Abstract
In insects, the boundary between the embryonic head and thorax is formed by the dorsal ridge, a fused structure composed of portions of the maxillary and labial segments. However, the mechanisms that promote development of this unusual structure remain a mystery. In Drosophila, mutations in the Hox genes Sex combs reduced and Deformed have been reported to cause abnormal dorsal ridge formation, but the significance of these abnormalities is not clear. We have identified three mutant allele classes of Cephalothorax, the Tribolium castaneum (red flour beetle) ortholog of Sex combs reduced, each of which has a different effect on dorsal ridge development. By using Engrailed expression to monitor dorsal ridge development in these mutants, we demonstrate that Cephalothorax promotes the fusion and subsequent dorsolateral extension of the maxillary and labial Engrailed stripes (posterior compartments) during dorsal ridge formation. Molecular and genetic analysis of these alleles indicates that the N terminus of Cephalothorax is important for the fusion step, but is dispensable for Engrailed stripe extension. Thus, we find that specific regions of Cephalothorax are required for discrete steps in dorsal ridge formation.
A defining feature of the insect body plan is the grouping of segments into three regions, or tagmata: the head, thorax, and abdomen. In most, if not all, insect species the boundary between the embryonic head and thorax is formed by the dorsal ridge, which develops by fusion of the dorsolateral components of the gnathal segments (post-oral head) (Rogers and Kaufman 1996). In some insects, the dorsal ridge appears as a discrete dorsal lobe between the head and thorax, while in others it is highly reduced and fused to the thorax, or not visible at all (Rogers and Kaufman 1997). In the fruit fly, Drosophila melanogaster, the larval head is dramatically reorganized during the process of head involution. A dorsal ridge still forms between the head and thorax but is subsequently internalized to form the dorsal pouch (Younossi-Hartenstein et al. 1993).
The initial development of the dorsal ridge is highly conserved among insects (Rogers and Kaufman 1996). The first evidence of dorsal ridge formation is the expression of the segment polarity gene engrailed (en) along the lateral edge of the anterior compartment of the labial segment. This unusual En domain connects the stripes of En expression in the posterior compartments of the maxillary and labial segments. A single En stripe then extends dorsally from this region as part of the dorsal ridge. In Drosophila, the Hox gene Deformed (Dfd) is expressed anterior to and in the anterior portion of this En stripe (presumably the maxillary portion of the dorsal ridge) (Rogers and Kaufman 1997). Sex combs reduced (Scr) is expressed in the posterior portion of the En stripe (Gorman and Kaufman 1995). Mutations in Dfd and Scr are reported to cause abnormal dorsal ridge formation (Rogers and Kaufman 1997), but no description of these abnormalities has been published. Interpretation of these phenotypes may be complicated by the internalization of the Drosophila dorsal ridge during head involution.
In contrast, head development in the red flour beetle, Tribolium castaneum, follows a more generalized plan. Unlike the internalized head of the Drosophila larva, the Tribolium larval head is articulated with the trunk and has external gnathal appendages, allowing the study of genetic regulatory interactions in more typical insect gnathal development. Thus, Tribolium provides an attractive system in which to analyze the regulation of dorsal ridge development. In this work, we first describe formation of the dorsal ridge during wild-type Tribolium development. Then, using a combination of genetic analysis and expression studies, we demonstrate that Cephalothorax (Cx), the Tribolium ortholog of Scr (Curtis et al. 2001), is essential for two discrete steps in dorsal ridge development: (1) fusion of the maxillary and labial En stripes and (2) extension of En stripes to the dorsolateral edges of the embryo. Furthermore, we find that the N-terminal domain of Cx is required for En stripe fusion, but is dispensable for En stripe extension.
MATERIALS AND METHODS
Genetic analysis:
CxE and Cx61 were isolated in an EMS mutagenesis of Eyeless-lethal free, a stock created by using the Eyeless balancer chromosome to isogenize and homozygose the region of LG2 containing the HOMC.
Stocks were maintained on whole wheat flour supplemented with 5% brewer's yeast (Beeman et al. 1989). The balancer chromosomes Abdominal Extra sclerite (AEs) and Eyeless (Ey) and the recessive lethal mutation Abdominal83 (A83) were used to facilitate maintenance of mutant lines. The following strains were used: Ga-1 (wild type), Cx6/AEs, Cx61/AEs, Cx20/AEs, CxE/AEs, mxpStm/A83, and Df(HOMC)/Ey.
After mating, eggs were collected at 2-day intervals and allowed to develop at 30°. Scanning electron micrographs were obtained as described by Curtis et al. (2001). For cuticle preps, newly hatched larvae were placed in lactic acid:ethanol (9:1), and after 7 days, all remaining unhatched eggs were placed in lactic acid:ethanol.
Southern analysis:
Genomic DNA was isolated from Ga-1 (wild-type) and Cx20/AEs beetles (Brown et al. 1990). DNA (2 μg) was digested with HindIII. The resulting DNA fragments were separated by field inversion gel electrophoresis and transferred to GeneScreen membrane (NEN Life Sciences). The membrane was hybridized with a radiolabeled 3-kb fragment containing the first exon of the Cx gene.
Molecular analysis of mutant alleles:
In preparation for inverse PCR, genomic DNA (2 μg) from Cx20 heterozygotes was digested with HindIII, purified with the High Pure PCR product purification kit (Roche Applied Science), diluted to ∼1 ng/μl, and ligated at 16° overnight. The ligation product (10 ng) was used as template for inverse PCR using Dynazyme EXT (Finnzymes-MJ Research). Amplified products were visualized by agarose gel electrophoresis. DNA from the two resulting bands (wild type and mutant) was ligated into the PCR 4-TOPO vector (Invitrogen, San Diego), and the plasmid inserts were sequenced at the Sequencing and Genotyping Facility (Department of Plant Pathology, Kansas State University).
