Abstract
In angiosperms, double fertilization of an egg cell and a central cell with two sperm cells results in the formation of a seed containing a diploid embryo and a triploid endosperm. The extent to which the embryo sac controls postfertilization events in the seed is unknown. The novel gametophytic maternal-effect maize mutation, baseless1 (bsl1) affects central cell development within the embryo sac, frequently by altering the position of the two polar nuclei. Despite this irregularity, fertilization is as efficient as in wild type. The spatial expression of basal endosperm-specific transcripts is altered in free-nuclear and cellular mutant endosperms. At later stages of seed development, bsl1 predominantly affects development of the basal endosperm transfer layer (BETL). When bsl1/+ diploid plants were pollinated by wild-type tetraploid plants, the BETL abnormalities observed in bsl1/bsl1/+/+ tetraploid endosperms were diverse and of variable severity. Moreover, the frequency of kernels with severely perturbed BETL development correlated with the percentage of severely affected bsl1 central cells. Therefore, BSL1 is likely required in the central cell before fertilization for correct BETL patterning to occur. These findings provide new genetic evidence that a maternal gametophytic component is necessary for correct endosperm patterning.
MOST flowering plants are sexually dimorphic, in that they possess both male and female reproductive organs. The male gametophyte (pollen) typically contains a vegetative nucleus, which contributes to pollen tube growth, and two male gametes (sperm cells) that participate in fertilization. The female gametophyte is usually of the Polygonum type, consisting of two synergid cells, some antipodal cells, and two female gametes—the egg cell and the larger central cell, which contains two polar nuclei (Drews and Yadegari 2002; Yadegari and Drews 2004).
The process of double fertilization is unique to the flowering plants and results in formation of the seed. For this to occur, the pollen tube must first grow toward the ovule, where it is guided to the micropyle and enters the female gametophyte through one of the two synergids. Recent studies have shown that pollen tube growth and guidance are largely controlled by the female gametophyte and in particular by the synergids (Higashiyama et al. 2001; Huck et al. 2003; Rotman et al. 2003; Marton et al. 2005). Under wild-type conditions, the pollen tube penetrates the female gametophyte through a degenerated synergid, after which the pollen tube tip ruptures, releasing the two sperm cells. The sperm cells subsequently fuse individually with the egg and central cell to typically form a diploid (1 maternal:1 paternal) embryo and a triploid (2 maternal:1 paternal) endosperm, respectively. Although both seed components are typically identical genetically, their developmental fates diverge significantly. While the life cycle of the embryo is extended to the next generation—where it forms the mature plant—the endosperm life cycle is confined to the seed stage, where it provides nutritional and structural support to the growing embryo (Walbot and Evans 2003; Costa et al. 2004).
In many flowering plants, the endosperm undergoes a nuclear-type mode of development (Olsen 2004). Strikingly similar to early Drosophila embryogenesis, the fertilized proendosperm triploid nucleus divides through a series of mitoses that are uncoupled to cell wall formation, to form a free-nuclear structure or syncytium with a single layer of cortical nuclei suspended in the peripheral cytoplasm (Olsen 2001). Following syncytial development, cellularization occurs and various cell types eventually differentiate within the fully cellular endosperm (Costa et al. 2004; Olsen 2004).
To date, relatively little is known about the mechanisms regulating early development of the endosperm. On the basis of current genetic evidence, endosperm patterning occurs in two main phases (Costa et al. 2004). The first, pivotal phase leads to the establishment of the proximal–distal axis, which is reflected by the polarized localization of transcripts (Doan et al. 1996; Gomez et al. 2002; Drea et al. 2005; Ingouff et al. 2005a), and, in Arabidopsis, the cytological organization of syncytial endosperm domains (Boisnard-Lorig et al. 2001; Brown et al. 2003). These early events are most likely regulated by several maternally required genes (for example, Springer et al. 2000; Grini et al. 2002; Holding and Springer 2002), including members of the Polycomb Group complex (Pc-G). The identification and characterization of the Arabidopsis Pc-G medea (mea) mutant first demonstrated the principle of gametophytic maternal effects on seed development in plants (Grossniklaus et al. 1998). Mutations in MEA and in two other Arabidopsis Pc-G genes, Fertilization Independent Endosperm (FIE) (Ohad et al. 1996) and Fertilization Independent Seed2 (FIS2) (Chaudhury et al. 1997), cause irregular nuclear proliferation in the unfertilized central cell as well as a delay in the developmental progression of the fertilized endosperm (Grossniklaus et al. 1998; Ingouff et al. 2005b). As a consequence of the latter, ectopic chalazal (posterior) endosperm-specific gene expression becomes manifest in inappropriately anterior positions (Sørensen et al. 2001; Guitton et al. 2004). Similarly, development of the chalazal endosperm is perturbed in two other Arabidopsis Pc-G mutants, borgia and medicis/multicopy suppressor of IRA1 (Guitton et al. 2004). Parallel studies have demonstrated that alterations in the 2 maternal:1 paternal genomic balance in the endosperm also strongly influence early development of the Arabidopsis chalazal endosperm and the maize basal endosperm transfer layer (BETL). In both species, development of these domains is more dramatically perturbed in 2 maternal (m):2 paternal (p) endosperms derived from crosses between diploid females and tetraploid males (reviewed in Costa et al. 2004).
Here we report the comprehensive characterization of baseless1 (bsl1)—a novel gametophytic maternal-effect mutant in maize. Before fertilization, bsl1 mutants displayed defects in the central cell within the mature female gametophyte. The two polar nuclei were frequently displaced in bsl1 central cells, yet, despite this irregularity fertilization was achieved, suggesting that sperm were able to efficiently locate and fuse with the displaced polar nuclei. Resulting mutant seeds displayed irregular distributions of transcripts specific to the basal region of syncytial and cellular endosperms. Mutant cellular endosperms also exhibited aberrant BETL development. BETL abnormalities were dramatically enhanced in seeds resulting from crosses between bsl1/+ females and wild-type tetraploid males. Our analysis of bsl1 mutants provides new genetic evidence in plants that essential endosperm patterning component(s) are present in the central cell before fertilization.
MATERIALS AND METHODS
Plant material and growth conditions:
The baseless1 mutant was originally isolated from a self-pollination of a W22 inbred maize (Zea mays) plant carrying a novel mutable allele, r1-m-Bolivia, of the R1 locus and was given the provisional designation dex-4299* (Kermicle 1978). The mutant was typically propagated as heterozygous by transmission through the female and selection for viable bsl1 defective kernels. Plants were grown in summer field conditions or in greenhouses under 16 hr light:8 hr dark cycles. bsl1 heterozygous (bsl1/+) and wild-type (+/+) plants used for controls were grown under the same conditions for each experiment. For characterization of the effects of bsl1 on seed development, bsl1/+ plants were pollinated with wild-type pollen. Segregating wild-type and mutant siblings were then taken from the same middle-third portion of the ear, as standard. With the exception of the mapping, all characterizations were performed in a standard W22 inbred background. Male transmission (Mt) of bsl1 was calculated on the basis of both linkage to and transmission of a linked genetic marker. Mtlinked marker equals the transmission of nonrecombinant bsl1 pollen grains and recombinant Bsl1+ pollen grains. This was calculated as Mtbsl1 times the frequency of nonrecombinants (1 − R) between bsl1 and the marker plus the frequency of transmission of Bsl1+ (1 − Mtbsl1) times the frequency of recombinants between bsl1 and the marker (R). Solving for Mtbsl1 gives Mtbsl1 = (Mtlinked marker − R)/(1.0 − 2R), where R equals the recombination frequency between bsl1 and the linked marker, and Mtlinked marker equals the transmission of the allele linked to bsl1.
Mapping of bsl1 was performed in crosses between bsl1/+, W22/W23 hybrid females and W23 wild-type males. Only bsl1 defective kernels were used for the mapping population, as incomplete penetrance causes some normal kernels to carry bsl1, necessitating progeny testing to ensure that they were wild type. DNA was extracted from seedlings from these defective kernels and maize simple sequence repeat (SSR) markers were analyzed as described previously (Evans and Kermicle 2001).
