Abstract
A novel role for Rad53 in the initiation of DNA replication that is independent of checkpoint or deoxynucleotide regulation is proposed. Rad53 kinase is part of a signal transduction pathway involved in the DNA damage and replication checkpoints, while Cdc7-Dbf4 kinase (DDK) is important for the initiation of DNA replication. In addition to the known cdc7-rad53 synthetic lethality, rad53 mutations suppress mcm5-bob1, a mutation in the replicative MCM helicase that bypasses DDK's essential role. Rad53 kinase activity but neither checkpoint FHA domain is required. Conversely, Rad53 kinase can be activated without DDK. Rad53's role in replication is independent of both DNA and mitotic checkpoints because mutations in other checkpoint genes that act upstream or downstream of RAD53 or in the mitotic checkpoint do not exhibit these phenotypes. Because Rad53 binds an origin of replication mainly through its kinase domain and rad53 null mutants display a minichromosome loss phenotype, Rad53 is important in the initiation of DNA replication, as are DDK and Mcm2–7 proteins. This unique requirement for Rad53 can be suppressed by the deletion of the major histone H3/H4 gene pair, indicating that Rad53 may be regulating initiation by controlling histone protein levels and/or by affecting origin chromatin structure.
ELABORATE regulatory mechanisms have evolved in eukaryotic cells to ensure that DNA replication occurs only once per cell cycle (for recent reviews see Bell and Dutta 2002; Diffley 2004). First, a multiprotein prereplication complex (pre-RC) is assembled onto origins of replication during the G1 phase of the cell cycle. Origins are bound throughout the cell cycle by a six-member protein complex known as the origin recognition complex (ORC). Cdc6, which is produced in the G1 phase, together with Cdt1 protein loads the multimeric minichromosome maintenance (MCM) complex onto the ORC. The pre-RC is then activated by two independent protein kinases: cyclin-dependent kinase (CDK) (Cdk1-Clb) and Dbf4-dependent kinase (DDK) (Cdc7-Dbf4). Both CDK and DDK are activated in late G1 phase by the binding of unstable regulatory subunits, the Clb5,6 and Dbf4 proteins, respectively.
Both CDK and DDK are needed for Cdc45 protein to load DNA polymerases and other replication proteins onto origins during S-phase (Zou and Stillman 1998; Aparicio et al. 1999). DDK phosphorylates Mcm2 in vitro (and likely in vivo) as part of the MCM complex (Lei et al. 1997; Oshiro et al. 1999; Weinreich and Stillman 1999). Because the MCM complex is believed to act as the replicative helicase (Labib and Diffley 2001), it is thought that inhibition of the helicase activity is abolished by phosphorylation, perhaps by allosteric change of the MCM complex (Sclafani et al. 2002, 2004; Fletcher et al. 2003; Chen et al. 2005). Hence, both events are necessary for producing the binding of DNA replication proteins and fork movement (Jares et al. 2000; Lei and Tye 2001). The essential DDK step in DNA replication initiation can be bypassed in budding yeast cells carrying the mcm5-bob1 mutation (Jackson et al. 1993; Hardy et al. 1997), which produces constitutive Cdc45 loading (Sclafani et al. 2002). In this regard, the mcm5-bob1 encoded protein may mimic the allosteric change required for the MCM helicase to become an active complex (Sclafani et al. 2002, 2004; Fletcher et al. 2003; Chen et al. 2005). At the same time, additional CDK activity is still needed to initiate DNA replication. Thus, cells that carry either a clb2Δ or a clb5Δ mutation are ineffective in mcm5-bob1-dependent bypass of DDK (Sclafani et al. 2002). In this manner, clb2Δ and clb5Δ mutations suppress the mcm5-bob1 mutation (Sclafani et al. 2002).
Concomitantly, DNA checkpoint mechanisms have evolved to monitor the successful completion of cell cycle events involving DNA replication and mitosis (see reviews in Foiani et al. 2000; Nyberg et al. 2002; Kastan and Bartek 2004). In Saccharomyces cerevisiae, Rad53 is an essential dual specificity protein kinase that is part of a signal transduction cascade involved in the response to DNA damage or stalled DNA replication forks. In this cascade, Mec1 and to a lesser extent Tel1 protein kinases activate Rad53 to prevent cell cycle progression, stabilize replication forks, and facilitate repair. Sensor proteins that bind Rad53 through its two fork head-associated (FHA) domains are needed either for the damage, e.g., Rad9 (Sun et al. 1998a), or for the replication response, e.g., Mrc1 (Alcasabas et al. 2001a). Mutations in both FHA1 and FHA2 domains of Rad53 ablate most checkpoint responses (Schwartz et al. 2003). Thus, Mec1 and Rad53 are needed for both responses, while Rad9 and Mrc1 are needed only for DNA damage or replication responses, respectively. These responses are important medically as defects in the human homologs of Rad53 (Chk2) or Mec1 (ATM/ATR) lead to human cancer by increasing genomic instability (Kastan and Bartek 2004).
The essential function of Rad53 or Mec1 kinases can be rescued by overexpression of the large subunit of ribonucleotide reductase encoded by RNR1 or by downregulation of the Sml1 protein, which when bound to Rnr1 protein holds it in check (Sanchez et al. 1996; Desany et al. 1998; Zhao et al. 1998, 2000, 2001; Zhao and Rothstein 2002). Because an increase in deoxyribonucleotide levels can rescue loss of the RAD53 or MEC1 genes, an essential function of these kinases is to upregulate deoxyribonucleotide levels (Chabes et al. 2003). Failure to remove Sml1 in rad53Δ or mec1Δ mutants results in incomplete DNA replication, defective mitochondrial DNA propagation, decreased dNTP levels, and cell death (Zhao et al. 2001; Cha and Kleckner 2002).
Another one of Rad53's functions appears to be monitoring the stability of replication forks. RAD53 mutants accumulate unusual replication structures at replication forks when treated with hydroxyurea (Lopes et al. 2001; Sogo et al. 2002). Replication fork catastrophe occurs in checkpoint mutants presented with DNA-damaging agents such as MMS (Tercero and Diffley 2001) or agents that stall replication such as HU (Sogo et al. 2002).
RAD53 is likely involved in S-phase progression control. Reduced origin firing was seen in a rad53 mutant using 2D-gel analysis (Lopes et al. 2001). The firing of late origins is also advanced in rad53 and mec1 mutants (Santocanale and Diffley 1998), resulting in a late origin becoming early replicating (Shirahige et al. 1998). The accelerated S-phase is thought to be a consequence of inappropriate initiation events (Santocanale et al. 1999). Rad53 may also modulate origin firing on the basis of growth conditions to optimize the rate of S-phase progression (Sidorova and Breeden 2002). These results suggest that Rad53 controls the timing of DNA replication during normal cell growth.
Rad53 is also involved in the degradation of excess, nonnucleosomal soluble histones, which are shown to accumulate in rad53Δ sml1Δ strains (Gunjan and Verreault 2003). Because deletion of the major copy of the H3/H4 histone gene pair, HHT2-HHF2, suppresses some of the phenotypes of rad53Δ sml1Δ but not of mec1Δ tel1Δ sml1Δ mutants, excess histones may account for the slow growth and chromosome loss phenotype that occurs in the absence of Rad53. In a recent genomewide study of the DNA integrity network (Pan et al. 2006), it was suggested that Rad53 protein kinase might also play a more direct role in maintenance of chromatin structure. However, the role of the histone chaperones Asf1, Cac-1 (CAF1-3 genes), and Hir1/2 complexes (Kaufman et al. 1997; Tyler et al. 1999; Sharp et al. 2001) in this process is less clear (Gunjan and Verreault 2003; Quivy and Almouzni 2003; Pan et al. 2006). These rad53 phenotypes are consistent with an effect on histone homeostasis and DNA replication independent of the DNA checkpoint or deoxynucleotide levels.
Our previous studies indicated a link between the initiation of DNA replication and Rad53 (Dohrmann et al. 1999). We found that cdc7 and rad53 mutations were synthetically lethal and that rad53 mutants have reduced Dbf4 protein levels. Our original hypothesis was that this lethality results from reduced protein levels of Dbf4, the regulatory subunit of DDK. Consistent with this hypothesis, Rad53 binds (Dohrmann et al. 1999; Duncker et al. 2002) and phosphorylates Dbf4 (Weinreich and Stillman 1999; Kihara et al. 2000). However, we show in this report that the original hypothesis is incorrect and propose a new role for Rad53 in the initiation of DNA replication that is independent of Rad53's known roles in checkpoint or deoxynucleotide regulation.
MATERIALS AND METHODS
Yeast strains, media, and plasmids:
Yeast strains and recombinant plasmids (Table 1) were grown and manipulated as described (Dohrmann et al. 1999; Sclafani et al. 2002; Pessoa-Brandao and Sclafani 2004). Standard genetic crosses were used for strain construction and tetrad analysis (Sherman et al. 1986; Burke et al. 2000).
TABLE 1.
