displays two blocks of hybridization of the 1.686 sequence. Due to In(1)sc8, one of the three inversions that composes FM7, the 1.686 satellite sequence was separated into two parts on this balancer chromosome: the larger region of satellite sequence located near the distal tip of the chromosome and the smaller region of 1.686 satellite sequences located at the centromere region (see Figure 2). Thus, as shown in Figure 3, in prometaphase oocytes the FM7 and X chromosomes can be easily differentiated because the normal-sequence X chromosome has one large block of 1.686 signal near its centromere, while FM7 has a small block of 1.686 signal at its base (near the centromere) and a larger block of 1.686 signal near its distal tip. The position of the long arm of the Y chromosome (YL) was assessed by hybridization using the AATAC repeats, which are located medially on the long arm on the Y chromosome (Figure 2). The y+Y chromosome also bears the tip of the In(1)sc8 chromosome appended to the distal tip of YL. This fragment of X contains a large block of X heterochromatin, including a large block of 1.686 satellite sequence, resulting in both X and Y signals being observed for the y+Y chromosome.
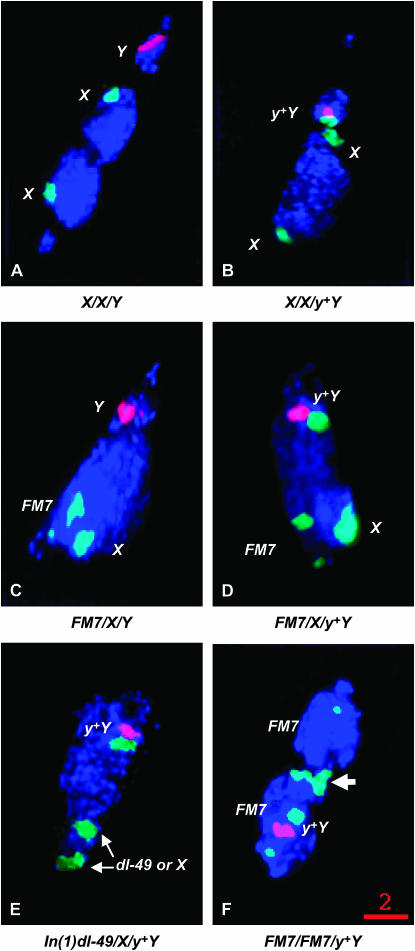
Figure 3.—
Centromere co-orientation in prometaphase/metaphase oocytes as detected with FISH. In XXY (A) and X/X/y+Y (B) oocytes, in which the X chromosomes were free to crossover, the centromeres of two X chromosomes were always at the ends of the main mass of chiasmate chromosomes, with the two homologous centromeres oriented toward opposite poles. The Y chromosome is virtually always observed between the main mass and one of the two poles of the spindle. However, in oocytes in which X chromosomal exchange is fully suppressed by the presence of the FM7 balancer chromosome, both the X and FM7 chromosomes are often oriented toward one pole with the Y chromosome oriented toward the other. Such orientations will result in XX ↔ Y segregations. (C) Depicts such a metaphase figure from an FM7/X/Y oocyte. (D) Depicts a similar metaphase figure from an FM7/X/y+Y oocyte. In both figures, the two X's and the Y chromosome are oriented toward opposite poles. The frequencies of XX ↔ Y orientations for each genotype studied are presented in Table 2. As shown in E, XX ↔ y+Y segregational events are also commonly observed in In(1)dl-49/X/Y oocytes. As shown in F, in FM7/FM7/y+Y females in which the FM7 chromosomes were free to crossover (Zhang and Hawley 1990), the centromeres of two X chromosomes were always oriented toward opposite poles. However, as indicated by the arrow in F, the distal heterochromatic blocks of the two homologs usually remained associated at the metaphase plate (see text). The Y chromosome is seen associated with either of the two poles. Red bar, 2 μm.
The 359-bp sequence of 1.686 satellite and (AATAC)6 were used for probe preparation. The 30-nucleotide oligos for both strands of the 359-bp sequence and (AATAC)6 for AATAC repeats were generated by Integrated DNA Technologies. The synthesized oligos for the 359-bp sequence were pooled in equal molar amounts and a total 10 μg of pooled oligos or (AATAC)6 were used for fluorescent probe labeling. The probes were labeled by using terminal deoxynucleotidyl transferase (Roche) to incorporate 5-(3-aminoallyl)-dUTP (Molecular Probes, Eugene, OR) onto the 3′-terminus as described in Dernburg et al. (1996) and Dernburg (2000). The 5-(3-aminoallyl)-labeled oligos were conjugated to a reactive fluorescent dye by using the ARES Alexa Fluor DNA labeling kit (Molecular Probes) followed by column purification as described in the kit. For probes of the 359-bp sequence on the X chromosome, Alexa Fluor 488 dye was used. For probes of (AATAC)6 on the Y chromosome, Alex Fluor 647 dye was used. The labeled probes were diluted in TE buffer at a concentration of 50–100 ng/μl and kept at −80° for in situ hybridization use.
