Abstract
The ability to process two or more rapidly presented, successive, auditory stimuli is believed to underlie successful language acquisition. Likewise, deficits in rapid auditory processing of both verbal and nonverbal stimuli are characteristic of individuals with developmental language disorders such as Specific Language Impairment. Auditory processing abilities are well developed in infancy, and thus such deficits should be detectable in infants. In the studies presented here, converging methodologies are used to examine such abilities in infants with and without a family history of language disorder. Behavioral measures, including assessments of infant information processing, and an EEG/event-related potential (ERP) paradigm are used concurrently. Results suggest that rapid auditory processing skills differ as a function of family history and are predictive of later language outcome. Further, these paradigms may prove to be sensitive tools for identifying children with poor processing skills in infancy and thus at a higher risk for developing a language disorder.
Keywords: auditory processing, language impairment, development, infants
From birth, infants possess remarkably sophisticated acoustic capabilities allowing the perception of speech as well as nonspeech sounds (Aslin & Hunt, 2001; Werner & Rubel, 1992). One of the skills critical to the acquisition of language is the ability to process and categorize brief, rapidly changing auditory stimuli (signals) occurring within tens of milliseconds. These acoustic temporospectral cues are critically important for identifying and distinguishing formant transitions within speech, and are discriminated by infants well before speech is produced (for reviews, see Jusczyk, 1997; Kuhl, 1987). On the basis of neurophysiological, neuroanatomical, and behavioral evidence, we argue that differences as well as deficits in these abilities are evident from early infancy (Benasich & Tallal, 1996, in press Fitch, Read, & Benasich, 2001). The studies presented here examine cognitive and language development prospectively and longitudinally to determine what impact early differences in rapid auditory processing abilities may have on emergent language as well as on general cognitive abilities. The use of converging methodologies that focus on each child’s behavioral and electrophysiological responses across time allows for the examination of individual differences in developmental trajectories across concurrent and successive measures. Thus, one may begin to examine how these interactive areas change in relation to one another, a necessity to answer the questions posed.
This article addresses a number of longstanding and intensely debated questions regarding the relation between rapid auditory processing (RAP) abilities and language-based learning impairments. First, do RAP deficits simply co-occur with the difficulties in phonological and syntactic decoding seen in children with developmental language-based learning impairments, or do these deficits precede and predict language outcomes? Second, what can the study of infant information processing contribute to longstanding questions as to the etiology and pattern of deficits seen in children with these types of disorders? Finally, we consider the role that efficient processing of basic acoustic properties early in life plays in subsequent language competence.
About 5 to 10% of children are reported to show developmental speech or language disorders (for review, see Leonard, 1998). Children with Specific Language Impairment (SLI) are characterized as having significantly delayed acquisition of language without the presence of mental retardation, impaired hearing or vision, childhood schizophrenia, infantile autism, or neurological causes such as seizures or motor paralysis. Children with SLI have extreme difficulty in acquiring language while other cognitive abilities appear to remain relatively intact. Delays in learning the semantic and syntactic rules critical to the development of language have most often been proposed as a primary determinant of SLI, although other factors are increasingly being implicated including poor phonological processing (Bird, Bishop, & Freeman, 1995; for review, see Leonard, 1998). In fact, a more basic difficulty in processing rapidly presented, brief auditory stimuli, for both speech and nonspeech stimuli, may underlie the poor phonological skills so frequently observed in SLI populations (Leonard, 1998; Tallal, Merzenich, Miller, & Jenkins, 1998; Wright, Bowen, & Zecker, 2000).
A large and growing literature suggests that impaired perception and discrimination of auditory stimuli that involve two or more rapidly presented, transient elements may hinder development of normal language and reading abilities (Godfrey, Syrdal-Lasky, Millay, & Knox, 1981; Kraus, McGee, Carrell, Zecker, Nicol, & Koch, 1996; McAnally & Stein, 1997; Nagarajan et al., 1999; Reed, 1989; Snowling, Goulandris, Bowlby, & Howell, 1986; Stark & Heinz, 1996a, 1996b; Werker & Tees, 1987; Wright et al., 2000). Children with deficits in RAP hear normally and can sequence sounds, but are selectively impaired in their ability to both perceive and produce those speech sounds which are characterized by brief or rapidly changing temporal cues (for reviews, see Fitch, Miller, & Tallal, 1997; Tallal & Benasich, in press). Much of the sensory processing necessary for language comprehension and production occurs within a brief window of time. Thus, to process speech it is necessary to hear and respond to auditory cues (in the tens of milliseconds) that signal what words are being produced. Such information is carried by elements of the speech sound that are called “formants,” which represent sound waveforms across time. The rapid transitional cues that facilitate the decoding of language are contained within the consonants, in particular, stop consonants such as /p/, /b/, /d/, and /g/. These critical transitional cues are short (~40 ms) and must be processed if speech is to be perceived accurately. Therefore, RAP limitations may directly interfere with adequate perception of spoken language and may disrupt efficient processing of the speech stream.
Problems decoding linguistic stimuli often co-occur with difficulties in nonspeech RAP. It has been hypothesized that the difficulties described earlier in processing brief, rapid, successive auditory cues could impair or delay the formation of distinct, phonological representations and thus possibly play a causal role in SLI (Benasich & Read, 1998; Tallal, Miller, Jenkins, & Merzenich, 1997). However, this hypothesis has not been universally supported (e.g., Martin, 1995; Mody, Studdert-Kennedy, & Brady, 1997, but see Denenberg, 1999, 2001; Leonard, 1998). At the heart of this theoretical controversy are issues of linguistic specificity and neural modularity for language development Liberman, 1996). In other words, are there dedicated neuroanatomical “modules” in the human brain that allow processing and computation of speech, or can more general sensory and learning mechanisms account for the development of language? Although a number of studies have failed to find links between RAP deficits and language or reading deficits, differences among study outcomes may arise for a number of reasons including a very basic one—the use of different subject selection criteria. Various studies have focused on children with SLI, children with dyslexia, children with reading abilities at the lower end of a normal distribution compared to more skilled readers, and adults with a childhood history of reading or language problems (Denenberg, 1999). Thus, it is not particularly surprising that language outcomes as well as perceptual profiles differ across such diverse groups.
Nonetheless, the high incidence of concomitant deficits in the auditory, visual, and motor systems seen in individuals with SLI is consistent with the notion that some underlying, more basic impairment impacts multiple systems (Fawcett, Nicolson, & Dean, 1996; Nicolson et al., 1999; Stein, in press). Research has shown that individuals with developmental language disorders exhibit rapid processing deficits in other modalities, including motor coordination, and tactile and visual perception (for a review, see Stein & Talcott, 1999; Tallal, 2000; Witton et al., 1998).1 Of particular interest are studies of visual processing with dyslexic individuals revealing that the ability to process fast, low-contrast, visual stimuli is impaired relative to controls. The system responsible for processing this type of information is called the magnocellular pathway of the visual system. This magnocellular deficit is evident in behavioral assessment (e.g., Witton et al., 1998) and also is reflected in neurophysiological measurements. Functional brain imaging of dyslexic subjects using event-related potentials (ERPs)2 and fMRI to rapidly presented visual stimuli reveals longer latencies and less activation than controls, reflecting a slower neural response (Demb, Boynton, & Heeger, 1997; Eden, VanMeter, Rumsey, Maisog, et al., 1996; Livingstone, Rosen, Drislane, & Galaburda, 1991; Neville, Coffey, Holcomb, & Tallal, 1993; Temple et al., 2000). However, other research has shown that these deficits are not as straightforward as first assumed and that they may not be unique to children with specific reading disability or be limited solely to left hemisphere dysfunction (e.g., Eden, VanMeter, Rumsey, & Zeffiro, 1996).
