Abstract
The International HapMap Project aims to generate detailed human genome variation maps by densely genotyping single-nucleotide polymorphisms (SNPs) in CEPH, Chinese, Japanese, and Yoruba samples. This will undoubtedly become an important facility for genetic studies of diseases and complex traits in the four populations. To address how the genetic information contained in such variation maps is transferable to other populations, the Korean government, industries, and academics have launched the Korean HapMap project to genotype high-density Encyclopedia of DNA Elements (ENCODE) regions in 90 Korean individuals. Here we show that the LD pattern, block structure, haplotype diversity, and recombination rate are highly concordant between Korean and the two HapMap Asian samples, particularly Japanese. The availability of information from both Chinese and Japanese samples helps to predict more accurately the possible performance of HapMap markers in Korean disease-gene studies. Tagging SNPs selected from the two HapMap Asian maps, especially the Japanese map, were shown to be very effective for Korean samples. These results demonstrate that the HapMap variation maps are robust in related populations and will serve as an important resource for the studies of the Korean population in particular.
THE International HapMap Project is an international effort to document the common DNA sequence variations in the human genome to facilitate genetic studies of common diseases and complex traits (Gibbs et al. 2003) and apply them to other populations. The phase I mapping of this project, which genotypes samples from the Yoruba in Ibadan, Nigeria (YRI); the Japanese in Tokyo (JPT); the Han Chinese in Beijing (CHB); and Utah residents with ancestry from northern and western Europe (CEU) at a density of 1 single-nucleotide polymorphism (SNP) every 5 kb, has been completed (International Hapmap Consortium 2005). The HapMap has been widely regarded as an important resource for disease association and population studies (Dudbridge and Koeleman 2004; Evans et al. 2004; Thomas et al. 2004; Morton 2005; Pittman et al. 2005). Doubts and controversy, however, have also surrounded the project from the outset (Terwilliger et al. 2002; Nielsen et al. 2004). One of the most critical questions is how robust such dense common variation maps produced for the four populations are to other populations.
Linkage disequilibrium (LD) patterns are known to vary across the human genome and across populations (Gabriel et al. 2002; Phillips et al. 2003; Ke et al. 2004a). Two important questions arise from these observations, i.e., how LD patterns are maintained between a HapMap population and a population that the HapMap is applied to and how strong is a population to which the HapMap is applied. These two questions are related and can often be assessed by choosing tagging SNPs (Johnson et al. 2001; Gibbs et al. 2003; Stram 2004) in one population sample and applying them to another population (Ke et al. 2004b; Ahmadi et al. 2005; Mueller et al. 2005). In a recent study involving four gene regions in several European populations (Mueller et al. 2005), tagging SNPs selected from the HapMap CEU data were found to perform very efficiently in local European samples in two of the four regions because of high conservation of LD. In the other two regions, however, restricted applicability of CEU-derived tagging SNPs was observed due to significant variation in LD among populations (Mueller et al. 2005).
For Asian populations, there are two HapMaps available, CHB and JPT. We are interested in the cross-population robustness of the HapMap in general and the applicability of JPT and CHB maps to the Korean population, in particular. We are also interested in potential advantages of having two related Asian HapMaps. To this end, the Korean government, industries, and academic institutions launched the Korean HapMap project in 2003, which involved the fine mapping of 7 of the 10 HapMap Encyclopedia of DNA Elements (ENCODE) regions.
MATERIALS AND METHODS
DNA samples and SNP selection:
The samples used for the study comprised 90 Korean samples randomly selected from the cohort samples preserved in the Korean National Institute of Health without family history of major diseases. SNP sites were selected from dbSNP database build 119 (http://www.ncbi.nlm.nih.gov/SNP) after applying repeat masking.
Genotyping:
Multiplex SNP analyses were performed for ENr213, ENr232, and ENr321 with the GenomeLab SNPstream genotyping platform (Beckman Coulter) and its accompanying SNPstream software suite as described by Denomme and Van Oene (2005). For ENr131 as well as for ENr112, ENr113, and ENm013, Sequenom's MassARRAY system was used with standard conditions as described before (Bansal et al. 2002) except the amount of genomic DNA (2.5 ng) was decreased with multiplexing assays. All genotypes were confirmed by an operator for final genotype calls.