List of S. cerevisiae strains and recombinant plasmids used in this study
| Strain or plasmid | Genotype | Source/reference/alias |
|---|---|---|
| PDY037 | MATaura3 his3 leu2 cdc7Δ1∷HIS3 mcm5-bob1-1 3HA-RAD53∷URA3 | Pessoa-Brandao and Sclafani (2004) |
| PDY201 | MATα cdc7-1 rad53-31 ade2 ade3 leu2 trp1 ura3 (pPD7 = YCp CDC7-ADE3) | Dohrmann et al. (1999) |
| PDY253 | MATacdc7-4 ade2 ura3 | This study |
| PDY258 | MATα rad53-11 pep4Δ∷URA3 ura3 | |
| PDY294 | MATα rad53Δ∷hisG-URA3-hisG his3 lys2 leu2 trp1 ura3 (pGAP-RNR1-2μ TRP) | This study |
| PDY297 | MATα rad53Δ∷hisG his3 lys2 leu2 trp1 ura3 (pGAP-RNR1-2μ TRP) | This study |
| PDY376 | MATaura3 leu2 trp1 3HA-RAD53∷URA3 | Pessoa-Brandao and Sclafani (2004) |
| PDY420 | MATα cdc7-1 mcm5-bob1-2 rad53-31 ade2 ade3 leu2 trp1 ura3 (pPD7 = YCp CDC7-ADE3) | Dohrmann et al. (1999) |
| PDY423 | MATacdc7-4 rad53-11 pep4Δ∷URA3 mcm5-bob1-1 ura3 | This study |
| PDY425 | MATahis3 cdc7-4 mcm5-bob1-2 ura3 rad53-11 pep4Δ∷URA3 | Dohrmann et al. (1999) |
| PDY449 | MATα rad53Δ∷hisG URA3∷ lacZ vector his3 lys2 leu2 trp1 (pGAP-RNR1-2μ TRP) | This study |
| PDY477 | MATα rad53Δ∷hisG URA3∷ARS1UAS-lacZ his3 lys2 leu2 trp1 ura3 (pGAP-RNR1-2μ TRP) | This study |
| PDY478 | MATα rad53Δ∷hisG URA3∷1xF.ARS1UAS lacZ his3 lys2 leu2 trp1 ura3 (pGAP RNR12μ TRP) | This study |
| PDY480 | MATaleu2 trp1 rad53Δ∷hisG URA3∷ARS.2xR (pGAP-RNR1-2μ TRP) | This study |
| P101 | MATα leu2 his7 tyr1 cdc7-3 mcm5-bob1-1 lys2 | Jackson et al. (1993); Hardy et al. (1997) |
| P138 | MATα leu2 ura3 cdc7-1 mcm5-bob1-1 cyh2 | Jackson et al. (1993); Hardy et al. (1997) |
| P253 | MATaura3 can1 trp1 his3 cdc7-1 bob1-1 cyh2 | Jackson et al. (1993); Hardy et al. (1997) |
| P142 | MATaleu2 ura3 cdc7-1 mcm5-bob1-1 | Jackson et al. (1993); Hardy et al. (1997) |
| 1815-4C | MATaura3 mad2-1 | P. Megee |
| RSY299 | MATα his3 leu2 trp1 ura3 | Jackson et al. (1993); Hardy et al. (1997) |
| RSY 311 | MATatrp1 leu2 ura3 can1 his6 bar1 | Sclafani et al. (2002) |
| RSY465 | MATaleu2 ura3 trp1 his7 rad9Δ∷LEU2 | 7859-7-4 (T. Weinert) |
| RSY847 | MATaleu2 trp1 his3 ura3 cdc7-7 mcm5-bob1-2 | Pessoa-Brandao and Sclafani (2004) |
| RSY870 | MATahis3 trp1 mec1-1 sml1-1 | TWY304 (T. Weinert) |
| RSY1060 | MATahis3 leu2 trp1 ura3 sml1Δ∷hygro rad53Δ∷kanMX4 | JKT010 rad53 (J. Tyler)b |
| RSY1064 | MATaleu2 trp1 rad53Δ∷hisG URA3∷ARS.2xR (pGAP-RNR1-2μ LEU2) | This study |
| RSY1104 | MATamrc1Δ∷kanMX4 leu2 lys2 ura3 his3 | YCL060C (Open Biosystems)c |
| RSY1109 | MATα cdc7-1 trp1-289 leu2 ura3 his3 mcm5-bob1-1 mrc1Δ∷kanMX4 | This studyd |
| RSY1111 | MATα cdc7-1 trp1-289 leu2 ura3 his3 mrc1Δ∷kanMX4 | This studyd |
| RSY1113 | MATachk1Δ∷URA3 his3 leu2 trp1 ura3 | YS152 Sanchez et al. (1996)b |
| RSY1128 | MATαleu2ura3cdc7-1mcm5-bob1-1cyh2 | P138 X RSY1060 (This study)a |
| MATaleu2 ura3 + + + | ||
| rad53Δ∷kanMX4his3trp1sml1Δ∷hygro | ||
| + + + + | ||
| RSY1139 | MATaleu2 ura3 his3 cdc7-7 tel1Δ∷kanMX4trp1 mcm5-bob1-2 | This studya |
| RSY1141 | MATα mec1-1 sml1-1 cdc7-1 leu2 his3 ura1 ura3 cyh2 | This study |
| RSY1142 | MATacdc7-1 leu2 ura3 rad53Δ∷kanMX4 his3 sml1Δ∷hygro (YCp URA3 RAD53) | This studya |
| RSY1143 | MATacdc7-1 leu2 ura3 rad53Δ∷kanMX4 his3 smlΔ1∷hygro (pARS CEN URA3 LEU2 RAD53) | This studya |
| RSY1145 | MATα cdc7-1 mcm5-bob1-1 leu2 ura3 his3 rad53Δ∷kanMX4sml1Δ∷hygro (pARS CEN URA3 RAD53) | This studya |
| RSY1157 | MATacdc7-1 leu2 ura3 rad53Δ∷kanMX4 his3 sml1Δ∷hygrohht2Δhhf2Δ∷HIS3 (pARS CEN URA3 RAD53) | This studya |
| RSY1158 | MATα cdc7-1 mcm5-bob1-1 leu2 ura3 his3 rad53Δ∷kanMX4sml1Δ∷hygro hht2Δhhf2Δ∷HIS3 (pARS CEN URA3 RAD53) | This studya |
| RSY1161 | MATα cdc7-1 mcm5-bob1-1 arg4 ura3 can1 cyh2 his3 ade1clb5∷ARG4 hht2Δhhf2Δ∷HIS3 | This study |
| RSY1162 | MATaade2 his3 leu2 trp1 ura3 asf1Δ∷kanMX4 | JLY1017 (J. Tyler)b |
| RSY1163 | MATaade2 his3 leu2 trp1 ura3 cac1Δ∷LEU2 | JLY1018 (J. Tyler)b |
| RSY1164 | MATatel1Δ∷kanMX4 ura3 his3 leu2 met15 | YBL088C (Open Biosystems)c |
| RSY1177 | MATacdc7-1 leu2 rad9Δ∷LEU2 mrc1Δ∷kanMX4 | This studyd |
| RSY1179 | MATα ura3 his3 lys2 leu2 dun1Δ∷kanMX4 | YDL101C (Open Biosystems)c |
| RSY1195 | MATα cdc7-1 mcm5-bob1-1 leu2 ura3 his3 rad53Δ∷kanMX4sml1Δ∷hygro (YCp LEU2 RAD53) | This studya |
| RSY1199 | MATα leu2 his3 cdc7-1 cac1Δ∷LEU2 ade2 ura3 | This studya |
| pPD1 | pRS316-CDC7 (URA3) | Dohrmann et al. (1999) |
| pPD7 | pRS314-CDC7-ADE3 (TRP1) | Dohrmann et al. (1999) |
| pPD60 | pRS316-RAD53+ (URA3) | Dohrmann et al. (1999) |
| pRS423 | HIS3 2μ yeast shuttle vector | Christianson et al. (1992) |
| pGAP-RNR1 | pRNR1 from GAP pro (TRP1) | T. Weinert |
| pPD348 | pRS423-GAP-RNR1 (HIS3) | This study |
| pPD348 | pRS425-GAP-RNR1 (LEU2) | This study |
| pRAS490 | mcm5-bob1-2 (URA3) | Pessoa-Brandao and Sclafani (2004) |
| pDK243 | CEN4 (1x ARS) (LEU2) | Hogan and Koshland (1992) |
| pDK368-7 | CEN4 (8x ARS) (LEU2) | Hogan and Koshland (1992) |
| p305.2 | ARS305 CEN5 (URA3) | Friedman et al. (1996) |
| pARS1 | ARS1 CEN5 (URA3) | Friedman et al. (1996) |
| p12 | ARS1412 CEN5 (URA3) | Friedman et al. (1996) |
| pLG-ARS1+ | ARS1-lacZ one-hybrid vector (URA3) | Dowell et al. (1994) |
| pPD339 | pLGΔ178 (URA3, Δ2μ seq) | Guarente and Mason (1983) |
| pPD342 | pLG-ARS1+ (URA3, Δ2μ seq) | This study |
| pPD357 | pLG-1xF.ARS1 (URA3, Δ2μ seq) | This study |
| pPD358 | pLG-1xR.ARS1 (URA3, Δ2μ seq) | This study |
| pPD359 | pLG-2xR.ARS1 (URA3, Δ2μ seq) | This study |
| pGAD-2F | pGAL4AD (LEU2) | Chien et al. (1991) |
| pUNI10 | Univector Plasmid System | Liu et al. (1998) |
| pACT2-lox | GAL4AD-fusion vector | Liu et al. (1998) |
| pPD206 | pUNI10-RAD53+ | This study |
| pPD233 | pACT2-lox-RAD53AD(LEU2) | This study |
| pRS315 | LEU2 yeast shuttle vector | Sikorski and Hieter (1989) |
| pPD361 | pRS315-RAD53 (LEU2) | This study |
| pY2 | pGAL4DB (TRP1) | Sadowski et al. (1992) |
| pPD94 | pGAL4DB-RAD53 (TRP1) | Dohrmann et al. (1999) |
| pRS316-RAD53 | RAD53+ (URA3) | Sun et al. (1996, 1998a,b) |
| pRS316-rad53kd | rad53 K227A D339A (URA3) | Sun et al. (1996) |
| pRS316-rad53-NVS | rad53 NVS/AAA (655-657) (URA3) | Sun et al. (1998a,b) |
| pRS316-rad53-H622A | rad53 H622A (URA3) | Sun et al. (1998a,b) |
| pRS315-RAD53 | RAD53+ (LEU2) | Schwartz et al. (2003) |
| pRS315-rad53-fha1 | rad53 R70A N107A (LEU2) | Schwartz et al. (2003) |
| pRS315-rad53-fha1,2 | rad53 R70A N107A NVS-AAA (655–657) (LEU2) | Schwartz et al. (2003) |
| pJG46 | B42AD (TRP1) vector | Duncker et al. (2002) |
| pRAD53 | B42AD-RAD53+(TRP1) | Duncker et al. (2002) |
| FHA1 | B42AD-rad53(I4-S165) (TRP1) | Duncker et al. (2002) |
| KIN | B42AD-rad53(G177-N599) (TRP1) | Duncker et al. (2002) |
| FHA2 | B42AD-rad53(M497-S821) (TRP1) | Duncker et al. (2002) |
| fha1 | B42AD-rad53(I4-S165; R70A) (TRP1) | Duncker et al. (2002) |
| fha2 | B42AD-rad53(M497-S821; R605A) (TRP1) | Duncker et al. (2002) |
| pRM102 | Gal10p-HHT2 Gal1p-HHF2 (URA3) | Mann and Grunstein (1992) |
All yeast strains are congenic with A364a except if indicated otherwise:
W303/A364a hybrid.