Postmortem analyses of the brains of humans with dyslexia have contributed information suggesting that neuropathological differences might originate in the prenatal period during neuronal migration. Tiny cortical malformations including ectopias and microgyric lesions have been reported (Cohen, Campbell, & Yagmai, 1989; Galaburda & Kemper, 1979; Galaburda, Sherman, Rosen, Aboitiz, & Geschwind, 1985; Humphreys, Kaufmann, & Galaburda, 1990). Further, Galaburda and colleagues have shown smaller and fewer magnocellular neurons in the visual processing area of the thalamus (lateral geniculate nucleus; LGN) of dyslexic human brains as compared to control brains (Galaburda & Livingstone, 1993; Galaburda, Menard, & Rosen, 1994; Livingstone et al., 1991). Similar results were obtained in the medial geniculate nucleus (MGN), the auditory nucleus of the thalamus; Galaburda and colleagues found more small and fewer large neurons in the left MGN of the brains of dyslexic individuals as compared to controls (Galaburda et al., 1994). These findings fit well with the psychophysical deficits seen in both dyslexic and SLI individuals (Cornelissen, Richardson, Mason, Fowler, & Stein, 1995; Lovegrove, Garzia, & Nicholson, 1990; Stein & Talcott, 1999; Stein & Walsh, 1997). The neuropathological abnormalities described in the MGN and cortex of dyslexic humans have been suggested as a viable explanation for poor discrimination of rapidly presented auditory stimuli observed in animal models of SLI (Fitch, Tallal, Brown, Galaburda, & Rosen, 1994; Herman, Fitch, Galaburda, & Rosen, 1995).
Adding to these findings are results of brain imaging studies that suggest neuroanatomic variance between individuals with SLI or dyslexia and normally developing controls (Cowell, Jernigan, Denenberg, & Tallal, 1995; Hagman et al., 1992; Tallal et al., 1990). For example, a series of imaging studies on children with SLI support the view that anomalies in anatomic asymmetry in frontal and posterior temporal regions may be related to the inability of individuals with developmental language disorders to sufficiently activate these same areas (Hagman et al., 1992; Jernigan, Hesselink, Sowell, & Tallal, 1991; Neville et al., 1993; Rumsey et al., 1992; for a review, see Plante, Shenkman, & Clark, 1996).
A number of more recent studies have failed to find significant differences in planum asymmetry (Heiervang et al., 2000; Rumsey et al., 1997). However, one of these studies suggests that morphological differences may be seen in other regions, specifically frontal and parietal areas (Robichon, Levrier, Farnarier, & Habib, 2000), as previously reported by Jernigan and colleagues (Jernigan et al., 1991). Interestingly, in many of these studies, group differences for both anatomical and functional measures were predicted by performance differences on tasks requiring the analysis of rapid acoustic change, regardless of whether the information was verbal or nonverbal. In addition, reduced cortical and subcortical volume in children with SLI (Jernigan et al., 1991) has been reported. In sum, neuroimaging and electrophysiological studies are consistent with the hypothesis that rapid temporal processing mechanisms may subserve speech processing systems in the brain, specifically those that are lateralized to the left hemisphere.
If, as hypothesized, compromised RAP skills that support language indeed underlie difficulties in phonological and linguistic processing, it becomes feasible to examine low-level, non-species-specific, sensory-processing abilities using rodent models. Animal models of impaired RAP capture several critical characteristics of language-based learning disorders in humans. The neuroanatomical abnormalities found in the brains of human dyslexics described earlier can be reproduced in animals: A strain of autoimmune deficient mice (BXSB) spontaneously develop ectopias (Sherman, Galaburda, & Geschwind, 1985; Sherman, Galaburda, Behan, & Rosen, 1987), and cortical microgyria that are histologically similar to those found in the brains of human dyslexics can be induced in rats (Dvoràk & Feit, 1977; Dvoràk, Feit, & Jurankova, 1978; Humphreys, Rosen, Press, Sherman, & Galaburda, 1991; Rosen, Press, Sherman, & Galaburda, 1992). These animals exhibit neuroanatomical changes, behavioral RAP deficits, and electrophysiological components analogous to those found in humans with language impairments.
Studies using the rat model of induced microgyric lesions have revealed that these animals exhibit significant deficits in RAP, both with nonspeech (Clark, Rosen, Tallal, & Fitch, 2000a; Fitch et al., 1997; Fitch et al., 1994; Herman, Galaburda, Fitch, Carter, & Rosen, 1997) and speech-like stimuli (Clark, Tallal, Rosen, Peiffer, & Fitch, 2000b), and exhibit anomalies in the MGN (fewer large and more small neurons), paralleling findings in the thalamus of humans with dyslexia (Galaburda et al., 1994; Herman et al., 1997). Furthermore, ERPs using both surface electrodes and implanted electrodes in mice with ectopias document altered RAP (Frenkel, Sherman, Bashan, Galaburda, & LoTurco, 2000). The animal research summarized here suggests that cortical and subcortical abnormalities observed in animal subjects could contribute to RAP deficits, and human studies seem to parallel those findings (e.g., Herman et al., 1997; Jernigan et al., 1991; Robichon et al., 2000; Tallal, Jernigan, & Trauner, 1994).
To summarize, there is substantial evidence across the literature reviewed here that a basic deficit in processing rapidly presented and brief auditory stimuli, whether speech or nonspeech, could well underlie the poor language skills seen in children with SLI. Moreover, if an error in neuronal migration is implicated, as the neuropathological and animal studies suggest, neural mechanisms that might produce such RAP deficits are likely to occur quite early in life, perhaps as early as the third trimester of prenatal development (Barth, 1987; Njiokiktjien, 1994). This hypothesis is further supported by a large literature reporting that children born to families with a history of SLI are at much higher risk for the disorder than children from families with no such a history (for a review, see Leonard, 1988; for a review of the genetics of language and methodology, see Brzustowicz, 1996). However, to date, no one has examined infants born into families with a history of SLI for deficits in the ability to process brief, rapid, successive auditory information. Given the extraordinary ability of very young infants to discriminate both speech and nonspeech acoustic input, differences in RAP thresholds should be detectable in infants and might serve as a predictor of later language competence (Benasich & Tallal, 1996).
The studies we now describe were designed to systematically examine the contribution of RAP abilities to developing language, and to then determine their relation to subsequent language outcome. In the prospective longitudinal studies summarized here, performance on tasks requiring accurate processing of brief, rapidly presented, successive auditory stimuli and perceptual-cognitive measures were evaluated in two groups of infants: normal control infants and infants with a family history of a language disorder. Children were recruited to participate in one of two ongoing projects: a series of behavioral studies or a combined behavioral and EEG/ERP experiment.
METHODS
Participants Across Studies
Controls (FH−)
Healthy, full-term (≥ 37 weeks gestational age), normal birth weight infants (> 2,500 g), with no history of hearing dysfunction, recent occurrences of otitis media, or family history of congenital hearing loss, speech, language, or reading disorder, were recruited to participate in our studies. The FH− control sample was matched to the experimental samples on age at test, socioeconomic status, and gender.
Family History of LI (FH+)
It is well established that SLI aggregates in families. Children born to families with affected first-degree relatives are approximately four times more likely to develop SLI as compared to children from control families (Bishop, North, & Donlan, 1995; Choudhury & Benasich, 2002; Robinson, 1987; Spitz, Tallal, Flax, & Benasich, 1997; Tallal, Ross, & Curtiss, 1989). Furthermore, behavioral-genetics research has shown that SLI has a highly heritable component (for reviews, see Brzustowicz, 1996; Leonard, 1988) and have reported higher concordance rates for SLI in monozygotic twins (range .70–.96) as compared to dizygotic twins (range .46–.69) (Bishop et al., 1995; Lewis & Thompson, 1992; Tomblin & Buckwalter, 1998).
Accordingly, children born into families with a history of specific language impairments were recruited to participate in the studies described next. All were healthy, full-term infants, with the exception that they had a documented family history of SLI (questionnaire and/or interview data) in a first-degree family member (for further descriptive data on sample characteristics, see Benasich & Tallal, 1996; Choudhury & Benasich, 2002).
Behavioral Studies
Children are typically seen in the laboratory at 6, 9, 12, 16, 24, and 36 months of age, and in some studies, children will be followed through the acquisition of early reading at age 7 years. A perceptual-cognitive battery of tasks, including reinforced conditioned head-turning, habituation, and recognition memory, is used to assess auditory processing of speech and nonspeech stimuli as well as more global measures of information processing in infants. These paradigms tap processing speed as well as memory and discrimination—abilities critical for both linguistic and more general cognitive development. Children’s cognitive development at each age as well as receptive and expressive vocabulary and phonemic discrimination at 12 months of age and older also were assessed.