To amplify the exons of mutant alleles, individual homozygous or hemizygous larvae were homogenized as described by Gloor et al. (1993). These homogenates were used as templates for Ready-To-Go bead (Amersham Pharmacia Biotech) or DyNAzyme EXT (Finnzymes) PCR reactions under the conditions described by Gloor et al. (1993). Amplified products were purified with the QIAquick PCR purification kit (QIAGEN, Chatsworth, CA) and then directly sequenced with internal primers. Two sequences, one for each exon of the Cx gene, were submitted to GenBank for each mutant allele (see results for accession numbers).
Putative translation start codons were identified with the ATGpr program created by Salamov et al. (1998). ATGpr is found online at http://www.hri.co.jp/atgpr/.
Expression analysis:
Immunostaining of 0- to 96-hr embryos was performed as described by Carroll et al. (1988). To detect Tc Engrailed expression (hereafter referred to simply as Engrailed), we used 4D9, a monoclonal antibody to Drosophila Engrailed/Invected developed by Corey Goodman (Patel et al. 1989), which was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, Iowa. A cross-reacting polyclonal antibody to Drosophila Scr, α-DmScr (a gift from Thomas Kaufman), was used to detect Cx. After staining, embryos were dissected from yolk and documented using bright-field or differential interference contrast illumination.
Tc hedgehog (Tc hh) riboprobe was synthesized from a cDNA clone kindly provided by Yoshinori Tomoyasu. In situ hybridization with Tc hh and immunostaining with MAb 4D9 were performed as described by Nagaso et al. (2001).
RESULTS
Dorsal ridge development in Tribolium:
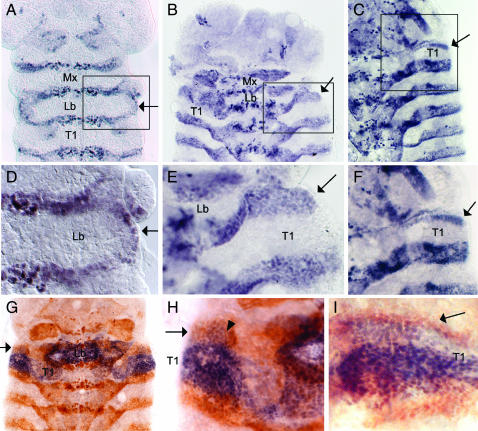
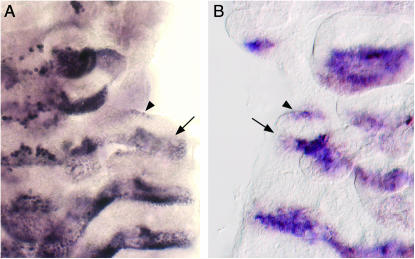
To follow the events of dorsal ridge development in Tribolium, we used a cross-reacting antibody to Drosophila Invected to detect the Tribolium Engrailed (En) protein, which is expressed in the posterior part of each segment (Brown et al. 1994). At the extended germband stage, En expression appears along the lateral edge of the anterior compartment of the labial segment (Figure 1, A and D). As has been described for the milkweed bug and cricket (Rogers and Kaufman 1996), En expression appears simultaneously in the entire row of cells and connects the maxillary and labial En stripes. As the embryo begins to dorsally close, the thoracic and abdominal En stripes extend dorsolaterally, such that they eventually encircle the embryo. Likewise, the single fused dorsal ridge En stripe extends dorsolaterally, initially as a broad stripe (Figure 1, B and E), but later becomes much narrower (Figure 1, C and F). The mechanisms underlying dorsal closure are not well understood in Tribolium. It is thought that at least some of the dorsolateral expansion of the embryo is due to cell division, but it is quite possible that cell rearrangements also play a role in this process. Because of this uncertainty, we have chosen the term “extension” rather than “growth” to describe the behavior of the dorsal ridge and trunk En stripes.
Figure 1.—
Dorsal ridge development in wild-type embryos. En expression in the dorsal ridge is indicated by an arrow in A–I. Relevant segments are labeled as follows: maxillary (Mx), labial (Lb), prothoracic (T1). (A–F) En expression (purple) in the wild-type dorsal ridge. (A) An extended germband-stage embryo viewed from the yolk side to provide a clearer view of Engrailed expression along the lateral edges of the labial segment. (B) As dorsal closure begins, a patch of En expression (apparently representing a fusion of the maxillary and labial En stripes) extends dorsally. (C) As dorsal closure continues, the dorsal ridge En stripe becomes significantly narrower than the other extending En stripes. (D) An enlarged view of the region boxed in A. (E) An enlarged view of the region boxed in B. (F) An enlarged view of the region boxed in C. (G–I) Cx expression (purple) and En expression (brown) in the wild-type dorsal ridge. (G) Early in the dorsal closure process, Cx is expressed in a subset of the En-expressing dorsal ridge cells. (H) An enlarged view of G showing the dorsal ridge cells that coexpress Cx and En (arrowhead). (I) As the dorsal ridge En stripe extends and narrows, Cx expression is confined to the posterior half of the En stripe.
In Drosophila, the Hox genes Dfd and Scr are expressed in portions of the dorsal ridge (Gorman and Kaufman 1995; Rogers and Kaufman 1997). Brown et al. (1999) observed expression of the Tribolium Dfd ortholog in the dorsal ridge. We have examined expression of Cephalothorax (Cx), the Tribolium ortholog of Scr, and find that, similar to Scr, it is expressed in the posterior region of the developing dorsal ridge (Figure 1, G–I).
Cx mutant phenotypes suggest a possible role in dorsal ridge development:
Two classes of Cx mutations that affect the boundary between head and thorax in Tribolium embryos have been described (Beeman et al. 1993). In one class, the segmental groove between the two tagma is absent, while in the other a supernumerary dorsal segment appears between the head and thorax. These phenotypes, in combination with expression data, suggest that Cx may be required for normal development of the dorsal ridge. To address this possibility, we have performed phenotypic, molecular, and expression analyses of four Cx alleles, placing particular emphasis on their effects on dorsal ridge development.