W303.
S288C.
S288C/A364a hybrid.
To construct pPD339 and pPD342, pLGΔ178 (Guarente and Mason 1983) and pLG-ARS+ (Dowell et al. 1994), respectively, were subjected to partial digestion with HindIII to remove ∼8000 bp containing the 2μ sequences. pPD357 was constructed by amplifying ARS1 sequences from pPD342 with primers ARS1-forward (5′ AAG CTT GCA TGC CTG CAG G 3′) and ARS1-reverse (5′ CCG CTC GAG AAT TCG AGC TCG GTA CCC 3′) digested with ApaI for integration at the URA3 locus. PDY477 through PDY480 were also constructed by integrating the indicated ARS1-lacZ plasmids at the URA3 locus.
The RAD53-activation domain fusion plasmid pPD233 (pACT2-lox-RAD53AD) was constructed using the universal plasmid fusion system (Liu et al. 1998). Plasmid pRAS574 was constructed from plasmid pPD233 by replacement of a 1076-bp SacI DNA restriction fragment from plasmid pPD335, which contains the rad53-11 mutation, cloned by gap repair of SstI-digested pPD94 (Dohrmann et al. 1999) using a rad53-11 strain.
The hht2-hhf2Δ∷HIS3 (Mann and Grunstein 1992) construct was amplified by PCR using the “D” primers for each gene (Issel-Tarver et al. 2002) as the genes are divergently transcribed. The resultant 2.4-kb PCR product was used to select His+ transformants. All hht2-hhf2Δ∷HIS3 strains were verified by use of the “A” and D primers of each gene, which yield no product, and the D primers, which yield a 2.4-kb product (Issel-Tarver et al. 2002).
Random spore analysis:
cyh2R/CYH2S diploids were sporulated in liquid 0.3% potassium acetate (KAC) and the resultant asci were digested with snail glusulase at 10% for 30 min at room temperature (Dohrmann et al. 1999; Sclafani et al. 2002; Pessoa-Brandao and Sclafani 2004). The digested spores were washed extensively with distilled water and then diluted 1/50 to 1/100-fold and vortexed for 2 min. The dilutions were plated on selective medium that also contained cycloheximide to select for haploid progeny. The phenotype of the progeny was then tested by picking and patching the colonies to master plates and then replica plating. For diploid strain RSY1128 (Table 1), YPD plates with G418 (100 μg/ml) and cycloheximide (5 μg/ml) were used to select rad53Δ∷kanMX4 cyh2 colonies. As expected, all rad53Δ∷kanMX4 colonies also contained sml1Δ∷hygro as rad53Δ∷kanMX4 SML1+ colonies are inviable (Zhao et al. 1998). In dropout medium, monosodium glutamate (1 g/liter) was substituted for (NH4)2SO4 and G418 was used at 400 μg/ml.
Plasmid shuffle assays:
For LEU2 or URA3 plasmid loss, at least 200 cells were plated on nonselective YPD plates and then the resultant colonies were replica plated to −Leu or −Ura dropout media to identify Leu− or Ura− clones, respectively. Alternatively, 5-fluoroorotic acid (5-FOA) was used to select Ura− colonies (Burke et al. 2000).
Minichromosome loss:
Mitotic loss rates were determined (Lengronne and Schwob 2002) in cells carrying plasmids pDK247 (LEU2 ADE3) with one ARS or eight ARSs (pDK368-7) (Hogan and Koshland 1992) or plasmids bearing early- (p305.2), middle- (pARS1), or late-firing (p12) ARSs (Ferguson et al. 1991; Friedman et al. 1996). All loss rates are percentage per generation and were determined in triplicate.
One-hybrid and two-hybrid assays:
Liquid culture β-galactosidase and X-Gal assays were done as previously described (Shellman et al. 1998; Dohrmann et al. 1999).
Rad53 protein immunoblots:
Strains with a 3XHA-Rad53 construct (pPD328) integrated at the Rad53 locus were analyzed with anti-HA antibody as described (Pessoa-Brandao and Sclafani 2004).
RESULTS
Bypass of cdc7 is dependent upon RAD53:
The mcm5-bob1 mutation bypasses the essential function of the DDK in that all cdc7ts and dbf4ts mutants are suppressed by mcm5-bob1 (Tables 1 and 2) and mcm5-bob1 cdc7Δ, mcm5-bob1 dbf4Δ, and mcm5-bob1 cdc7Δ dbf4Δ mutants are viable (Jackson et al. 1993; Hardy et al. 1997; Sclafani et al. 2002). Bypass of DDK by mcm5-bob1 also functions in all common genetic backgrounds that were tested (W303, A364a, and S288C—data not shown) (Weinreich and Stillman 1999). If the synthetic lethality of cdc7 rad53 mutants is due to reduced Dbf4 protein levels, which would compromise the mutant cdc7 protein kinase as proposed (Dohrmann et al. 1999), than mcm5-bob1 should suppress it as it bypasses deletions of both essential CDC7 and DBF4 genes. We used a “plasmid sectoring assay” (Dohrmann et al. 1999) to test the idea. In this case, a cdc7 rad53 strain is kept alive with a plasmid-borne copy of CDC7. The synthetic lethality can be monitored by a red/white colony-sectoring assay. In this case, strain PDY201 cdc7 rad53 requires the CDC7 plasmid for viability and demonstrates a red nonsectored colony morphology. If one complements the cdc7 rad53 strain with either another CDC7 or the RAD53 plasmids, then the strain can lose CDC7 plasmid pPD7, which results in a red/white sectored colony morphology. The mcm5-bob1 mutation was introduced into strain PDY201 by transformation and recombination using plasmid pRAS490 (Table 1) (Fletcher et al. 2003; Pessoa-Brandao and Sclafani 2004), generating the triple-mutant strain PDY420 cdc7 rad53 mcm5-bob1. Surprisingly, the cdc7 rad53 mcm5-bob1 strain was unable to lose the CDC7 plasmid and demonstrated the nonsectoring colony morphology (100/100 clones tested). This indicates that mcm5-bob1 is unable to suppress the cdc7 rad53 synthetic lethality (Table 2).
TABLE 2.
Summary of phenotypes of cdc7, rad53, mcm5-bob1, and hht2-hhf2 strains
| Genotype | Viability | Tsma |
|---|---|---|
| cdc7-1, cdc7-4 | + | − |
| rad53-11 or rad53Δ sml1Δ | + | + |
| cdc7-1 rad53-11 or cdc7-1 rad53Δ sml1Δ | − | NAc |
| cdc7-xbmcm5-bob1 | + | + |
| cdc7-1 rad53-11 mcm5-bob1 or | − | NAc |
| cdc7-1 rad53Δ sml1Δ | ||
| cdc7-1 rad53Δ sml1Δ hht2-hhf2Δ | + | − |
| cdc7-1 rad53Δ sml1Δ mcm5-bob1 hht2-hhf2Δ | + | + |
| cdc7-4 rad53-11 | + | − |
| cdc7-4 rad53-11 mcm5-bob1 | + | − |
| cdc7-1 mcm5-bob1 clb5Δ | + | − |
| cdc7-1 mcm5-bob1 clb5Δ hht2-hhf2Δ | + | − |
Temperature sensitivity.
x, any allele including cdc7Δ.
Not applicable.