All infants received habituation and recognition memory tasks. Paradigms for assessing habituation and recognition memory are based on the tendency of infants to differentially fixate novel as compared to familiar visual stimuli. Measures of speed or efficiency of habituation and recognition memory in infants have been shown to predict language as well as general cognitive outcomes (for review, see Bornstein & Sigman, 1986; Columbo, 1993; McCall & Carriger, 1993; Sigman, Cohen, & Beckwith, 1997).
A standard infant-control habituation and recognition memory test using face stimuli was administered (Bornstein & Benasich, 1986). During the habituation phase, the stimuli were continuously available to the infant (until he or she looked away); thus, this task measured overall processing requirements, but did not require rapid processing of transiently presented stimuli.
Infants also received a series of auditory-visual (AV) habituation and recognition memory tasks (same habituation procedure described above) to index RAP abilities. In these paradigms, the infant was habituated to a static visual stimulus (an abstract pattern) coupled with an auditory stimulus (either a 400–400 Hz tone pair, or synthetic consonant vowel syllable /ba/).3 After habituation, the infant received a series of test trials during which the visual stimulus was unchanged but was paired with a novel auditory stimulus (400–600 Hz tone pair, or synthetic consonant vowel syllable /da/) on 50% of the trials. A significant novelty preference (the dependent measure) represents the infant’s ability to discriminate between two auditory stimuli. By varying the characteristics of the tone pair (i.e., lengthening or shortening the amount of time between the tones) or the speech stimulus, we are able to assess auditory discrimination and RAP abilities.
Conditioned Head-Turn Tasks
We used infant conditioning paradigms, also referred to as visually reinforced conditioned head-turn paradigms (for a review, see Kuhl, 1985; Morrongiello, 1990) to assess processing of nonlinguistic auditory stimuli. Two different paradigms were used: a two-alternative forced-choice head turn (2-AFC) task and a go/no go operant head-turn procedure. In both paradigms, infants were operantly conditioned to make a head turn following the presentation of a target tone pair.4 Performance on these tasks is based on the ability to learn a contingency and discriminate between target and nontarget auditory stimuli.
During the 2-AFC task, infants were trained to make one of two behavioral responses (a head turn to the left or right) to receive visual reinforcement in response to one of two tone pairs. By training the infant with a large interstimulus interval (ISI) between the tones of 500 ms, dropping that ISI to 300 ms when the contingency is acquired, and then gradually decreasing the interval between the tones using an up-down adaptive procedure, the point at which the infant can no longer discriminate between the two sequences may be identified (the infant’s auditory processing threshold) (for further procedural details, see Benasich & Tallal, 1996).
The go/no go procedure is a shorter, less demanding variant of the previous technique. Infants are operantly trained to make a head turn to the left in response to a target tone sequence embedded within a repeating standard tone-pair sequence. As with the 2-AFC paradigm, infants are trained with a supra-threshold ISI (500 ms) until the contingency is acquired. The test phase consists of 20 trials that are presented in blocks of different ISIs (300, 180, 70, 40, or 10 ms): Ten are target trials, and 10 are no go trials using the standard sequence, but scored for the incidence of head turns to give a baseline. The dependent measure used in this task is d′ (the difference between the hit and false alarm distributions), which reflects the rate of head turning to target trials as compared with baseline levels.
Standardized Language and Cognitive Measures
Children’s cognitive and language outcomes were assessed with a series of standardized tests according to age. From 12 to 24 months, children’s cognitive outcome was assessed with the Bayley Scales of Infant Development-Mental Scale (Bayley, 1993). At older ages, cognitive skills were assessed using the Stanford-Binet Intelligence Scale (4th ed.) (Thorndike, Hagen, & Sattler, 1986). Children’s language skills were assessed from 12 to 24 months by parental report using the MacArthur Communicative Development Inventories (CDI; Fenson et al., 1993). Language also was assessed in the laboratory from age 16 months using the Preschool Language Scale-3 (PLS-3; Zimmerman, Steiner, & Pond, 1992). In all assessments, testers were unaware of experimental group membership (i.e., family history of the child).
Findings From Behavioral Studies
In our initial prospective longitudinal study, we examined RAP skills in two groups of infants from 6 to 10 months of age (32 control FH− infants and 11 FH + infants) using a 2-AFC operantly conditioned head-turn task (Benasich & Tallal, 1996). Robust differences in mean RAP threshold were obtained between these two groups of infants at a mean age of 7.5 months. Infants from families with a positive history of SLI had significantly lower mean thresholds than control infants. Mean threshold for the FH− group was 71 ms (SD = 25) as compared with 148 ms (SD = 11) for the FH+ group, t(41) = −5.04, p <.0001. Moreover, a subset of infants showed a distinctive pattern of response to tone pairs with gradually decreasing ISIs; 5 of the 11 FH+ infants (45%) tested dropped to a chance level of response once the ISI fell below 150 ms (see Figure 1). Our data also revealed that the shapes of the distribution of RAP thresholds significantly differed between FH− and FH + groups, and that processing thresholds for rapidly presented auditory stimuli (in the tens-of-milliseconds range) vary even among normal control infants.
FIGURE 1.
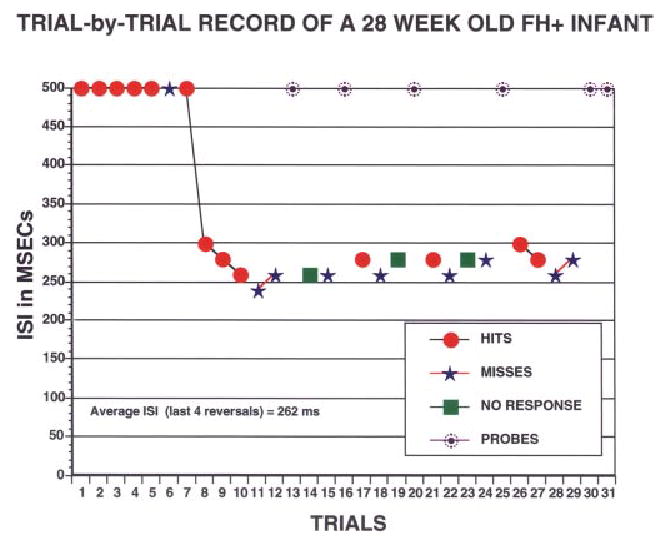
Trial-by-trial recording for the test phase of the RAP go/no go task from a 28-week-old infant with a family history of language-based learning disability. At 500-ms ISI, this infant had no difficulty discriminating one tone sequence from another, but once the ISI dropped below 250 ms, discrimination dropped to chance levels. The RAP threshold is 262 ms.
Prospective follow-up of this group of FH + children and their FH− controls revealed a robust predictive relationship between the RAP thresholds in infancy and language outcomes through 36 months of age (Benasich & Tallal, 1998, in press). Relations across time were examined using hierarchical linear regressions. Language comprehension and expression at 16, 24, and 36 months were significantly related to RAP thresholds assessed at a mean age of 7.5 months. Discriminant function analyses also were computed to assess how sensitive the infant measures were to individual differences in outcome at 36 months. Individual RAP thresholds in infancy were not only related to later language development but were found to be the single best predictor of expressive and receptive language outcome in both FH+ and FH− groups. Gender was the next best predictor while speed of processing and percent of novelty preference in the habituation task contributed to prediction at some ages (Benasich & Tallal, in press).
One of the most compelling findings in this follow-up study was that RAP rates significantly predicted language acquisition regardless of family history. Specifically, those infants that demonstrated the lowest (best) thresholds for brief, rapidly presented, successive tone doublets went on to demonstrate superior language development. On the other hand, infants who showed the highest thresholds (slower processing) went on to demonstrate poorer language outcomes. Overall, those children who had the most elevated thresholds as infants were most likely to be significantly delayed in acquiring language.