Pollen tube growth analysis:
Pollen was analyzed from five bsl1/+ heterozygotes and five homozygous wild-type plants. Old anthers were removed from plants the day before pollen collection. The following day, one newly extruded anther was picked from each plant prior to dehiscence, and anthers were quickly dissected to liberate pollen onto germination plates. In vitro pollen tube growth measurements were performed as described previously (Evans and Kermicle 2001).
Histology:
For examination of pollen morphology, pollen grains were collected in the same manner as for pollen tube growth measurements, except that anthers were dissected directly on slides. Pollen was mounted under coverslips in iodide potassium iodine to visualize starch or stained with hematoxylin/ferric ammonium sulfate to visualize nuclei (Kindiger 1994). Pollen grains were analyzed with a Nikon Eclipse E600 microscope.
For analysis of embryo sacs, samples collected from homozygous wild-type and heterozygous bsl1/+ plants were fixed in 5% formaldehyde, 5% acetic acid, 45% ethanol under vacuum for 15 min, followed by overnight fixation in fresh fixing buffer at 4°. Samples were rinsed and stored in 70% ethanol. Ovules and developing kernels were bisected along the longitudinal axis of the ear, dehydrated through a standard ethanol series, and cleared in methyl salicylate. Samples were mounted in methyl salicylate and visualized on a Bio-Rad (Hercules, CA) laser scanning confocal microscope. Excitation was performed at 488 nm and emission was collected at both 522- and 585-nm wavelengths and combined. Image files were opened with NIH Image v. 1.62 and handled in Adobe Photoshop v. 5.0.
For epifluorescence microscopy of early endosperms, kernels were collected from wild-type ears and ears segregating for the bsl1 mutation at 1–3 days after pollination (dap) and then fixed as described by Costa et al. (2003). DNA content of nuclei from 3-dap wild-type and bsl1 endosperms was measured using a Newcastle Photometric Systems photon counting photometer attached to a side port of a Nikon Diaphot TMD inverted microscope. Samples were viewed with a Zeiss 100× oil immersion objective using an ultraviolet filter set with a excitation filter (360 nm, bandpass 10 nm), a dichroic mirror (400 nm), and a barrier filter (420 nm). For each measurement, individual calibration curves were prepared on the basis of a linear regression analysis as the relative fluorescence units (RFU) of DAPI-stained nuclei in proportion to DNA content (Coleman and Goff 1985).
mRNA in situ hybridization:
In situ hybridization was performed on kernels as described previously (Costa et al. 2003). Probes for MRP1 (Gomez et al. 2002) and the maize homolog of barley END1 (Doan et al. 1996) were generated by reverse transcriptase amplification (RT–PCR) with gene-specific oligonucleotides: MRP1.FOR 5′-ATGAATCCCAACTTCAACAGTGTGTG-3′, MRP1.REV 5′-TATCGGTTA TATATCTGGCTCTCC-3′, END1.FOR 5′-ATGAAACGAGAGTGCAAGCAGTTTGAG-3′, and END1.REV 5′-CATACTAAGAGGAACTGTATAACTCC-3′. Slides were viewed with a Zeiss Axiophot microscope under bright field optics and images were digitally recorded with a coolpix Nikon camera.
Transgenic reporter gene analysis:
To analyze BETL-specific reporter gene expression in mutant and wild-type seeds, bsl1/+ plants were crossed as females by males carrying one of two BETL transgenic reporters: ProBet1:GUS (Hueros et al. 1999a) or ProMeg1:GUS (Gutierrez-Marcos et al. 2004). Kernels were cut along the longitudinal axis and stained for GUS activity as previously described (Costa et al. 2003; Gutierrez-Marcos et al. 2004). To analyze the combined effects of bsl1 and parental genomic imbalance in the tetraploid endosperm on BETL-specific reporter gene expression, plants heterozygous for bsl1 and homozygous for ProMeg1:GUS transgene were crossed by wild-type (W22) tetraploid plants.
RESULTS
baseless1 is a novel maternal-effect mutation in maize:
The baseless1 mutant arose spontaneously as a sector on a female inflorescence (ear) of a wild-type W22 inbred maize plant. One side of the ear had 70 normal seeds; the other side of the ear had 40 defective seeds and 58 normal seeds. Fortuitously, the viability of some abnormal seeds allowed recovery and propagation of the mutant when transmitted maternally. bsl1 was initially mapped to chromosome 5 using waxy1 (wx1)-marked reciprocal translocations. This location was confirmed by linkage to the purple aleurone1 (pr1) gene (49/226 = 21.7%; P < 0.0001). Mapping with SSR markers placed bsl1 on the short arm of chromosome 5 in proximity to the centromere—between umc1110 and bnlg603.
After pollinating bsl1/+ plants with wild-type pollen, 45.7 ± 2.6% of the seeds were visibly defective (Figure 1 and Table 1). Progeny testing of phenotypically normal seeds revealed that 8.4 ± 2.3% were also carrying the bsl1 defective allele, so that the percentage of bsl1/+ kernels was always ∼50% (Table 1). Progeny testing of the defective seeds revealed that they almost exclusively (238/241 individuals) segregated defective kernels in the next generation (i.e., due to bsl1 inheritance). Further, the penetrance and severity of the bsl1/+ seed phenotype varied from cross to cross within the same genetic background (Figure 1, B and C), suggesting that the mutant phenotype is most likely influenced by extrinsic environmental factors. Mature mutant seeds fell into different phenotypic classes, including reduced endosperm, loose pericarp, empty pericarp, and germless (Figure 1, D–H). Consequently, the most severely affected seeds failed to germinate (27/98), while those that did displayed root or shoot defects (18/71), including fasciations and/or absence of the root and shoot axes.
Figure 1.—
The bsl1 seed phenotype. (A) Ear from a homozygous wild-type plant pollinated with pollen from a bsl1/+ heterozygous plant, exhibiting normal kernels. (B) Ear from a bsl1/+ heterozygous female pollinated with homozygous wild-type pollen, showing a strong bsl1 maternal effect. Approximately half of the kernels have reduced or aborted endosperms with loose pericarps (arrowhead). (C) Ear from a bsl1/+ heterozygous female pollinated with homozygous wild-type pollen, showing a mild bsl1 maternal effect. Approximately half of the kernels have slightly reduced endosperms (arrowhead). (D–H) Phenotypic classes of kernels produced in test crosses and self-crosses of bsl1/+ plants (germinal face of kernels on the left and median longitudinal sections of kernels on the right). The germinal face of sectioned kernels is oriented to the left for all kernels. (D) Wild type. (E–H) bsl1 phenotypic classes. (E) Reduced endosperm. (F) Germless. (G) Loose pericarp. (H) Empty pericarp.
TABLE 1.
Phenotypic classes of kernel progeny from pollinations with wild-type and bsl1/+ plants
| Type of cross | Defective +/+ and bsl1/+ (%) | Morphologically normal bsl1/+ (%) | Morphologically normal +/+ (%) |
|---|---|---|---|
| wt × wt (844 kernels/4 ears) | 2.4 ± 1.1 | 0 | 97.6 ± 1.1 |
| bsl1/+ × wt (1282 kernels/8 ears) | 45.7 ± 2.6 | 8.4 ± 2.3ab | 45.9 ± 0.8 |
| wt × bsl1/+ (1719 kernels/11 ears) | 2.0 ± 0.7 | 12.0 ± 2.3ab | 86.0 ± 2.4 |
| bsl1/+ × bsl1/+ (1414 kernels/8 ears) | 45.3 ± 1.4 | 14.4 ± 1.2a | 40.3 ± 2.0 |
Percentages given are the average of each kernel class per cross ± the standard error of the mean.
In some crosses the transmission of bsl1 was calculated from the transmission and linkage of the genetic markers purple aleurone1 (pr1), or a waxy1 (wx1) marked reciprocal translocation T5-9c, linked in repulsion phase to bsl1. Male transmission rates of bsl1 were calculated as Mtbsl1 = (Mtlinked marker − R)/(1 − 2R) (see materials and methods).
In those crosses without an endosperm marker, the transmission rate of bsl1 in morphologically normal kernels was inferred by sample progeny testing.