One possible explanation for this result is that the rad53 mutation suppresses mcm5-bob1 bypass similarly to clb5 or clb2 mutations (Sclafani et al. 2002). When mcm5-bob1 is suppressed, it can no longer suppress cdc7ts mutations, and thus the strains become temperature-sensitive mutant (Tsm)− again. To test the effect of a rad53 mutation directly on mcm5-bob1 bypass, we exploited the fact that the “leaky” cdc7-4 allele is not synthetically lethal with rad53 mutations (Weinert et al. 1994; Dohrmann et al. 1999) (Table 2). We crossed strain PDY258 rad53-11 pep4Δ∷URA3 with strain PDY253 cdc7-4 mcm5-bob1. Because the pep4Δ∷URA3 marker is tightly linked to the rad53 locus (Dohrmann et al. 1999) and pep4 mutations alone have no effect on cdc7 or mcm5-bob1 mutations (Jackson et al. 1993), it can be used to follow rad53. Because CDC7 (chromosome IV) and MCM5 (chromosome XII) are unlinked, the meiotic products of a single ascus will display predominantly 3+:1− Tsm phenotype segregation indicative of an extragenic suppressor (Hardy et al. 1997). Our results demonstrated a decrease of 3+:1− tetratype (T) asci (37 vs. 53.3 expected) and a concomitant increase of nonparental ditype (NPD) 2+:2− asci (36 vs. 13.3 expected; P < 0.01). As seen for clb5 or clb2 suppression (Sclafani et al. 2002), the increase in 2+:2− asci is the result of suppression of mcm5-bob1, which in this case causes a cdc7 mcm5-bob1 rad53 strain to become Tsm−. Indeed, a survey of 25 individual cdc7 rad53 Tsm− colonies indicated that 11/25 (46%) contained the mcm5-bob1 mutation as expected. The presence of mcm5-bob1 was determined by backcrossing each spore clone to cdc7ts tester strains and checking whether the resulting cdc7/cdc7 +/mcm5-bob1 diploid papillated to Tsm+ (Hardy et al. 1997). As mcm5-bob1 is recessive, papillation results from the production of mcm5-bob1/mcm5-bob1 homozygotes by mitotic recombination in 5–10% of the cells. Thus, while strain P138 carrying the cdc7-4 mcm5-bob1 mutations is Tsm+, strain PDY423 with the cdc7-4 mcm5-bob1 rad53 mutations is Tsm− (Tables 1 and 2). Neither plasmid pPD348 (pGAP-RNR1) nor addition of the sml1 mutation, both of which increase deoxyribonucleotide levels and bypass a rad53Δ (Sanchez et al. 1996; Desany et al. 1998; Zhao et al. 1998), had any effect (Table 2 and data not shown). We conclude that bypass or suppression of cdc7 by mcm5-bob1 is dependent upon RAD53 and this effect is independent of deoxyribonucleotide levels.
The triple-mutant strain PDY425 cdc7-4 mcm5-bob1 rad53 was temperature sensitive (Table 2) and arrested as large-budded cells (>85%) after 4 hr at the restrictive temperature. The majority of these large-budded cells (>90%) had the nucleus at the mother-bud neck (data not shown). This terminal phenotype is similar to that of cdc7 mutants (Hartwell 1973). Because mcm5-bob1 is suppressed by a rad53 mutation and cannot suppress the cdc7ts mutation, a cdc7ts and a cdc7ts mcm5-bob1 rad53 strain have the same phenotype (Table 2).
Rad53 kinase activity but neither FHA domain is needed for both cdc7 viability and mcm5-bob1 suppression:
Our previous results (Dohrmann et al. 1999) and those above used rad53 hypomorphic alleles. We produced a series of strains that had either cdc7 rad53Δ∷kanMX4 sml1Δ∷hygro or cdc7 rad53Δ∷kanMX4 sml1Δ∷hygro mcm5-bob1 genotypes (Table 1) and a complementing RAD53+ plasmid by performing random spore analysis with diploid strain RSY1128 (Table 1). Resultant cdc7ts segregants could be identified as Tsm− and cdc7ts mcm5-bob1 segregants as Trp+ Tsm+ because cdc7ts is tightly linked to TRP1+ (2 cM). Because CDC7 and MCM5 are unlinked, 50% of TRP1+ colonies will be Tsm+ because mcm5-bob1 suppresses cdc7ts (Hardy et al. 1997). If RSY1128 alone or RSY1128 with a URA3 vector pRS316 was used no cdc7 rad53Δ∷kanMX4 sml1Δ∷hygro or cdc7 rad53Δ∷kanMX4 sml1Δ∷hygro mcm5-bob1 isolates were obtained in 100 rad53Δ∷kanMX4 sml1Δ∷hygro colonies analyzed. If a URA3 RAD53 complementing plasmid was included and also selected in the spores, the expected 50:50 ratio of cdc7 rad53Δ∷kanMX4 sml1Δ∷hygro and cdc7 rad53Δ∷kanMX4 sml1Δ∷hygro mcm5-bob1 genotypes was obtained. The presence of mcm5-bob1 in these strains was also confirmed by complementation test with cdc7 tester strains (Hardy et al. 1997; Sclafani et al. 2002). These results indicate that cdc7-1 is synthetically lethal with rad53Δ and this lethality cannot be bypassed by mcm5-bob1.
To test additional rad53 mutations, we used a “plasmid shuffle” assay with rad53Δ sml1Δ cdc7 (pRAD53) strain RSY1142 and rad53Δ sml1Δ cdc7 mcm5-bob1 strain RSY1145 obtained from this cross or by “shuffle” of these strains with a LEU2 RAD53 plasmid to produce strains RSY1143 and RSY1195 (Table 1). Different rad53 mutant plasmids (Tables 1 and 3) were transformed into these strains and tested for complementation by determining if the original pRAD53 plasmid could be lost.
TABLE 3.
Plasmid “shuffle” assay with rad53 mutations in cdc7ts and cdc7ts mcm5-bob1 strains
|
cdc7ts mcm5-bob1ab
|
||||
|---|---|---|---|---|
| Plasmid for “shuffle” | Mutant domain(s) | cdc7tsa | 22° | 36° |
| Vector | NA | − | − | − |
| RAD53+ | None | + | + | + |
| rad53 R70A N107A | FHA1 | + | + | + |
| rad53 NVS/AAA | FHA2 | + | + | + |
| rad53 H622A | FHA2 | + | + | + |
| rad53 K227A D339A | Kinase | − | − | − |
| rad53 R70A N107A NVS/AAA | FHA1 FHA2 | + | + | + |
Strains RSY1142 and RSY1143 rad53Δ sml1Δ cdc7 (pRAD53) were transformed with the indicated plasmids (Table 1). + or − indicates whether the initial pRAD53 plasmid could or could not be shuffled and replaced with the listed plasmid, respectively, at the permissive temperature of 22°.
RSY1145 and RSY1195 rad53Δ sml1Δ cdc7 mcm5-bob1 (pRAD53) were transformed with the indicated plasmids (Table 1). + or − indicates whether the resultant rad53Δ sml1Δ cdc7 mcm5-bob1 strain with the shuffled plasmid listed could grow at the restrictive temperature of 36°. Growth at 36° indicates mcm5-bob1 suppression of cdc7ts.
Mutations in the FHA1 domain (R70A N107A), in the FHA2 domain (NVS/AAA and H622A), or in both the FHA1 and the FHA2 domains (R70A N107A NVS/AAA) (Sun et al. 1998b; Schwartz et al. 2003) complemented (Table 3). Only the rad53 K227A D339A kinase dead mutant failed to complement. All these rad53 mutants except for the kinase-dead allele also complemented the rad53Δ sml1Δ cdc7 mcm5-bob1 strains. In addition, they became Tsm+, indicating that mcm5-bob1 function was also restored. We conclude that Rad53 protein kinase activity but not its ability to mediate DNA checkpoint signaling via the two FHA domains (Pike et al. 2003; Schwartz et al. 2003) is needed for both cdc7 viability and mcm5-bob1 suppression.
Mutations in most other checkpoint genes have no effect on cdc7 mutant viability and mcm5-bob1 suppression:
We determined whether the DNA replication, damage, or mitotic checkpoints are important for the effects we have observed by crossing different checkpoint mutants with a cdc7ts mcm5-bob1 strain (Tables 1 and 4). Again, we used the tightly linked TRP1 marker to identify cdc7 mcm5-bob1 strains. Our results using tetrad analysis show that in contrast to the results with rad53 mutants, both cdc7 (Tsm−) and cdc7 mcm5-bob1 (Tsm+) strains containing null mutations in mec1, tel1, chk1, mrc1, rad9, dun1, and mad2 were easily obtained (Tables 1 and 4). Furthermore, all cdc7 mcm5-bob1 strains with these checkpoint mutations were Tsm+, indicating that mcm5-bob1 bypass of cdc7 was not affected. Similar results were also found for mec1 tel1 and rad9 mrc1 strains, which are defective in all DNA replication and damage checkpoint signaling (Sanchez et al. 1996; Alcasabas et al. 2001b). We conclude that loss of the DNA checkpoint per se in rad53 mutants is not responsible for the effects we have observed.
TABLE 4.
Only RAD53 is required for both cdc7 viability and mcm5-bob1 suppression
| Cross | Query gene | Tsm+ (cdc7ts mcm5-bob1) | Tsm− (cdc7ts) | n |
|---|---|---|---|---|
| RSY311 × P101 | None | 50 | 50 | 34 |
| RSY1060 × P138 | rad53 | 0 | 0 | 48 |
| P142 × RSY870 | mec1 | 59 | 41 | 22 |
| RSY1164 × 847 | tel1 | 58 | 42 | 26 |
| RSY1113 × P138 | chk1 | 50 | 50 | 43 |
| P253 × RSY1104 | mrc1 | 42 | 58 | 27 |
| RSY847 × 1815-4C | mad2 | 41 | 59 | 20 |
| RSY1139 × RSY1141 | tel1 mec1 | 42 | 58 | 20 |
| RSY1111 × RSY465 | rad9 | 45 | 55 | 20 |
| RSY1179 × P253 | dun1 | 48 | 52 | 21 |
| JLY1018 × P142 | asf1 | 53 | 47 | 17 |
| JLY018 × P142 | cac1 | 50 | 50 | 24 |
| RSY1111 × RSY465 | rad9 mrc1 | — | 40 | 20 |
| RSY1109 × RSY1177 | rad9 mrc1 | 43 | — | 23 |
All numbers are the percentage of colonies with the query mutant gene(s) that also have cdc7 mcm5-bob1 or just cdc7 mutations. Because all genes are unlinked, 50% of colonies with the cdc7 mutation will be Tsm−. Tsm+ cdc7 colonies contain mcm5-bob1 (Hardy et al. 1997); n, number of colonies in tetrads examined. cdc7ts mutation was followed by the Tsm− phenotype or by linkage to TRP1.