We were able to extend and replicate these findings in two additional longitudinal samples. In one study using the go/no go head-turn technique, a new sample of 44 infants (22 FH+ infants and 22 matched FH− controls) was tested at 6, 9, 12, 16, and 24 months of age. Although we are still collecting data for this group, preliminary analyses show that at 6 and 9 months, RAP skills, as measured by d′, significantly differ between the groups at 70 ms ISI or less, but at 300 to 500 ms ISI, the groups perform comparably (Benasich & Leevers, in press). Infants also received an AV habituation task that evaluated each infant’s ability to discriminate consonant vowel pairs (/ba/ vs. /da/) and a visual habituation task that did not require processing of rapidly presented stimuli. The FH+ children as a group did poorly on this AV habituation task as compared to FH− controls, with discrimination scores not only significantly different but only at chance levels (see Figure 2) No significant group differences emerzged on static visual stimuli (face discrimination). Moreover, performance on the AV discrimination task was highly correlated with d′ scores on the head-turn task at age 6 months (r = .66, p < .0001) as well as at age 9 months (r = .52, p < .001). Systematic correlations among concurrent RAP, habituation, and recognition memory also were seen, suggesting that these measures tap some common processes.
FIGURE 2.
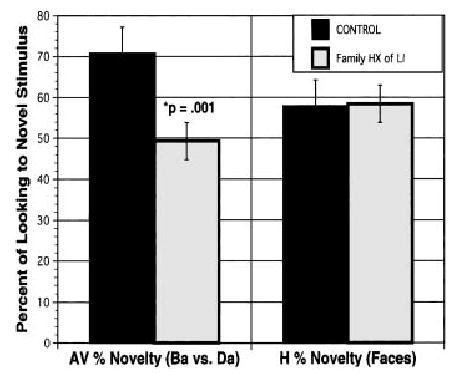
Discrimination of consonant vowel syllables (/ba/ vs /da/) as compared to face discrimination in infants with a family history of SLI and matched controls (n = 44).
Thus, the processing of rapidly changing auditory cues in speech as well as in nonspeech sounds are related during early infancy. These data replicate and extend the findings of our earlier (2-AFC) study suggesting that difficulties in processing successive, brief, rapidly changing auditory stimuli can be demonstrated early in infancy, well before verbal language is in place. Moreover, this processing difficulty, thus far, is predictive of later language outcomes.
Converging Evidence From Two Behavioral Paradigms Assessing RAP Abilities
In a study with 16 FH− infants, we investigated the association between performance on the HT task and the AV task and assessed possible differences in performance as a function of age and/or the demand characteristics of the tasks. As described previously, the stimuli were complex tone pairs designed to assess RAP abilities. A two-condition block design was used for the HT task (70 and 300 ms ISI) while two separate AV habituation and recognition memory tasks (70 and 300 ms ISI) were used. First, consistent with our previous results, the data revealed that 6- and 9-month-old infants were able to differentiate pitch changes in complex tone-pairs occurring at both slow and fast rates (ISIs of 300 and 70 ms) in both tasks. However, performance on the two tasks varied as a function of the demands of the paradigms. Correlational analyses showed that slow- and fast-rate performances were associated for the AV task at 6 months, but these associations only occurred for the conditioned HT task at 9 months. On both tasks, fast-rate performance improved with age; however, age-dependent changes were seen for slow-rate performance only on the more difficult HT task. Finally, at both ages, there was an association between latency for hits (correct head turns) and the slope of the habituation function (learning curve) for the 70 ms stimuli (Figure 3). These findings suggest that both paradigms do in fact provide converging evidence for RAP abilities in infancy and may share a common mechanism that partly underlies performance on both tasks (possibly speed of encoding). However, the more cognitively demanding HT task might be more sensitive to subtle differences in RAP abilities.
FIGURE 3.
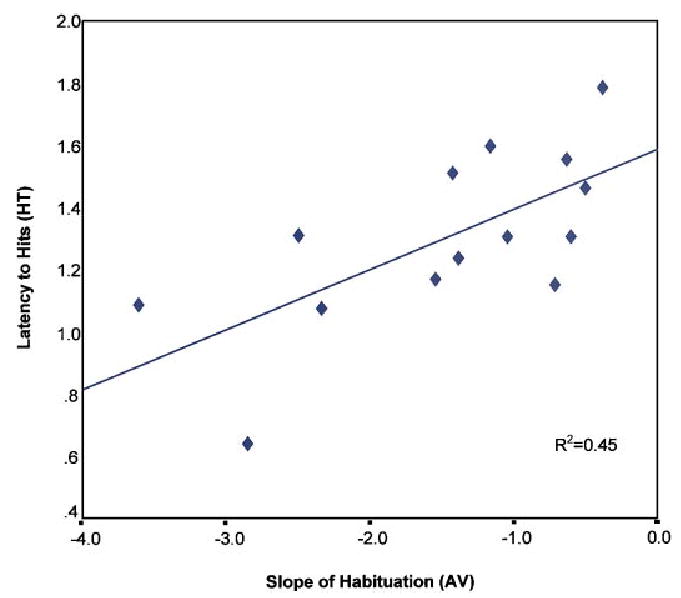
Association between latency to hits and slope of habituation function for 70-ms ISI at 6 months.
Electrophysiological Studies
ERPs are voltage deflections of scalp-recorded EEG, which are time locked to a particular stimulus event (see Taylor & Baldeweg, 2002 for a methodological description of ERPs in a parallel special issue of Developmental Science on imaging methods). The ERP components measuring auditory and speech processing, which have most consistently differentiated between SLI an normal groups, are N1, mismatch negativity (MMN), and P3. Of these, N1 and MMN do not require active attention to stimulus events to be generated (for a review, see Leppänen, Choudhury, Benasich, & Lyytinen, in press; Lyytinen, Blomberg, & Näätänen, 1992; Näätänen, 1992) and are particularly suitable for studying automatic (preattentive) discrimination and perception processes in young subjects (see Alho and Cheour, 1997; Csepe, 1995; Cheour, Leppänen, & Kraus, 2000).
In our ongoing longitudinal ERP study, two groups of infants (FH− and FH+) were assessed at 6, 9, 12, 16, and 24 months to examine the development and maturation of electrocortical brain responses to rapidly changing auditory cues through the first 2 years of life.
ERPs were recorded from 62 scalp sites (64 in adults) using the Geodesic Sensor Net (Electrical Geodesics, Inc., Eugene, OR) (Figure 4). Auditory stimuli were presented free field from speakers located to the child’s left and right. The stimuli were complex tone pairs5 presented in a passive oddball paradigm. To assess the infants’ ability to process rapid changes in acoustic information as a function of tone-presentation rate, the infant brain-response to a deviant stimulus (100–300 Hz, occurring 15% of the time) was compared to responses for the standard stimulus (100–100 Hz, occurring 85% of the time). The ISI between the tones in the pair varied in different conditions (either 70 or 300 ms for infants, and 10, 70, or 300 ms for adults).
FIGURE 4.
Photograph of a 6-month-old child seated on his mother’s lap during an ERP testing session.
We also have studied the effects of sound-presentation rate in the mature brain (Leppänen, Choudhury, Leevers, & Benasich, 2000). Our findings with adult subjects suggest that both tones in a pair evoke distinct response patterns (for both deviant and standard stimuli). However, the responses to the two tones are more clearly separated for the 300 ms ISI than the 70 or 10 ms ISI. ERPs to the second tone for the shorter ISIs (i.e., 70 ms) become merged with the responses to the first tone (see Figure 5A, upper panel). This seems to suggest that this tone pair is processed as a unitary sensory event (cf. Tervaniemi, Maury, & Näätänen, 1994; Yabe, Tervaniemi, Reinikainen, & Näätänen, 1997). The negative response for the second deviant tone at 492 ms (122 ms from the time point where the deviant and standard pairs differ) reflects mainly MMN with a typical fronto-central scalp distribution (although the obligatory N1 overlaps with it), indicating that the brain has automatically detected the stimulus difference (Leppänen et al., 2000). However, when the second tone appears rapidly after the first one in a sound sequence, as in the case of the shorter 70-ms-ISI pair, two responses are likely to summate: one to the change in the characteristics of the tone pair pattern (low-low vs. low-high) and the other to the pitch change for the second tone (low vs. high).