When wild-type females were crossed with bsl1/+ heterozygous males, the frequency of defective kernels did not significantly differ from that observed in wild-type crosses (Table 1). These findings indicate that bsl1 male transmission alone has no detrimental effect on seed development and that bsl1 is not dominant. Moreover, progeny testing of the offspring of bsl1/+ males revealed that the bsl1 mutant allele was transmitted through pollen less efficiently than the wild-type allele. The rate of bsl1 male transmission was calculated as 12.0 ± 2.3%, both by progeny testing bsl1/+ males and by segregation distortion of the linked marker pr1 or a waxy1 (wx1) marked reciprocal translocation, T5-9c wx1 (Table 1). These data indicate that the deficit of bsl1 heterozygotes after male transmission was caused by reduced transmission of the bsl1 allele rather than by frequent reversion of bsl1 to wild type.
We self-pollinated bsl1/+ heterozygotes in an attempt to generate bsl1/bsl1 homozygous progeny. If bsl1 homozygous seeds were viable, they would be expected to occur at an average frequency of 6% (i.e., on the basis of the probability of 12% male transmission and 50% female transmission of bsl1 alleles). Furthermore, 12% of the resulting defective kernels (i.e., those with maternal bsl1) are predicted to have inherited paternal bsl1 and be homozygous. With this in mind, all resulting defective kernels were selected, and plants from these seeds were grown to maturity. These plants did not exhibit any morphological abnormalities, and they were subsequently progeny tested for homozygosity of bsl1 on the basis of the frequency of defective seed production (i.e., from ∼50% defective seeds to ∼100% defective seeds). However, of 103 plants tested, none were homozygous for bsl1, indicating that the homozygotes were seed lethal (likelihood that bsl1/bsl1 plants were missed by random chance, P < 0.0001). No novel kernel phenotypes were visible in self-pollinations of bsl1/+ heterozygotes compared to pollinations of bsl1/+ females by wild type, suggesting that the bsl1/bsl1 individuals are not phenotypically distinct from heterozygous individuals carrying maternal bsl1 alleles. To confirm this finding we carried out test crosses and self-pollinations of bsl1 Wx1+/Bsl1+ T5-9c wx1 plants, which enabled us to distinguish kernels carrying wild-type or bsl1 alleles. Progeny derived from self-pollinations of bsl1/+ plants exhibited a higher proportion of lethal phenotypic classes (18.9%) compared to those derived from test crosses (7%) (Table 2). Therefore the rise in seed lethality following self-pollinations can be accounted for by the presence of bsl1 homozygotes in these populations.
TABLE 2.
Phenotypic classes of kernels produced in test crosses and self-crosses of bsl1/+ plants marked with T5-9c wx1 in repulsion to bsl1
|
bsl1/+ and bsl1/bsl1b
|
+/+: | ||||||
|---|---|---|---|---|---|---|---|
| Ear parent | Pollen parent | Empty pericarpa (%) | Loose pericarpa (%) | Germlessa (%) | Reduced endosperm (%) | Normal morphology (%) | Normal morphology (%) |
| bsl1 Wx1+ + T5-9c wx1 (199 kernels/3 ears) | wx1 | 1.9 | 3.5 | 1.6 | 33.6 | 12.8 | 46.6 |
| bsl1 Wx1+ + T5-9c wx1 (352 kernels/5 ears) | bsl1 Wx1+ + T5-9c wx1 | 3.2 | 10.3 | 5.4 | 28.1 | 8.7 | 43.9 |
bsl1 phenotypic classes are listed from most to least severe (empty pericarp > loose pericarp > germless > reduced endosperm > normal).
Lethal phenotypic classes.
Include +/+ defective kernels.
Taken together, these genetic data indicate that bsl1 confers a maternal effect on seed development. That only half of the seeds are affected is consistent with either a gametophytic maternal effect or an incompletely penetrant sporophytic maternal effect. The correlation between kernel phenotype and the inheritance of bsl1 in the progeny of these crosses demonstrates that the presence of the mutation in the embryo sac rather than the diploid parent is critical, thus supporting the view that the bsl1 maternal effect is gametophytic.
baseless1 affects development of the central cell within the female gametophyte:
The reduced male transmission of bsl1 and linked markers from bsl1/+ heterozygotes suggests that mutant bsl1 pollen grains are at a competitive disadvantage to wild-type pollen. Thus it is possible that bsl1 causes either an increased frequency of aborted and abnormal pollen or more subtle defects, such as in pollen tube growth rate, its ability to target embryo sacs, or the fertilization process, any of which could result in bsl1 pollen grains achieving less efficient fertilization than wild type. We therefore examined pollen from heterozygous bsl1/+ plants and compared them to pollen from wild-type plants (see materials and methods). No abnormalities in pollen morphology, starch filling, or nuclear number were apparent in pollen from bsl1/+ heterozygous plants (Table 3), suggesting that bsl1 pollen grains develop normally to maturity. Further, germination and tube growth rates of pollen from bsl1/+ heterozygous plants and wild-type plants were compared in vitro (see materials and methods) and were found to be identical (Table 3), indicating that pollen tube growth is not perturbed by bsl1.
TABLE 3.
Phenotypes of pollen from wild-type and bsl1/+ plants
| Pollen morphology
|
In vitro pollen analysis
|
|||
|---|---|---|---|---|
| Plant genotype | Aborted (%) | Normal (%) | Germination frequency (after 30 min) (%) | Pollen tube length (after 3.5 hr) (μm) |
| Wild type | 8.5 (n = 398) | 91.5 (n = 398) | 65 (n = 834) | 463 ± 13a (n = 168) |
| bsl1/+ | 8.0 (n = 387) | 92.0 (n = 387) | 67 (n = 397) | 451 ± 12a (n = 212) |
±1 standard error of the mean.
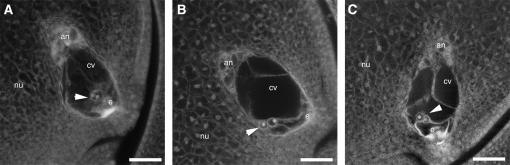
We also investigated the possible effects of the bsl1 mutation on female gametophyte development. Cell number, position, and morphology within embryo sacs of bsl1/+ plants were as in wild type, with discrepancies observed only in the central cell. In wild-type plants, the two polar nuclei of the central cell were always positioned adjacent to the egg cell, along the central longitudinal axis of the embryo sac (Figure 2A). However, in bsl1/+ plants we found that the two polar nuclei were not properly positioned within the central cell in 38% (n = 142) of embryo sacs examined (Table 4). In 16.9% of embryo sacs, the two polar nuclei were found apposed to the abgerminal wall of the central cell, whereas the polar nuclei were situated proximal to the adgerminal wall in only 0.7% of embryo sacs (Figure 2B, Table 4). In contrast, the two polar nuclei were situated off center, in that they were abgerminal to the central longitudinal axis of the central cell, in 20.4% of embryo sacs from bsl1/+ heterozygotes—a phenotype rarely seen in wild-type plants (Figure 2C). In all cases, the central cytoplasmic strands that extend from the antipodal cells to the polar nuclei, and from the polar nuclei to the egg, were also displaced in mutant embryo sacs compared to wild type. Because the observed frequency of abnormal embryo sacs in bsl1/+ heterozygotes was significantly lower than the expected 50% (χ2 = 8.14, P < 0.01), we predicted that 12% of embryo sacs must have been defective for bsl1, yet were indistinguishable from wild type (i.e., 24% of bsl1 embryo sacs are normal in appearance). Therefore the effects of bsl1 on polar nuclei positioning are not fully penetrant. Interestingly, we also observed that 31.8% (n = 91) of embryo sacs from +/+ W22 plants contained polar nuclei that had fused prior to fertilization and that 31.6% (n = 142) of embryo sacs from bsl1/+ W22 plants also contained fused polar nuclei—a phenomenon that has not been previously reported in maize.
Figure 2.—
Embryo sac morphologies in bsl1/+ plants. (A) Wild-type embryo sac with the two central cell polar nuclei centrally located above the egg cell. (B) bsl1 mutant embryo sac with the polar nuclei located adjacent to the abgerminal wall of the central cell. (C) bsl1 mutant embryo sac showing polar nuclei situated off center, abgerminal to the central longitudinal axis. Arrowheads point to the polar nuclei. an, antipodals; cv, central vacuole; e, egg cell; nu, nucellus. Bar, 25 μm.