Egg chamber dissection and fixation:
Approximately 30–40 females, which had enclosed 2–3 days previously, were mated to 5–10 males and fed on yeast for 3 days prior to egg chamber dissection. These females were then anesthetized and the abdomens were ruptured one by one with forceps. Whole ovaries were collected and kept in 1× Robb's solution during the dissection. The ovaries were then transferred to 1.0 ml prewarmed fixation solution (4% formaldehyde, 100 mm Na–cacodylate, pH 7.2, 10 mm EGTA) on a dissecting plate to fix for 4 min. Oocytes were staged according to their morphology as described in Spradling (1993). Basically, the oocytes that composed less than one-third of the volume of their egg chamber were deemed to be in midprophase (stages 2–9). The oocytes that composed one-half or more of the volume of the egg chambers, but which lacked dorsal filaments, were considered to be in late prophase (stages 10–12). The oocytes in which the dorsal filaments were visible, and for which few or no nurse-cell nuclei remained, were considered to be in prometaphase–metaphase (stages 13–14). During the fixation, ovaries were teased apart using forceps. After fixation, the egg chambers were quickly transferred to 2× SSCT (0.3 m NaCl, 0.03 m Na–citrate, pH 7.2, 0.1% Tween-20) and washed with 2× SSCT four times for 10 min each wash.
Fluorescent in situ hybridization and microscopy:
FISH was performed as described in Dernburg (2000) with only the following slight modifications. Fixed egg chambers were incubated successively in 20, 40, and 50% formamide-containing 2× SSCT for 10 min each. The egg chambers were then transferred to fresh 2× SSCT with 50% formamide (Fluka) and incubated at 37° for at least 1 hr. The solution was aspirated carefully and the egg chambers were left at the bottom. Two microliters of each X and Y chromosome fluorescence-labeled DNA probe solution was combined with 36 μl of hybridization solution containing 10% dextran sulfate (Fluka), 3× SSCT, and 50% formamide (Fluka) and the solution was added to the egg chambers. The egg chambers with probes were denatured at 94° for 2 min followed by incubation for hybridization at 30° overnight. When hybridization was finished, the egg chambers were washed in 2× SSCT with 50% formamide three times at 37° for 10 min/wash, followed by two washes in 2× SSCT with 40 and 20% formamide, respectively, at room temperature. The egg chambers were then washed twice in 2× SSCT for a total of 30 min, stained for 10 min in 2× SSCT with 0.5 μg/ml DAPI, and rewashed four times in 2× SSCT for a total of 40 min. The egg chambers were mounted on slides in Vectashield (Vector Laboratories, Burlingame, CA) for analysis.
Microscopy was conducted using a DeltaVision microscopy system (Applied Precision, Issaquah, WA) equipped with an Olympus IX70 inverted microscope and high-resolution CCD camera. The image data were deconvolved using the softWoRx v.25 software (Applied Precision) and projected with multiple stacks. In all prophase oocytes examined, the two X chromosomes were observed as a single bright mass of hybridization, and thus, as previously shown by Dernburg et al. (1996), X heterochromatin remains paired throughout prophase. (Individual X chromosomes were not observed as separate entities until prometaphase; see Figure 3.) However, as shown in Figure 4, the Y chromosome can be shown to be either physically associated or not associated with the X chromosome during prophase I by examining the colocalization of the 1.686 and AATAC hybridization signals.
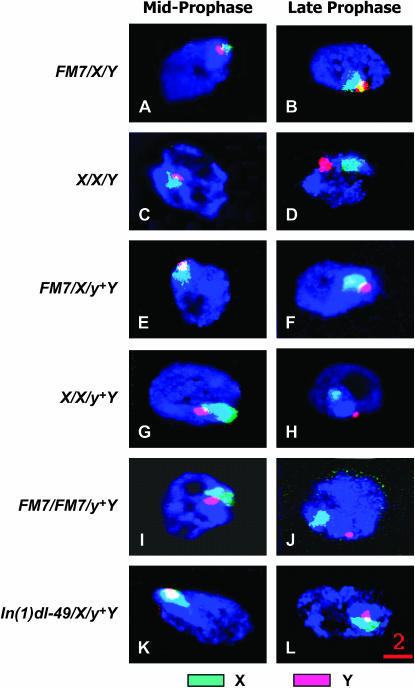
Figure 4.—
XXY associations in mid- and late meiotic prophase. FISH was used to determine the positions of the X and Y chromosomes in both midprophase (left column) and late prophase (right column) oocytes. The frequency of XXY associations for each genotype at mid- and late prophase is quantified in Table 3. Although the X and Y chromosomes were associated as a single mass in more than half of the midprophase oocytes examined for all genotypes, the highest frequencies of XXY associations (∼70%) were observed in oocytes heterozygous for the FM7 balancer chromosome. These XXY associations persisted until late prophase at high frequencies in oocytes in which X chromosomal crossing over was suppressed by inversion heterozygosity [i.e., in FM7/X/Y, FM7/X/y+Y, or In(1)dl-49/X/y+Y oocytes]. Indeed, in either FM7 or In(1)dl-49 heterozygotes the frequency of XXY associations in late prophase remained virtually unchanged from the frequency observed in the mid-prometaphase. However, when exchange-competent oocytes were examined in late prophase, the frequency of XXY associations had diminished by approximately fivefold, demonstrating that XXY associations are frequently dissolved in exchange-competent oocytes. Red bar, 2 μm.