Rad53 can be activated by HU in the absence of Cdc7:
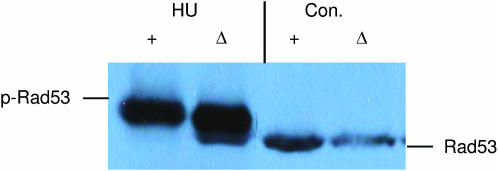
Our results indicate that DDK's role is compromised in the absence of Rad53. Conversely, we tested if Rad53 function was compromised by the absence of DDK. Previously, we found that in cdc7Δ mcm5-bob1 strains, the DNA damage checkpoint is intact and Rad53 can be activated by UV-induced DNA damage (Pessoa-Brandao and Sclafani 2004). The DNA replication checkpoint is also intact in these strains in that survival is high even when DNA replication is blocked with HU (Weinreich and Stillman 1999). We tested whether Rad53 can be activated in the absence of Cdc7 protein when DNA replication is blocked with HU (Figure 1). When Rad53 is activated, slower-migrating forms appear in the gel due to autophosphorylation and phosphorylation by Mec1 kinase (Pellicioli et al. 2001). Cells were arrested in S-phase with HU at 200 mm for 3.5 hr. As we have seen with UV damage, Rad53 protein can be activated by HU treatment in a cdc7Δ mcm5-bob1 strain. However, the response is partially attenuated with somewhat less Rad53 protein being shifted. The amount of Rad53 protein that is activated must be sufficient because these cdc7Δ mcm5-bob1 cells remain viable in HU even after 12 hr as seen for wild-type cells (data not shown). Therefore, Rad53 kinase can function in the DNA checkpoint even in the absence of DDK.
Figure 1.—
Rad53 protein kinase is activated by HU even in the absence of Cdc7 protein. Wild-type (+) or cdc7Δ mcm5-bob1 (Δ) cells with the tagged 3XHA-RAD53 gene were treated with 200 mm HU for 3.5 hr. Untreated control log-phase cells (Con.) were also examined. Immunoblots were prepared from cell extracts and probed with anti-HA antibody to detect the 3XHA-Rad53-tagged protein. The positions of the Rad53 (Rad53) and activated phosphorylated Rad53 (p-Rad53) proteins are indicated.
rad53Δ mutants demonstrate a minichromosome loss phenotype:
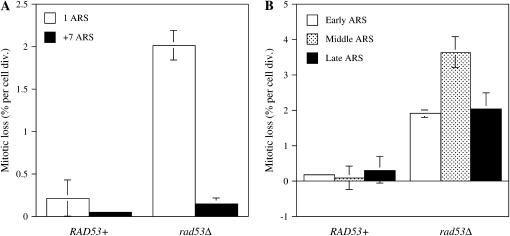
Because DDK is important for the initiation of DNA replication, we tested Rad53's role in this process. Many mutations in the genes involved in the initiation of DNA replication such as mcms and orcs show a minichromosome loss phenotype (Maine et al. 1984) that is suppressible by addition of multiple ARS elements (Hogan and Koshland 1992; Foss et al. 1993; Loo et al. 1995; Hardy 1996). Strain PDY256 RAD53+ or PDY294 rad53Δ was transformed with plasmids containing either one ARS (pDK243) or eight ARSs (pDK368-7). Mitotic loss rates were calculated by fluctuation tests and were found elevated 20-fold in the rad53Δ strain (Figure 2A). Also as seen in other initiation mutants, the loss rate could be suppressed completely by the addition of multiple ARS elements.
Figure 2.—
rad53Δ mutants exhibit a minichromosome loss phenotype. (A) Mitotic loss rates were measured, as in materials and methods, in wild-type (PDY256) and rad53Δ (PDY294) cells that were transformed with either pDK243 (1 ARS) or pDK368-7 (+7 ARS). Shown are mean values from three independent experiments. (B) Mitotic rates were measured as in A for WT (PDY256) and rad53Δ (PDY294) cells transformed with one of three plasmids: p305.2 (early-firing ARS305), pARS1 (early/middle-firing ARS1), or p12 (late-firing ARS1412).
The minichromosome loss phenotype was not limited to just ARS1. We tested the minichromosome loss phenotype on individual plasmids that carry individual ARSs that fire at different times during S-phase: ARS305 (early firing), ARS1 (early/middle firing), and ARS1412 (late firing) (Ferguson et al. 1991; Friedman et al. 1996). In each case, elevated minichromosome loss phenotypes were seen in the rad53Δ mutant strain (Figure 2B).
Rad53 is targeted to ARS1 through its kinase domain:
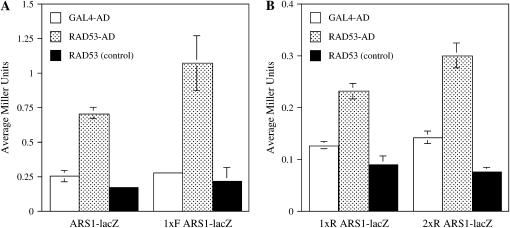
Due to the increased minichromosome loss rate in rad53Δ strains, we hypothesize that Rad53 protein may bind to origins of replication. Binding of Rad53 protein to origins by chromatin immunoprecipiation (ChIP) has been shown (Katou et al. 2003). However, this binding was barely detectable as compared to Mrc1 or Tof1 proteins and required checkpoint activation. Therefore, we used the one-hybrid system as an alternative. This strategy has been used successfully to detect proteins that interact with a yeast origin in vivo, including Orc6 and Dbf4 proteins (Li and Herskowitz 1993; Dowell et al. 1994). Briefly, the reporter gene lacZ is fused downstream of a protein binding site in the promoter region. Hybrid proteins containing transcriptional activation domains (AD) that recognize this site, either directly or indirectly, can activate transcription of the reporter gene. In this case, the yeast ARS1 origin of replication was fused in front of the lacZ reporter (Dowell et al. 1994) and integrated in single copy at the URA3 locus. Because overexpression of RAD53-AD from the constitutive ADH promoter dramatically inhibits cell growth in RAD53+ strains (Sun et al. 1996), these experiments were carried out in rad53Δ reporter strains PDY477, PDY478, and PDY480 (Table 1). Several reporters were tested with one or two copies of ARS1 in different orientations (for example, 1xF.ARS1UAS indicates one copy of ARS1 in the forward orientation) (Figure 3A). Only the RAD53-AD fusion activated transcription two- to fourfold above background in three different ARS1-lacZ reporter strains (1xF, 1xR, and 2xR). A RAD53 construct lacking the activation domain (pPD361; Table 1) failed to activate transcription, similar to the vector alone control. Only background activity was also detected with the RAD53-AD fusion in a control strain PDY449 (Table 1) with only the lacZ vector (data not shown). We conclude that Rad53, like Orc6 and Dbf4, can be targeted to the ARS1 origin of replication.
Figure 3.—
Rad53 is targeted to origin of replication ARS1. (A and B) Quantitative liquid β-galactosidase assays. The indicated activation domain plasmids GAL4-AD (pGAD-2F), RAD53-AD (pPD233), and RAD53 (pPD361 control without AD) were transformed into reporter strains PDY477 and PDY478 containing indicated versions of the integrated ARS1-lacZ reporter (Table 1). 1xF.ARS1UAS indicates one copy of ARS1 in the forward orientation (materials and methods), while 1xR.ARS1UAS and 2xR.ARS1UAS indicate one or two copies of ARS1 in forward or reverse orientation, respectively. Assays shown are mean values from three independent colonies.
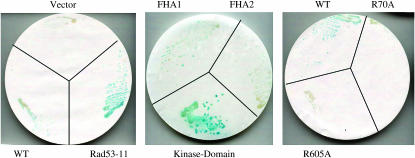
We determined the domain of Rad53 that binds ARS1-lacZ by using plasmids with different fragments of Rad53 fused to the B42 transcriptional AD (Duncker et al. 2002), using reporter strain RSY1064 (Table 1). All these plasmids produce Rad53-AD fusion proteins in similar amounts (Duncker et al. 2002). We tested full-length RAD53+, kinase domain (G177-N599), and FHA1 (I4-S165) and FHA2 (M497-S821) domain fusions as well as the FHA1 (R70A) and FHA2 mutants (R605A) (Figure 4). We used the more sensitive X-Gal plate assay. The strongest signal was obtained with the Rad53 kinase domain fusion, while reduced signal was seen with the FHA1 domain and the full-length RAD53+ fusion. The FHA1 signal was ablated by the R70A mutation, which destroys binding to Dbf4 protein (Duncker et al. 2002).
Figure 4.—
Rad53 kinase domain binds ARS1. Strain PDY480 with an ARS-lacZ reporter containing different Rad53-AD fusions was assayed for β-galactosidase activity using the X-Gal filter assay. Plates were grown for 3 days and filters were developed for similar times. Fusions used were full-length wild-type Rad53 protein (821 residues), full-length Rad53-11 mutant (G653E), kinase domain (G177-N599), FHA1 (I4-S165), FHA2 (M497-S821), and mutant FHA1-R70A and mutant FHA2-R605A domains.
We also tested the rad53-11 mutation, which is also known as mec2-1, as it is checkpoint defective (Weinert et al. 1994), has undetectable kinase activity in vivo (Sun et al. 1996) and in vitro (Sidorova and Breeden 2003), is synthetically lethal with cdc7, and suppresses mcm5-bob1 (Tables 1 and 2). We cloned and sequenced this allele (materials and methods). A point mutation at position 1958 in the coding region results in a single-amino-acid change, G653E. This Rad53-11 fusion protein is still able to bind ARS1 (Figure 4). One possibility is that the Rad53-11 mutant protein can still bind the origin but is unable to phosphorylate an important substrate or that kinase activity is not important for origin binding. We conclude that Rad53 binds the ARS1 origin mainly through its kinase domain. Because our reporter strains are rad53Δ (Table 1), ARS1 binding by the Rad53 kinase domain fusion is checkpoint independent.