TABLE 4.
Position of central cell polar nuclei in embryo sacs of wild-type and bsl1/+ plants
| Plant genotype | Central | Apposed to abgerminal wall | Off center, abgerminally positioned | Apposed to adgerminal wall |
|---|---|---|---|---|
| Wild type | 85 | 0 | 6 | 0 |
| bsl1/+ | 88 | 24 | 29 | 1 |
Given that a significant number of maternal-effect mutations cause precocious endosperm development in the absence of fertilization, we investigated whether bsl1 had a similar effect. Following examination of mature, nonfertilized embryo sacs from 20 bsl1/+ plants, we found no evidence of abnormal nuclear proliferation or autonomous endosperm development. On the other hand, controlled pollinations of embryo sacs from heterozygous bsl1/+ plants revealed that they could be fertilized as efficiently as in wild type, despite the displacement of polar nuclei in some mutant embryo sacs. Thus, bsl1 is required for correct development of the central cell within the female gametophyte. Despite the low male transmission of bsl1, the mutation does not appear to affect male gametophyte morphology, suggesting that bsl1 pollen grains might instead be defective in a later critical function, such as interaction with the stigma (silk), targeting to the embryo sac, or delivery of the sperm cells.
Mutation in bsl1 causes maternal-effect abnormalities on seed development:
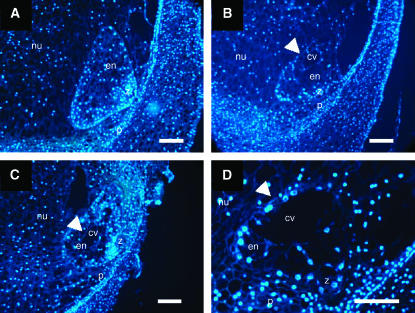
Because bsl1 causes a maternal effect on seed development, we determined the stage at which developmental abnormalities were first manifest in mutant kernels. For this, we analyzed 1- and 2-dap embryo and syncytial endosperm sections from wild-type plants and plants carrying the bsl1 mutation. However, no abnormalities were detected in embryos or in number of nuclei and their arrangements within syncytial endosperms at these stages (data not shown). In wild-type maize, syncytial endosperms commonly undergo coordinated cellularization, initiating from the periphery of the syncytium and progressing centripetally until a fully cellular structure is formed (Costa et al. 2003). In this study, fully cellular wild-type endosperms were formed by 3 dap (Figure 3A). By contrast, ∼40% of 3-dap endosperms examined from bsl1/+ plants exhibited delayed cellularization, which was indicated by the continued presence of the central vacuole at a time when other sibling endosperms were fully cellular (Figure 3, B–D). To determine the ploidy level of these endosperms, we performed a microspectrofluorometric analysis. We found no differences in the nuclear fluorescence intensity (RFU) of these endosperms, indicating that the ploidy levels of sibling wild-type (145.5 ± 5.3 RFU, n = 98) and bsl1 mutant (152.6 ± 4.5 RFU, n = 127) endosperms were similar.
Figure 3.—
Developing 3-dap endosperms segregating from bsl1/+ plants pollinated with wild-type pollen. (A) Wild-type fully cellular endosperm. (B–D) Segregating bsl1 mutant endosperms showing delayed cellularization—as indicated by the prolonged presence of the central vacuole (arrowheads), occurring irregularly, in a noncentripetal fashion. Endosperm sections were stained with DAPI and viewed under fluorescence microscopy. cv, central vacuole; en, endosperm; nu, nucellus; p, pericarp; z, zygote. Bar, 50 μm.
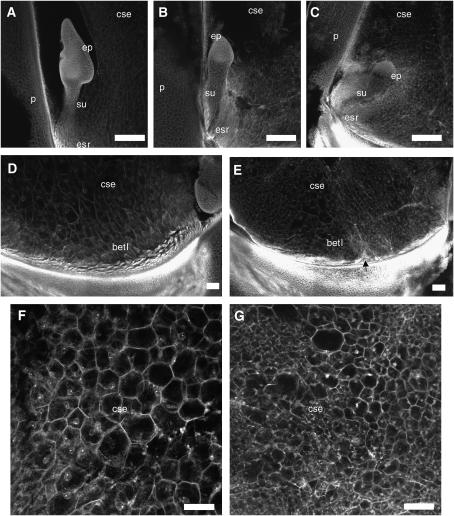
By 9 dap, bsl1 mutant kernels were macroscopically distinguishable from their wild-type siblings. We therefore examined the cellular organization of 9-dap sibling mutant and wild-type kernels from bsl1/+ plants to determine which tissues were most affected by the mutation at this stage. Our 9-dap wild-type embryos were at the coleoptile developmental stage (Figure 4A), whereas mutant sibling embryos were retarded and had reached only the transition stage (Figure 4, B and 4C). These embryos typically possessed a morphologically normal suspensor and embryo proper (Figure 4B), although some of the more severe mutants had a smaller than typical embryo proper (Figure 4C).
Figure 4.—
Confocal microscopy analysis of 9-dap embryos and endosperms from bsl1/+ plants pollinated with wild-type pollen. (A, D, and F) Wild type. (B, C, E, and G) bsl1. (A) Wild-type coleoptile stage embryo with prominent shoot apical meristem and (B and C) sibling mutant embryos lagging at the transition stage. (D and E) BETL endosperm morphology. (D) Wild-type endosperm with BETL consisting of approximately three layers of slightly elongated cells (autofluorescent) located above the pedicel (intense autofluorescent signal). (E) Typical bsl1 endosperm possessing an irregular BETL containing fewer cell layers. Arrow points to region of basal endosperm devoid of BETL. (F and G) Starchy endosperm morphology. Cells in the central endosperm are large and regularly spaced in wild-type endosperms (F), but appear smaller and of variable size in bsl1 endosperms (G). betl, basal endosperm transfer layer; cse, central starchy endosperm; ep, embryo proper; esr, embryo surrounding region; p, pericarp; su, suspensor. Bar, 100 μm.
In mutant endosperms, the most notable and consistent defects were observed in the organization of the BETL. Wild-type endosperms regularly possessed several layers of elongated and angular BETL cells in the basal region (Figure 4D), whereas we observed patchy distribution of BETL-like cells across fewer cell layers along the basal endosperm of mutant kernels (Figure 4E). Interestingly, bsl1 mutants displayed subtle or no morphological defects in the epidermal aleurone layer or embryo surrounding region (ESR) (data not shown). Abnormalities were, however, noted in the central starchy endosperm (CSE) in more severely perturbed bsl1/+ kernels, which contained smaller and more irregularly sized cells compared to wild type (Figure 4, F and G).
In summary, our findings show that after fertilization, bsl1 causes retarded growth of embryo and endosperm. Furthermore, bsl1 predominantly confers aberrations to the BETL tissue in mutant endosperms.
baseless1 alters the spatial distribution of transcripts specific to the basal syncytial and cellular endosperm:
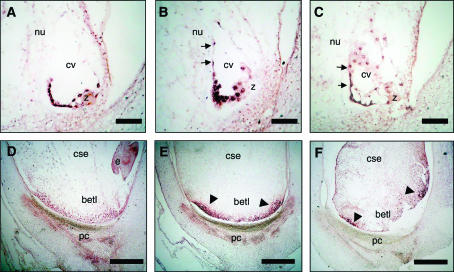
To further investigate the effects of the bsl1 mutation on early endosperm development, we performed mRNA in situ hybridization analysis of known transcripts expressed in the syncytial endosperm. We examined the localization of two basal-specific transcripts, maize end1 (see materials and methods) and MRP1 (Gomez et al. 2002), in wild-type syncytia and in syncytia isolated from ears segregating the bsl1 mutant allele. Maize end1 transcript was strongly detected along the entire basal portion of syncytial endosperms from wild-type plants (Figure 5A). By contrast, end1 transcript was localized in the basal region of ∼50% (n = 78) of syncytial endosperms examined from plants segregating for bsl1. The remainder exhibited distribution of end1 transcript either in patches along the basal portion of the syncytium or in the basal medio-lateral margins of the syncytial endosperm (Figure 5, B and C). We found similar results with MRP1, which was weakly expressed in both wild-type and mutant syncytial endosperms examined (data not shown).