RESULTS
Genetic analysis of secondary nondisjunction in the genotypes used in this study:
The results of measuring X chromosomal nondisjunction in both XX and XXY females are reported in Table 1. The crosses reported here involve three different X chromosomes, whose structures are portrayed in Figure 2: a normal-sequence X chromosome, the FM7 balancer chromosome, and a chromosome bearing the entirely euchromatic inversion In(1)dl-49. Multiple lines of evidence suggest that FM7 fully blocks exchange when heterozygous, while heterozygosity for In(1)dl-49 appears to reduce the total frequency of X chromosomal exchange to ∼70–80% of normal (Novitski and Braver 1954; Roberts 1962; Theurkauf and Hawley 1992; Gong et al. 2005). We also used two separate Y chromosomes: a normal-sequence Y chromosome derived from our Oregon-R stock and the y+Y chromosome.
TABLE 1.
Comparision of nondisjunction in X/X and X/X/Y Drosophila females
| Oocyte genotype | Sperm genotypea | X/Xb | X/X/Y | X/X/y+Y | FM7/X | FM7/X/Y | FM7/X/y+Y | dl-49/Xb | dl-49/X/y+Y | FM7/FM7/y+Y |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | ||||||||||
| X; 4 | XY; 44 | 970 | 582 | 743 | 614 | 244 | 684 | 3761 | 127 | 792 |
| X; 4 | 0; 44 | 1707 | 573 | 1333 | 584 | 232 | 672 | 2111 | 231 | 363 |
| X nondisjunction | ||||||||||
| 0; 4 | XY; 44 | 1 | 7 | 19 | 1 | 270 | 409 | 16 | 94 | 7 |
| XX; 4 | 0; 44 | 2 | 12 | 21 | 2 | 307 | 668 | 12 | 68 | 11 |
| Total progeny | 2680 | 1174 | 2128 | 1201 | 1054 | 2433 | 5900 | 521 | 1181 | |
| Adjusted total | 2683 | 1193 | 2168 | 1204 | 1631 | 3502 | 8039 | 683 | 1199 | |
| % nullo-X | 0.1 | 1.2 | 1.8 | 0.2 | 33.1 | 23.4 | 0.4 | 19.9 | 1.2 | |
| % diplo-X | 0.1 | 2 | 1.9 | 0.3 | 37.7 | 37.9 | 0.3 | 27.5 | 1.8 | |
| Total % X nondisjunction | 0.2 | 3.2 | 3.7 | 0.5 | 70.8 | 61.3 | 0.7 | 47.4 | 3.0 |
The genotype of male tester is attached-XY, y+ v f B; C(4), ci eyR.
The control data are from Zitron and Hawley (1989).
As shown in Table 1, the frequency of secondary nondisjunction is low (∼3%) in XXY females in which the X chromosomes are free to undergo crossing over, but quite high in FM7/X/Y females in which exchange is absent. The frequencies of X nondisjunction that are observed in XXY and X/X/y+Y females are 3.2 and 3.7%, respectively. Twenty-fold higher frequencies of X nondisjunction are observed in FM7/X/Y and FM7/X/y+Y females (70.8 and 61.3%, respectively). These frequencies of Y-induced nondisjunction can be compared to the frequency of X chromosomal nondisjunction in either genetically normal XX females or exchange-suppressed FM7/X females, which are usually in the range of 0.1–0.2% (Ashburner et al. 2005; Gong et al. 2005). The presence of the marked y+Y chromosome in X/X/y+Y and FM7/X/y+Y females allows us to conclude that nearly all cases of X nondisjunction in XXY females were due to XX ↔ Y segregations. For example, 100% of the instances of X nondisjunction observed in X/X/y+Y females and 99.6% instances of X nondisjunction observed in FM7/X/y+Y females reflected XX ↔ Y segregations.
Similarly, in females heterozygous for In(1)dl-49, which reduces exchange by ∼70–80%, the frequency of X nondisjunction is quite low (∼0.7%) (Novitski and Braver 1954; Roberts 1962; Zitron and Hawley 1989; Whyte et al. 1993). However, a 50-fold higher frequency of X nondisjunction is observed in In(1)dl-49/X/y+Y females (47.4%). The presence of the marked y+Y chromosomes reveals that all of the 162 instances of X nondisjunction observed in In(1)dl-49/X/y+Y females were the result of XX ↔ Y segregations, and no offspring derived from XXY- or 0-bearing oocytes were obtained.
We should note that while the frequency of secondary nondisjunction in In(1)dl-49/X/y+Y females observed here (47.4%) is similar to the frequency of secondary nondisjunction observed in In(1)dl-49/X/Y females by Sturtevant and Beadle (1936) and by Zitron and Hawley (1989) (45.6 and 56.4%, respectively); it is substantially lower than the frequency of 70% obtained for In(1)dl-49/X/Y females by Cooper (1948). However, Cooper also obtained a frequency of secondary nondisjunction of 80% in In(1)dl-49/In(1)AM/Y females, exceeding the values of 61–70% that we observe in FM7/X/Y females, in which exchange is fully suppressed. The basis for these differences is not understood, but it presumably reflects the effect of genetic background on the efficacy of the Y chromosome in directing XX ↔ Y segregations. Indeed, Neuhaus (1941) noted that different wild-type Y chromosomes yielded different frequencies of secondary nondisjunction for the same X chromosomal pairs.