FIGURE 5.
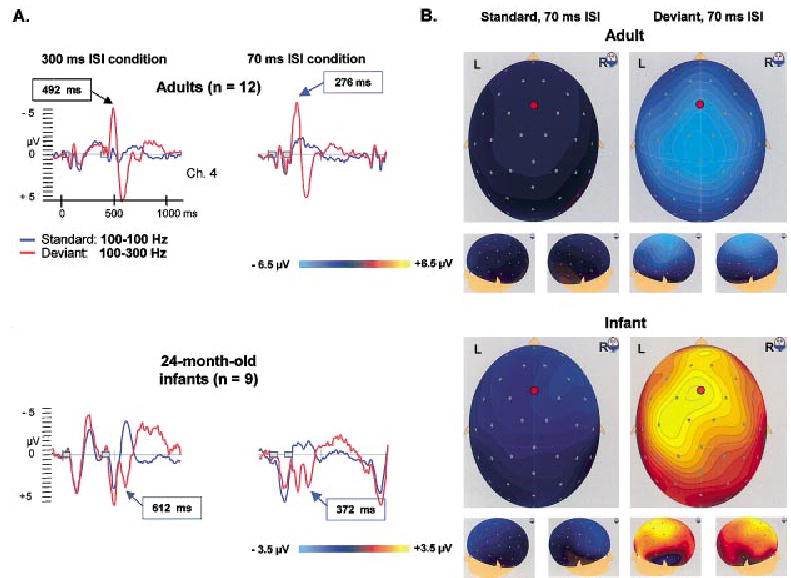
Panel A: Brain electrical responses (averaged across participants) for tone pairs with the within-pair intervals of 70 and 300 ms in adults (n = 12) and 24-month-old infants (n = 9). The stimuli presentations are represented by two gray boxes. Panel B: Scalp surface maps of the standard and deviant responses at the time point of the peaks indicated by the blue arrows in Panel A. The red circles on the surface maps indicate the electrode site from which the ERPs displayed in Panel A are taken. Note the different scaling of the potential maps for the ERPs of adults and infants.
Our preliminary analyses of child data are promising, suggesting that young children process similar pitch changes in paired stimuli and that their ERPs, though different from adults, also are affected by presentation rate. This is evident from our example from the ERPs of 24-month-old children (see Figure 5A, lower panel). Distinct responses are generated by both tones in the pair with 300-ms ISI. The waveforms are similar for the standard and deviant tone pairs until the positive obligatory response to the second tone at about 500 ms. However, at about 610 ms (240 ms from the stimulus difference) the deviant response has an “additional” positive peak and differs from the standard response (p < .01)6 at several fronto-central electrodes. The ERP pattern is more fused for the tone pair with the shorter ISI. The response to the standard pair shows only one clear obligatory positive response. The response pattern for the second (deviant) tone has, as also seen in adults, merged with the response to the first tone. The positive peak at about 370 ms (230 ms from the stimulus difference) corresponds to the positive peak at 610 ms for 300-ms ISI. The response to the deviant tone significantly differs from the standard response (p < .01) and is seen across similar scalp areas. Whether the differential ERPs for the deviant as compared to the standard tones in this study correspond to the automatic change detection response, suggested to be reflected in MMN-like responses reported in infants (Cheour et al., 2000), will be determined in further analyses.
The waveforms and the scalp-distribution maps in Figure 5 highlight developmental differences in ERPs between infants and adults (Dehaene-Lambertz & Baillet, 1998). Infant ERP waveform differences (Dehaene-Lambertz & Dehaene, 1994; Kurtzberg, Vaughan, Kreuzer, & Fliegler, 1995; Leppänen, Eklund, & Lyytinen, 1997) are due to a variety of maturational factors, such as the anatomy of the auditory areas, myelination, synaptic density and other synaptic properties (the location, type, and geometric relations of pre- and postsynaptic elements), the response variability of neural ensembles, and refractory periods. While considering age-related differences, our findings suggest that both mature and developing brains show similar processing of rapidly occurring auditory changes. The integrity of such processes may be critical to the formation of accurate speech-sound representations in the brain. As we continue to analyze this dataset, the relationship between ERPs and performance on behavioral measures will allow examination of converging evidence for neural substrates of speech and language processing, and perhaps differentiate infants at high risk for SLI from controls.
DISCUSSION AND CONCLUSIONS
The use of converging methodologies described here has allowed the investigation of a number of specific research questions exploring the relations between rapid auditory-processing skills in early infancy and later language and cognitive outcome. We are exploiting methodologies, including EEG/ERPs and converging behavioral studies, to address our main research questions as well as to assess the relationship between passively elicited ERPs and active assessment of RAP with conditioned head-turn and auditory-visual tasks. Across studies, infants with a family history of language impairment performed more poorly on measures of RAP as compared to control groups (Benasich & Tallal, 1996, in press; Spitz et al., 1997). In a longitudinal follow-up study, we found that the RAP thresholds measured in infancy were significantly related to later language comprehension and production at 16 to 36 months of age (Benasich, Spitz, Flax, & Tallal, 1997; Benasich & Tallal, 1998, in press). Our findings to date strongly suggest that our behavioral tasks elicit differential performance in infants from families with a history of specific language impairment and that these infants have a 45 to 50% incidence of language impairment by the age of 36 months.
Follow-up data from both behavioral RAP paradigms suggest that linguistic outcomes are significantly related to early RAP thresholds in both normal control populations and in infants at higher risk for such impairments as a function of family history. Added to these findings are reports of predictive associations between early auditory-processing skills and language performance later in life in normally developing samples (see Benasich & Tallal, in press). For example, in a retrospective study, Trehub and Henderson (1996) found that children who had performed above, as compared with below, the median on a variety of gap-detection tasks at 6 or 12 months were subsequently reported to have more sophisticated language skills at 16 to 29 months of age. Thus, it is evident that RAP skills are critical to timely mounting of early language skills and that deficits in these abilities may be demonstrated well before expressive language emerges. The ongoing impact of deviant early RAP abilities is yet to be assessed. However, these studies provide suggestive evidence that RAP deficits can serve as a behavioral “marker” of SLI in young infants and could be used in early identification of language disorders.
While we do not yet have enough data to present comparative EEG/ERP findings from controls and children with a family history of a language impairment, we can speculate that the differences between normal and “at-risk” infant waveforms, at the group level, may be more obvious for short ISI conditions than for long conditions (for discussion on SLI and rapid rate auditory processing, see Tallal, 2000). Given that ERPs do not require overt attention, motor, or verbal responses, they may provide a more neutral index of perceptual discrimination and may be a useful clinical and/or research tool, even at the individual level. Further, ERPs combined with behavioral indices will reveal specific activation patterns which may be related to poor speech and linguistic skills. Together, these techniques may have the predictive power necessary for studying populations at risk for language-related disorders. Currently, there is increasing interest in developing a combined assessment procedure for early identification of SLI in populations at highest risk for the disorder. This would allow such children to receive well-focused intervention at critical stages of language development.
Further support for the notion that infant ERPs may be used to predict later language skills comes from a number of studies measuring brain ERPs to auditory stimuli in normally developing populations. For example, Molfese and colleagues were able to identify ERP waveform features in normal newborns which discriminated between children with high and low language skills at the ages of 3 and 5 years (Molfese & Betz, 1988; Molfese & Molfese, 1994, 1997). In a recent review, Mills and Neville (1997) reported differences on P100 asymmetry between children (from 13 to 30 months of age) with “high” and “low” productive vocabularies, with an L > R pattern for speech sounds in high producers. These ERP findings and the preliminary data we report here are promising because they indicate that the ERP technique can reveal new and useful information about underlying brain functions that are related to rapid auditory processing in young infants.