Figure 5.—
mRNA in situ hybridization of basal endosperm-specific transcripts in sibling wild-type and mutant kernels. (A–C) 2-dap syncytial endosperms. (D–F) 9-dap cellular endosperms. (A) ZmEND1 transcript is confined to the basal portion of the wild-type free-nuclear endosperm. (B and C) In sibling mutant endosperms, ZmEND1 expression is displaced from the basal portion of the syncytial endosperm to more lateral regions (arrows). (D) Wild-type meg1 expression is restricted to the BETL, whereas in bsl1 endosperms (E and F) meg1 transcript is localized in discrete areas of the endosperm (arrowheads). betl, basal endosperm transfer layer; cse, central starchy endosperm; e, embryo; pc, placento-chalazal region; cv, central vacuole; nu, nucellus; z, zygote. Bar, 100 μm.
In situ hybridization was also performed on 9-dap cellular endosperms to detect a range of transcripts specific to the different domains. Our analysis revealed that several transcripts specific to the aleurone and ESR were correctly localized in wild-type and mutant sibling kernels (data not shown). In stark contrast, we discovered that BETL-specific transcripts were not properly localized in mutant endosperms. In wild type, meg1 transcript was detected throughout the BETL tissue (Figure 5D), whereas in bsl1 mutants, this transcript was present only in portions of the abgerminal and adgerminal basal endosperm (Figure 5, E and F). Thus our data indicate that basal endosperm-specific gene expression is severely perturbed during free-nuclear and cellular endosperm development by mutation in bsl1.
Baseless1 is necessary for the establishment of correct BETL-patterning components:
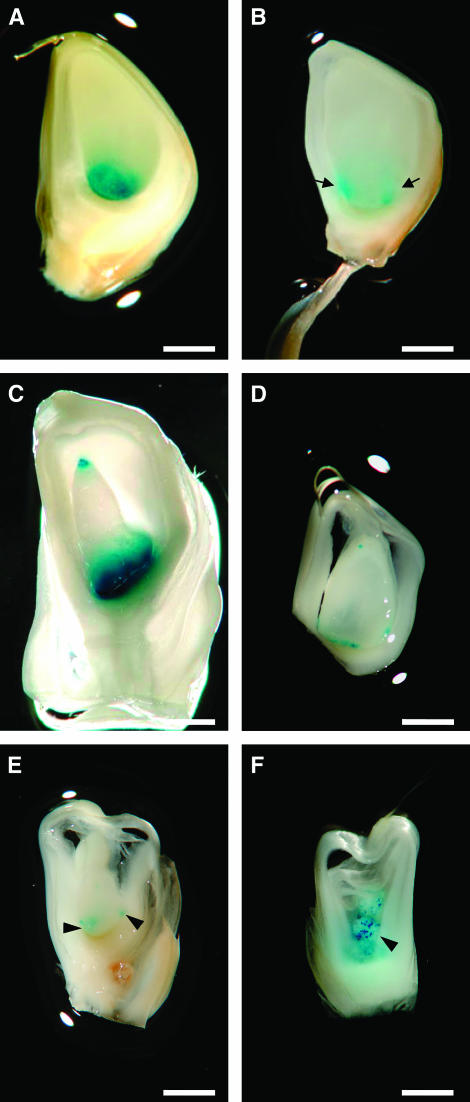
Previous work in maize has shown that interploidy crosses interfere with the normal postfertilization developmental program of the BETL (Charlton et al. 1995; Gutierrez-Marcos et al. 2003). We therefore decided to cross diploid wild-type and bsl1/+ plants as female by wild-type tetraploid plants to investigate whether the maternal defects caused by bsl1 could lead to further alterations in BETL development in resulting tetraploid endosperms. By following this strategy, we predicted one of three different outcomes: the expected defects in the BETL of tetraploid bsl1/bsl1/+/+ endosperms would be similar to those observed in triploid bsl1/bsl1/+ endosperms, be enhanced, or be suppressed. To facilitate this analysis, bsl1/+ plants were initially crossed as females by plants carrying one of two BETL-specific promoter GUS fusions, ProMeg1:GUS (Gutierrez-Marcos et al. 2004) or ProBet1:GUS (Hueros et al. 1999a). In wild-type kernels resulting from crosses between bsl1/+ female heterozygotes and ProMeg1:GUS-carrying plants, uniform GUS staining was observed in several layers of the BETL (Figure 6A). As expected from our in situ analysis of basal-specific endosperm transcripts, mutant sibling kernels displayed small patches of GUS precipitate in the lateral margins of the basal endosperm, denoted “mirror image” (Figure 6B). Similar GUS staining patterns were observed when using pollen from transgenic lines carrying the ProBet1:GUS reporter to pollinate bsl1/+ plants (data not shown). bsl1/+, ProMeg1:GUS and +/+, ProMeg1:GUS diploid females were then crossed by tetraploid males and resulting tetraploid endosperms were stained for GUS at different developmental stages. From ears carrying only wild-type Bsl1 alleles, all kernels were morphologically similar and GUS staining was often present in discrete clusters along the basal, and in some instances apical, endosperm and denoted “top–bottom” (Figure 6C). By contrast, three distinct classes of seed phenotype were found in ears segregating the bsl1 defective allele. Kernels of the first class (top–bottom: 55.8 ± 6.4%, n = 691) were similar to kernels examined from plants carrying Bsl1 wild-type alleles (Figure 6D). In contrast, kernels of the second and third classes (44.1 ± 3.1%) were reduced in size and showed much more unusual and varied GUS staining patterns. We therefore assumed that these kernels were defective for bsl1. The majority of these kernels (25.5 ± 4.1%) exhibited small patches of GUS staining in the basal endosperm (mirror image) and, at times, in the apical endosperm (Figure 6E), while others (18.6 ± 6.5%) displayed intensely staining puncta scattered throughout the entire endosperm (Figure 6F). Additionally, we noted that the more dramatic GUS staining patterns were observed in the most severely reduced endosperms. Taken together, these findings suggest that not only do the prefertilization defects caused by bsl1 greatly perturb BETL development, but also these abnormalities are enhanced in the tetraploid endosperm.
Figure 6.—
Effects of bsl1 on BETL-specific transgenic reporter expression in triploid and tetraploid endosperms. (A and B) 10-dap sibling kernels taken from a bsl1/+, ProMeg1:GUS/ProMeg1:GUS plant pollinated by a wild-type diploid plant. (A) Wild-type triploid endosperm with uniform ProMeg1:GUS expression (blue) distributed along the basal endosperm. (B) Typical mirror-image GUS expression confined to discrete basal areas of a typical bsl1/bsl1/+ endosperm (indicated by arrows). (C) 10-dap tetraploid endosperm expressing ProMeg1:GUS in the basal and apical endosperm (denoted “top–bottom”). Tetraploid endosperms were generated from crosses between wild-type ProMeg1:GUS/ProMeg1:GUS diploid plants and wild-type tetraploid plants. (D–F) Three distinct classes of 10-dap sibling kernels segregating in an ear from a bsl1/+, ProMeg1:GUS/ProMeg1:GUS plant pollinated by a wild-type tetraploid plant. (D) Typical kernel of the first class showing top–bottom ProMeg1:GUS expression patterns similar to those found in tetraploid endosperms shown in C. (E) Abnormal kernel of the second class exhibiting irregular mirror-image GUS staining patterns in the basal endosperm. (F) Kernel typical of the third class showing highly scattered ProMeg1:GUS staining throughout the endosperm (arrowheads). Bar, 200 μm.
DISCUSSION
bsl1 is a novel gametophytic mutant in maize:
To gain further understanding of plant reproductive development, efforts have been made in recent years to identify mutations affecting development and function of the male and female gametophytes (Feldmann et al. 1997; Christensen et al. 1998, 2002; Howden et al. 1998; Ebel et al. 2004; Johnson et al. 2004; Pagnussat et al. 2005). For instance, a range of mutations affecting aspects of pollen development, such as the first asymmetric mitotic division, pollen tube growth, or sperm delivery, have been identified thus far (reviewed in McCormick 2004).