The average genotyping error rate was estimated at ∼0.1% by routinely running duplicated sample wells in the plate. The genotyping results in the study are available for download from http://www.ngic.re.kr:8080/khapmap/.
LD analysis:
All analyses in this study were based on the HapMap ENCODE genotype downloaded at February 2005, unless otherwise stated. Only markers that were polymorphic in the Chinese, Japanese (http://www.hapmap.org/), and Korean samples were used (“shared data sets” thereafter). This selection of markers, therefore, allowed direct comparison of LD structure between population samples and all other analyses except tagging. For the sliding-window analysis, average pairwise r2 was calculated from 10- to 50-kb interspaced SNPs in 50-kb sliding windows (5-kb increment between windows). Haplotype blocks were defined using Haploview (Barrett et al. 2005) based on block definition of Gabriel et al. (2002). Haplotypes and their frequencies in block regions were estimated using snphap (http://www-gene.cimr.cam.uk/clayton/software/).
Fst analysis:
Fst was calculated according to the Wright's F-statistic (Wright 1951). Because Fst estimates for SNPs in LD are correlated (Weir et al. 2005), here we selected SNPs with pairwise r2 all <0.20 from the CHB + JPT combined set.
Recombination analysis:
Phase 2.1 (Li and Stephens 2003; Crawford et al. 2004) was used to analyze the shared data sets as described above.
Tagging analysis:
Tagging SNPs were selected from the full-density CHB and JPT maps (as well as the combined CHB + JPT map) using Tagger (de Bakker et al. 2005; http://www.broad.mit.edu/mpg/tagger/) in aggressive tagging mode (r2 or haplotype r2 ≥ 0.80, minor allele frequency cutoff = 5%, and other settings at default value). Tagging efficiency was defined as n/nh, where nh is the number of tagging SNPs selected to cover the region and n is the total number of markers genotyped (Ke et al. 2004b). The performance of those tagging SNPs was evaluated again using Tagger with the same settings by applying tags to the 90 Korean (KR) samples. A marker is “captured” if the pairwise r2 or haplotype r2 with the tags is ≥0.80.
RESULTS
SNP genotyping:
The Korean HapMap project undertook fine mapping of 7 of the 10 HapMap ENCODE regions using DNA samples from 90 individuals from South Korea (Table 1, supplemental Figure 1 at http://www.genetics.org/supplemental/). Marker density varied from different levels of an average 1 SNP/1.88 kb in ENr113 to having the lowest 1 SNP/330 bp in ENr131 (Table 1).
TABLE 1.
Summary of genotyping data
| Region | Chromosome location | No. of SNPs with genotypes | Average marker spacing | Regions chosen for analysis and no. of polymorphic SNPs shared by KR, JPT, and CHB (spacing, 1 kb) |
|---|---|---|---|---|
| ENr112 | 2p16.3 | 345 | 1.45 | |
| ENr131 | 2q37.1 | 1513 | 0.33 | 410 (1.20) |
| ENr113 | 4q26 | 266 | 1.88 | |
| ENm013 | 7q21.13 | 640 | 1.72 | |
| ENr321 | 8q24.11 | 653 | 0.77 | 258 (1.94) |
| ENr232 | 9q34.11 | 445 | 1.12 | |
| ENr213 | 18q12.1 | 557 | 0.90 | 218 (2.25) |
ENr131, ENr213, and ENr321 were the three most densely genotyped regions and were used to investigate the applicability of Japanese and Chinese HapMap to the Korean population (Table 1). As described in detail in the following sections, ENr131 had a low level of LD and ENr213 had a high level of LD, whereas ENr321 had an intermediate level of LD. Findings from these three dense maps, we hope, would shed light on studies of genomewide scale. To match the sample sizes of JPT and CHB samples in the International HapMap Project, 45 KR individuals were randomly selected from the 90 Koreans and used for comparisons of the three populations, unless stated otherwise.