Thus our observations reprise those of Bridges (1916), Gershenson (1935), Sturtevant and Beadle (1936), and Cooper (1948) in demonstrating that the frequency of secondary nondisjunction increases with the fraction of E0 tetrads (achiasmate X chromosomes) and that virtually all cases of secondary nondisjunction reflect XX ↔ Y segregations. Finally, on the basis of estimates of E0 frequencies in XX, FM7/X, and dl-49/X females (8–10, 100, and 70–80%, respectively; Ashburner et al. 2005), it seems clear that a high fraction of nonexchange X chromosome pairs participate in secondary nondisjunction.
Direct cytological visualization of XX ↔ Y co-orientation in prometaphase and metaphase:
In oocytes in which the X chromosomes are free to undergo crossing over, an analysis of chromosome orientation in stage 13 (prometaphase) to stage 14 (metaphase) oocytes reveals that the two X chromosomes are usually oriented toward opposite poles of the meiotic spindle with the Y or y+Y chromosome usually located on one of the two half-spindles (see Figure 3, A and B, and Table 2). In all of the XXY and X/X/y+Y oocytes examined, the two centromeric regions of the two X chromosomes are observed at opposite ends of the main mass of chiasmate chromosomes, with the Y or y+Y chromosome usually located between the main mass and one of the two poles (see Figures 3, A and B, and Table 2). No cases were observed in which the X chromosomes appeared to be segregating from the Y chromosome (N = 72). However, given that the observed frequency of secondary nondisjunction in these crosses is only 3.0% (see Table 1), our failure to observe such events is perhaps not surprising.
TABLE 2.
Centromere co-orientation during secondary nondisjunction
| % XX ↔ Y segregation
|
|||||
|---|---|---|---|---|---|
| Genotype | Total | XY ↔ X | XX ↔ Y | As assayed cytologically | As assayed genetically |
| FM7/X/Y | 68 | 21 | 47 | 69.1 | 70.8 |
| X/X/Y | 37 | 37 | 0 | 0.0 | 3.2 |
| FM7/X/y+Y | 76 | 24 | 51 | 67.1 | 61.3 |
| X/X/y+Y | 35 | 35 | 0 | 0.0 | 3.7 |
| In(1)dl-49/X/y+Y | 31 | 17 | 14 | 45.2 | 47.4 |
| FM7/FM7/Y | 23 | 23 | 0 | 0.0 | 3.2 |
However, cases in which the two X chromosomes were oriented to one pole with the Y chromosome oriented toward the opposite pole were frequently observed when X chromosome exchange is prevented by heterozygosity for FM7 (see Figures 3, C and D, and Table 2). As noted in Table 2, the frequencies of XX ↔ Y orientations in FM7/X/Y and FM7/X/y+Y oocytes (69.1 and 67.1%, respectively) correspond well to the frequencies of XX ↔ Y segregations observed genetically (70.8 and 61.3%). To ensure that the high levels of XX ↔ Y co-orientation events observed in FM7/X/Y females were not simply a consequence of the structural aberrations associated with the FM7 balancer chromosome, we also examined prometaphase orientation in FM7/FM7/y+Y oocytes, which have near normal levels of recombination (Zhang and Hawley 1990). In all 23 FM7/FM7/y+Y oocytes imaged, the centromeric regions of the two FM7 chromosomes are associated with opposite poles of the spindle (see Figure 3F).
Moreover, high levels of XX ↔ Y co-orientation events were also observed in X exchange-suppressed females that carried homologous blocks of structurally normal X heterochromatin [In(1)dl-49/X/y+Y]. As shown in Figure 3E and Table 2, XX ↔ Y co-orientations were commonly observed in In(1)dl-49/X/y+Y oocytes. This cytologically observed frequency of XX ↔ Y co-orientations is comparable to the frequency of secondary nondisjunction (47.4%) obtained genetically. While the frequencies of XX ↔ Y co-orientation and segregation events observed in In(1)dl-49/X/y+Y oocytes are lower than the frequencies with which such events are observed in FM7/X/Y and FM7/X/y+Y, it must be remembered that, unlike FM7, the In(1)dl-49 chromosome still allows a substantial frequency of X exchange (Sturtevant and Beadle 1936; Novitski and Braver 1954; Roberts 1962). Thus, the nondisjunctional events observed by genetic tests appear to be an accurate reflection of the real frequency of XX ↔ Y segregational events.
To determine whether or not these XX ↔ Y segregations were presaged by XXY pairings during meiotic prophase as predicted by Cooper (1948), we set out to examine X and Y chromosomal associations prior to nuclear envelope breakdown. As shown below, such associations are common in oocytes of all the tested genotypes in early to late prophase, but are maintained only until the end of prophase in exchange-suppressed oocytes.