Our analysis of bsl1 mutants revealed that transmission of the bsl1 mutant allele through the male was reduced to 12%, which would imply that male gametophyte development and/or function is perturbed by mutation in bsl1. However, no obvious cellular defects in pollen grain morphology or any deficiency in pollen tube growth were detected, suggesting that bsl1 pollen is not subject to a fundamental developmental or metabolic defect. Instead, we postulate that bsl1 male gametophyte function might be affected either during pollination (e.g., interaction of mutant pollen with silks) or during fertilization (e.g., in delivery and/or function of the sperm cells), where, similarly to maize rop2, maternal effect lethal1, and aberrant pollen transmission1 mutant pollen (Evans and Kermicle 2001; Arthur et al. 2003; Xu and Dooner 2006), it is at a competitive disadvantage. By contrast, we noted that bsl1 caused abnormal development of the female gametophyte. Thus far, an increasing number of female gametophytic mutations affecting most stages of embryo sac development, such as general nuclear division and migration, cellularization, and/or fusion of the polar nuclei, have been identified (Moore et al. 1997; Christensen et al. 1998, 2002; Ebel et al. 2004; Pagnussat et al. 2005). The majority of these mutations consequently impair fertilization. Because correct development of the egg apparatus (especially the synergids) is key to successful fertilization (Higashiyama et al. 2001; Huck et al. 2003; Rotman et al. 2003; Marton et al. 2005), it is likely that the egg apparatus is unaffected by the bsl1 mutation, as fertilization of bsl1 embryo sacs is achieved, although subtle defects cannot be ruled out. However, we found notable defects in bsl1 central cells, which exhibited abnormalities in the position of the polar nuclei, while other cells of the female gametophyte did not show any such phenotypes. Interestingly, the proportion of fused polar nuclei in embryo sacs from wild-type and bsl1/+ plants was similar, as was the fertilization efficiency of wild-type and mutant central cells, together suggesting that most central cell functions are not impaired by the bsl1 mutation. Given that the penetrance of the misplacement of polar nuclei in mutant central cells was only 38% (n = 142), it is probable that the bsl1 maternal effect on seed development is not a direct consequence of misplacement of the polar nuclei per se. Instead, this result points to underlying developmental abnormalities in the bsl1 central cell, which lead to both the maternal effect and misplacement of the polar nuclei. Nonetheless, it is unclear how the sperm are able to locate and fuse with the misplaced polar nuclei in bsl1 embryo sacs as efficiently as in wild type. While a great deal of experimental evidence points to an active role of the female gametophyte in targeting the pollen tube to the synergids for sperm cell release, very little is known about how the sperm nuclei are subsequently targeted to the egg and central cell nuclei to achieve karyogamy after fertilization. One possibility is that this second step is a passive process that depends on the surrounding cytoskeleton to effectively deliver the sperm cells to the appropriate nuclear positions within the embryo sac, a process that in turn is dependent on embryo sac architecture. The displaced cytoplasmic strands in bsl1 central cells support this view, although an alternative explanation of there being an active attraction between the sperm and central cell nuclei cannot be ruled out.
Maternal-effect baseless1 predominantly confers developmental abnormalities to the basal endosperm transfer tissue:
The bsl1 mutation caused ∼50% seed abnormalities when plants carrying the bsl1 defective allele were crossed by wild-type pollen, but not when wild-type plants were crossed by bsl1 pollen, indicating that the bsl1 mutation has a maternal effect on seed development rather than a dominant effect. Maternal effects can be caused by mutations in genes expressed either in the surrounding sporophytic tissue (for examples see Felker et al. 1985; Ray et al. 1996; Colombo et al. 1997) or in the female gametophyte [examples include capulet1 and capulet2 mutants (Grini et al. 2002), prolifera1 (Springer et al. 2000; Holding and Springer 2002), and maternal effect lethal1 (Evans and Kermicle 2001)] and whose gene products are necessary for correct development of embryo and endosperm. The cosegregation of bsl1 alleles with the defective kernels demonstrates maternal gametophytic—rather than sporophytic—inheritance.
A distinct class of maternal-effect genes comprises members of the Pc-G complex. MEA, FIE, and FIS2 are expressed in the central cell before fertilization, where they are required for suppression of autonomous endosperm development until fertilization occurs (Ohad et al. 1996; Chaudhury et al. 1997; Grossniklaus et al. 1998). During early endosperm development these genes are maternally expressed and paternally silenced. Mutant mea, fie, and fis2 endosperms all exhibit retarded growth, while embryos are inviable. Similar to bsl1 mutant endosperms, the region thought to be responsible for nutrient transfer is also affected in mea, fie, and fis2 endosperms (Sørensen et al. 2001; Guitton et al. 2004; Ingouff et al. 2005b). However, the nature of the aberrant maternal effects of bsl1 on seed development differs from that reported for defects in Pc-G class mutants, in that bsl1 does not induce parthenogenic development of the endosperm. In addition, when bsl1 plants were self-fertilized we observed an increase in the proportion of kernels with severe (lethal) developmental abnormalities, suggesting that paternally contributed Bsl1 also has a role in kernel development. Because paternally contributed Bsl1 alone was not able to rescue defects caused by maternal Bsl1 deficiency, it is possible that the paternal contribution either acts to reinforce the maternal gametophytic function or performs another role during seed development.
The maternal effect of bsl1 is unique in that the mutation specifically causes detrimental effects on BETL development. Because the BETL is the primary site for the transfer of solutes from the mother plant to the seed (Thompson et al. 2001), an impaired BETL would produce significant changes to nutrient influx, resulting in pleiotropic effects on seed development. This phenomenon has been observed in other zygotic mutants possessing a defective BETL (Cheng et al. 1996; Maitz et al. 2000; Costa et al. 2003) and in maternal-effect mutants with defective connecting maternal sporophytic tissue (Felker et al. 1985). Moreover, an impaired BETL would certainly account for the slower growth and delayed programmed cell death in bsl1 kernels when compared to wild-type siblings (data not shown). Similar to reduced grain filling1 mutants in maize (Maitz et al. 2000), expression of BETL-specific genes was downregulated in bsl1 mutants, although BETL-specific transcripts were often confined to discrete portions of the adgerminal and abgerminal basal endosperm. Although we are currently uncertain of the direct effects of the bsl1 mutation on embryo development, it is likely that the retarded growth of bsl1/+ embryos is caused by the inability of compromised bsl1/bsl1/+ endosperms to support their growth. This has been shown also to be the case for most zygotic defective kernel mutants by using chromosomal translocations to produce nonconcordant seeds with mutant endosperms and wild-type embryos (Neuffer and Sheridan 1980; Chang and Neuffer 1994).
BETL development is under maternal gametophytic control but is also regulated biparentally after fertilization:
It is currently held that cell fate specification of the maize BETL occurs during early syncytial endosperm development (Costa et al. 2003). This is most likely the case in other cereals and thus far a growing number of future transfer cell-specific transcripts that are localized in a polar fashion within barley and wheat syncytial endosperms have been identified (Doan et al. 1996; Drea et al. 2005). Interestingly, we found irregular distributions of basal-specific endosperm transcripts in syncytia segregating the bsl1 mutation, yet we did not detect any morphological abnormalities in these endosperms. In addition, irregular localization of BETL-specific transcripts was observed in bsl1 mutant cellular endosperms, which also possessed aberrant BETL tissue. These findings therefore indicate that BSL1 is necessary for correct BETL patterning.