Population difference and LD conservation:
The three ENCODE regions had a different level of average LD at a broad scale, with ENr131 being the lowest and ENr213 the highest (Figure 1a). LD patterns in all three regions were similar among Korean, Japanese, and Chinese in general although some differences were apparent in ENr213 and ENr321. Average Fst-values between Korean and Japanese and between Korean and Chinese were 0.0060, 0.0044, and 0.0062 and 0.0064, 0.0075, and 0.0095 for ENr131, ENr213, and ENr321, respectively. This observation indicates that the Korean population is very close to Japanese and Chinese populations in general and, perhaps, closer to Japanese than to Chinese. This observation was reflected in the sliding-window analysis showing more departures of Korean samples from Chinese samples in ENr213 and ENr321 than ENr131 (Figure 1a). The close relatedness of the haplotypes of the Korean, Japanese, and Chinese populations was further confirmed by phylogenetic analysis (supplemental Figure 2 at http://www.genetics.org/supplemental/) (Akey et al. 2002).
Figure 1.—
Comparison of Korean, Japanese, and Chinese in patterns of LD, haplotype block structure, and recombination rate in ENCODE regions (ENr131, ENr213, and ENr321). (a) Patterns of LD in sliding windows: dark blue lines denote the HapMap Chinese sample (CHB); red and pink lines denote the HapMap Japanese sample (JPT); and the yellow, green, and brown lines denote three random Korean (KR) samples of 45 individuals. (b–d) Haplotype block structure. (e) Recombination rate: blue lines denote CHB, red lines denote the JPT, the green lines denote average of three random KR samples of 45 individuals.
The haplotype block structures of the three regions were generally concordant among Korean, Japanese, and Chinese. Block structure had the highest concordance in ENr131 (Figure 1, b–d) although the average LD in this region was lower than in the other two (Figure 1a). This was consistent with the mean LD trends, where the lines representing three different samples within the Korean population as well as three Asian populations overlapped tightly. Differences were observed, however, in ENr213 and ENr321, which were consistent with the departures observed in the broad scale of LD patterns (Figure 1, Table 2).
TABLE 2.
Comparison of block structure and SNP tagging efficiency
| Marker spacing (kb): | Region
|
|||
|---|---|---|---|---|
| ENr131 1.2 | ENr213 2.25 | ENr321 1.94 | ||
| Block structure, sequence coverage of blocks (%) | ||||
| KRa | 69 | 65 | 54 | |
| JPT | 69 | 67 | 62 | |
| CHB | 69 | 72 | 55 | |
| KRb | 76 | 76 | 57 | |
| CHB + JPT | 75 | 74 | 69 | |
| Block structure, average block size (kb) | ||||
| KRa | 11.3 | 18.8 | 9.3 | |
| JPT | 9.5 | 18.4 | 14.8 | |
| CHB | 10.3 | 19.7 | 9.0 | |
| KRb | 11.3 | 20.9 | 10.2 | |
| CHB + JPT | 10.3 | 22.6 | 11.1 | |
| SNP tagging efficiency | ||||
| KRa | 3.8 | 4.2 | 4.4 | |
| JPT | 3.9 | 5.3 | 4.4 | |
| CHB | 3.7 | 5.5 | 4.7 | |
| KRb | 3.9 | 4.2 | 4.2 | |
| CHB + JPT | 4.2 | 5.7 | 4.7 | |
Average of three random samples of 45 individuals.
All 90 individuals.
Comparison of the major haplotypes in haplotype blocks of the ENr131, ENr213, and ENr321 regions between KR and JPT/CHB showed that close to 90% of their haplotypes in block regions defined in JPT and CHB were major haplotypes, and this number was still >85% when haplotypes were reconstructed for the same regions using the Korean samples (Table 3). Furthermore, the majority of the major haplotypes as defined in JPT and CHB remained major haplotypes in KR, demonstrating a high degree of haplotype conservation across the three populations (Table 3). In ENr321, where a higher concordance of block structure was observed between KR and CHB than between KR and JPT (Figure 1, b–d, Table 2), the major haplotypes were found, however, to be more conserved between KR and JPT (0.749) than between KR and CHB (0.662). This indicates that the Koreans and Japanese are perhaps more closely related in this particular region.
TABLE 3.