Cx null mutants lack the boundary between head and thorax:
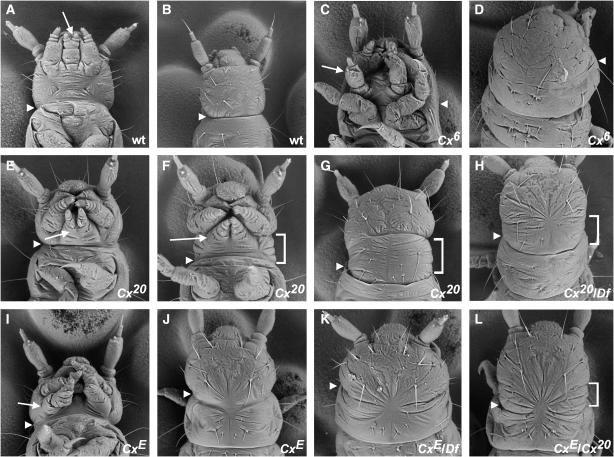
Beeman et al. (1993) described a class of Cx alleles that cause fusion of the first thoracic segment (T1) with the head (cephalization), as well as transformation of the larval labial appendages to antennae. These alleles were deemed likely to be nulls, since identical effects were observed in homozygotes and hemizygotes [individuals carrying one copy of the mutant allele and one copy of Df(HOMC), a deficiency that removes Cx and several other Hox genes]. We have characterized two alleles in this class: Cx6 (Beeman et al. 1989) and Cx61 (this work). Our analysis of Cx6 (Figure 2) and Cx61 (data not shown) confirms that the homozygous (Figure 2, C and D) and hemizygous (data not shown) phenotypes of these mutations are identical, indicating that these are null alleles.
Figure 2.—
Scanning electron micrographs of wild-type and Cx mutant Tribolium larvae. The boundary between head and thorax (approximated in cephalized mutants) is denoted by an arrowhead in A–L, and the labial appendages are indicated by an arrow in all ventral views. (A) Wild type, ventral view. (B) Wild type, dorsal view. (C) Cx6 homozygote, ventral view. Note the transformation of labial appendages to antennae (arrow) and the fusion of T1 with the head. (D) Cx6 homozygote, dorsal view. T1 is fused with the head dorsally as well as ventrally. (E) Cx20 homozygote, ventral view. The phenotype of Cx20 homozygotes is variable. In this mildly affected individual, the labial appendages are more posteriorly located than in wild type and only the most proximal segment of each appendage is fused. The extra dorsal segment between the head and T1 is not visible from the ventral side. (F) Cx20 homozygote, ventral view. In a more severely affected individual, the labial appendages completely fail to fuse. The extra dorsal segment between the head and T1 (bracket) can be seen from the ventral side. (G) Cx20 homozygote, dorsal view. Note the extra segment (bracket) between the head and T1. (H) Cx20 hemizygote [Cx20/Df(HOMC)], dorsal view. An extra segment (bracket) is present, although partly obscured by dorsal closure defects. (I) CxE homozygote, ventral view. The labial appendages are positioned laterally, in a similar orientation to the maxillary appendages. Note the triangular head shape. (J) CxE homozygote, dorsal view. A segmental groove is present between the head and thorax, but does not appear as deep as in wild type. (K) CxE hemizygote [CxE/Df(HOMC)], dorsal view. T1 is fused with the head. The head has the abnormal triangular shape seen in CxE homozygotes. (L) CxE/Cx20, dorsal view. An extra segment is present (bracket) although not as distinct as in Cx20 homozygotes. Head shape is apparently normal.
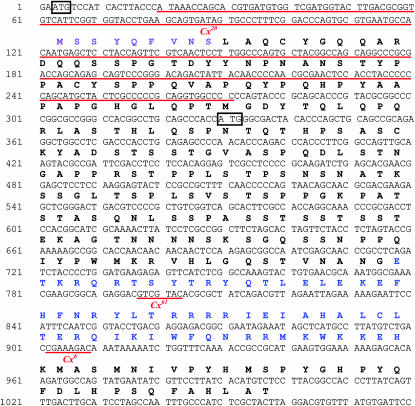
We sequenced the exons of the null alleles described above and identified sequence changes associated with each allele. The Cx61 allele (GenBank accession nos. AY055845 and AY055848) contains a 7-bp deletion within the homeobox (Figure 3). The resulting frameshift should produce a prematurely truncated protein lacking all but a few residues of the homeodomain. The Cx6 allele (GenBank accession nos. AY057858 and AF426395) contains a similar lesion—an 8-bp deletion within the homeobox (Figure 3) that would also result in a truncated protein lacking much of the homeodomain. Thus, the deduced Cx61 and Cx6 proteins are predicted to have no DNA-binding activity and to be nonfunctional.
Figure 3.—
Molecular lesions associated with Cx mutant alleles. The sequence of the Cx 5′-UTR and coding region is annotated with locations of Cx mutant lesions. The encoded amino acids are written above the nucleotide sequence. Significant motifs are color coded: octapeptide (purple) and homeodomain (blue). The nucleotides deleted in various Cx mutant alleles are underlined in red. Two methionine-encoding codons (ATG) that are in frame in the Cx20 allele and could serve as alternative translation initiation sites are boxed in black.
Cx null mutants are defective in both fusion and dorsal extension of dorsal ridge Engrailed stripes:
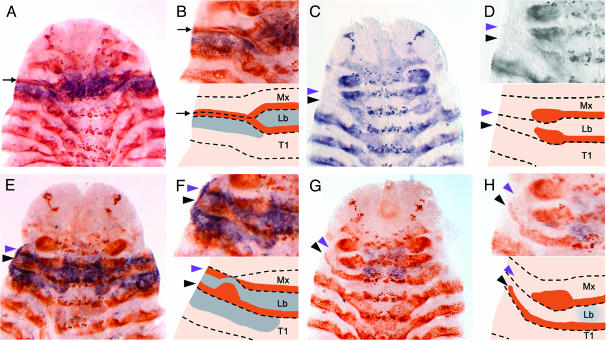
One of the most striking abnormalities found in Cx null larvae is cephalization of the first thoracic segment, due to the absence of a segmental groove between the head and thorax (compare Figure 2, A and B, with Figure 2, C and D). To determine whether this phenotype results from improper development of the dorsal ridge during embryogenesis, we used En expression in Cx null embryos to follow dorsal ridge formation. We found that dorsal ridge development is abnormal (or nonexistent) in Cx null embryos. In these embryos, the maxillary and labial En stripes fail to fuse (Figure 4, C and D). Furthermore, although the embryo continues to grow dorsally, the maxillary and labial En stripes do not. Thus, the dorsal material in the maxillary and labial region of Cx null embryos apparently originates only from the anterior compartment (and not from the En-expressing posterior compartment) of one or both of these segments. An alternative, but rather unlikely, possibility is that the posterior compartment cells continue to extend dorsally, but stop expressing En.