Studies on interploidy crosses in maize have shown that the transfer tissue is particularly sensitive to alterations in the 2 m:1 p parental genomic balance in the endosperm (Charlton et al. 1995; Hueros et al. 1999b; Gutierrez-Marcos et al. 2003), thus reflecting the antagonistic influences exerted by maternal and paternal genomes following fertilization. When we pollinated bsl1/+ plants with pollen from tetraploid plants, we found that the introduction of an extra paternal Bsl1 wild-type allele was still not sufficient to compensate for the BETL abnormalities caused by maternal transmission of bsl1. Although this finding may be indicative of BSL1 function not being dependent on dosage, the issue remains difficult to resolve since tetraploidy per se causes patterning defects in the endosperm. Notably, we found more severe defects in the BETL tissue of bsl1/bsl1/+/+ tetraploid endosperms than in either bsl1/bsl1/+ triploid endosperms or +/+/+/+ tetraploid endosperms. Moreover, these abnormalities ranged in severity, with the frequency of severely defective bsl1/bsl1/+/+ endosperms (18.6%) roughly correlating with the percentage of severely affected central cells in bsl1/+ plants (i.e., 17.6%, as indicated by displaced polar nuclei). This correlation favors the idea that maternal gametophytic contribution of Bsl1, rather than the number of functional Bsl1 alleles in the endosperm, is important for kernel phenotype. Moreover, these data indicate that the prefertilization gametophytic effects of bsl1 lead directly to the formation of a more aberrant BETL. On this basis, it appears that intrinsic information for BETL patterning is present in the central cell prior to fertilization (see proposed model, Figure 7). Our findings thus contribute significantly to the model currently held for BETL development, which is believed to occur in an irreversible lineage-dependent manner in response to a combination of developmental cues from the maternal sporophyte and from within the endosperm (Costa et al. 2003). These data support the proposed model (Birchler 1993) that regulation of maize endosperm development resembles that of the Drosophila blastoderm. Early patterning of the maize endosperm transfer tissue is under strong maternal control and hence resembles embryonic patterning events in Drosophila, which is achieved before fertilization via the asymmetric localization of maternal determinants within a common cytoplasm (Johnstone and Lasko 2001; reviewed in Ephrussi and St. Johnston 2004). Certainly, molecular data in Arabidopsis and maize show that there is a significant contribution of maternal products to endosperm, as well as embryo (Vielle-Calzada et al. 2000; Grimanelli et al. 2005). In addition, current genetic data highlight a large number of female gametophytic mutants that exhibit only postfertilization defects (Pagnussat et al. 2005). Our data further support the notion that maternally contributed factors interact with information from within the endosperm (Birchler 1993), in this case to direct BETL patterning and development.
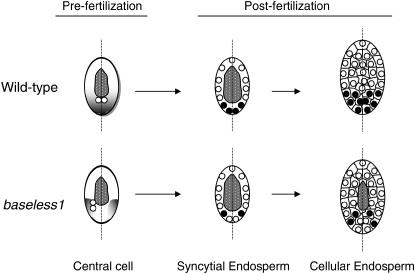
Figure 7.—
Proposed model for the maternal regulation of BETL development in wild type and bsl1. In wild type, maternal factor(s) required for correct BETL patterning (shading) are putatively distributed along the proximal–distal axis (dashed line) of the central cell before fertilization. Mutation in bsl1 affects development of the central cell, as indicated by the displacement of the polar nuclei (open circles) away from the central longitudinal (proximal–distal) axis. We propose that these maternal factor(s) are also displaced in bsl1 central cells, thus altering BETL patterning. Following fertilization in wild type, BETL specification of basal nuclei (solid circles) takes place in the syncytial endosperm, with subsequent daughter nuclei and BETL cells developing in a lineage-dependent fashion (Costa et al. 2003). Despite fertilization of bsl1/+ plants with wild-type pollen, BETL specification occurs abnormally in bsl1 syncytial endosperms, such that some basal nuclei remain undifferentiated. This leads directly to decreased BETL cell distribution and to subsequent poor nutrient uptake. As a consequence, cellularization is delayed and is indicated by the prolonged presence of the central vacuole (stippled area) in mutant endosperms, at a time when sibling wild-type endosperms are fully cellular. The future germinal side of the kernel is oriented to the right.
Analysis of bsl1 central cells and syncytial endosperms further suggests that polarity is perturbed in both structures. We therefore favor a hypothesis in which BSL1 is required for the establishment and/or maintenance of polarity in these reproductive structures. As such, mutation in bsl1 might affect temporal and/or spatial distribution of BETL factor(s) within the common cytoplasm of the central cell and syncytial endosperm, until endosperm cellularization takes place (see proposed model, Figure 7). However, it remains unknown how the original asymmetry is generated.
In summary, through the study of bsl1 we have found that correct BETL patterning is predetermined in the central cell before fertilization. This information is most likely maintained within the syncytial endosperm, during which time BETL development becomes finely regulated by both maternally and paternally contributed factors.
Acknowledgments
We thank Jerry L. Kermicle for advice and support on this project, as well as for seeds of the bsl1 mutant. We also thank Hugh G. Dickinson for his support with this work and Richard D. Thompson for seeds carrying the ProBet1:GUS reporter construct.
References
- Arthur, K. M., Z. Vejlupkova, R. B. Meeley and J. E. Fowler, 2003. Maize ROP2 GTPase provides a competitive advantage to the male gametophyte. Genetics 165: 2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., 1993. Dosage analysis of maize endosperm development. Annu. Rev. Genet. 27: 181–204. [DOI] [PubMed] [Google Scholar]
- Boisnard-Lorig, C., A. Colon-Carmona, M. Bauch, S. Hodge, P. Doerner et al., 2001. Dynamic analyses of the expression of the HISTONE∷YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13: 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R. C., B. E. Lemmon and H. Nguyen, 2003. Events during the first four rounds of mitosis establish three developmental domains in the syncytial endosperm of Arabidopsis thaliana. Protoplasma 222: 167–174. [DOI] [PubMed] [Google Scholar]
- Chang, M. T., and M. G. Neuffer, 1994. Endosperm-embryo interactions in maize. Maydica 39: 9–18. [Google Scholar]
- Charlton, W. L., C. L. Keen, C. Merriman, P. Lynch, A. J. Greenland et al., 1995. Endosperm development in Zea mays: implication of gametic imprinting and paternal excess in regulation of transfer layer development. Development 121: 3089–3097. [Google Scholar]
- Chaudhury, A. M., L. Ming, C. Miller, S. Craig, E. S. Dennis et al., 1997. Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94: 4223–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W. H., E. W. Taliercio and P. S. Chourey, 1996. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8: 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, C. A., S. Subramanian and G. N. Drews, 1998. Identification of gametophytic mutations affecting female gametophyte development in Arabidopsis. Dev. Biol. 202: 136–151. [DOI] [PubMed] [Google Scholar]
- Christensen, C. A., S. W. Gorsich, R. H. Brown, L. G. Jones, J. Brown et al., 2002. Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. Plant Cell 14: 2215–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, A. W., and L. J. Goff, 1985. Mithramycin and 4′,6-diamidino-2-phenylindole (DAPI) as vital stains and for quantitation of nuclear DNA. Stain Technol. 60: 145–154. [DOI] [PubMed] [Google Scholar]
- Colombo, L., J. Franken, A. Van der Krol, P. E. Wittich, H. J. M. Dons et al., 1997. Down-regulation of ovule-specific MADS box genes from Petunia results in maternally controlled defects in seed development. Plant Cell 9: 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, L. M., J. F. Gutierrez-Marcos, T. P. Brutnell, A. J. Greenland and H. G. Dickinson, 2003. The globby1–1 (glo1–1) mutation disrupts nuclear and cell division in the developing maize seed causing alterations in endosperm cell fate and tissue differentiation. Development 130: 5009–5017. [DOI] [PubMed] [Google Scholar]
- Costa, L. M., J. F. Gutierrez-Marcos and H. G. Dickinson, 2004. More than a yolk: the short life and complex times of the plant endosperm. Trends Plant Sci. 9: 507–514. [DOI] [PubMed] [Google Scholar]