Haplotype conservation across populations
| Population 1 | Population 2 | Region | Total frequency of major haplotypes in population 1 | Total frequency of major haplotypes in population 2 | Total frequency of major haplotypes in population 1 conserved as major haplotypes in population 2 | Frequency correlation coefficient of major haplotypes between populations (P-value) |
|---|---|---|---|---|---|---|
| CHB | KR | ENr131 | 0.887 ± 0.07 | 0.859 ± 0.09 | 0.714 ± 0.132 | 0.900 (<0.0001) |
| CHB | KR | ENr213 | 0.926 ± 0.05 | 0.841 ± 0.09 | 0.735 ± 0.156 | 0.800 (0.0003) |
| CHB | KR | ENr321 | 0.881 ± 0.101 | 0.858 ± 0.09 | 0.662 ± 0.115 | 0.838 (0.0003) |
| CHB | KR | Total | 0.897 ± 0.006 | 0.854 ± 0.008 | 0.707 ± 0.018 | 0.866 (<0.0001) |
| JPT | KR | ENr131 | 0.874 ± 0.06 | 0.882 ± 0.06 | 0.759 ± 0.138 | 0.927 (<0.0001) |
| JPT | KR | ENr213 | 0.892 ± 0.07 | 0.791 ± 0.138 | 0.710 ± 0.161 | 0.769 (0.0154) |
| JPT | KR | ENr321 | 0.894 ± 0.07 | 0.845 ± 0.09 | 0.749 ± 0.096 | 0.898 (<0.0001) |
| JPT | KR | Total | 0.884 ± 0.004 | 0.852 ± 0.009 | 0.745 ± 0.017 | 0.911 (<0.0001) |
| CHB + JPT | KR | ENr131 | 0.869 ± 0.066 | 0.847 ± 0.093 | 0.727 ± 0.096 | 0.750 (<0.0001) |
| CHB + JPT | KR | ENr213 | 0.879 ± 0.076 | 0.813 ± 0.082 | 0.742 ± 0.121 | 0.893 (<0.0001) |
| CHB + JPT | KR | ENr321 | 0.841 ± 0.090 | 0.793 ± 0.127 | 0.704 ± 0.126 | 0.785 (<0.0001) |
| CHB + JPT | KR | Total | 0.864 ± 0.075 | 0.823 ± 0.102 | 0.724 ± 0.110 | 0.770 (<0.0001) |
Haplotypes and their frequencies were estimated in high-LD regions (i.e., Gabriel blocks of five or more markers defined by Haploview) for ENr131, ENr213, and ENr321 from a starting population sample, i.e., population 1. They were then compared with haplotypes estimated in the same regions (exact boundaries) of a test population sample, i.e., population 2. Major haplotypes were those with frequency ≥0.10. Phasing was done separately for the starting and test populations using snphap.
Different patterns and behaviors were still observed between populations when the combined CHB + JPT data sets were compared with the complete whole Korean sample of sets containing 90 individuals (Table 2). In ENr321, the sequence coverage of haplotype blocks was 69% for CHB + JPT combined samples and 57% for Korean samples. This difference was primarily due to the difference between the Japanese and Korean samples (Table 2). It was also revealed that major haplotype conservation between CHB + JPT and KR was in general between what was obtained by comparing CHB and JPT separately with KR, except for ENr213 where higher conservation was achieved in CHB + JPT samples (Table 3).
Recombination rate:
The pattern of estimated recombination rate in ENr131 was very simple compared to the other two regions and remarkably similar between KR, JPT, and CHB (Figure 1e). In contrast, recombination rates of ENr213 and ENr321 regions were more varied across regions as well as among populations (Figure 1e). This was consistent with the broad view of LD and the primary reason for the highest similarity of block structure in ENr131 and less concordance among the three populations in the ENr213 and ENr321 regions although they had a higher average LD (Figure 1). In ENr213, the amplitude of recombination rate variations was smaller than in ENr131 and ENr321, in agreement with the fact that this region had the highest LD (Figure 1).