The heterochromatic regions of the X and Y chromosomes are physically associated during early to midprophase in all genotypes studied:
To examine X–Y associations in early prophase, we asked whether or not the Y-specific AATAC probe colocalized with the 1.686 X chromosomal probe in the germarial cells of FM7/X/Y, FM7/X/y+Y, and X/X/y+Y females (N = 91, 99, and 89, respectively). In each of these cases, the Y chromosome was physically associated with the X chromosomes in at least 93% of the cells (data not shown). Cases in which the Y chromosome signal was clearly separated from the X chromosomes were observed in <7% of the oocytes for all three genotypes.
As noted above, following synaptonemal complex dissolution at the end of pachytene, meiotic chromosomes in Drosophila enter an extended and unusual diplotene-like phase where euchromatic regions desynapse but heterochromatic regions remain tightly paired (Dernburg et al. 1996). To determine whether the X and Y chromosomes remained associated during this period of middle prophase, we examined X and Y chromosomal associations during stages 2–9 of oogenesis. As shown in Figure 4 and quantified in Table 3, XXY associations during midprophase were observed in at least 50% of the oocytes in all genotypes studied. In oocytes in which X chromosomal exchange has been suppressed [those of FM7/X/Y, FM7/X/y+Y, and In(1)dl-49/X/y+Y females], the observed frequencies of such associations (67–69%) correlate well with the observed frequencies of secondary nondisjunction (71–73%). However, for exchange-competent oocytes (XXY, X/X/y+Y), the observed frequencies of XXY associations in mid-prometaphase (50–55%) are far higher than would be predicted on the basis of observed frequencies of secondary nondisjunction (∼3–4%). To test the possibility that XXY associations might be dissolved prior to the end of prophase in oocytes in which the X chromosomes had undergone crossing over, but maintained in oocytes in which X chromosomal exchange had been suppressed, we next examined XXY associations in late prophase oocytes (stages 10–12), which precede nuclear envelope breakdown.
TABLE 3.
XXY association during mid- to late prophase
| Midprophase
|
Late prophase
|
|||||
|---|---|---|---|---|---|---|
| Total | XY association | % | Total | XY association | % | |
| FM7/X/Y | 82 | 60 | 73.2 | 69 | 48 | 69.6 |
| X/X/Y | 91 | 50 | 54.9 | 95 | 11 | 11.6 |
| FM7/X/y+Y | 76 | 54 | 71.1 | 88 | 60 | 68.2 |
| X/X/y+Y | 88 | 44 | 50.0 | 68 | 8 | 11.8 |
| dl-49/X/y+Y | 45 | 23 | 51.5 | 39 | 18 | 46.2 |
| FM7/FM7/y+Y | 56 | 37 | 66.1 | 44 | 11 | 25.0 |
The association of X and Y chromosomes is greatly diminished by the end of prophase in exchange-competent oocytes but maintained in oocytes in which X chromosomal exchange has been suppressed:
As shown in the right column of Figure 4 and quantified in Table 3, in oocytes in which X chromosomal crossing over was suppressed by inversion heterozygosity [either FM7a or In(1)dl-49], the frequencies of XXY associations in late prophase (stages 10–12) remained virtually unchanged from the frequencies observed in midprophase. This demonstrates that in the absence of exchange these XXY associations are stable throughout prophase. However, when XXY associations were observed in late prophase in X chromosome exchange-competent oocytes, their frequency had diminished substantially (see Table 3). Indeed, in X/X/Y or X/X/y+Y oocytes the frequency of such associations diminished by approximately fivefold to 11–12%. Since many of our oocytes were in stage 10, we suspect that this value may be an overestimate of the true frequency of such associations that will still exist at nuclear envelope breakdown.
The fact that XXY associations are maintained in In(1)dl-49/X/y+Y oocytes suggests that the maintenance of stable XXY associations observed in FM7/X/y+Y oocytes is not simply a consequence of the heterochromatic rearrangements associated with the FM7 chromosome. By the same token, the dissolution of XXY associations in FM7/FM7/y+Y females, in which the homozygous balancer chromosomes are free to undergo exchange, reveals that even in this genotype the frequency of XXY associations diminishes as prophase continues. Thus, the maintenance of XXY associations throughout prophase that is observed in FM7/X/Y and FM7/X/y+Y oocytes cannot be a consequence of heterochromatic rearrangement, but rather must reflect the fact that XXY associations are stabilized throughout prophase only in the absence of crossing over.
The X chromosomal sequences at the distal tip of y+Y often remained associated with the X chromosomes even when the X and Y chromosomes were no longer paired:
In FM7/X/y+Y or X/X/y+Y oocytes in which the medial region of YL was not associated with the two X chromosomes (as judged by the failure of the AATAC probe to colocalize with the X chromosomal 1.686 signal), the 1.686 sequences located on the distal tip of the y+Y chromosome often remained paired with the X chromosomes. Indeed, for all y+Y-bearing genotypes examined, the 1.686 signal located on the y+Y tip could be resolved only from the main X signal in 14–17% of the oocytes in which the AATAC YL-specific sequences were clearly separated from the X chromosomes. Thus, although the association of Y-specific heterochromatic sequences with the X chromosomes appears to be dissolved in the presence of chiasmata, pairing between the X chromosomal 1.686 sequences located at the tip of the Y and the homologous 1.686 sequences on the X chromosomes appears to persist.
