Abstract
Isolated lymphoid follicles (ILFs) are organized intestinal lymphoid structures whose formation can be induced by luminal stimuli. ILFs have been demonstrated to act as inductive sites for the generation of immune responses directed toward luminal stimuli; however, the phenotype of the immune response initiated within ILFs has largely been uninvestigated. To gain a better understanding of the immune responses initiated within ILFs, we examined phenotypic and functional aspects of the largest cellular component of the murine ILF lymphocyte population, B lymphocytes. We observed that murine ILF B lymphocytes are composed of a relatively homogenous population of follicular B-2 B lymphocytes. Consistent with their proximity to multiple stimuli, ILF B lymphocytes displayed a more activated phenotype compared with their counterparts in the spleen and Peyer’s patch (PP). ILF B lymphocytes also expressed higher levels of immunomodulatory B7 and CD28 family members B7X and programmed death-1 compared with their counterparts in the spleen and PP. ILF B lymphocytes preferentially differentiate into IgA-producing plasma cells and produce more IL-4 and IL-10 and less interferon-γ compared with their counterparts in the spleen. Immunoglobulin repertoire analysis from individual ILFs demonstrated that ILFs contain a polyclonal population of B lymphocytes. These findings indicate that murine ILFs contain a polyclonal population of follicular B-2 B lymphocytes with a phenotype similar to PP B lymphocytes and that, in unchallenged animals, ILFs promote immune responses with a homeostatic phenotype.
Keywords: intestine, immunoglobulin A
THE PRESENCE of organized mononuclear cell aggregates resembling Peyer’s patches (PPs) or lymph nodes (LNs) in the human intestine has been well documented (13, 20, 27). The identification of isolated lymphoid follicles (ILFs), the murine homolog of these aggregates, has greatly facilitated studies into the genesis and function of these structures. These aggregates also exist in the human and murine colon (24, 45) but are less well understood in part due to the complexity of distinguishing ILFs from PP equivalents in the colon. ILFs share some features of PPs. ILFs contain a loosely organized germinal center with peanut agglutinin (PNA)+ B lymphocytes, and ILFs can have a follicle-associated epithelium (FAE) containing M cells. However, in contrast to PPs, ILFs lack a discrete T lymphocyte zone (15, 29). The mature forms of ILFs, which possess a FAE and loosely organized PNA+ cells, have a cellular composition similar to that of PPs containing B lymphocytes (up to 70% of the population), CD4+ T cell receptor (TCR)αβ+ T lymphocytes (~10% of the population), CD8+ TCR)αβ+ T lymphocytes (~3% of the population), CD11c+ dendritic cells (~10% of the population), and IL-7 receptor+ c-kit+ cells (~15% of the population) (15, 29, 30).
The function of ILFs has only recently been investigated. The FAE of ILFs facilitates the translocation of the luminal pathogen Yersinia enterocolitica to the underlying lymphocyte aggregate, similar to the FAE of PPs (30). ILFs were noted to support class switch of immunoglobulins to IgA (42), and a further study (30) has demonstrated that ILFs can initiate immune responses to luminal T lymphocyte-dependent antigens, resulting in the production of antigen-specific IgA. These functions are likely restricted to the mature forms of ILFs, which contain PNA+ cells and a FAE, because mice possessing only immature ILFs, which lack these features, were deficient in the generation of antigen-specific IgA responses to luminal Salmonella typhimurium (30). These studies only assessed the production of IgA, and, consequently, they lack a more detailed analysis of the phenotype of the immune response initiated within ILFs. The preferential production of other immunoglobulin isotypes, particularly IgG2a, would be less consistent with the phenotype of a mucosal immune response and would suggest that ILFs can act as inductive sites mediating immune responses that lack a mucosal phenotype and could be damaging to the intestinal mucosa.
We observed that ILFs can have the characteristics of tertiary lymphoid structures. Tertiary lymphoid structures are inducible, organized, lymphocyte aggregates that share many features with secondary lymphoid structures. These structures generally have a similar composition and architecture to secondary lymphoid structures, including the presence of high endothelial venules and germinal centers (19, 23, 43). However, the pathways leading to secondary and tertiary lymphoid structure formation are distinct. Like tertiary lymphoid structures in other tissues, ILFs can be formed de novo in adult animals by pathways that are distinct from those of secondary lymphoid structure formation (29). Similar to tertiary lymphoid structures formed in other tissues, ILFs can be ectopically positioned compared with the relatively strict antimesenteric positioning of PPs (intestinal secondary lymphoid structures) (29, 39). Notably, the formation and presence of tertiary lymphoid structures is associated with a number of autoimmune and chronic inflammatory conditions, including those affecting the intestine, and these structures have been proposed to be sites initiating or propagating the inappropriate immune responses seen in these conditions (8, 14, 18, 20, 48). Together, these observations suggest that ILFs may play a more sinister role in promoting inappropriate immune responses such as those seen in inflammatory bowel disease.