Figure 4.—
Expression patterns of En and Cx in wild-type and Cx mutant embryos. All embryos are shown in ventral view and have begun the process of germband retraction. In schematic views, brown represents En expression and blue represents Cx. Segments are labeled as follows: maxillary (Mx), labial (Lb), and prothoracic (T1). (A) Wild type, En (brown), and Cx (purple) expression. The maxillary and labial En stripes have fused and the dorsal ridge En stripe (black arrow) extends dorsally. Cx is expressed ventrally in the labial appendages, the anterior compartment of the labial segment, and the posterior compartment of the maxillary segment, as well as dorsolaterally in the anterior compartment of T1. Cx is also expressed in some cells of the developing dorsal ridge. (B) Enlarged view of A (top) and schematic representation of En and Cx expression (bottom). (C) Cx6 homozygote, En expression (purple). The maxillary (purple arrowhead) and labial (black arrowhead) En stripes have not fused and neither extends dorsally. The apparent contact between stripes is likely due to continued proliferation of En-positive cells, resulting in thickening of the ends of the En stripes. (D) Enlarged view of C (top) and schematic representation of En and Cx expression (bottom). (E) Cx20 homozygote, En (brown), and Cx (purple) expression. The maxillary (purple arrowhead) and labial (black arrowhead) En stripes have not fused. Instead, two stripes extend to the dorsal edge of the embryo. Cx is expressed within its normal domain, and additionally in the ectopic dorsolateral material between the two En stripes. (F) Enlarged view of E (top) and schematic representation of En and Cx expression (bottom). (G) CxE homozygote, En (brown), and Cx (purple) expression. The maxillary (purple arrowhead) and labial (black arrowhead) En stripes have not fused. Only the labial En stripe extends dorsally. Cx is expressed in the CNS of the maxillary and labial segments, but is absent or very faint in the ectoderm. (H) Enlarged view of G (top) and schematic representation of En and Cx expression (bottom).
Extra segment formation in Cx20 mutants is due to partial loss of Cx function:
Beeman et al. (1993) described another Cx allele, Cx1, which has been renamed Cx20. Cx20/Cx20 larvae have a very different phenotype from Cx null larvae. The labial appendages retain a palp-like morphology, but are abnormal in size and position. In the most mildly affected individuals (Figure 2E), fusion of the labial appendages is abnormal (only the most basal segment, the mentum, is fused) and the position of the labial appendages is more posterior than in wild type. The most severely affected larvae (Figure 2F) have larger-than-normal labial appendages that are completely unfused and occupy a more posterior and lateral position, such that they more closely resemble the maxillary appendages. In all of these larvae, the labial appendages are closer than normal to the posterior edge of the head. This may result from reduction of the posterior ventral head, or it may simply reflect the failure of the maxillary and labial palps to migrate anteriorly during development. The abnormal orientation of the maxillary palps is probably an indirect effect of the labial appendage defects. Defects in dorsal closure (evidenced by puckering of the cuticle at the dorsal midline) are also common in Cx20 mutants.
The most striking feature of Cx20 homozygotes is an extra dorsal segment that forms between the head and T1. This supernumerary segment was previously interpreted as a duplication of the dorsal portion of T1 (Beeman et al. 1993). However, the data that we present below suggest an alternative explanation for the presence of additional dorsal material.
The size of the extra segment in Cx20 homozygotes (denoted by brackets in Figure 2, F and G) is somewhat variable. Individuals with more severe labial appendage abnormalities typically have a more extensive extra segment (compare Figure 2E with Figure 2F). All Cx20 effects are completely recessive, since Cx20 heterozygotes are phenotypically normal (data not shown).
To further characterize the nature of the Cx20 allele, we analyzed its effect in the presence of other Cx alleles. Larvae hemizygous for Cx20 (Figure 2H) or heteroallelic for Cx20 and the hypomorphic allele CxE (Figure 2L) (see below) develop the extra segment, suggesting that this phenotype is seen only when the Cx20 allele is present and wild-type Cx function is either absent or reduced. These larvae also show dorsal closure defects and apparent reduction of the posterior head. In addition, the labial appendages of Cx20/CxE and Cx20/Df larvae (data not shown) are more severely affected than those of Cx20/Cx20 larvae in that they are larger and positioned more laterally (i.e., more closely resembling the maxillary appendages). These results suggest that the Cx20 phenotype is due to loss-of-function effects. We discuss in greater detail below our conclusion that Cx20 is an unusual hypomorphic allele.
Cx20 encodes a protein lacking the normal N terminus:
To identify the molecular lesion associated with Cx20, we performed Southern analysis of Cx20/+ and wild-type genomic DNA using a probe from the 5′-end of the Cx transcription unit. We identified a HindIII restriction fragment length polymorphism (RFLP) (2.6 kb from Cx20 vs. 2.9 kb from wild type) associated with the Cx20 mutation (data not shown). We cloned these fragments using inverse PCR and sequenced them to determine the cause of the RFLP. The Cx20-specific fragment has a 252-bp deletion in the 5′ region of the transcription unit that removes 149 bp of coding region, including the probable translation start site deduced by Curtis et al. (2001) from sequence conservation (Figure 3). We confirmed this deletion by sequencing the Cx exons from the Cx20 allele (GenBank accession nos. AF416772 and AF416773). No additional changes affecting the encoded amino acid sequence were found.