The present findings suggest candidate causal mechanisms of disorders of RAP and subsequent language-based learning impairments in humans. Given that RAP deficits seen across infant populations predict deficient emerging language in the toddler years, a low-level, more general sensory mechanism becomes a good contender for this role. Neurobiological and neuropathological evidence also is consistent with this hypothesis (for a review, see Fitch et al., 2001). Moreover, animal research demonstrates that such altered processing and related behavioral outcomes can be induced in both microgyric rat and ectopic mouse models (Frenkel et al., 2000; Thomas, Clark, Benasich, & Fitch, 2001).
Prospective studies beginning in early infancy that assess sensory, perceptual, cognitive, and linguistic development concurrently as well as longitudinally have the potential to better address the interactive processes most essential for language development. It is clear, however, that critical areas of knowledge remain elusive. For example, the role of specific linguistic experience and attentional processes in shaping brain structures that support language in the prenatal and infancy period is not yet well understood. Continuing study of differences in early processing of speech as compared to nonspeech (tones, frequency sweeps, etc.) stimuli in normally developing and clinical populations as well as the advent of exciting new converging methodologies (e.g., dense array EEG/ERP, MRI, Optical Imaging, and Near Infrared Spectroscopy) provide opportunities to advance our understanding of the neural underpinnings of early language.
Footnotes
NOTES
This research was supported by NICHD Grant RO1-HD29419 to A.A.B., with additional support from the Human Frontier Science Program, the Don and Linda Carter Foundation and the Elizabeth H. Solomon Center for Neurodevelopmental Research. We thank the parents and children who participated in these studies.
In SLI children, it has been proposed that some of these deficits may be the result of poor attention span (not explicitly assessed by traditional IQ tests), and indeed a relatively high proportion of SLI children meet the criteria for attention deficit disorder.
Measurement of brain activity using an EEG and time-locked cortical event-related potentials (ERPs).
The speech stimuli were constructed using the SynSpeech system to have three formants (F1: 200–700 Hz; F2:838–1102 Hz; F3:2172–2478 Hz) and a fundamental frequency (74–140 Hz). The stimuli differed in F2 and F3 during the initial 43-ms transition period, which was followed by a 207-ms steady-sate vowel sound. The nonspeech stimuli were tone pairs constructed as noted earlier.
Complex tones with a fundamental frequency of 100 and 300 Hz with 15 harmonics (6 dB roll-off per octave) are presented at 75 dB SPL. Each tone is 70 ms long. The tones are paired: The standard stimulus is 100–100 Hz tone pair, and the target (oddball) stimulus is a 100–300 Hz tone pair.
Stimuli were constructed in the same way as those in the head-turn and auditory-visual habituation tasks.
The difference was considered significant if at least five consecutive data points (i.e., 20-ms period) around the peak differed in two-tailed t tests at the level of p < .01.
Contract grant sponsor: NICHD the and the
Contract grant number: RO1-HD29419
Contract grant sponsor: Human Frontier Science Program
Contract grant sponsor: Don and Linda Carter Foundation
Contract grant sponsor: Elizabeth H. Solomon Center for Neurodevelopmental Research
References
- Alho K, Cheour M. Auditory discrimination in infants as revealed by the mismatch negativity of the event-related brain potential. Developmental Neuropsychology. 1997;13:157–165. [Google Scholar]
- Aslin RN, Hunt RH. Development, plasticity, and learning in the auditory system. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. pp. 205–220. [Google Scholar]
- Barth PG. Disorders of neuronal migration. Canadian Journal of Neurological Science. 1987;14:1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- Bayley NB. 2nd ed. San Antonio, TX: Psychological Corporation; 1993. Bayley Scales of Infant Development. [Google Scholar]
- Benasich AA, Leevers HJ. Processing of rapidly presented auditory cues in infancy: Implications for later language development. In: Fagan J, Haynes H, editors. Progress in infancy research. Vol. 3. Mahwah, NJ: Erlbaum; in press. [Google Scholar]
- Benasich AA, Read H. Representation: Picture or process? In: Sigel IE, editor. Theoretical perspectives in the development of representational (symbolic) thought. Mahwah, NJ: Erlbaum; 1998. pp. 33–160. [Google Scholar]
- Benasich AA, Spitz RV, Flax J, Tallal P. Early auditory temporal processing abilities and later language among children with a family history of language impairment. Paper presented at the annual meeting of the Cognitive Neuroscience Society; April; Boston. 1997. [Google Scholar]
- Benasich AA, Tallal P. Auditory temporal processing thresholds, habituation, and recognition memory over the first year. Infant Behavior and Development. 1996;19:339–357. [Google Scholar]
- Benasich AA, Tallal P. Infant processing of auditory temporal information: Links to family history and later language outcome. Society for Neuroscience Abstracts. 1998;24:819. [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues: Links to family history and later language outcome. Behavioural Brain Research. doi: 10.1016/s0166-4328(02)00098-0. (in press) [DOI] [PubMed] [Google Scholar]
- Bird D, Bishop D, Freeman N. Phonological awareness and literacy development in children with expressive phonological impairments. Journal of Speech and Hearing Research. 1995;38:446–462. doi: 10.1044/jshr.3802.446. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, North T, Donlan C. Genetic basis of specific language impairment: Evidence from a twin study. Developmental Medicine and Child Neurology. 1995;37:56–71. doi: 10.1111/j.1469-8749.1995.tb11932.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Benasich AA. Infant habituation: Assessments of individual differences and short-term reliability at 5 months. Child Development. 1986;57:87–99. doi: 10.1111/j.1467-8624.1986.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Brzustowicz L. Looking for language genes: Lessons from complex disorder studies. In: Rice ML, editor. Towards a genetics of language. Mahwah, NJ: Erlbaum; 1996. pp. 3–25. [Google Scholar]
- Cheour M, Leppänen PHT, Kraus N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology. 2000;111:4–16. doi: 10.1016/s1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Benasich AA. Familial aggregation in a sample of infants born into families with a history of language-based learning impairments. 2002 Manuscript submitted for publication. [Google Scholar]
- Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria. Journal of Cognitive Neuroscience. 2000a;12:828–839. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Clark MG, Tallal P, Rosen GD, Peiffer AM, Fitch RH. Impaired perception of speech stimuli in rats with induced microgyria. Society for Neuroscience Abstracts. 2000b;26:74. [Google Scholar]
- Cohen M, Campbell R, Yagmai F. Neuropathological abnormalities in developmental dysphasia. Annals of Neurology. 1989;25:567–570. doi: 10.1002/ana.410250607. [DOI] [PubMed] [Google Scholar]
- Columbo J. Infant cognition: Predicting later intellectual functioning. Newbury Park, CA: Sage; 1993. [Google Scholar]
- Cornelissen P, Richardson A, Mason A, Fowler S, Stein J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Research. 1995;35:1483–1494. doi: 10.1016/0042-6989(95)98728-r. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Jernigan TL, Denenberg VH, Tallal P. Language and learning impairment and prenatal risk: An MRI study of the corpus callosum and cerebral volume. Journal of Medical Speech-Language Pathology. 1995;3:1–13. [Google Scholar]
- Csepe V. On the origin and development of the mismatch negativity. Ear and Hearing. 1995;16:91–104. doi: 10.1097/00003446-199502000-00007. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lamberzt G, Baillet S. A phonological representation in the infant brain. NeuroReport. 1998;9:1885–1888. doi: 10.1097/00001756-199806010-00040. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S. Speed and cerebral correlates of syllable discrimination in infants. Nature. 1994;370:292–295. doi: 10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Brain activity in visual cortex predicts individual differences in reading performance. Proceedings of the National Academy of Sciences. 1997;94:13363–13366. doi: 10.1073/pnas.94.24.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH. A critique of Mody, Studdert-Kennedy, and Brady’s “Speech perception deficits in poor readers: Auditory processing or phonological coding? Journal of Learning Disabilities. 1999;32:379–383. doi: 10.1177/002221949903200502. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. More power to them—Statistically, that is: A commentary on Studdert-Kennedy, Mody, and Brady’s criticism of a critique. Journal of Learning Disabilities. 2001;32:379–383. doi: 10.1177/002221940103400401. [DOI] [PubMed] [Google Scholar]