To gain insight into the role the ILF plays in the mucosal immune system, we examined the characteristics of the largest lymphocyte population within ILFs, B lymphocytes. B lymphocytes participate in the immune response in multiple ways. B lymphocytes have a well-identified role as the precursors to antibody-producing plasma cells. B lymphocytes may also act as antigen-presenting cells, promoting T lymphocyte-dependent immune responses. Importantly, mucosal B lymphocytes have been observed to play both pathogenic roles, promoting the propagation of inappropriate inflammatory responses, as well as homeostatic roles, preventing the development of intestinal inflammation (11, 35, 38). The previous observations regarding ILFs, and tertiary lymphoid structures in general, could be consistent with either of these possibilities.
In the present study, we examined the B lymphocyte population within ILFs and found that this population is predominantly comprised of a homogenous population of B lymphocytes with the phenotype of mature follicular B-2 B lymphocytes. These B lymphocytes preferentially differentiated into IgA-producing, as opposed to IgG or IgM-producing, plasma cells and produced a profile of immunoglobulins most similar to that found in the diffuse lamina propria. ILF B lymphocytes have a phenotype consistent with activation and express higher levels of the immunomodulatory members of the B7 and CD28 family compared with splenic B lymphocytes. ILF B lymphocytes were also found to express higher levels of IL-4 and IL-10 and low to absent levels of interferon (IFN)-γ compared with splenic B lymphocytes. The immunoglobulin repertoire of ILF B lymphocytes was found to be diverse, consisting of many different VH genes and their VDJ rearrangements. These findings indicate that ILFs contain a polyclonal population of follicular B-2 B lymphocytes with a predisposition to initiate immune responses with a mucosal phenotype.
MATERIALS AND METHODS
Mice
The female BALB/c mice (8–12 wk old; Jackson Laboratory, Bar Harbor, MN) used for this study were housed in a specific pathogen-free facility and fed a routine chow diet. Animal procedures and protocols were carried out in accordance with the institutional review board at the Washington University School of Medicine.
Isolation of cellular populations from the spleen, PP, and ILF
Spleens and PPs were removed from BALB/c mice and disrupted by mechanical dissociation. Intestines were removed from mice, flushed with cold PBS, opened along the mesenteric border, and mounted with the lumen facing up in cold PBS. ILFs could be identified as pale nodules with a domelike appearance scattered throughout the small intestine. For illustration purposes, a low-power photomicrograph of a BALB/c mouse intestine containing ILFs stained with lectin from Ulex europaeus (Sigma-Aldrich, St. Louis, MO), as previously described (29), is shown in Fig. 1. Using the dissecting microscope and a 26-gauge needle and syringe, the contents of multiple mature ILF were aspirated and placed in cold PBS. Red blood cells were lysed from cellular suspensions and then utilized for flow cytometric analysis, ELISPOT, and ELISA analysis (as described below). The average yield of viable mononuclear cells as determined by trypan blue exclusion was 5 × 105 ILF cells, 2.5 × 106 PP cells, and 5 × 107 splenocytes per mouse. The percentage of viable cells falling into the lymphocyte gate, as based on forward and side scatter, was 82%, 75%, and 72% for the spleen, PP, and ILF cellular populations, respectively.
Fig. 1.
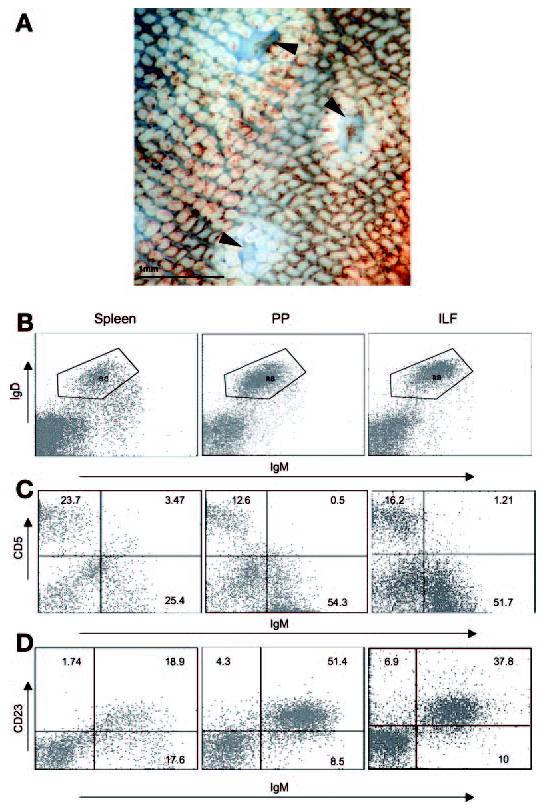
Isolated lymphoid follicules (ILFs) contain mature follicular B-2 B lymphocytes. Flow cytometry analysis was performed on cellular populations from the spleen (SP), Peyer’s patch (PP), and mature ILF as outlined in MATERIALS AND METHODS . A low-power photomicrograph of an intestine from a Balb/c mouse stained with lectin from Ulex Europaeus demonstrated the appearance of mature ILFs (arrowheads in A). Mature ILFs contained a population of B lymphocytes that was sIgDhi sIgMlo (B), CD5− (C), and CD23+ (D). Mature ILF B lymphocytes have a cell surface phenotype that is most similar to that of PP B lymphocytes as opposed to splenic B lymphocytes. Flow cytometry analysis of ILFs cellular populations was performed on a pooled population from 3 mice. The percentages of positive cells in each quadrant are shown, and the plots are representative of 1 of 3 experiments. Original magnification of A: ×20.