The absence of the putative Cx translation start site in Cx20 mutants presents an interesting dilemma. Since Cx20 is not a null allele, and immunostaining (see below) indicates that Cx protein is produced, translation must initiate at an alternative site. Two methionine codons in frame with the YPWM-encoding region (one upstream and one downstream of the predicted start) are obvious candidates (Figure 3). We used the ATGpr program (Salamov et al. 1998) to analyze the likelihood of each of these codons being used for initiation. The probable wild-type initiation codon received the highest score (64%). The more 3′ ATG received a slightly higher score (56%) than the 5′ ATG (49%). Either, or both, of these sites could conceivably serve as a translation initiation site for the Cx20 allele.
In addition to the normal translation start site, the Cx20 deletion removes the region encoding the highly conserved octapeptide motif (Zhao et al. 1996) (purple in Figure 3). This sequence is found in all known Scr family members, including those of vertebrates, although an additional amino acid is present in the insect orthologs. Another motif shared by the Tribolium, Bombyx mori, and Apis mellifera orthologs, but not by the known dipteran or vertebrate family members (Curtis et al. 2001 and data not shown), is also deleted.
An extra Engrailed stripe extends dorsally in Cx20 homozygotes:
The presence of an extra segment between the head and the thorax of Cx20 homozygotes is suggestive of a dorsal ridge defect distinct from the one seen in Cx null homozygotes. Indeed, En expression in Cx20 homozygotes is abnormal. As in Cx null larvae, the maxillary and labial En stripes do not fuse. In this case, however, two stripes (rather than a single dorsal ridge stripe) extend to the dorso-lateral edges of the gnathal region of the embryo (Figure 4, E and F). One of these stripes is clearly an extension of the labial En stripe (Figure 5A). The second stripe, however, appears to arise de novo just anterior to the labial En stripe (Figure 5A). The presence of two En stripes correlates with the presence of two segmental grooves (one on either side of the additional dorsal segment) in Cx20 larvae. In Drosophila, segmental groove formation requires coexpression of En and Hedgehog (Hh) (Larsen et al. 2003). Therefore, we used in situ hybridization to examine the expression of the Tribolium ortholog of hh (Tc hh) at the head/thorax boundary in wild-type and Cx20 embryos. Tc hh is coexpressed with En, both in the wild-type dorsal ridge (data not shown) and in the two En stripes of Cx20 homozygotes (Figure 5B). Thus there is a correlation between the expression of En and Tc hh and the number of segmental grooves at the head/thorax boundary.
Figure 5.—
Expression of En and Tc hh in Cx20 embryos. (A) En expression in a Cx20 homozygote. The labial En stripe extends to the dorsolateral edge of the embryo (arrow). A second stripe of En expression (arrowhead) appears anterior to the labial En stripe. This new expression domain is discontinuous with but roughly parallel to the maxillary En stripe. (B) Tc hh and En expression in a Cx20 homozygote. Tc hh (purple) and En (brown) are coexpressed in the labial segment (arrow) and in the more anterior expression domain (arrowhead).
We also compared Cx expression in Cx20 homozygotes and wild-type embryos using an antibody to Drosophila Scr that cross-reacts with Cx protein (Curtis et al. 2001). As the dorsal ridge forms in wild-type embryos, En expression at the lateral edge of the labial segment links the maxillary and labial En stripes, forming a ring of En expression that encompasses Cx expression in the anterior compartment of the labial segment (Figure 4, A and B). Some cells in the posterior maxillary segment and the extending dorsal ridge appear to express both Cx and En. As described above, the maxillary and labial En stripes of Cx20 homozygotes do not fuse, so the ring of En expression does not form. Instead, the labial En stripe and a second, more anterior En stripe of uncertain origin extend to the dorsal edge of the embryo. Cx is expressed between these two En stripes, suggesting that this region is made up of cells from the anterior compartment of the labial segment.
CxE represents a new class of Cx allele:
In an EMS mutagenesis, we isolated a new allele of Cx whose phenotype differs from the other Cx alleles. Larvae homozygous for this allele, CxE, have labial appendages that resemble maxillary appendages in size and position, but lack the basal endite lobes characteristic of maxillary appendages (Figure 2I). CxE homozygotes have a segmental groove between the head and T1 (Figure 2J) but it is not as well defined as that of wild-type larvae. No extra segment is present. CxE homozygotes also display posterior head reduction and dorsal closure defects similar to those associated with Cx20. In addition, these individuals have abnormally shaped heads (Figure 2, I and J) that resemble those of TcDfd mutants (Brown et al. 2000) in that the head is triangular and the labrum is pointed and angled upwards (S. J. Brown, unpublished results). When CxE is heterozygous with Cx20, head shape is apparently normal, although these individuals have dorsal closure defects and the extra segment associated with Cx20 (Figure 2L) as well as maxillary-like labial appendages and reduced posterior head structures (data not shown). CxE hemizygotes have labial appendages similar, if not identical, to CxE homozygotes and CxE/Cx20 individuals (data not shown). They also display the abnormal head shape and dorsal closure defects associated with CxE (Figure 2K). But unlike homozygotes, CxE hemizygotes exhibit T1 cephalization (Figure 2K). It is somewhat difficult to classify the CxE allele on the basis of these phenotypes. The labial appendage phenotype of CxE homozygotes and hemizygotes and the cephalization of hemizygotes suggest that CxE is a loss-of-function allele. However, the triangular head shape is a novel effect not seen in Cx RNA interference (RNAi) (Curtis et al. 2001) or in known loss-of-function alleles (see above). We sequenced the exons of CxE (GenBank accession nos. AY057859 and AF426396) and found no changes that would alter the amino acid sequence of the encoded protein.
Cx expression and dorsal ridge formation are abnormal in CxE homozygotes:
Since the other two classes of Cx alleles showed dorsal ridge abnormalities, we examined En expression in CxE homozygotes. Interestingly, these individuals show yet another type of dorsal ridge defect. As in the other allele classes, the maxillary and labial En stripes do not fuse. In this case, however, a patchy labial En stripe extends laterally (Figure 4, G and H).