The Histone H3/H4 deletion suppresses rad53 effects on cdc7 and mcm5-bob1:
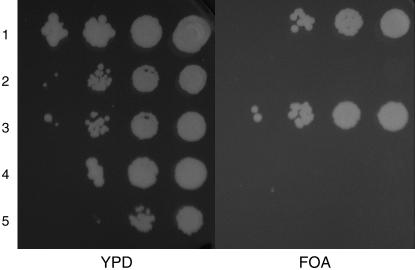
Partial suppression of the rad53Δ phenotype by deletion of the major copy of the histone H3/H4 gene pair, HHT2-HHF2, has been shown (Gunjan and Verreault 2003). The suppression results in increased resistance to low levels of HU (3 mm), an increased growth rate, and a reduction in chromosome loss rate of rad53Δ sml1Δ strains. These effects are similar to the effects of rad53 mutations on cdc7 viability and mcm5-bob1 suppression described herein in that they are both dependent on Rad53 kinase activity but are independent of mec1, mrc1, mec1 tel1, and sml1 null mutations and hence checkpoint function. Furthermore, neither deletion of HHT2-HHF2 (Gunjan and Verreault 2003) nor the mcm5-bob1 mutation (Dohrmann et al. 1999) can suppress the lethality of a rad53Δ. Therefore, a hht2-hhf2Δ∷HIS3 deletion (Mann and Grunstein 1992) was produced by transformation of both cdc7 sml1Δ rad53Δ (pRAD53 URA3) strain RSY1142 and cdc7 mcm5-bob1 sml1Δ rad53Δ (pRAD53 URA3) strain RSY1145 to His+. These strains cannot lose the complementing RAD53 plasmid because of the cdc7-rad53 synthetic lethality (Tables 2 and 3). If suppression of rad53Δ occurs then both strains will be able to lose the pRAD53 URA3 plasmid and become resistant to 5-FOA, that is, become Ura−. This is indeed the case (Figure 5; Tables 1 and 2). In addition, the resultant cdc7 mcm5-bob1 rad53Δ hht2-hhf2Δ∷HIS3 strain RSY1158 also becomes Tsm+, indicating that mcm5-bob1 suppression of cdc7 also is rescued by the H3/H4 deletion (Table 2). Thus, deletion of the major histone H3/H4 gene pair suppresses the effect of rad53Δ on cdc7 mutant viability and mcm5-bob1 suppression. The effect is specific to rad53 suppression as hht2-hhf2Δ∷HIS3 does not suppress the effect of clb5Δ on mcm5-bob1 in strain RSY1161 (Table 2) (Sclafani et al. 2002).
Figure 5.—
Deletion of histone H3/H4 gene pair 2 suppresses the cdc7-rad53 synthetic lethality. Strain RSY1142 cdc7 rad53 sml1 his3 ura3 (pRAD53 URA3) (rows 2 and 5) or RSY1145 cdc7 rad53 sml1 mcm5-bob1 his3 ura3 (pRAD53 URA3) (row 4) cells were transformed with a hht2-hhf2Δ∷HIS3 PCR fragment to His+ to produce strains RSY1157 (row 3) and RSY1158 (row 1), respectively. Colonies were diluted serially in 10-fold increments and spotted on either YPD or 5-FOA plates. Only cells with the hht2-hhf2Δ∷HIS3 deletion (rows 1 and 3) can lose the pRAD53 URA3 plasmid and grow on the 5-FOA plates after incubation at 22° for 3 days.
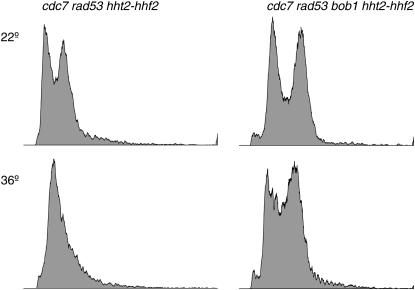
To further analyze the effect of the H3/H4 deletion, cdc7ts rad53Δ hht2-hhf2Δ∷HIS3 strain RSY1157 and cdc7ts mcm5-bob1 rad53Δ hht2-hhf2Δ∷HIS3 strain RSY1158 were grown at both restrictive (36°) and permissive (22°) temperatures (Figure 6). The DNA content was analyzed by flow cytometry (Ostroff and Sclafani 1995). As expected for loss of DDK function (Ostroff and Sclafani 1995; Sclafani 2000), the cdc7ts rad53Δ hht2-hhf2Δ∷HIS3 strain arrested at the G1/S boundary with a G1 content of DNA while the cdc7ts mcm5-bob1 rad53Δ hht2-hhf2Δ∷HIS3 strain continued to cycle, yielding both S and G2/M peaks. However, these cdc7ts mcm5-bob1 cells progress into S-phase slower at the restrictive temperature because mcm5-bob1 bypass of cdc7 is inefficient (Weinreich and Stillman 1999; Pessoa-Brandao and Sclafani 2004). Because the cells have the expected phenotypes, the hht2-hhf2Δ∷HIS3 histone deletion suppresses all the observed effects of rad53Δ on cdc7 viability and mcm5-bob1 bypass.
Figure 6.—
Flow cytometry of the DNA content of cdc7 rad53 mutant cells suppressed by histone H3/H4 deletion. Strains RSY1157 cdc7ts rad53Δ hht2-hhf2Δ∷HIS3 and RSY1158 cdc7ts mcm5-bob1 rad53Δ hht2-hhf2Δ∷HIS3 were grown at both restrictive (36°) and permissive (22°) temperatures in YPD medium for 4 hr and then processed for flow cytometry (Ostroff and Sclafani 1995). y-axis is cell number and x-axis is DNA content.
DISCUSSION
In this report, we provide both genetic and molecular evidence that Rad53 kinase and DDK interact to regulate the initiation of DNA replication independently of Rad53's role in the DNA damage or replication checkpoints (Tables 2–4). In contrast, DDK has little if any role in the DNA checkpoint as Rad53 kinase can be activated by HU in the absence of DDK (Figure 1) and both checkpoints remain intact in cdc7Δ mcm5-bob1 strains (Weinreich and Stillman 1999; Pessoa-Brandao and Sclafani 2004). We have demonstrated that Rad53 kinase activity is required for the viability of cdc7 mutants and for mcm5-bob1 to bypass the function of DDK (Table 3). Moreover, cells lacking Rad53 exhibit minichromosome loss defects that are suppressible by increased origin dosage (Figure 2), similar to known initiation mutants (Hogan and Koshland 1992; Foss et al. 1993; Loo et al. 1995; Hardy 1996). Finally, through one-hybrid experiments, we have demonstrated that Rad53 can be targeted to the ARS1 origin of DNA replication, mainly through its kinase domain (Figure 3 and 4).
We propose that Rad53 can bind to an origin independently of Dbf4 protein. The Rad53 kinase domain, which does not bind Dbf4 protein (Duncker et al. 2002), is the most effective at binding ARS1 (Figure 4). Rad53 kinase activity is needed for both cdc7 viability and mcm5-bob1 bypass (Table 4). In contrast, mutations in both FHA1and FHA2 domains of Rad53 have no effect (Table 3). These double FHA1 and FHA2 mutants have been shown to abolish Rad53 activation either by DNA replication inhibitors or by DNA-damaging agents and to ablate downstream checkpoint functions (Pike et al. 2003; Schwartz et al. 2003). Yet they have no effect on cdc7 mutant viability or mcm5-bob1 suppression (Table 3). Similarly, mutations in genes required for all aspects of the DNA checkpoint also have no effect (Table 4). These results demonstrate that neither the DNA damage nor the replication checkpoint response is required for Rad53's function in this context.
We can also rule out the hypothesis that in cdc7 and cdc7Δ mcm5-bob1 mutants replication is defective in some manner leading to stalled replication forks or DNA damage and that the DNA checkpoint “saves” these cells either by stabilizing the stalled forks or by preventing the cells from entering mitosis before S-phase is complete. Even mec1 tel1 double mutations, which eliminate both DNA damage and replication checkpoints (Nyberg et al. 2002) have no effect. Previous studies also showed that cdc7-1 mec1Δ double mutants were viable (Desany et al. 1998).
Furthermore, there are not enough damage or stalled replication forks in the cdc7Δ mcm5-bob1 mutant to constitutively activate Rad53 (Figure 1) (Pessoa-Brandao and Sclafani 2004). In fact, the cdc7Δ dbf4Δ mcm5-bob1mutant is sensitive to DNA damage because of a lack of translesion synthesis from the Rev3 (DNA pol ζ) pathway and not because of a defective DNA damage checkpoint (Pessoa-Brandao and Sclafani 2004). Therefore, DDK is not an important target of checkpoint control in S. cerevisiae, although it could be in other eukaryotes including fission yeast (Takeda et al. 1999), Xenopus (Costanzo et al. 2003), and humans (Dierov et al. 2004). However, recent results using Xenopus extracts have shown that DDK is not important in DNA damage checkpoint control (Petersen et al. 2006).
We propose that Rad53 through its kinase activity helps to stabilize replication complexes at origins during initiation. In the absence of Rad53, more DDK activity is needed for initiation, which can explain the cdc7-rad53 synthetic lethality. Furthermore, Mcm5-bob1 protein, which may act by pushing out the A domain of Mcm5 to attract Cdc45 protein (Sclafani et al. 2002, 2004; Fletcher et al. 2003; Chen et al. 2005), is inefficient and may fail when the initiation complex is compromised by a lack of Rad53 function. This hypothesis is similar to one we proposed to explain the suppression of mcm5-bob1 by loss of Cdk1-Clb5 kinase (Sclafani et al. 2002), a known regulator of initiation (Zou and Stillman 1998). In this scenario, reduced initiation at ARS1 occurs on the minichromosome (Figure 2) because initiation complexes are unstable or infrequently activated. By increasing the number of origins, “mass action” results in the activation of at least one active origin, thereby suppressing the defect.