Flow cytometric analysis
Single cell suspensions, obtained as above, were used for flow cytometric analysis. The reagents used for analysis are listed in Table 1. Briefly, single cell suspensions were resuspended in PBS with 1% BSA (Fisher Scientific, Pittsburgh, PA) and 1 mg/ml human IgG (Sandoz Pharmaceuticals, East Hanover, NJ) at 2 × 107 cells/ml. Cells were incubated with directly conjugated or biotin-conjugated antibodies for 30 min on ice in the dark. Cellular suspensions with biotin-conjugated primary antibodies were washed twice in PBS containing 1% BSA and then incubated with streptavidin-Per CP (BD Biosciences) for 30 min on ice. Cells were washed twice as described above and fixed with 1% paraformaldehyde in PBS. Flow cytometry acquisition was done on a Becton Dickinson FACS Caliber (BD Bioscience) and analysis was performed on a Macintosh G3 using the CellQuest program (BD Bioscience). Dead cells were excluded based on forward and side light scatter. Gates for positive staining were defined such that 1% of the analyzed population stained positive with the appropriate isotype control antibodies (all from BD Biosciences).
Table 1.
Antibodies used in this study
| Antibody Against | Clone No. | Isotype | Vendor |
|---|---|---|---|
| Mouse IgM | 11/41 | Rat IgG2a, κ | eBioscience |
| Mouse IgD | 11–26c (11–26) | Rat IgG2a, κ | eBioscience |
| Mouse CD44 | IM7 | Rat IgG2b, κ | Pharmingen |
| Mouse CD62L | MEL-14 | Rat IgG2a, κ | BD bioscience |
| Mouse CD69 | H1.2F3 | Hamster IgG | BD bioscience |
| Mouse MHC II | NIMR-4 | Rat IgG | Southern Biotech |
| Mouse CD86 | PO3.1 | Rat IgG2a, κ | eBioscience |
| Mouse PD-1 | J43 | Hamster IgG | eBioscience |
| Mouse B7S1 | 188 | Rat IgG2a, κ | eBioscience |
| Mouse B7-H1 | MIH5 | Rat IgG2a, λ | eBioscience |
| Mouse CD23 | B3B4 | Rat IgG2a, κ | eBioscience |
| Mouse CD 80 | 16–10AI | Hamster IgG | eBioscience |
| Mouse CD5 | 53–7.3 | Rat IgG2a, κ | eBioscience |
ELISA for antibody production
Cellular populations from the spleen, PP, and ILF isolated as described above were cultured at a density of 106 cells/ml in RPMI 1640 media (BioWhittaker, Walkersville, MD) containing 10% FCS (Hyclone, Logan, UT), 2 mM glutamax (Life Technologies, Grand Island, NY), 10 mM HEPES (BioWhittaker), 1 mM sodium pyruvate (BioWhittaker), 50 U/ml penicillian-50 mg/ml streptomycin (Life Technologies), and 50 mM 2-mercaptoethanol (2-ME) (Fisher Scientific) in a 96-well plate for 7 days at 37°C and 5% CO2. After 7 days, the culture supernatants were collected, and the concentrations of IgA, IgG, and IgM were determined as described below.
Cell culture supernatants or IgA, IgG, and IgM standards (Southern Biotechnology Associates, Birmingham, AL) diluted in PBS containing 1% BSA and 0.05% Tween 20 (Sigma-Aldrich) were incubated in 96-well Immuno 4 plates (Fisher Scientific) that had been previously coated with 10 μg/ml goat anti-mouse Ig (Southern Biotechnology Associates) and blocked with PBS containing 5% BSA and 0.05% Tween 20 at room temperature for 2 h. Plates were washed three times with PBS containing 0.05% Tween 20, and goat anti-mouse IgA, IgG, and IgM alkaline phosphatase-conjugated antibodies (Southern Biotechnology Associates) diluted in PBS containing 1% BSA and 0.05% Tween 20 were added to the plate and incubated for 2 h at room temperature. Plates were washed three times with PBS containing 0.05% Tween 20, and p-nitrophenyl phosphate alkaline phosphatase substrate (Sigma-Aldrich) was added. Plates were read at 405 nm using a Bio-Tek Instruments Microplate Reader (Bio-Tek Instruments, Winooski, VT). Each sample was measured in duplicate in at least three dilutions.