Examination of Cx expression in CxE/CxE embryos reveals an abnormal expression pattern that is first evident during germband elongation. In wild-type embryos of this stage, Cx is expressed throughout the posterior compartment of the maxillary segment (coincident with the En stripe) as well as in the anterior compartment of the labial segment. In CxE homozygotes, posterior maxillary expression is limited to cells near the ventral midline (data not shown). Expression in the labial segment is apparently normal. Later, during germband retraction, Cx expression is faint, or absent, in the ectoderm, but remains detectable in the central nervous system (CNS) (Figure 4, G and H). Since all these changes in the Cx expression pattern represent loss of normal expression rather than gain of novel expression, we conclude that CxE is a hypomorphic allele. These expression changes, as well as the absence of a molecular lesion within the Cx exons, suggest that the CxE mutation probably affects transcriptional regulation of Cx.
DISCUSSION
Overview:
We have identified three classes of Cx mutations on the basis of their effects on the labial appendages and dorsal ridge. Since null alleles, by definition, lack all function, we can use this class to infer the normal functions of Cx in these structures. We can gain additional insight by examining which functions are affected by the partial loss-of-function alleles and correlating this information with what is known about the nature of these mutations.
CxE appears to be a regulatory mutation that causes diminished levels of Cx expression in portions of its normal domain. Thus, as we discuss below, the CxE phenotype may help us to understand the role Cx expression plays in these regions.
The Cx20 allele is associated with a deletion that removes the region encoding the N terminus of the protein. There are several conceivable ways in which this lesion could produce a mutant phenotype. The resulting protein could have acquired a new function. However, most gain-of-function mutations are dominant, while the extra segment phenotype produced by Cx20 is recessive. Furthermore, the labial appendage abnormalities seen in Cx20 homozygotes are reminiscent of those seen in larvae mildly affected by Cx RNAi (Curtis et al. 2001). This observation might suggest an overall reduction in Cx function. Such a reduction could occur if removal of the preferred translation start site leads to decreased levels of protein production, or if loss of the N-terminal region of the protein causes a reduction in protein function or stability. However, not all aspects of the Cx20 phenotype can be explained by a general reduction in Cx level or function. The extra segment phenotype is never seen in Cx RNAi individuals. For this reason, we favor the hypothesis that the N terminus of the Cx protein is specifically required for a particular subset of Cx functions and that the Cx20 phenotype reflects the presence of a protein that retains some Cx functions but is completely deficient in others (see below).
Labial appendage development:
Previous studies have implicated both Cx and mxp in specification of the labial appendages (Beeman et al. 1993; Shippy et al. 2000; DeCamillis et al. 2001). DeCamillis et al. (2001) showed that Cx regulates transcription of mxp, either directly or indirectly, such that removal of Cx eliminates mxp function as well. Loss of both gene functions results in transformation of the labial appendages to antennae. Weaker Cx mutations (Cx20 and CxE) do not cause transformation to antennae, but rather result in unfused labial appendages that more closely resemble the maxillary appendages in size, shape, and lateral position. The unmistakably palp-like morphology of these appendages probably reflects the presence of mxp, given the essential role of mxp in maxillary and labial palp formation (Shippy et al. 2000). Thus, the level of Cx function in these mutants is apparently sufficient to activate mxp expression.
The wild-type labial appendages of Tribolium larvae differ from the other pairs of appendages in that their proximal segments are fused at the ventral midline. This fusion follows the movement of the labial appendage primordia ventrally and anteriorly from their original lateral position to their final location between the maxillary appendages. Labial appendage migration and fusion do not require mxp function. The labial appendages of mxp null mutants are fully transformed to legs, but are basally fused and occupy their usual location (Shippy et al. 2000). In contrast, the labial appendages of Cx nulls (which are transformed to antennae) are not fused and remain in a lateral position. The labial appendages are also unfused in larvae carrying weaker Cx alleles, although their position is quite variable. These observations suggest that Cx is responsible for the migration and fusion of the wild-type labial appendages. A similar role has been suggested for orthologs of Cx in other insects (Pattatucci et al. 1991; Rogers et al. 1997; Hughes and Kaufman 2000).
Dorsal ridge development:
Dorsal ridge development, as visualized by expression of the posterior compartment marker En, has been assayed in a number of insects and appears to be relatively well conserved (Rogers and Kaufman 1996, 1997). Very little is known, however, about the regulation of steps involved in this developmental process. Below we discuss how our analysis of Tribolium Cx mutants has provided insight into several aspects of dorsal ridge development.
Engrailed stripe fusion:
The first evidence of dorsal ridge formation in wild-type embryos is the connection of the maxillary and labial En stripes by the appearance of En expression along the lateral edges of the anterior compartment of the labial segment. Since this “fusion” does not occur in Cx null embryos, we conclude that Cx is responsible for the unique behavior of these En stripes. The maxillary and labial En stripes also fail to fuse in Cx20 and CxE homozygotes. Thus, this event apparently requires the N terminus of Cx, as well as expression of Cx in at least one of the domains affected in CxE mutants. At the time the maxillary and labial En stripes begin to fuse, Cx is expressed in the posterior compartment of the maxillary segment and the anterior compartment of the labial segment. CxE homozygotes have little or no Cx expression in the lateral regions of the posterior maxillary segment, but apparently normal expression in the anterior compartment of the labial segment. This could mean that maxillary Cx expression is required for fusion of the En stripes. Taken together, the phenotypes produced by each of these allele classes indicate that fusion of the maxillary and labial En stripes is an essential step in normal head development. One unresolved issue is the mechanism by which these En stripes fuse. Do En-expressing posterior compartment cells move along the edges of the labial segment? If so, do the migrating cells originate from the maxillary or labial segment, or both? Or does En expression appear de novo in anterior compartment cells instead? There have been previous reports of en expression in the anterior compartment of Drosophila wing discs, but in that case hh expression remains limited to the posterior compartment (Blair 1992; Strigini and Cohen 1997). Likewise, En is expressed in the anterior compartment of the eighth abdominal segment in Drosophila, but hh is not (Merabet et al. 2005). We have observed that Tc hh is coexpressed with En along the edges of the labial segment (data not shown), suggesting that these cells derive from the posterior compartment. However, the simultaneous appearance of En expression along the entire anterior compartment might suggest that “de novo” expression is more likely.