The continued association of these blocks of 1.686 satellite sequence confirms the observations of Dernburg et al. (1996) that heterochromatic pairings can perdure until metaphase. However, the fact that the frequency of secondary nondisjunction observed in FM7/X/ y+Y females is in fact somewhat lower than the frequency of secondary nondisjunction observed in FM7/X/Y females (see Table 1) argues that such pairings are not sufficient to interfere with the segregation of nonexchange X chromosomes or to promote XX ↔ Y segregational events. Moreover, the presence of these 1.686 sequences on the tip of the y+Y chromosome also appears to have little or no effect on the maintenance of XX–YL associations as assayed by hybridization using the Y chromosome-specific AATAC probe (see Table 3). Thus, we conclude that the expulsion of the Y chromosome from the XXY trivalent in crossover oocytes occurs in such a fashion as to allow the 1.686 sequences appended to the tip of YL to maintain their association with the X chromosome.
DISCUSSION
A new model of secondary nondisjunction:
Our data suggest that XXY pairings are common, if not universal, in early meiotic prophase. As first reported by Dernburg et al. (1996), the pairing of homologous regions of X chromosomal heterochromatin is maintained throughout prophase, regardless of whether or not crossing over occurs. We saw no instances in which the two X chromosomes were visible as separate entities prior to NEB in any of the genotypes reported here. However, the association of the two X chromosomes with the Y chromosome is maintained only at high frequency in those oocytes in which the X chromosomal crossing over is suppressed. To explain these data, we propose, contrary to previous models, that XXY pairings occur early in prophase, coincident with or even prior to, the initiation of synapsis. We further propose that the occurrence of X chromosomal crossing over alters the spatial relationship between the heterochromatin of the Y and X chromosomes, and more specifically, their centromeres, in a fashion that limits the associations of the X centromeric regions with each other and dissolves their association with the less homologous Y chromosome.
Support for exactly such a role of crossing over in restricting centromeric associations comes from recently published work in yeast by Tsubuchi and Roeder (2005). These authors demonstrated that during early meiotic prophase in wild-type yeast, centromeric pairings usually involve the centromeres of nonhomologous chromosomes. However, during the process of synapsis and exchange these nonhomologous centromere couplings undergo switching until all couples involve homologs. The transition to purely homologous centromere pairings requires Spo11, a protein required for the initiation of meiotic recombination. Similar observations supporting a role for centromeric associations in mediating the segregation of nonexchange chromosomes in yeast cells carrying either two homologous or two homeologous chromosomes and a competing but nonhomologous, CEN-bearing, yeast artificial chromosome (YAC) have also been published by Kemp et al. (2004). In the absence of exchange in cells bearing the two homeologous chromosomes, the YAC centromere paired at high frequency with the centromeres of two homeologous chromosomes. Pairings involving the YAC-born CEN sequence were much less frequent in cells carrying two homologous chromosomes that were free to crossover. In that case, the plasmid or YAC-born centromere was excluded from pairing with the homologous centromeres.
We propose that a similar set of processes occur in XXY females in Drosophila oocytes, which is to say that XXY pairing (and perhaps synapsis) is common in early prophase, but that the process of X exchange promotes the co-orientation of the two X centromeres prior to spindle formation and dissolves the association of the X chromosome centromeric region with the Y chromosome. Unlike Cooper's model, which suggests that the association of the Y with the X chromosomes occurs subsequent to their failure to undergo crossing over, we propose that the XXY associations occur in early prophase in all oocytes and that crossing over between the X chromosomes acts to dissolve the connection between the Y and the two X chromosomes. However, as did Cooper (1948), we propose that the XXY trivalents that are maintained until prometaphase can and do direct XX ↔ Y segregational events.
Why don't all nonexchange X bivalents participate in secondary nondisjunction?
It is curious that only some (60–80%) of the E0 tetrads undergo secondary nondisjunction. Cooper (1948) envisioned a model in which the trivalent could occasionally line up as a linear structure on the metaphase spindle, with the two X chromosomes pointed toward opposite poles and unoriented Y in the middle (see Figure 2b of Cooper 1948). However, our observation that the fractions of XXY associations in late prophase are similar to the frequencies of secondary nondisjunction as assayed genetically and cytologically in both FM7/X/Y and In(1)dl-49/X/Y oocytes (see Tables 1 and 2) suggests that most XXY associations that persist until the end of prophase do result in the formation of classic trivalents and that in these trivalents the metacentric Y chromosome orients the two acrocentric X chromosomes to opposite poles of the spindle. Thus, we propose that 20–40% of nonexchange X chromosomes that fail to segregate from the Y do so because of an occasional failure of the Y chromosome to maintain its association with the achiasmate X chromosomes during early to midprophase.