ELISPOT assays for antibody production
The antibody-forming cells in the spleen, PP, ILF, and lamina propria were measured from freshly isolated cellular populations as previously described (36). Ninety-six-well multiscreen-HA plates (Millipore, Bedford, MA) were coated with goat anti-mouse Ig (Southern Biotechnology Associate) overnight at room temperature. Plates were washed three times in PBS, blocked with PBS containing 5% newborn calf serum (Hyclone) for 1 h at 37°C, and washed, and spleen, PP, ILF, and lamina propria cell suspensions of different concentrations in IMDM (Bio-Whittaker) containing 5% FCS (Hyclone), 2 mM glutamax (Life Technologies), and 50 mM 2-ME (Fisher Scientific) were added to the plates. Plates were incubated at 37°C and 5% CO2 overnight, washed with PBS containing 0.05% Tween 20, and incubated with alkaline phosphatase-conjugated goat anti-mouse IgA, IgG, IgM antibodies (Southern Biotechnology Associates) overnight at 4°C. Plates were washed with PBS and exposed to 5-bromo-4-chloro-3-indolyl phosphatase-nitroblue tetrozloium substrate (Sigma-Aldrich), and spot-forming cells were counted under a dissecting microscope.
ELISPOT assays for cytokine production
Cellular populations isolated as described above were flow cytometrically sorted for sIgDhi sIgMlo B lymphocytes and stimulated with 5 ng/ml PMA (Sigma-Aldrich) and 1.25 μM calcium ionophore A-23187 (Sigma-Aldrich) in RPMI 1640 media (BioWhittaker) containing 10% FCS (Hyclone), 2 mM glutamax (Life Technologies), 10 mM HEPES (BioWhittaker), 1 mM sodium pyruvate (BioWhittaker), 50 U/ml penicillian-50 mg/ml streptomycin (Life Technologies), and 50 mM 2-ME (Fisher Scientific) for 4 days. IL-4, IL-10, and IFN-γ production were measured by ELISPOTS using BD ELISPOT mouse IL-4, IL-10, and IFN-γ ELISPOT pairs (BD Bioscience). Briefly, 96-well multiscreen-HA plates (Millipore) coated with anti-mouse IL-4, anti-mouse IL-10, or anti-mouse IFN-γ antibodies overnight at 4°C were blocked with PBS containing 10% FBS for 2 h at room temperature. Splenic, PP, and ILF cell suspensions in the above media containing PMA and calcium ionophore A-23187 were added to each well and cultured for 4 days at 37°C and 5% CO2. The wells were washed three times with PBS containing 0.05% Tween 20, detection antibody was then added, and suspensions were incubated for 2 h at room temperature. Plates were washed three times with PBS containing 0.05% Tween 20 and incubated with streptavidin-horseradish peroxidase for 1 h at room temperature. Plates were washed four times with PBS containing 0.05% Tween 20 and twice with PBS alone and then exposed to BD ELISPOT AEC substrate solution (BD Bioscience) for 1 h. The plates were air dried overnight at room temperature, and spot-forming cells were counted under a dissecting microscope.
Immunoglobulin heavy chain repertoire analysis
Single cell populations from the spleen and PP and individual ILFs were isolated as described above, and DNA was isolated using a QIAamp DNA Micro Kit (Qiagen). The immunoglobulin heavy chain repertoire was analyzed using a PCR amplification of the rearranged genes as previously described (7). PCR was performed on 100 ng of DNA from each tissue using a sense primer specific for the VH family and an antisense primer directed to the immunoglobulin enhancer region. Amplification was performed in a GeneAmp PCR System 2700 (Applied Biosystems) and consisted of a denaturation time for 2 min at 94°C, followed by 40 cycles consisting of 30 s at 94°C, 25 s at 60°C, and 25 s at 72°C and an elongation at 72°C for 4 min. The products were run on a 5% Tris-boric acid-EDTA gel, stained with ethidium bromide, and photographed. The following primers were used for this analysis: J558 VH family (sense) 5′-AAGGCCACACTGACTGTAGAC-3′, Q52 VH family (sense) 5′-AGACTGAGCATCAGCAAAGAC-3′, 7183 VH family (sense) 5′-GCGAATTCGATTCATCATCTCCAGAGAC-3′, and immunoglobulin heavy chain enhancer (antisense) 5′-GCAGGCTCCACCAGAACCT-3′.
Statistical analysis
Data analysis using Student’s t-test and one-way ANOVA followed by Tukey’s multiple-comparison posttest was performed using GraphPad Prism (GraphPad Software). A value of P < 0.05 was used as the cutoff for statistical significance.