Transferability of tagging SNPs:
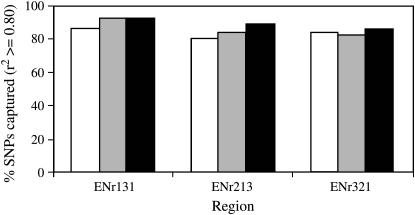
We selected tagging SNPs from CHB, JPT, or CHB + JPT samples and applied them to the Korean population. At the same marker densities, tagging efficiency was very similar among the three populations in ENr131 and ENr321 with some difference in ENr213 (Table 2). When tagging SNPs were selected from full-density sets of CHB, JPT, and CHB + JPT, higher tagging efficiencies were obtained as expected (4.5, 5.8, and 5.2 in ENr131; 7.0, 7.0, and 7.7 in ENr213; 5.8, 5.9, and 6.1 in ENr321). In all three regions, at least 80% of SNPs in the Korean samples can be captured by tagging SNPs selected from the CHB, JPT, and CHB + JPT HapMap samples (Figure 2). In ENr131 and ENr213, tagging SNPs selected from JPT were better at capturing SNPs in the Korean samples and more effective than those selected from CHB. Some additional benefit was also observed when tagging SNPs were selected from CHB + JPT than those selected from JPT alone (e.g., ENr213 and ENr321). Since higher tagging efficiency was observed for JPT and CHB + JPT than for CHB, the advantage of using JPT (particularly in ENr131) or CHB + JPT as reference for the Korean population was more apparent. The difference of JPT and CHB + JPT seems to correspond well to the local LD feature of a region. In ENr131, little variation of LD was observed between populations as well as within the Korean population (Figure 1a). As a result, JPT alone seems to be good enough to capture the variations in Korean samples. Whereas in ENr321 and especially in ENr213 more variations were observed between as well as within populations, and the combined CHB + JPT sets seems to perform better than JPT alone.
Figure 2.—
Evaluation of tagging performance. Tagging SNPs were selected from HapMap Chinese (CHB), Japanese (JPT), and the combined samples using Tagger at full density and applied to the 90 Korean individuals. An aggressive tagging mode with the same settings in Tagger was used for both selection of tagging SNPs and a test of their performance. Open bars denote CHB, shaded bars JPT, and solid bars the combined set of Chinese and Japanese samples (CHB + JPT).
We have also examined some extreme cases of population difference in SNP allele frequency, which could affect tagging performance. If a common SNP in a reference population is monomorphic in the test population and this SNP happens to be selected as a tagging SNP, the power of the whole tagging SNP sets may drop in the test population. On the other hand, if a SNP is monomorphic in a reference population but polymorphic in the test population, the tagging SNP sets selected from the reference population could be unprepared for this particular SNP and may fail to tag it. As shown in supplemental Table 1 (http://www.genetics.org/supplemental/), overall these extreme cases were rare in regions and samples analyzed in this study whether the comparison was made against the early version of HapMap ENCODE data (February 2005) or the latest (October 2005). This demonstrated again that Korean, Japanese, and Chinese are indeed closely related populations and tagging SNPs selected from the CHB/JPT HapMap are highly transferable to the Korean population. It is interesting, however, to observe that ENr213 did show more variations than ENr131 and ENr321 among the three populations (i.e., cases where SNPs were monomorphic in KR but polymorphic in CHB/JPT or vice versa. This is consistent with observations made about LD and recombination.
DISCUSSION
LD patterns can be refined at a local level using haplotype block analysis resulting in delineation of haplotype blocks for high-LD and nonblocks for low-LD regions (Daly et al. 2001; Gabriel et al. 2002; Phillips et al. 2003). Haplotype blocks are generally associated with limited haplotype diversity, such that a few major haplotypes explain the majority of the diversity in a block (Daly et al. 2001; Gabriel et al. 2002; Phillips et al. 2003). The Korean population is known to be very close to the Chinese and Japanese. The haplotype block structures of the three ENCODE regions, ENr131, ENr213, and ENr321, were generally concordant among Korean, Japanese, and Chinese, although some differences were observed in ENr213 and ENr321. Block structure in ENr131 had the highest concordance. ENr213 had the highest sequence coverage by blocks, especially when density difference was taken into account. In ENr321, block structure was more similar between Korean and Chinese than between Korean and Japanese. Because haplotype blocks were defined using LD thresholds, certain variations may change the block boundaries in particular regions (Ke et al. 2004b), especially when the regions are small.