Because rad53Δ sml1Δ strains grow slower than mec1Δ sml1Δ strains (Zhao et al. 2001; Gunjan and Verreault 2003) and histone suppression occurs only for rad53Δ sml1Δ strains (Gunjan and Verreault 2003), there must be a mec1-independent function of Rad53 that is important for cell cycle progression and does not involve the checkpoint function (Zhao et al. 2001; Gunjan and Verreault 2003). A Rad53-dependent surveillance mechanism that balances the rate of DNA replication and histone synthesis during S-phase and in response to DNA damage has been proposed to explain these effects (Gunjan and Verreault 2003). Rad53 may be involved in the degradation of excess soluble histones as these accumulate in rad53Δ sml1Δ strains and a reduction of H3/H4 levels with hht2-hhf2Δ mutation partially suppresses some rad53 mutant phenotypes. We find that the hht2-hhf2Δ mutation also suppresses the effect of rad53Δ on both cdc7 mutant viability and mcm5-bob1 suppression (Figures 5 and 6). These excess histones may titrate important replication factors such as Cdc45 or Sld3 (Diffley 2004) and thereby reduce the efficiency of DDK and hence Mcm-helicase function. Thus, loss of Rad53 could reduce DDK and mcm5-bob1 efficiency, both of which affect Cdc45 function (Zou and Stillman 2000; Sclafani et al. 2002). Rad53 may function to remove these inhibitory histones and increase the efficiency of initiation. Therefore, Rad53 kinase may maintain a balance of histones at origins of DNA replication and at replication forks. However, overexpression of Cdc45 or Sld3 has no effect (data not shown).
Alternatively, Rad53 may also affect chromatin structure (Pan et al. 2006) at the origin and reduce the efficiency of binding of a number of replication proteins. Probably, histone chaperones (Kaufman et al. 1997; Tyler et al. 1999; Sharp et al. 2001) are not involved in this process as cac1 and asf1 also have no effect (Table 4). Furthermore, a reduction in histone expression by hht2-hhf2Δ is not phenocopied by a reduction in histone chaperones as cac1Δ cannot suppress the effect of rad53Δ on both cdc7 mutant viability and mcm5-bob1 suppression (data not shown).
In summary, our results support the hypothesis that Rad53 has an important role in the cell cycle that is independent of its checkpoint function, which has been proposed by a number of others (Zhao et al. 2001; Gunjan and Verreault 2003; Pan et al. 2006). Our results show that this role is most likely the regulation of the initiation of DNA replication because of genetic interactions of Rad53 protein kinase with DDK (Cdc7-Dbf4 kinase) and the MCM helicase, both of which act in replication. Further support for the hypothesis is provided by demonstration of origin binding by Rad53 (Figures 3 and 4) and elevated minichromosome loss in rad53 mutants (Figure 2).
Acknowledgments
We thank Walt Fangman, John Diffley, Chris Hardy, Joachim Li, Bernie Duncker, David Stern, Ted Weinert, Yolanda Sanchez, Lew Pizer, and Jessica Tyler for plasmids and/or strains and Marianne Tecklenburg for superb technical assistance. We also thank Judith Jaehning, Paul Megee, and Mingxia Huang for critically reading the manuscript. The DNA samples were sequenced by the University of Colorado Cancer Center DNA Sequencing and Analysis Core Facility, which is supported by the National Institutes of Health/National Cancer Institute cancer core support grant (CA46934). This work was supported by U.S. Public Health Service grant GM35078 awarded to R.A.S.
References
- Alcasabas, A. A., A. J. Osborn, J. Bachant, F. Hu, P. J. Werler et al., 2001. a Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 3: 958–965. [DOI] [PubMed] [Google Scholar]
- Alcasabas, A. A., A. J. Osborn, J. Bachant, F. Hu, P. J. Werler et al., 2001. b Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 3: 958–965. [DOI] [PubMed] [Google Scholar]
- Aparicio, O. M., A. M. Stout and S. P. Bell, 1999. Differential assembly of Cdc45p and DNA polymerases at early and late origins of DNA replication. Proc. Natl. Acad. Sci. USA 96: 9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, S. P., and A. Dutta, 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71: 333–374. [DOI] [PubMed] [Google Scholar]
- Burke, D., D. Dawson and T. Stearns, 2000. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Cha, R. S., and N. Kleckner, 2002. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297: 602–606. [DOI] [PubMed] [Google Scholar]
- Chabes, A., B. Georgieva, V. Domkin, X. Zhao, R. Rothstein et al., 2003. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112: 391–401. [DOI] [PubMed] [Google Scholar]
- Chen, Y. J., X. Yu, R. Kasiviswanathan, J. H. Shin, Z. Kelman et al., 2005. Structural polymorphism of Methanothermobacter thermautotrophicus MCM. J. Mol. Biol. 346: 389–394. [DOI] [PubMed] [Google Scholar]
- Chien, C. T., P. L. Bartel, R. Sternglanz and S. Fields, 1991. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein or interest. Proc. Natl. Acad. Sci. USA 88: 9578–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero and P. Hieter, 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. [DOI] [PubMed] [Google Scholar]
- Costanzo, V., D. Shechter, P. J. Lupardus, K. A. Cimprich, M. Gottesman et al., 2003. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell 11: 203–213. [DOI] [PubMed] [Google Scholar]
- Desany, B. A., A. A. Alcasabas, J. B. Bachant and S. J. Elledge, 1998. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 12: 2956–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierov, J., R. Dierova and M. Carroll, 2004. BCR/ABL translocates to the nucleus and disrupts an ATR-dependent intra-S phase checkpoint. Cancer Cell 5: 275–285. [DOI] [PubMed] [Google Scholar]
- Diffley, J. F., 2004. Regulation of early events in chromosome replication. Curr. Biol. 14: R778–R786. [DOI] [PubMed] [Google Scholar]
- Dohrmann, P. R., G. Oshiro, M. Tecklenburg and R. A. Sclafani, 1999. RAD53 regulates DBF4 independently of checkpoint function in Saccharomyces cerevisiae. Genetics 151: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell, S. J., P. Romanowski and J. F. Diffley, 1994. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science 265: 1243–1246. [DOI] [PubMed] [Google Scholar]
- Duncker, B. P., K. Shimada, M. Tsai-Pflugfelder, P. Pasero and S. M. Gasser, 2002. An N-terminal domain of Dbf4p mediates interaction with both origin recognition complex (ORC) and Rad53p and can deregulate late origin firing. Proc. Natl. Acad. Sci. USA 99: 16087–16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, B. M., B. J. Brewer, A. E. Reynolds and W. L. Fangman, 1991. A yeast origin of replication is activated late in S phase. Cell 65: 507–515. [DOI] [PubMed] [Google Scholar]
- Fletcher, R. J., B. E. Bishop, R. P. Leon, R. A. Sclafani, C. M. Ogata et al., 2003. The structure and function of MCM from archaeal M. thermoautotrophicum. Nat. Struct. Biol. 10: 160–167. [DOI] [PubMed] [Google Scholar]
- Foiani, M., A. Pellicioli, M. Lopes, C. Lucca, M. Ferrari et al., 2000. DNA damage checkpoints and DNA replication controls in Saccharomyces cerevisiae. Mutat. Res. 451: 187–196. [DOI] [PubMed] [Google Scholar]
- Foss, M., F. J. McNally, P. Laurenson and J. Rine, 1993. Origin recognition complex (ORC) in transcriptional silencing and DNA replication in S. cerevisiae. Science 262: 1838–1844. [DOI] [PubMed] [Google Scholar]
- Friedman, K. L., J. D. Diller, B. M. Ferguson, S. V. Nyland, B. J. Brewer et al., 1996. Multiple determinants controlling activation of yeast replication origins late in S phase. Genes Dev. 10: 1595–1607. [DOI] [PubMed] [Google Scholar]
- Guarente, L., and T. Mason, 1983. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell 32: 1279–1286. [DOI] [PubMed] [Google Scholar]
- Gunjan, A., and A. Verreault, 2003. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell 115: 537–549. [DOI] [PubMed] [Google Scholar]
- Hardy, C. F., 1996. Characterization of an essential Orc2p-associated factor that plays a role in DNA replication. Mol. Cell. Biol. 16: 1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, C. F., O. Dryga, S. Seematter, P. M. Pahl and R. A. Sclafani, 1997. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc. Natl. Acad. Sci. USA 94: 3151–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell, L. H., 1973. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J. Bacteriol. 115: 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, E., and D. Koshland, 1992. Addition of extra origins of replication to a minichromosome suppresses its mitotic loss in cdc6 and cdc14 mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89: 3098–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issel-Tarver, L., K. R. Christie, K. Dolinski, R. Andrada, R. Balakrishnan et al., 2002. Saccharomyces Genome Database. Methods Enzymol. 350: 329–346. [DOI] [PubMed] [Google Scholar]
- Jackson, A. L., P. M. Pahl, K. Harrison, J. Rosamond and R. A. Sclafani, 1993. Cell cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol. Cell. Biol. 13: 2899–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jares, P., A. Donaldson and J. J. Blow, 2000. The Cdc7/Dbf4 protein kinase: Target of the S phase checkpoint? EMBO Rep. 1: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan, M. B., and J. Bartek, 2004. Cell-cycle checkpoints and cancer. Nature 432: 316–323. [DOI] [PubMed] [Google Scholar]