RESULTS
ILF B lymphocytes are mature follicular B-2 B lymphocytes
B lymphocytes are the largest lymphocyte population within ILFs (15, 29, 30), and therefore identification of the type(s) or subset(s) of B lymphocytes within ILFs yields insight into the function of ILFs. Comparing ILF B lymphocytes to PP B lymphocytes, a well-studied organized intestinal lymphoid structure, as well as splenic B lymphocytes, a component of the systemic immune system, also provides insight into the role of the ILF in mucosal immune responses. ILF, PP, and splenic cellular populations were examined with three-color flow cytometry. ILFs and PPs harbored a single population of B lymphocytes, which were sIgDhi sIgMlo, consistent with a follicular B lymphocyte phenotype (Fig. 1B). In comparison, the spleen contained both sIgDhi sIgMlo B lymphocytes and sIgMhi sIgDlo B lymphocytes, consistent with the phenotype of follicular and transitional B lymphocytes, respectively (Fig. 1B).
CD5 is a member of the scavenger receptor cysteine-rich protein superfamily. CD5 is expressed by a subset of peripheral B lymphocytes that represents a distinct B lymphocyte lineage, B-1a B lymphocytes (2, 22, 47). Consistent with previous observations, ILFs and PPs contained very few CD5+ IgM+ cells, indicating that ILFs and PPs do not contain B-1a B lymphocytes (Fig. 1C). The spleen contained a few CD5+ IgM+ cells, consistent with the presence of a small B-1a B lymphocyte population (Fig. 1C). CD23, the low-affinity IgE Fc receptor (FcεRII), is expressed on mature resting B-2 B lymphocytes but not on B-1 B lymphocytes (5, 46). Consistent with the lack of CD5 expression and our previous observations of the lack of CD11b expression (29), IgM+ cells from ILFs and PPs were CD23+ (Fig. 1D). The regulation of CD23 expression in B-2 B lymphocytes is complex, and decreased expression of CD23 can be a marker of activation (41). About one-half of the IgM+ cells from the spleen were CD23+, which is most consistent with both the presence of a small B-1 B lymphocyte population as well as decreased regulated expression of CD23 by splenic B-2 B lymphocytes (Fig. 1D). On the basis of the differential staining of IgM, IgD, CD5, and CD23, our findings suggest that ILFs contain predominantly follicular B-2 B lymphocytes. ILF B lymphocytes are phenotypically similar to PP B lymphocytes, suggesting ILF B lymphocytes and PP B lymphocytes have similar functions.
ILF B lymphocytes express higher levels of CD69 and CD80 and higher levels of immunomodulatory B7 and CD28 family members
To further characterize ILF B lymphocytes, we examined the levels of expression of cell surface markers associated with activation, major histocompatibility complex II (MHC II), and B7/CD28 family members. For a more accurate comparison, analysis was performed by gating on the sIgDhi sIgMlo B lymphocyte population from each tissue, as shown in Fig. 1B.
CD44, CD69, and CD62L expression are regulated after lymphocyte activation, with CD44 and CD69 expression increasing after activation and CD62L expression decreasing after activation (3, 4, 25, 32, 37, 44, 50). While changes in the cell surface expression of these molecules are most accepted as markers for T lymphocyte activation, parallel changes are seen in B lymphocytes. We observed that ILF B lymphocytes expressed high levels of CD69, which were greater than those seen in PP and splenic B lymphocytes (Fig. 2). ILF B lymphocytes expressed lower levels of CD44 and intermediate levels of CD62L compared with PP and splenic B lymphocytes (Fig. 2).
Fig. 2.
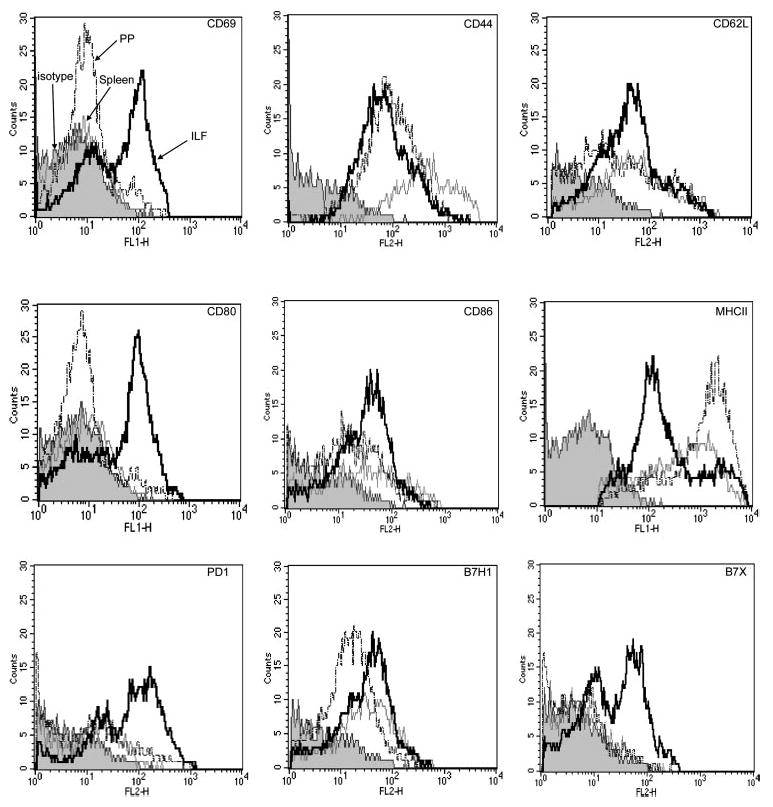
Mature ILF B lymphocytes express higher levels of CD69 and CD80 and higher levels of B7 family members. Three-color flow cytometry analysis was performed on cellular populations from the SP, PP, and mILF as outlined in MATERIALS AND METHODS. Analysis was performed by gating on sIgDhi sIgMlo follicular B-2 B lymphocytes from each tissue, as shown in Fig. 1B. The majority of ILF B lymphocytes (solid trace) expressed CD44, CD62L, CD86, major histocompatibility complex II (MHC II), and B7H1. Among all the markers, the ILF expressed much higher CD69, CD80, B7X, and PD-1 compared with the SP (shaded trace) and PP (dotted trace). Isotype control staining is designated by the filled shaded trace. Flow cytometry analysis of ILF cellular populations was performed on a pooled population from 3 mice. Data shown are representative 1 of 2 experiments.