Engrailed stripe extension:
During dorsal closure in wild-type embryos, En stripes in the thorax and abdomen extend to the dorsolateral edges of the embryo. Likewise, the fused dorsal ridge En stripe (apparently composed of the maxillary and labial En stripes) extends to the dorsolateral edge of the embryo. The maxillary and labial En stripes fail to fuse in all three Cx mutant classes, but the subsequent fate of these stripes differs in each class. In Cx null mutants, neither En stripe extends dorsally. This indicates that Cx is required for dorsolateral extension of En in the maxillary/labial region. The ability to promote dorsolateral En expression seems to be a function shared by many Tribolium Hox genes, since in Df(HOMC) homozygotes (which lack most of the Hox genes) all of the En stripes fail to extend (T. D. Shippy, unpublished results).
In CxE homozygotes, the labial En stripe extends, but the maxillary En stripe does not. This difference seems to correlate with the more severe reduction in Cx expression in the posterior maxillary segment in CxE mutants. In Cx20 homozygotes, the labial En stripe extends dorsolaterally to the edges of the embryo, thus behaving like the thoracic and abdominal En stripes. In addition, a second En stripe appears anterior to the labial En stripe and extends dorsolaterally. This suggests that the Cx protein missing its N terminus cannot promote fusion of the maxillary and labial En stripes, but is sufficient for En stripe extension.
Function of the Cx N-terminal domain:
The simplest interpretation of our data is that the N-terminal region of Cx includes a domain that controls maxillary and labial En stripe fusion. Interestingly, the region missing from the Cx20 protein contains the octapeptide motif that is present in many Hox genes. Zhao et al. (1996) reported that deletion of the N terminus of Hox-a5 (a mouse Scr homolog) decreased its transactivation ability in in vitro assays and its ability to produce homeotic transformations when ectopically expressed in Drosophila. Furthermore, Tour et al. (2005) recently showed that Ultrabithorax lacking its N terminus can repress target genes when expressed in Drosophila, but its ability to activate target genes is greatly reduced. Likewise, deletion of the Scr N terminus reduces its ability to activate target genes. Extrapolating from these observations, our current model is that the N-terminally truncated protein produced from the Cx20 allele is capable of repression but not activation of target genes. This model is consistent with the loss of particular functions that we see in Cx20 mutants and predicts that fusion of the maxillary and labial En stripe during dorsal ridge formation requires activation of target genes, while dorsolateral extension of En stripes requires repression of target genes (perhaps genes that negatively regulate en).
Origin and identity of the extra segment in Cx20:
In wild-type embryos, the anterior compartment of the labial segment (marked by Cx expression) does not extend dorsolaterally after En expression appears along its lateral edges. Rather, it is restricted to a ventral position encircled by En expression. However, in Cx20 homozygotes, the anterior compartment of the labial segment (visible as a Cx-expressing domain between the two En stripes) clearly extends dorsally. Thus we conclude that Cx function is required to prevent dorsal extension of the anterior compartment of the labial segment and that the N terminus of Cx is required for this function. We believe that the dorsal growth or movement of these cells is responsible for the extra dorsal material observed in Cx20 homozygotes. The additional hh/En stripe that forms at the anterior border of this region probably produces the segmental groove at the anterior border of the extra segment, while the extension of the labial En stripe seems to correlate with the more posterior segmental groove. This interpretation sheds new light on the identity of the extra segment, suggesting that it is not a duplication of dorsal T1, but rather the uncharacteristic formation of a dorsal labial segment. Morphological similarities to dorsal T1 can be attributed to the expression of Cx in both regions. It is likely that the labial segment also has a dorsal component in Cx null and CxE homozygotes, but no labial segment-specific marker is currently available to test this possibility. The presence of extra dorsal material could contribute to the abnormal head shape seen in CxE homozygotes.
The mechanism responsible for the repression of the dorsal component of the anterior labial segment is unclear. Cx might actively suppress dorsal extension of the anterior compartment, either by preventing cell division and/or migration or by inducing apoptosis. Alternatively, the repression may be an indirect result of En stripe fusion (which we have shown to be dependent on Cx function). Numerous studies in Drosophila have shown that posterior compartment cells (which express En) and anterior compartment cells (which do not express En) segregate from one another (reviewed in Dahmann and Basler 1999). Thus, the En-expressing cells surrounding the anterior compartment might act as a barrier against dorsolateral migration, effectively imprisoning the anterior compartment cells.
Conclusion:
We have shown that function of the homeotic gene Cx is crucial for formation of the dorsal ridge in Tribolium. Given that the events of dorsal ridge development are conserved in a wide variety of insects, it will be interesting to determine whether the upstream factors governing this process are also conserved. Closer analysis of dorsal ridge formation in Drosophila Scr mutants will be a first step, but perturbation of Scr ortholog function(s) in other insects (perhaps by RNAi) will be important as well.
Acknowledgments
We are grateful to Kathy Leonard, Sue Haas, Kay Hummels, Deane Lehmann, and Michelle Coleman for technical assistance and to Yoshinori Tomoyasu for the Tribolium hedgehog clone as well as for helpful discussions and critical reading of the manuscript. We thank Thom Kaufman for the kind gift of the antibody to Scr. We thank Kent Hampton and the Kansas Agricultural Experiment Station Scanning Electron Microscope Laboratory (administered by the Department of Entomology at Kansas State University) for assistance with the SEMs. This work was supported by grants to R.E.D., S.J.B., and R.W.B. from the National Institutes of Health (R01HD029594) and the National Science Foundation (IBN-0321882).