The human genome is known to be delimited by recombination into hotspot and coldspot regions (Gabriel et al. 2002; Phillips et al. 2003; Crawford et al. 2004; McVean et al. 2004). Coldspot regions usually correspond to haplotype blocks, whereas hotspots typically occur where haplotype blocks are expected to break down (Gabriel et al. 2002; Phillips et al. 2003). The recombination rate was very simple in ENr131 showing low LD, while recombination rates of ENr213 and ENr321 were more varied. In general, the patterns of LD and recombination are highly conserved between the Koreans and Chinese and Japanese. Having two related East Asian population HapMaps enables us to examine a region in close detail via comparison. If the Japanese and Chinese maps are highly concordant, as in ENr131, a Korean sample would likely share similar patterns of LD and recombination (Figure 1). On the other hand, if differences are observed between Japanese and Chinese maps, as in ENr213 and ENr321, a Korean sample might be expected to reveal greater variability within and between populations. This understanding could assist in interpreting disease associations in a particular region.
A more practical use of human genome variation maps is perhaps to design tagging SNPs for related populations in regional or genomewide association studies (Johnson et al. 2001; Gibbs et al. 2003; Stram 2004). The present results also show that tagging SNPs selected from the Chinese and particularly from the Japanese samples are highly transferable to our Korean samples. Even in regions where differences were observed among the three groups, tagging SNPs from the Japanese performed at least as effectively as those from Korean samples. These observations suggest that the Japanese and Chinese HapMaps will be robust for the Korean population and serve as an important resource for the association and population studies of the Koreans and possibly other Asian populations.
Acknowledgments
The authors gratefully acknowledge the Korean National Institute of Health for providing DNA samples and the Korean HapMap consortium. This work was supported by grants from the Korea Ministry of Science and Technology, the National Research and Development Program, the Korean Haplotype Information Development Program, the Samsung Corporate Research Fund, and the DNA Link Research Fund. X.K. was supported by the Wellcome Trust. L.R.C. was supported by the Wellcome Trust and National Institutes of Health (NIH). B.S.W. was supported by the NIH.
References
- Ahmadi, K. R., M. E. Weale, Z. Y. Xue, N. Soranzo, D. P. Yarnall et al., 2005. A single-nucleotide polymorphism tagging set for human drug metabolism and transport. Nat. Genet. 37(1): 84–89. [DOI] [PubMed] [Google Scholar]
- Akey, J. M., G. Zhang, K. Zhang, L. Jin and M. D. Shriver, 2002. Interrogating a high-density SNP map for signatures of natural selection. Genome Res. 12(12): 1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal, A., D. van den Boom, S. Kammerer, C. Honisch, G. Adam et al., 2002. Association testing by DNA pooling: an effective initial screen. Proc. Natl. Acad. Sci. USA 99: 16871–16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, J. C., B. Fry, J. Maller and M. J. Daly, 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2): 263–265. [DOI] [PubMed] [Google Scholar]
- Crawford, D. C., T. Bhangale, N. Li, G. Hellenthal, M. J. Rieder et al., 2004. Evidence for substantial fine-scale variation in recombination rates across the human genome. Nat. Genet. 36(7): 700–706. [DOI] [PubMed] [Google Scholar]
- Daly, M. J., J. D. Rioux, S. F. Schaffner, T. J. Hudson and E. S. Lander, 2001. High-resolution haplotype structure in the human genome. Nat. Genet. 29(2): 229–232. [DOI] [PubMed] [Google Scholar]
- de Bakker, P. I., R. Yelensky, I. Pe'er, S. B. Gabriel, M. J. Daly et al., 2005. Efficiency and power in genetic association studies. Nat. Genet. 37(11): 1217–1223. [DOI] [PubMed] [Google Scholar]