The ability to function in antigen presentation is directly related to the expression of MHC II and costimulatory molecules. We observed that ILF B lymphocytes expressed high levels of CD80 and lower levels of MHC II compared with PP and splenic B lymphocytes. The expression of CD86 was comparable between the three populations examined (Fig. 2). Among the other B7 and CD28 family members examined, ILF B lymphocytes expressed higher levels of the immunomodulatory receptors B7X and PD-1 compared with splenic and PP B lymphocytes; the expression of B7H1 was comparable between the three groups (Fig. 2).
ILF B lymphocytes preferentially differentiate into IgA-producing plasma cells
B lymphocytes play a unique role in immune responses as the precursors to antibody-producing cells. Different isotypes of antibodies play varying roles within the immune response distinguished by functional differences including the ability to fix complement and the ability to be transported across epithelial barriers. Moreover, the isotypes of antibodies produced in an immune response parallels the phenotype of the immune responses. T helper 1 type (Th1) cytokines preferentially induce a class switch to IgG3 and IgG2a isotypes, whereas T helper 2 type (Th2) cytokines preferentially induce a class switch to IgG1, IgG2b, IgE, and IgA.
To gain insight into the function of ILFs, we examined immunoglobulin production by ILF cellular populations. As shown in Fig. 3A, ILF B lymphocytes preferentially produced IgA as opposed to other immunoglobulin isotypes; this profile was similar to that produced by PPs and intestinal lamina propria cells and dissimilar to that produced by the spleen or that seen in the serum. ELISPOT analysis confirmed that ILF B lymphocytes preferentially differentiated into IgA-producing plasma cells; this profile was most similar to that seen in the PP and lamina propria and least similar to that seen in the spleen (Fig. 3B). Notably, the ILF cell population contained a higher number of IgA-producing cells on a per cell basis compared with the spleen. We did observe a significant number of IgM-producing plasma cells within ILFs by ELISPOT assay; however, only a modest amount of IgM was secreted into the culture supernatant, as detected by ELISA. Consistent with this, the IgM+ spots were smaller than the IgA+ spots in the ILF ELISPOT assays. Together, these findings indicate that ILFs preferentially generate IgA-producing plasma cells, and, given that the class switch to IgA is favored by non-Th1 cytokines IL-5 and transforming growth factor (TGF)-β, this suggests that ILFs promote non-Th1 immune responses.
Fig. 3.
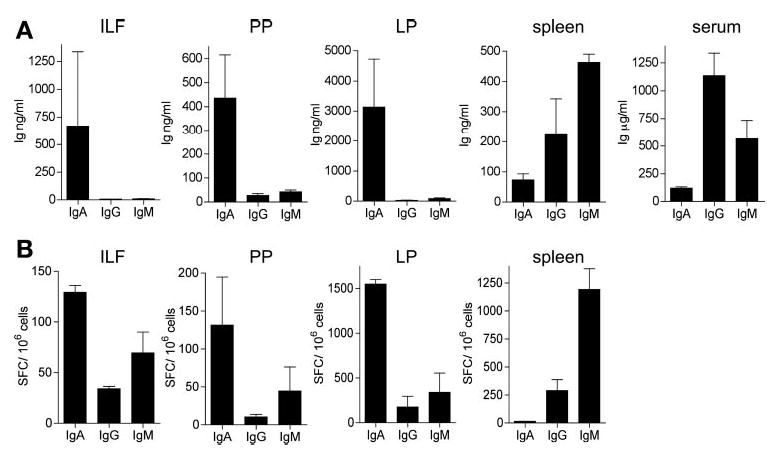
ILF B lymphocytes preferentially differentiate into IgA-producing plasma cells. The level of secreted Ig was quantified by ELISA (A), and the number of antibody-forming cells was quantified by ELISPOTs (B) as described in MATERIALS AND METHODS . ILF cellular populations preferentially produced IgA as opposed to other immunoglobulin isotypes; this profile was similar to that produced by PPs and intestinal lamina propia (LP) cells and dissimilar to that produced by the sP or that seen in the serum (A). ELISPOT analysis confirmed that ILF B lymphocytes preferentially differentiate into IgA- and IgM-producing plasma cells; this profile was most similar to that seen in the PP and LP and least similar to that seen in the SP (B). ILF cell populations contained higher number of IgA-producing cells on a per cell basis compared with the SP using a standard Student’s t-test (P < 0.05); no differences were noted when the IgA-producing cell populations from the PP and ILF were compared. Pooled cellular populations from 3 or 4 mice were used to measure immunoglobulin production by ELISA or ELISPOT assay. Data are represented as means ± SE from 3 independent experiments.