- Dvoràk K, Feit J. Migration of neuroblasts through partial necrosis of the cerebral cortex in newborn rats—Contribution to the problems of morphological development and developmental period of cerebral microgyria. Histological and autoradiographical study. Acta Neuropathologia (Berlin) 1977;38:203–212. doi: 10.1007/BF00688066. [DOI] [PubMed] [Google Scholar]
- Dvoràk K, Feit J, Jurankova Z. Experimentally induced focal microgyria and status verrucosus deformis in rats—Pathogenesis and interrelation. Histological and autoradiographical study. Acta Neuropathologia (Berlin) 1978;44:121–129. doi: 10.1007/BF00691477. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996;4:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Zeffiro TA. The visual deficit theory of developmental dyslexia. Neuroimage. 1996;4:S108–S117. doi: 10.1006/nimg.1996.0061. [DOI] [PubMed] [Google Scholar]
- Fawcett AJ, Nicolson RI, Dean P. Impaired performance of children with dyslexia on a range of cerebellar tasks. Annals of Dyslexia. 1996;46:259–283. doi: 10.1007/BF02648179. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. Technical Manual for the MacArthur Communicative Development Inventory. San Diego: Singular Press; 1993. [Google Scholar]
- Fitch RH, Miller S, Tallal P. Neurobiology of speech perception. Annual Review of Neuroscience. 1997;20:331–353. doi: 10.1146/annurev.neuro.20.1.331. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Read H, Benasich AA. Neurophysiology of speech perception in normal and impaired systems. In: Jahn A, Santos-Sacchi J, editors. Physiology of the ear. 2nd ed. San Diego: Singular Press; 2001. pp. 651–672. [Google Scholar]
- Fitch RH, Tallal P, Brown CP, Galaburda AM, Rosen GD. Induced microgyria and auditory temporal processing in rats: A model for language impairment? Cerebral Cortex. 1994;4:260–270. doi: 10.1093/cercor/4.3.260. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Sherman GF, Bashan KA, Galaburda AM, LoTurco JJ. Neocortical ectopias are associated with attentuated neurophysiological responses to rapidly changing auditory stimuli. Developmental Neuroscience. 2000;11:575–579. doi: 10.1097/00001756-200002280-00029. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Kemper TL. Cytoarchitectonic abnormalities in developmental dyslexia: A case study. Annals of Neurology. 1979;6:94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Livingstone M. Temporal information processing in the nervous system: Special reference to dyslexia and dysphasia. Vol. 682. New York: The New York Academy of Sciences; 1993. Evidence for a magnocellular defect in developmental dyslexia. ; pp. 70–82. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Menard MT, Rosen GD. Evidence for aberrant auditory anatomy in developmental dyslexia. Proceedings of the National Academy of Sciences, USA. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: Four consecutive cases with cortical anomalies. Annals of Neurology. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Godfrey JJ, Syrdal-Lasky AK, Millay KK, Knox CM. Performance of dyslexic children on speech perception tests. Journal of Experimental Child Psychology, 32. 1981;3:401–424. doi: 10.1016/0022-0965(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Hagman J, Wood F, Buchsbaum M, Flowers L, Katz W, Tallal P. Cerebral brain metabolism in adult dyslexics assessed with positron emission tomography during performance of an auditory task. Archives of Neurology. 1992;49:734–739. doi: 10.1001/archneur.1992.00530310082015. [DOI] [PubMed] [Google Scholar]
- Heiervang E, Hugdahl K, Steinmetz H, Inge Smievoll A, Stevenson J, Lund A, Ersland L, Lundervold A. Planum temporale, planum parietale, and dichotic listening in dyslexia. Neuropsychologia. 2000;38:1704–1713. doi: 10.1016/s0028-3932(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Herman A, Fitch RH, Galaburda AM, Rosen GD. Induced microgyria and its effects on cell size, cell number, and cell packing density in the medial geniculate nucleus. Society for Neuroscience Abstracts. 1995;21:1711. [Google Scholar]
- Herman A, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalmic cell size, cell number, and auditory temporal processing in male and female rats. Cerebral Cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Humphreys P, Kaufmann WE, Galaburda AM. Developmental dyslexia in women: Neuropathological findings in three cases. Annals of Neuroscience. 1990;28:727–738. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- Humphreys P, Rosen GD, Press DM, Sherman GF, Galaburda AM. Freezing lesions of the developing rat brain: A model for cerebrocortical microgyria. Journal of Neuropathology and Experimental Neurology. 1991;502:145–160. doi: 10.1097/00005072-199103000-00006. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Hesselink JR, Sowell E, Tallal P. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Archives of Neurology. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW. The discovery of spoken language. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems [see comments] Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Methods in the study of infant speech perception. In: Gottlieb G, Krasnegor NA, editors. Measurement of audition and vision in the first year of postnatal life: A methodological overview. Norwood, NJ: Ablex; 1985. pp. 223–249. [Google Scholar]
- Kuhl PK. The special-mechanisms debate in speech research: Categorization tests on animal and infants. In: Harnad S, editor. Categorical perception: The groundwork of cognition. Cambridge, England: Cambridge University Press; 1987. pp. 355–386. [Google Scholar]
- Kurtzberg D, Vaughan HG, Jr, Kreuzer JA, Fliegler KZ. Developmental studies and clinical application of mismatch negativity: Problems and prospects. Ear and Hearing. 1995;16:105–117. doi: 10.1097/00003446-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Leppänen PHT, Choudhury N, Benasich AA, Lyytinen H. Classification of developmental language disorders: Theoretical issues and clinical implications. In: Verhoeven L, van Balkom H, editors. Mahwah, NJ: Erlbaum; Neuroimaging measures in the study of specific language impairments in children. (in press) [Google Scholar]
- Leppänen PHT, Choudhury N, Leevers HJ, Benasich AA. Brain event-related potentials to tone pairs are modulated by rate and attention. Journal of Cognitive Neuroscience. 2000;32(Suppl) [Google Scholar]
- Leppänen PHT, Eklund KM, Lyytinen H. Event-related brain potentials to change in rapidly presented acoustic stimuli in newborns. Developmental Neuropsychology. 1997;13:175–204. [Google Scholar]
- Lewis BA, Thompson LA. A study of developmental speech and language disorders in twins. Journal of Speech and Hearing Research. 1992;35:1086–1094. doi: 10.1044/jshr.3505.1086. [DOI] [PubMed] [Google Scholar]
- Liberman AM. Speech: A special code. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proceedings of the National Academy of Sciences, USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyytinen H, Blomberg AP, Näätänen R. Event-related potentials and autonomic responses to a change in unattended auditory stimuli. Psychophysiology. 1992;29:2–14. doi: 10.1111/j.1469-8986.1992.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Martin R. Heterogeneity of deficits in developmental dyslexia and implications for methodology. Psychological Bulletin Reviews. 1995;2:494–500. doi: 10.3758/BF03210984. [DOI] [PubMed] [Google Scholar]
- Mc Anally KI, Stein JF. Scalp potentials evoked by amplitude-modulated tones in dyslexia. Journal of Speech Language and Hearing Research. 1997;40:939–945. doi: 10.1044/jslhr.4004.939. [DOI] [PubMed] [Google Scholar]
- McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Development. 1993;64:57–79. [PubMed] [Google Scholar]
- Mills DL, Neville HJ. Electrophysiological studies of language and language impairment. Seminars in Pediatric Neurology. 1997;4:125–134. doi: 10.1016/s1071-9091(97)80029-0. [DOI] [PubMed] [Google Scholar]
- Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: Auditory processing or phonological coding? Journal of Experimental Child Psychology. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Betz JC. Electrophysiological indices of the early development of lateralization for language and cognition, and their implications for predicting later development. In: Molfese DL, Segalowitz SJ, editors. Brain lateralization in children: Developmental implications. New York: Guilford Press; 1988. pp. 171–190. [Google Scholar]