One could argue that Cooper's proposal might apply to XXY females bearing normal-sequence X chromosomes, since the frequency of XXY associations observed in late prophase (11–12%) is threefold higher than the observed frequency of spontaneous nondisjunction (3–4%). However, unlike the frequency of XXY associations in X exchange-suppressed oocytes, which is similar in both mid- and late prophase oocytes, the frequency of XXY associations appears to continuously decline during the progression of prophase in XXY females. The frequency of such associations is 93% in early prophase, but declines to 50–55% in midprophase and 11–12% in late prophase oocytes. Because our estimate for the frequency of XXY associations in late prophase includes oocytes throughout this interval, we propose that the observed frequency of XXY associations (11–12%) is likely to be an overestimate of the frequency of XXY associations that will indeed persist beyond NEB. Thus, even in this genotype it seems likely that the XXY associations that do survive past NEB will direct XX ↔ Y segregational events.
Carpenter's two-step model of centromere co-orientation in XXY females:
Carpenter (1973) proposed that the co-orientation of the X chromosomes by the Y chromosome involves two separate steps: first, a mechanism that committed the two X chromosomes to cosegregate (i.e., to move to the same pole, perhaps as a single entity), and second, the co-orientation of that set of “locked” X chromosomes from the Y chromosome. She based this hypothesis of the analysis of secondary nondisjunction in females homozygous for a loss-of-function mutation (noda) in the nod gene. The nod gene encodes a “chromokinesin-like” protein that serves to hold achiasmate chromosomes on the developing spindle and prevent their precocious migration to the poles (Carpenter 1973; Cui et al. 2005). In the absence of functional Nod protein, achiasmate X chromosomes nondisjoin at high frequency and thus appear to segregate at random from their homolog (Carpenter 1973; Theurkauf and Hawley 1992).
Carpenter (1973) observed that the frequency of X chromosomal nondisjunction in noda/noda/Y females was much higher than that observed in noda/noda females and was, in fact, identical to that observed in nod+/nod+/Y controls. However, in the controls, the nondisjunctional progeny were of only two gamete types, namely XX and Y, while noda/noda/Y females produced ova with XX, XXY, Y, and 0 at equal frequencies. Carpenter interpreted these results to mean that the noda mutant allowed the proper commitment of the X chromosomes to cosegregate to the same pole, followed by defective disjunction of this pair from the Y chromosome, such that the two X chromosomes still cosegregate to the same pole while the Y disjoins at random.
In the images of XX ↔ Y co-orientation in Figure 3, we do not observe a physical association between two X's that are segregating toward the same pole. Thus, if the X's truly are somehow locked together during the process of setting up XX ↔ Y segregations, such associations do not usually persist into late prometaphase or metaphase oocytes. However, we do note that heterochromatic associations between the proximally located blocks of X heterochromatin can persist into prometaphase (Dernburg et al. 1996; but see also Gilliland et al. 2005). Moreover, associations between the distally located blocks of heterochromatin often persist even into late prometaphase/metaphase in FM7/FM7/y+Y females. Perhaps the persistence of such pairings, at least until early prometaphase, helps to facilitate the “commitment of the X chromosomes to co-segregate” step proposed by Carpenter (1973). In other words, when not acted on by forces that would normally drag the two X centromeres to opposite poles in XX oocytes, perhaps such pairings can persist long enough in nod oocytes to facilitate the observed cosegregation events.
Summary:
Our data both provide strong evidence for the XXY trivalent postulated by Cooper (1948) and disprove Bridges' (1916) model of competitive pairing between the X and Y chromosomes. But of greater importance, our data suggest a new model of secondary nondisjunction in which XXY associations are ubiquitous during early prophase in all oocytes of this genotype and then dissolve in mid- to late prophase following the occurrence of X chromosomal crossing over. These data suggest that that the association of homologous centromeres, and perhaps even their co-orientation, can be influenced or directed by the occurrence of crossing over long before NEB.
Acknowledgments
We gratefully acknowledge Abby Dernburg for consultation and guidance during the early phases of this project. We also thank members of the Hawley lab (especially Cathleen Lake and Susan Flynn) for valuable discussion and comments on the manuscript. This research was supported by funds from the Stowers Institute for Medical Research and by an American Cancer Society Research Professor Award to R.S.H.
This article is dedicated to Kenneth W. Cooper, who inspired the senior author by the high quality of his science throughout his career and by his unparalleled role as a teacher and mentor over 30 years ago at the University of California at Riverside.
References
- Ashburner, M., K. Golic and R. S. Hawley, 2005. Drosophila: A Laboratory Handbook, Ed. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Bridges, C. B., 1916. Non-disjunction as proof of the chromosome theory of heredity. Genetics 1: 1–52, 107–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, A. T. C., 1973. A mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73: 393–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, A. T. C., and L. Sandler, 1974. On recombination-defective meiotic mutants in Drosophila melanogaster. Genetics 76: 453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadov, B. F., 1981. Behavior of chromosomes during mitosis and meiosis and chromocentral organization of nuclei in Drosophila melanogaster. Proceedings of the XIV International Congress of Genetics, Vol. 3, Ithaca, NY, pp. 343–356.