ILF B lymphocytes produce higher levels of IL-4 and IL-10 and lower levels of IFN-γ
It is well known that B lymphocytes produce cytokines in response to diverse stimuli (31, 40). Cytokine-producing B lymphocytes have been found to play a critical role in several autoimmune disease models, including inflammatory bowel disease, experimental autoimmune encephalomyelitis, and collagen-induced arthritis (11, 35, 40). B lymphocytes can be identified as Be-1 and Be-2 cells, which can regulate the differentiation of naïve CD4 T cells to Th1 and Th2 cells through the production of polarizing cytokines such as IFN-γ and IL-4, respectively (16). In addition, B lymphocytes can act as regulatory cells in immunologically mediated inflammatory reactions by producing cytokines such as IL-10 (11, 35).
To investigate the cytokine profile of the ILF B cells, IL-4, IL-10, and IFN-γ secretion were measured by ELISPOTS after the stimulation of sIgDhi sIgMlo B lymphocytes. Compared with splenic B lymphocytes, ILF B lymphocytes produced more IL-4 and IL-10 and less IFN-γ. The cytokine production profile of ILF B lymphocytes was similar to PP B lymphocytes but with a higher IL-4 and IL-10 production (Fig. 4). Consistent with the observation that ILFs preferentially promote the production of IgA-producing plasma cells, this observation also indicates that the cytokine environment in ILFs promotes non-Th1 immune responses.
Fig. 4.
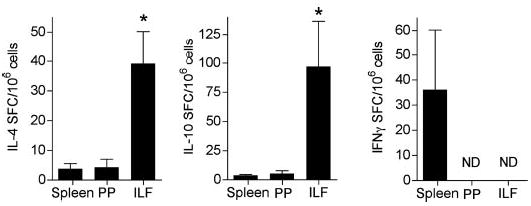
ILF B lymphocytes produce IL-4 and IL-10 and little interferon (IFN)-γ. IL-4, IL-10, and IFN-γ secretion by ILF B lymphocytes was measured by ELISPOT as described in MATERIALS AND METHODS. Compared with splenic and PP sIgDhi sIgMlo B lymphocytes, ILF B lymphocytes produced more IL-4 and IL-10. Compared with splenic B lymphocytes, IFN-γ-secreting cells were not detected among the ILF and PP sIgDhi sIgMlo B lymphocytes. Pooled cellular populations from 3 or 4 mice were used for the measurement of each cytokine. Data are represented as means ± SD from 2 or more independent experiments. ND, none detected. *P < 0.05 compared with cytokine production by ILFs with that of the PP or spleen using one-way ANOVA.
ILFs contain a population of polyclonal B lymphocytes
The development and function of ILFs are not completely understood. Previous observations are consistent with ILF development and function paralleling that of secondary lymphoid structures, and, accordingly, the population of B lymphocytes inhabiting ILFs would be representative of the entire immunoglobulin repertoire of B-2 B lymphocytes within an individual animal. Alternatively, ILFs may arise from a small number of B-2 B lymphocytes that have expanded in response to luminal stimuli and luminal antigens. In this scenario, the population of ILF B lymphocytes would be representative of a smaller subset of the potential pool of B lymphocytes, and the ILF immunoglobulin repertoire would be restricted with overrepresentation of some rearrangements compared with the entire immunoglobulin repertoire within an individual animal. This second possibility is supported by a studies showing that in aged activation-induced cytidine deaminase (AID)-deficient (9) mice, the predominant immunoglobulin VH gene used within individual ILFs varied, suggesting that different B lymphocytes expanded within individual ILFs (10). To differentiate between these possibilities, we analyzed the immunoglobulin heavy chain repertoire on DNA isolated from individual ILFs using a standard PCR-based approach (7). We observed that the immunoglobulin heavy chain repertoire of ILFs was diverse and comparable with that seen in the PP and spleen (Fig. 5A). We also observed that the spleen, PP, and all the individual ILFs examined displayed preferential usage of the largest VH family, J558 (Fig. 5B). These findings indicate that ILFs have a diverse immunoglobulin repertoire and are representative of the repertoire seen in other systemic and mucosal sites.