- Katou, Y., Y. Kanoh, M. Bando, H. Noguchi, H. Tanaka et al., 2003. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424: 1078–1083. [DOI] [PubMed] [Google Scholar]
- Kaufman, P. D., R. Kobayashi and B. Stillman, 1997. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 11: 345–357. [DOI] [PubMed] [Google Scholar]
- Kihara, M., W. Nakai, S. Asano, A. Suzuki, K. Kitada et al., 2000. Characterization of the yeast Cdc7p/Dbf4p complex purified from insect cells. Its protein kinase activity is regulated by Rad53p. J. Biol. Chem. 275: 35051–35062. [DOI] [PubMed] [Google Scholar]
- Labib, K., and J. F. Diffley, 2001. Is the MCM2–7 complex the eukaryotic DNA replication fork helicase? Curr. Opin. Genet. Dev. 11: 64–70. [DOI] [PubMed] [Google Scholar]
- Lei, M., and B. K. Tye, 2001. Initiating DNA synthesis: from recruiting to activating the MCM complex. J. Cell Sci. 114: 1447–1454. [DOI] [PubMed] [Google Scholar]
- Lei, M., Y. Kawasaki, M. R. Young, M. Kihara, A. Sugino et al., 1997. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev. 11: 3365–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengronne, A., and E. Schwob, 2002. The yeast CDK inhibitor Sic1 prevents genomic instability by promoting replication origin licensing in late G(1). Mol. Cell 9: 1067–1078. [DOI] [PubMed] [Google Scholar]
- Li, J. J., and I. Herskowitz, 1993. Isolation of ORC6, a component of the yeast origin recognition complex by a one-hybrid system. Science 262: 1870–1874. [DOI] [PubMed] [Google Scholar]
- Liu, Q., M. Z. Li, D. Leibham, D. Cortez and S. J. Elledge, 1998. The univector plasmid-fusion system, a method for rapid construction of recombinant DNA without restriction enzymes. Curr. Biol. 8: 1300–1309. [DOI] [PubMed] [Google Scholar]
- Loo, S., C. A. Fox, J. Rine, R. Kobayashi, B. Stillman et al., 1995. The origin recognition complex in silencing, cell cycle progression, and DNA replication. Mol. Biol. Cell 6: 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, M., C. Cotta-Ramusino, A. Pellicioli, G. Liberi, P. Plevani et al., 2001. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412: 557–561. [DOI] [PubMed] [Google Scholar]
- Maine, G. T., P. Sinha and B. K. Tye, 1984. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics 106: 365–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, R. K., and M. Grunstein, 1992. Histone H3 N-terminal mutations allow hyperactivation of the yeast GAL1 gene in vivo. EMBO J. 11: 3297–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, K. A., R. J. Michelson, C. W. Putnam and T. A. Weinert, 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36: 617–656. [DOI] [PubMed] [Google Scholar]
- Oshiro, G., J. C. Owens, Y. Shellman, R. A. Sclafani and J. J. Li, 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol. Cell. Biol. 19: 4888–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff, R. M., and R. A. Sclafani, 1995. Cell cycle regulation of induced mutagenesis in yeast. Mutat. Res. 329: 143–152. [DOI] [PubMed] [Google Scholar]
- Pan, X., P. Ye, D. S. Yuan, X. Wang, J. S. Bader et al., 2006. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124: 1069–1081. [DOI] [PubMed] [Google Scholar]
- Pellicioli, A., S. E. Lee, C. Lucca, M. Foiani and J. E. Haber, 2001. Regulation of Saccharomyces Rad53 checkpoint kinase during adaptation from DNA damage-induced G2/M arrest. Mol. Cell 7: 293–300. [DOI] [PubMed] [Google Scholar]
- Pessoa-Brandao, L., and R. A. Sclafani, 2004. CDC7/DBF4 functions in the translesion synthesis branch of the RAD6 epistasis group in Saccharomyces cerevisiae. Genetics 167: 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, P., D. M. Chou, Z. You, T. Hunter, J. C. Walter et al., 2006. Protein phosphatase 2A antagonizes ATM and ATR in a Cdk2- and Cdc7-independent DNA damage checkpoint. Mol. Cell. Biol. 26: 1997–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, B. L., S. Yongkiettrakul, M. D. Tsai and J. Heierhorst, 2003. Diverse but overlapping functions of the two forkhead-associated (FHA) domains in Rad53 checkpoint kinase activation. J. Biol. Chem. 278: 30421–30424. [DOI] [PubMed] [Google Scholar]
- Quivy, J. P., and G. Almouzni, 2003. Rad53: a controller ensuring the fine-tuning of histone levels. Cell 115: 508–510. [DOI] [PubMed] [Google Scholar]
- Sadowski, I., B. Bell, P. Broad and M. Hollis, 1992. GAL4 fusion vectors for expression in yeast or mammalian cells. Gene 118: 137–141. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., B. A. Desany, W. J. Jones, Q. Liu, B. Wang et al., 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271: 357–360. [DOI] [PubMed] [Google Scholar]
- Santocanale, C., and J. F. Diffley, 1998. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395: 615–618. [DOI] [PubMed] [Google Scholar]
- Santocanale, C., K. Sharma and J. F. Diffley, 1999. Activation of dormant origins of DNA replication in budding yeast. Genes Dev. 13: 2360–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. F., S. J. Lee, J. K. Duong, S. Eminaga and D. F. Stern, 2003. FHA domain-mediated DNA checkpoint regulation of Rad53. Cell Cycle 2: 384–396. [PubMed] [Google Scholar]
- Sclafani, R. A., 2000. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 113(12): 2111–2117. [DOI] [PubMed] [Google Scholar]
- Sclafani, R. A., M. Tecklenburg and A. Pierce, 2002. The mcm5-bob1 bypass of Cdc7p/Dbf4p in DNA replication depends on both Cdk1-independent and Cdk1-dependent steps in Saccharomyces cerevisiae. Genetics 161: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani, R. A., R. J. Fletcher and X. S. Chen, 2004. Two heads are better than one: regulation of DNA replication by hexameric helicases. Genes Dev. 18: 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, J. A., E. T. Fouts, D. C. Krawitz and P. D. Kaufman, 2001. Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol. 11: 463–473. [DOI] [PubMed] [Google Scholar]
- Shellman, Y. G., I. E. Schauer, G. Oshiro, P. Dohrmann and R. A. Sclafani, 1998. Oligomers of the Cdc7/Dbf4 protein kinase exist in the yeast cell. Mol. Gen. Genet. 259: 429–436. [DOI] [PubMed] [Google Scholar]
- Sherman, F., G. R. Fink and J. B. Hicks, 1986. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Shirahige, K., Y. Hori, K. Shiraishi, M. Yamashita, K. Takahashi et al., 1998. Regulation of DNA-replication origins during cell-cycle progression. Nature 395: 618–621. [DOI] [PubMed] [Google Scholar]
- Sidorova, J. M., and L. L. Breeden, 2002. Precocious S-phase entry in budding yeast prolongs replicative state and increases dependence upon Rad53 for viability. Genetics 160: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova, J. M., and L. L. Breeden, 2003. Rad53 checkpoint kinase phosphorylation site preference identified in the Swi6 protein of Saccharomyces cerevisiae. Mol. Cell. Biol. 23: 3405–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo, J. M., M. Lopes and M. Foiani, 2002. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297: 599–602. [DOI] [PubMed] [Google Scholar]
- Sun, Z., D. S. Fay, F. Marini, M. Foiani and D. F. Stern, 1996. Spk1/Rad53 is regulated by Mec1-dependent protein phosphorylation in DNA replication and damage checkpoint pathways. Genes Dev. 10: 395–406. [DOI] [PubMed] [Google Scholar]
- Sun, Z., J. Hsiao, D. S. Fay and D. F. Stern, 1998. a Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science 281: 272–274. [DOI] [PubMed] [Google Scholar]
- Sun, Z., J. Hsiao, D. S. Fay and D. F. Stern, 1998. b Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science 281: 272–274. [DOI] [PubMed] [Google Scholar]
- Takeda, T., K. Ogino, E. Matsui, M. K. Cho, H. Kumagai et al., 1999. A fission yeast gene, him1(+)/dfp1(+), encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage. Mol. Cell. Biol. 19: 5535–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercero, J. A., and J. F. Diffley, 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412: 553–557. [DOI] [PubMed] [Google Scholar]
- Tyler, J. K., C. R. Adams, S. R. Chen, R. Kobayashi, R. T. Kamakaka et al., 1999. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402: 555–560. [DOI] [PubMed] [Google Scholar]
- Weinert, T. A., G. L. Kiser and L. H. Hartwell, 1994. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 8: 652–665. [DOI] [PubMed] [Google Scholar]
- Weinreich, M., and B. Stillman, 1999. Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 18: 5334–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., and R. Rothstein, 2002. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc. Natl. Acad. Sci. USA 99: 3746–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., E. G. Muller and R. Rothstein, 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2: 329–340. [DOI] [PubMed] [Google Scholar]
- Zhao, X., B. Georgieva, A. Chabes, V. Domkin, J. H. Ippel et al., 2000. Mutational and structural analyses of the ribonucleotide reductase inhibitor Sml1 define its Rnr1 interaction domain whose inactivation allows suppression of mec1 and rad53 lethality. Mol. Cell. Biol. 20: 9076–9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., A. Chabes, V. Domkin, L. Thelander and R. Rothstein, 2001. The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 20: 3544–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L., and B. Stillman, 1998. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science 280: 593–596. [DOI] [PubMed] [Google Scholar]
- Zou, L., and B. Stillman, 2000. Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol. Cell. Biol. 20: 3086–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]