- Denomme, G. A., and M. Van Oene, 2005. High-throughput multiplex single-nucleotide polymorphism analysis for red cell and platelet antigen genotypes. Transfusion 45(5): 660–666. [DOI] [PubMed] [Google Scholar]
- Dudbridge, F., and B. P. Koeleman, 2004. Efficient computation of significance levels for multiple associations in large studies of correlated data, including genomewide association studies. Am. J. Hum. Genet. 75(3): 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, D. M., L. R. Cardon and A. P. Morris, 2004. Genotype prediction using a dense map of SNPs. Genet. Epidemiol. 27(4): 375–384. [DOI] [PubMed] [Google Scholar]
- Gabriel, S. B., S. F. Schaffner, H. Nguyen, J. M. Moore, J. Roy et al., 2002. The structure of haplotype blocks in the human genome. Science 296: 2225–2229. [DOI] [PubMed] [Google Scholar]
- Gibbs, R. A., J. W. Belmont, P. Hardenbol, T. D. Willis, F. Yu et al., 2003. The International HapMap Project. Nature 426: 789–796. [DOI] [PubMed] [Google Scholar]
- International Hapmap Consortium, 2005. A haplotype map of the human genome. Nature 437: 1299–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, G. C., L. Esposito, B. J. Barratt, A. N. Smith, J. Heward et al., 2001. Haplotype tagging for the identification of common disease genes. Nat. Genet. 29(2): 233–237. [DOI] [PubMed] [Google Scholar]
- Ke, X., S. Hunt, W. Tapper, R. Lawrence, G. Stavrides et al., 2004. a The impact of SNP density on fine-scale patterns of linkage disequilibrium. Hum. Mol. Genet. 13(6): 577–588. [DOI] [PubMed] [Google Scholar]
- Ke, X., C. Durrant, A. P. Morris, S. Hunt, D. R. Bentley et al., 2004. b Efficiency and consistency of haplotype tagging of dense SNP maps in multiple samples. Hum. Mol. Genet. 13(21): 2557–2565. [DOI] [PubMed] [Google Scholar]
- Li, N., and M. Stephens, 2003. A new multilocus model for linkage disequilibrium, with application to exploring variations in recombination rate. Genetics 165: 2213–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVean, G. A., S. R. Myers, S. Hunt, P. Deloukas, D. R. Bentley et al., 2004. The fine-scale structure of recombination rate variation in the human genome. Science 304(5670): 581–584. [DOI] [PubMed] [Google Scholar]
- Morton, N. E., 2005. Linkage disequilibrium maps and association mapping. J. Clin. Invest. 115(6): 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, J. C., E. Lohmussaar, R. Magi, M. Remm, T. Bettecken et al., 2005. Linkage disequilibrium patterns and tagSNP transferability among European populations. Am. J. Hum. Genet. 76(3): 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, R., M. J. Hubisz and A. G. Clark, 2004. Reconstituting the frequency spectrum of ascertained single-nucleotide polymorphism data. Genetics 168: 2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. S., R. Lawrence, R. Sachidanandam, A. P. Morris, D. J. Balding et al., 2003. Chromosome-wide distribution of haplotype blocks and the role of recombination hot spots. Nat. Genet. 33(3): 382–387. [DOI] [PubMed] [Google Scholar]
- Pittman, A. M., A. J. Myers, P. Abou-Sleiman, H. C. Fung, M. Kaleem et al., 2005. Linkage disequilibrium fine-mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J. Med. Genet. 42(11): 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stram, D. O., 2004. Tag SNP selection for association studies. Genet. Epidemiol. 27(4): 365–374. [DOI] [PubMed] [Google Scholar]
- Terwilliger, J. D., F. Haghighi and T. S. Hiekkalinna, 2002. Goring HHH A biased assessment of the use of SNPs in human complex traits. Curr. Opin. Genet. Dev. 12(6): 726–734. [DOI] [PubMed] [Google Scholar]
- Thomas, D., R. Xie and M. Gebregziabher, 2004. Two-stage sampling designs for gene association studies. Genet. Epidemiol. 27(4): 401–414. [DOI] [PubMed] [Google Scholar]
- Weir, B. S., L. R. Cardon, A. D. Anderson, D. M. Nielsen and W. G. Hill, 2005. Measures of human population structure show heterogeneity among genome regions. Genome Res. 15: 1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S., 1951. The genetical structure of populations. Ann. Eugen. 15: 323–354. [DOI] [PubMed] [Google Scholar]