- Molfese DL, Molfese VJ. Short-term and long-term developmental outcomes: The use of behavioral and electrophysiological measures in early infancy as predictors. In: Dawson, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford Press; 1994. pp. 493–517. [Google Scholar]
- Molfese DL, Molfese VJ. Discrimination of language skills at five years of age using event-related potentials recorded at birth. Developmental Neuropsychology. 1997;13:135–156. [Google Scholar]
- Morrongiello BA. Hillsdale, NJ: Erlbaum; 1990. The study of individual differences in infants: Auditory processing measures. [Google Scholar]
- Näätänen R. Attention and brain function. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Cortical auditory signal processing in poor readers. Proceedings of the National Academy of Science, USA. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville HJ, Coffey SA, Holcomb PJ, Tallal P. The neurobiology of sensory and language processing in language-impaired children. Journal of Cognitive Neuroscience. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Njiokiktjien C. Dyslexia: A neuroscientific puzzle. Acta Paedopsychiatrica: International Journal of Child and Adolescent Psychiatry. 1994;56:157–167. [PubMed] [Google Scholar]
- Plante E, Shenkman K, Clark MM. Classification of adults for family studies of developmental language disorders. Journal of Speech and Hearing Research. 1996;39:661–667. doi: 10.1044/jshr.3903.661. [DOI] [PubMed] [Google Scholar]
- Reed MA. Speech perception and the discrimination of brief auditory cues in reading disabled children. Journal of Experimental Child Psychology. 1989;48:270–292. doi: 10.1016/0022-0965(89)90006-4. [DOI] [PubMed] [Google Scholar]
- Robichon F, Levrier O, Farnarier P, Habib M. Developmental dyslexia: Atypical cortical asymmetries and functional significance. European Journal of Neurology. 2000;7:35–46. doi: 10.1046/j.1468-1331.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- Robinson RJ. The causes of language disorders. Association for all Speech Impaired Children; Proceedings of the 1st international symposium on specific speech and language disorders in children, Reading; England. 1987. 1987. pp. 1–19. [Google Scholar]
- Rosen GD, Press DM, Sherman GF, Galaburda AM. The development of induced cerebrocortical microgyria in the rat. Journal of Neuropathology and Experimental Neurology. 1992;51:601–611. doi: 10.1097/00005072-199211000-00005. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, Pikus A, Rapoport JL, Cohen RM. Failure to activate the left temporoparietal cortex in dyslexia. An oxygen 15 positron emission tomographic study. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donohue BC, Brady DR, Nace K, Giedd JN, Andreason PA. Magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives in Neurology. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Sherman G, Galaburda A, Behan P, Rosen G. Neuroanatomical anomalies in autoimmune mice. Acta Neuropathology (Berlin) 1987;74:239–242. doi: 10.1007/BF00688187. [DOI] [PubMed] [Google Scholar]
- Sherman G, Galaburda A, Geschwind N. Cortical anomalies in brains of New Zealand mice: A neuropathologic model of dyslexia? Proceedings of the National Academy of Science, USA. 1985;82:8072–8074. doi: 10.1073/pnas.82.23.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Cohen SE, Beckwith L. Why does infant attention predict adolescent intelligence? Infant Behavior and Development. 1997;20:133–140. [Google Scholar]
- Snowling M, Goulandris N, Bowlby M, Howell P. Segmentation and speech perception in relation to reading skill: A developmental analysis. Journal of Experimental Child Psychology. 1986;41:489–507. doi: 10.1016/0022-0965(86)90006-8. [DOI] [PubMed] [Google Scholar]
- Spitz RV, Tallal P, Flax J, Benasich AA. Look who’s talking: A prospective study of familial transmission of language impairments. Journal of Speech and Hearing Research. 1997;40:990–1001. doi: 10.1044/jslhr.4005.990. [DOI] [PubMed] [Google Scholar]
- Stark RE, Heinz JM. Vowel perception in children with and without language impairment. Journal of Speech and Hearing Research. 1996a;39:860–869. doi: 10.1044/jshr.3904.860. [DOI] [PubMed] [Google Scholar]
- Stark RE, Heinz JM. Perception of stop consonants in children with expressive and receptive-expressive language impairments. Journal of Speech and Hearing Research. 1996b;39:676–686. doi: 10.1044/jshr.3904.676. [DOI] [PubMed] [Google Scholar]
- Stein JF. The neurobiology of reading difficulties. In: Wolf M, editor. Dyslexia, Fluency and the Brain. M. Timonium, Maryland: York Press; 2001. pp. 3–23. [Google Scholar]
- Stein JF, Talcott J. Impaired neuronal timing in developmental dyslexia—The magnocellular hypothesis. Dyslexia. 1999;5:59–77. [Google Scholar]
- Stein JF, Walsh V. To see, but not to read: The magnocellular theory of dyslexia. Trends in Neuroscience. 1997;20:147–151. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Tallal P. Experimental studies of language learning impairments: From research to remediation. In: Bishop DM, Leonard LB, editors. Speech and language impairments in children: Causes, characteristics, intervention, and outcome. Philadelphia: Psychology Press; 2000. pp. 131–155. [Google Scholar]
- Tallal P, Benasich AA. Developmental language learning impairments. Development and Psychopathology. doi: 10.1017/s0954579402003097. in press. [DOI] [PubMed] [Google Scholar]
- Tallal P, Jernigan T, Trauner D. Developmental bilateral damage to the head of the caudate nuclei: Implications for speech-language pathology. Journal of Medical Speech Language Pathology. 1994;2:23–28. [Google Scholar]
- Tallal P, Merzenich MM, Miller S, Jenkins W. Language learning impairments: Integrating basic science, technology, and remediation. Experimental Brain Research. 1998;123:210–219. doi: 10.1007/s002210050563. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Jenkins B, Merzenich M. The role of temporal processing in developmental language-based learning disorders: Research and clinical implications. In: Blachman B, editor. Foundations of reading acquisition. Mahwah, NJ: Erlbaum; 1997. pp. 49–66. [Google Scholar]
- Tallal P, Ross R, Curtiss S. Familial aggregation in specific language impairment. Journal of Speech, Language, and Hearing Disorders, 54. 1989;2:167–173. doi: 10.1044/jshd.5402.167. [DOI] [PubMed] [Google Scholar]
- Tallal P, Wood F, Buchsbaum M, Flowers L, Brown I, Katz W. Decoupling of PET measured left caudate and cortical metabolism in adult dyslexics. Society for Neuroscience Abstracts. 1990;16:1241. [Google Scholar]
- Taylor M, Baldeweg T. Basic principles and applications of EEG, ERPs, and intracranial methods. Developmental Science. 2002;XX [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich MM, Gabrieli JD. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. Proceedings of the National Academy of Sciences, USA. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M, Maury S, Näätänen R. Neural representations of abstract stimulus features in the human brain as reflected by the mismatch negativity. NeuroReport. 1994;5:844–846. doi: 10.1097/00001756-199403000-00027. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. The Stanford Binet Intelligence Scale. 4th ed. Chicago: Riverside; 1986. [Google Scholar]
- Tomblin BJ, Buckwalter PR. Heritability of poor language achievement among twins. Journal of Speech, Language, and Hearing Research. 1998;41:188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Henderson JL. Temporal resolution in infancy and subsequent language development. Journal of Speech and Hearing Research. 1996;39:1315–1320. doi: 10.1044/jshr.3906.1315. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. Speech perception in severely disabled and average reading children. Canadian Journal of Psychology. 1987;41:48–61. doi: 10.1037/h0084150. [DOI] [PubMed] [Google Scholar]
- Werner LA, Rubel EW. Developmental psychoacoustics. Washington, DC: American Psychological Association; 1992. [Google Scholar]
- Witton C, Talcott J, Hansen P, Richardson A, Griffiths T, Rees A, Stein J, Green G. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Current Biology. 1998;8:791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- Wright BA, Bowen RW, Zecker SG. Nonlinguistic perceptual deficits associated with reading and language disorders. Current Opinion in Neurobiology. 2000;10:482–486. doi: 10.1016/s0959-4388(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Yabe H, Tervaniemi M, Reinikainen K, Näätänen R. Temporal window of integration revealed by MMN to sound omission. NeuroReport. 1997;8:1971–1974. doi: 10.1097/00001756-199705260-00035. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-3. New York: Psychological Corporation; 1992. [Google Scholar]