References
- Beeman, R. W., J. J. Stuart, M. S. Haas and R. E. Denell, 1989. Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev. Biol. 133: 196–209. [DOI] [PubMed] [Google Scholar]
- Beeman, R. W., J. J. Stuart, S. J. Brown and R. E. Denell, 1993. Structure and function of the homeotic gene complex (HOM-C) in the beetle, Tribolium castaneum. BioEssays 15: 439–444. [DOI] [PubMed] [Google Scholar]
- Blair, S. S., 1992. Engrailed expression in the anterior lineage compartment of the developing wing blade of Drosophila. Development 115: 21–33. [DOI] [PubMed] [Google Scholar]
- Brown, S. J., J. K. Henry, W. C. Black and R. E. Denell, 1990. Molecular genetic manipulation of the red flour beetle: genome organization and cloning of a ribosomal protein gene. Insect Biochem. 20: 185–193. [Google Scholar]
- Brown, S. J., N. H. Patel and R. E. Denell, 1994. Embryonic expression of the single Tribolium engrailed homolog. Dev. Genet. 15: 7–18. [DOI] [PubMed] [Google Scholar]
- Brown, S., S. Holtzman, T. Kaufman and R. Denell, 1999. Characterization of the Tribolium Deformed ortholog and its ability to directly regulate Deformed target genes in the rescue of a Drosophila Deformed null mutant. Dev. Genes Evol. 209: 389–398. [DOI] [PubMed] [Google Scholar]
- Brown, S., M. DeCamillis, K. Gonzales-Charneco, M. Denell, R. Beeman et al., 2000. Implications of the Tribolium Deformed mutant phenotype for the evolution of Hox gene function. Proc. Natl. Acad. Sci. USA 97: 4510–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, S. B., S. DiNardo, P. H. O'Farrell, R. A. White and M. P. Scott, 1988. Temporal and spatial relationships between segmentation and homeotic gene expression in Drosophila embryos: distributions of the fushi tarazu, engrailed, Sex combs reduced, Antennapedia, and Ultrabithorax proteins. Genes Dev. 2: 350–360. [DOI] [PubMed] [Google Scholar]
- Curtis, C. D., J. A. Brisson, M. A. DeCamillis, T. D. Shippy, S. J. Brown et al., 2001. Molecular characterization of Cephalothorax, the Tribolium ortholog of Sex combs reduced. Genesis 30: 12–20. [DOI] [PubMed] [Google Scholar]
- Dahmann, C., and K. Basler, 1999. Compartment boundaries: at the edge of development. Trends Genet. 15: 320–326. [DOI] [PubMed] [Google Scholar]
- DeCamillis, M. A., D. L. Lewis, S. J. Brown, R. W. Beeman and R. E. Denell, 2001. Interactions of the Tribolium Sex combs reduced and proboscipedia orthologs in embryonic labial development. Genetics 159: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor, G. B., C. R. Preston, D. M. Johnson-Schlitz, N. A. Nassif, R. W. Phillis et al., 1993. Type I repressors of P-element mobility. Genetics 135: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman, M. J., and T. C. Kaufman, 1995. Genetic analysis of embryonic cis-acting regulatory elements of the Drosophila homeotic gene sex combs reduced. Genetics 140: 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, C. L., and T. C. Kaufman, 2000. RNAi analysis of Deformed, proboscipedia and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the hemipteran head. Development 127: 3683–3694. [DOI] [PubMed] [Google Scholar]
- Larsen, C. W., E. Hirst, C. Alexandre and J.-P. Vincent, 2003. Segment boundary formation in Drosophila embryos. Development 130: 5625–5635. [DOI] [PubMed] [Google Scholar]
- Merabet, S., J. C. Hombria, N. Hu, J. Pradel and Y. Graba, 2005. Hox-controlled reorganisation of intrasegmental patterning cues underlies Drosophila posterior spiracle organogenesis. Development 132: 3093–3102. [DOI] [PubMed] [Google Scholar]
- Nagaso, H., T. Murata, N. Day and K. K. Yokoyama, 2001. Simultaneous detection of RNA and protein by in situ hybridization and immunological staining. J. Histochem. Cytochem. 49: 1177–1182. [DOI] [PubMed] [Google Scholar]
- Patel, N. H., T. B. Kornberg and C. S. Goodman, 1989. Expression of engrailed during segmentation in grasshopper and crayfish. Development 107: 201–212. [DOI] [PubMed] [Google Scholar]
- Pattatucci, A. M., D. C. Otteson and T. C. Kaufman, 1991. A functional and structural analysis of the Sex combs reduced locus of Drosophila melanogaster. Genetics 129: 423–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, B. T., and T. C. Kaufman, 1996. Structure of the insect head as revealed by the EN protein pattern in developing embryos. Development 122: 3419–3432. [DOI] [PubMed] [Google Scholar]
- Rogers, B. T., and T. C. Kaufman, 1997. Structure of the insect head in ontogeny and phylogeny: a view from Drosophila. Int. Rev. Cytol. 174: 1–84. [DOI] [PubMed] [Google Scholar]
- Rogers, B. T., M. D. Peterson and T. C. Kaufman, 1997. Evolution of the insect body plan as revealed by the Sex combs reduced expression pattern. Development 124: 149–157. [DOI] [PubMed] [Google Scholar]
- Salamov, A. A., T. Nishikawa and M. B. Swindells, 1998. Assessing protein coding region integrity in cDNA sequencing projects. Bioinformatics 14: 384–390. [DOI] [PubMed] [Google Scholar]
- Shippy, T. D., J. Guo, S. J. Brown, R. W. Beeman and R. E. Denell, 2000. Analysis of maxillopedia expression pattern and larval cuticular phenotype in wild-type and mutant tribolium. Genetics 155: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini, M., and S. M. Cohen, 1997. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124: 4697–4705. [DOI] [PubMed] [Google Scholar]
- Tour, E., C. T. Hittinger and W. McGinnis, 2005. Evolutionarily conserved domains required for activation and repression functions of the Drosophila Hox protein Ultrabithorax. Development 132: 5271–5281. [DOI] [PubMed] [Google Scholar]
- Younossi-Hartenstein, A., U. Tepass and V. Hartenstein, 1993. Embryonic origin of the imaginal discs of the head of Drosophila melanogaster. Roux's Arch. Dev. Biol. 203: 60–73. [DOI] [PubMed] [Google Scholar]
- Zhao, J. J., R. A. Lazzarini and L. Pick, 1996. Functional dissection of the mouse Hox-a5 gene. EMBO J. 15: 1313–1322. [PMC free article] [PubMed] [Google Scholar]