- Cooper, K. W., 1948. A new theory of secondary non-disjunction in female Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 34: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, W., L. R. Sproul, S. M. Gustafson, H. J. G. Matthies, S. P. Gilbert et al., 2005. Drosophila Nod protein binds preferentially to the plus ends of microtubules and promotes microtubule polymerization in vitro. Mol. Biol. Cell 16: 5400–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., 2000. In situ hybridization to somatic chromosomes, pp. 25–56 in Drosophila Protocol, edited by W. Sullivan, M. Ashburner and R. S. Hawley. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PubMed]
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Gershenson, S. M., 1935. The mechanisms of non-disjunction in the ClB stock of Drosophila melanogaster. J. Genet. 30: 115–125. [Google Scholar]
- Gilliland, W. D., S. M. Wayson and R. S. Hawley, 2005. The meiotic defects of mutants in the Drosophila mps1 gene reveals a critical role of Mps1 in the segregations of achiasmate homologs. Curr. Biol. 15: 672–677. [DOI] [PubMed] [Google Scholar]
- Gong, W. J., K. S. McKim and R. S. Hawley, 2005. All paired up with no place to go: pairing, synapsis, and DSB formation in a balancer heterozygote. PLoS Genet. 1: 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell, R. F., 1962. A new model for secondary nondisjunction: the role of distributive pairing. Genetics 47: 1737–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell, R. F., 1976. Distributive pairing, pp. 435–486 in Genetics and Biology of Drosophila, edited by E. Novitski and M. Ashburner. Academic Press, New York.
- Haaf, T., H. Winking and M. Schmid, 1989. Immunocytogenetics. III. Analysis of trivalent and multivalent configurations in mouse pachytene spermatocytes by human autoantibodies to synaptonemal complexes and kinetochores. Cytogenet. Cell Genet. 50: 14–22. [DOI] [PubMed] [Google Scholar]
- Harris, D., C. Orme, J. Kramer, L. Namba, M. Champion et al., 2003. A deficiency screen of the major autosomes identifies a single gene (matrimony) that is haplo-insufficient for achiasmate segregation in Drosophila oocytes. Genetics 165: 637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley, R. S., and W. Theurkauf, 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila melanogaster. Trends Genet. 9: 310–317. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., H. Irick, A. E. Zitron, D. A. Haddox, A. Lohe et al., 1993. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13(6): 440–467. [DOI] [PubMed] [Google Scholar]
- Karpen, G. H., M. H. Le and H. Le, 1996. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273: 118–122. [DOI] [PubMed] [Google Scholar]
- Kemp, B., R. M. Boumil, M. N. Stewart and D. S. Dawson, 2004. A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 18: 1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe, A. R., A. J. Hilliker and P. A. Roberts, 1993. Mapping simple repeated DNA sequences in heterochromatin of Drosophila melanogaster. Genetics 134: 1149–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüning, K.G., 1982. Genetics of inbred Drosophila melanogaster. VI. Crossing-over in secondary non-disjunction exceptionals. Hereditas 96: 161–174. [DOI] [PubMed] [Google Scholar]
- MacKnight, R. H., and K. W. Cooper, 1944. The synapsis of the sex chromosomes of Drosophila Miranda in relation to their directed segregation. Proc. Natl. Acad. Sci. USA 30: 384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam, J. R., 1967. The initiation of nonhomologous chromosome pairing before exchange in female Drosophila melanogaster. Genetics 57: 409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus, M., 1941. The influence of separate arms of the Y chromosome on secondary nondisjunction in the females of D. melanogaster. Dros. Inf. Ser. 15: 16. [Google Scholar]
- Novitski, E., and G. Braver, 1954. An analysis of crossing over within a heterozygous inversion in Drosophila melanogaster. Genetics 39: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa, J., 1982. Meiotic chromosome behavior influenced by mutation-altered disjunction in Drosophila melanogaster females. Genetics 102: 503–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro, J., and S. Nokkala, 1981. Secondary nondisjunction in multiple inversion heterozygotes as revealed by direct cytological and genetic tests. Proceedings of the 7th European Drosophila Research Conference, p. 87.
- Rutherford, S. L., and A. T. C. Carpenter, 1988. The effect of sequence homozygosity on the frequency of X-chromosomal exchange in Drosophila melanogaster females. Genetics 120: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling, A. C., 1993. Development genetics of oogenesis, pp. 1–71 in The Development of Drosophila melanogaster, edited by M. Bate and A. M. Arias. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sturtevant, A. H., and G. W. Beadle, 1936. The relations of inversions in the X chromosome of Drosophila melanogaster to crossing over and disjunction. Genetics 21: 554–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W. E., and R. S. Hawley, 1992. Meiotic spindle assembly in Drosophila females: behavior of non-exchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi, T., and G. S. Roeder, 2005. A synaptonemal complex protein promotes homology-independent centromere coupling. Science 308: 870–873. [DOI] [PubMed] [Google Scholar]
- Whyte, W., H. Irick, T. Arbel, G. Yasuda, R. L. French et al., 1993. The genetic analysis of distributive pairing in Drosophila melanogaster. III. The wild-type product of the Axs locus is required for achiasmate meiotic segregation. Genetics 134: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., and R. S. Hawley, 1990. The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics 125: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitron, A. E., and R. S. Hawley, 1989. The genetic analysis of distributive pairing in Drosophila melanogaster. I. Isolation and characterization of Aberrant X segregation, Axs, a mutant defective in chromosome partner choice. Genetics 122: 801–821. [DOI] [PMC free article] [PubMed] [Google Scholar]