- Doan, D. N., C. Linnestad and O. A. Olsen, 1996. Isolation of molecular markers from the barley endosperm coenocyte and the surrounding nucellus cell layers. Plant Mol. Biol. 31: 877–886. [DOI] [PubMed] [Google Scholar]
- Drea, S., D. J. Leader, B. C. Arnold, P. Shaw, L. Dolan et al., 2005. Systematic spatial analysis of gene expression during wheat caryopsis development. Plant Cell 17: 2172–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews, G. N., and R. Yadegari, 2002. Development and function of the angiosperm female gametophyte. Annu. Rev. Genet. 36: 99–124. [DOI] [PubMed] [Google Scholar]
- Ebel, C., L. Mariconti and W. Gruissem, 2004. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature 429: 776–780. [DOI] [PubMed] [Google Scholar]
- Ephrussi, A., and D. St. Johnston, 2004. Seeing is believing: the bicoid morphogen gradient matures. Cell 116: 143–152. [DOI] [PubMed] [Google Scholar]
- Evans, M. M. S., and J. L. Kermicle, 2001. Interaction between maternal effect and zygotic effect mutations during maize seed development. Genetics 159: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann, K. A., D. A. Coury and M. L. Christianson, 1997. Exceptional segregation of a selectable marker (KanR) in Arabidopsis identifies genes important for gametophytic growth and development. Genetics 147: 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker, F. C., D. M. Peterson and O. M. Nelson, 1985. Anatomy of immature grains of eight maternal effect shrunken endosperm barley mutants. Am. J. Bot. 72: 248–256. [Google Scholar]
- Gomez, E., J. Royo, Y. Guo, R. Thompson and G. Hueros, 2002. Establishment of cereal endosperm expression domains: identification and properties of a maize transfer cell-specific transcription factor, ZmMRP-1. Plant Cell 14: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimanelli, D., E. Perotti, J. Ramirez and O. Leblanc, 2005. Timing of the maternal-to-zygotic transition during early seed development in maize. Plant Cell 17: 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grini, P. E., G. Jurgens and M. Hulskamp, 2002. Embryo and endosperm development is disrupted in the female gametophytic capulet mutants of Arabidopsis. Genetics 162: 1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus, U., J. P. Vielle-Calzada, M. A. Hoeppner and W. B. Gagliano, 1998. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science 280: 446–450. [DOI] [PubMed] [Google Scholar]
- Guitton, A. E., D. R. Page, P. Chambrier, C. Lionnet, J. E. Faure et al., 2004. Identification of new members of Fertilisation Independent Seed Polycomb Group pathway involved in the control of seed development in Arabidopsis thaliana. Development 131: 2971–2981. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos, J. F., P. D. Pennington, L. M. Costa and H. G. Dickinson, 2003. Imprinting in the endosperm: a possible role in preventing wide hybridization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Marcos, J. F., L. M. Costa, C. Biderre-Petit, B. Khbaya, D. M. O'Sullivan et al., 2004. Maternally expressed gene1 is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell 16: 1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama, T., S. Yabe, N. Sasaki, Y. Nishimura, S. Miyagishima et al., 2001. Pollen tube attraction by the synergid cell. Science 293: 1480–1483. [DOI] [PubMed] [Google Scholar]
- Holding, D. R., and P. S. Springer, 2002. The Arabidopsis gene PROLIFERA is required for proper cytokinesis during seed development. Planta 214: 373–382. [DOI] [PubMed] [Google Scholar]
- Howden, R., S. K. Park, J. M. Moore, J. Orme, U. Grossniklaus et al., 1998. Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics 149: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck, N., J. M. Moore, M. Federer and U. Grossniklaus, 2003. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130: 2149–2159. [DOI] [PubMed] [Google Scholar]
- Hueros, G., E. Gomez, N. Cheikh, J. Edwards, M. Weldon et al., 1999. a Identification of a promoter sequence from the BETL1 gene cluster able to confer transfer-cell-specific expression in transgenic maize. Plant Physiol. 121: 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueros, G., J. Royo, M. Maitz, F. Salamini and R. D. Thompson, 1999. b Evidence for factors regulating transfer cell-specific expression in maize endosperm. Plant Mol. Biol. 41: 403–414. [DOI] [PubMed] [Google Scholar]
- Ingouff, M., J. N. Fitz Gerald, C. Guerin, H. Robert, M. B. Sorensen et al., 2005. a Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat. Cell Biol. 7: 374–380. [DOI] [PubMed] [Google Scholar]
- Ingouff, M., J. Haseloff and F. Berger, 2005. b Polycomb group genes control developmental timing of endosperm. Plant J. 42: 663–674. [DOI] [PubMed] [Google Scholar]
- Johnson, M. A., K. von Besser, Q. Zhou, E. Smith, G. Aux et al., 2004. Arabidopsis hapless mutations define essential gametophytic functions. Genetics 168: 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, O., and P. Lasko, 2001. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu. Rev. Genet. 35: 365–406. [DOI] [PubMed] [Google Scholar]
- Kermicle, J. L., 1978. Imprinting of gene action in maize endosperm, pp. 357–371 in Maize Breeding and Genetics, edited by D. B. Walden. John Wiley & Sons, New York.
- Kindiger, B., 1994. A staining procedure for pollen grain chromosomes of maize, pp. 476–480 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York/Berlin/Heidelberg, Germany.
- Maitz, M., G. Santandrea, Z. Zhang, S. Lal, L. C. Hannah et al., 2000. rgf1, a mutation reducing grain filling in maize through effects on basal endosperm and pedicel development. Plant J. 23: 29–42. [DOI] [PubMed] [Google Scholar]
- Marton, M. L., S. Cordts, J. Broadhvest and T. Dresselhaus, 2005. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307: 573–576. [DOI] [PubMed] [Google Scholar]
- McCormick, S., 2004. Control of male gametophyte development. Plant Cell 16(Suppl.): S142–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. M., J. P. Calzada, W. Gagliano and U. Grossniklaus, 1997. Genetic characterization of hadad, a mutant disrupting female gametogenesis in Arabidopsis thaliana. Cold Spring Harbor Symp. Quant. Biol. 62: 35–47. [PubMed] [Google Scholar]
- Neuffer, M. G., and W. F. Sheridan, 1980. Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95: 929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad, N., L. Margossian, Y. C. Hsu, C. Williams, P. Repetti et al., 1996. A mutation that allows endosperm development without fertilization. Proc. Natl. Acad. Sci. USA 93: 5319–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, O. A., 2001. Endosperm development: cellularization and cell fate specification. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 233–267. [DOI] [PubMed] [Google Scholar]
- Olsen, O. A., 2004. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16(Suppl.): S214–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat, G. C., H. J. Yu, Q. A. Ngo, S. Rajani, S. Mayalagu et al., 2005. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132: 603–614. [DOI] [PubMed] [Google Scholar]
- Ray, S., T. Golden and A. Ray, 1996. Maternal effects of the short integument mutation on embryo development in Arabidopsis. Dev. Biol. 180: 365–369. [DOI] [PubMed] [Google Scholar]
- Rotman, N., F. Rozier, L. Boavida, C. Dumas, F. Berger et al., 2003. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr. Biol. 13: 432–436. [DOI] [PubMed] [Google Scholar]
- Sørensen, M. B., A. M. Chaudhury, H. Robert, E. Bancharel and F. Berger, 2001. Polycomb group genes control pattern formation in plant seed. Curr. Biol. 11: 277–281. [DOI] [PubMed] [Google Scholar]
- Springer, P. S., D. R. Holding, A. Groover, C. Yordan and R. A. Martienssen, 2000. The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G(1) phase and is required maternally for early Arabidopsis development. Development 127: 1815–1822. [DOI] [PubMed] [Google Scholar]
- Thompson, R. D., G. Hueros, H. Becker and M. Maitz, 2001. Development and functions of seed transfer cells. Plant Sci. 160: 775–783. [DOI] [PubMed] [Google Scholar]
- Vielle-Calzada, J. P., R. Baskar and U. Grossniklaus, 2000. Delayed activation of the paternal genome during seed development. Nature 404: 91–94. [DOI] [PubMed] [Google Scholar]
- Walbot, V., and M. M. Evans, 2003. Unique features of the plant life cycle and their consequences. Nat. Rev. Genet. 4: 369–379. [DOI] [PubMed] [Google Scholar]
- Xu, Z., and H. K. Dooner, 2006. The maize aberrant pollen transmission 1 gene is a SABRE/KIP homolog required for pollen tube growth. Genetics 172: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadegari, R., and G. N. Drews, 2004. Female gametophyte development. Plant Cell 16(Suppl.): S133–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]