Fig. 5.
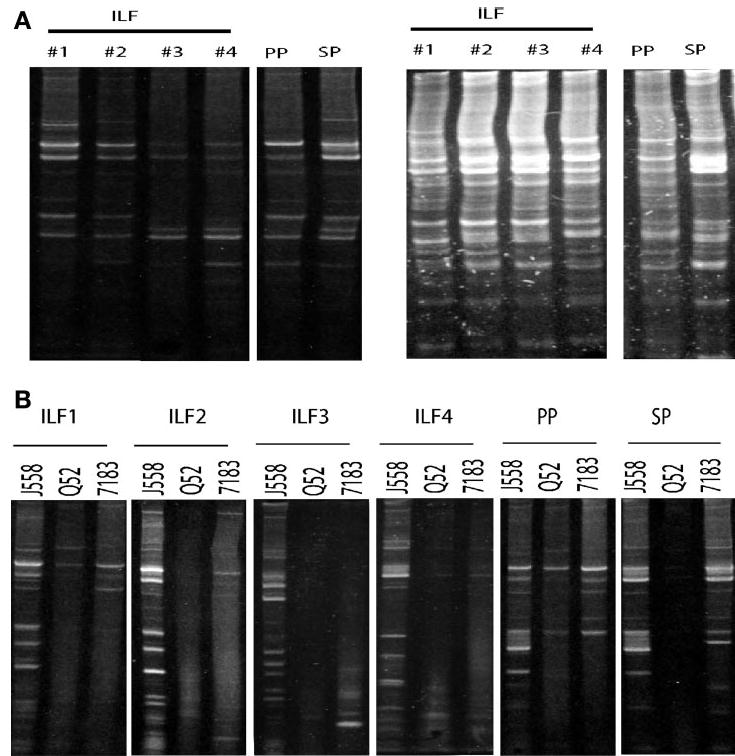
ILF B lymphocytes have a diverse immunoglobulin repertoire similar to the repertoire seen in the SP and PP. Immunoglobulin heavy-chain usage in the SP and PP and individual ILFs was analyzed using a PCR-based approach as described in MATERIALS AND METHODS. PCR was performed with primers specific for each of the three VH families and analyzed by combining the products for each family from an individual ILF, PP, or SP (A). Examination of the usage of three VH families revealed that the SP, PP, and individual ILF displayed a diverse immunoglobulin repertoire with multiple bands corresponding to unique rearrangements (A). When we examined the usage of individual VH families by the individual ILFs, SP, and PP, we observed that each tissue preferentially used J558 VH family members (B). J558 is the largest VH family in the mouse and is used by ~50% of immunoglobulin gene rearrangements. Preferential usage of this family by all of the individual ILFs examined is most consistent with ILFs containing a population of B lymphocytes reflective of the systemic pool as opposed to ILFs arising from a small number of B lymphocytes that have expanded in situ. The above results are representative of findings from the examination of 20 individual ILFs from multiple mice.
DISCUSSION
ILFs are recently appreciated members of the mucosal immune system (15). Previous studies (15, 29) have demonstrated that similar to PPs, ILFs contain PNA+ germinal centers and an overlying FAE, which contains M cells. Likewise, the cellular composition of ILFs resembles that of PPs, containing B lymphocytes, TCRαβ+ T lymphocytes, and dendritic cells (30). ILFs can also contain a significant proportion, up to 10% of the cellular population, of lineage marker negative (lin−) c-kit+ cells (15). However, unlike PPs, ILFs have been observed to form de novo in adult animals in response to luminal stimuli (29). Although the function of ILFs is still an area of active investigation, the inducible nature, cellular composition, and architecture of ILFs suggest that ILFs may act as reservoirs of inductive sites for mucosal immune responses. This suggestion is supported by studies documenting that ILFs can support IgA class switching (42) and that ILFs can act as inductive sites for mucosal immune responses to T cell-dependent antigens (30). These studies only investigated the ability of ILFs to propagate homeostatic immune responses such as the production of IgA. The predisposition of ILFs to initiate or propagate more inflammatory type responses is unknown but supported by parallel observations that document the presence of ectopic lymphoid structures in the target organs affected by autoimmune and chronic inflammatory conditions as well as the production autoantibodies within these structures.
B lymphocytes are the largest members of the ILF cellular population, comprising up to 70% of the lymphocyte population. B lymphocytes have a unique function in immune responses as the precursors to antibody-producing plasma cells. B lymphocytes can also contribute to other aspects of the immune response by acting as antigen-presenting cells and through the production of cytokines. More recent studies have suggested that mucosal B lymphocytes possess unique properties allowing them to mediate homeostatic or immunomodulatory responses. Given this background, we examined the phenotype of ILF B lymphocytes to gain insight into the role that the ILF plays in mucosal immune responses.
In this study, we examined the phenotypic and functional aspects of ILF B lymphocytes and compared these findings with those obtained from PP and splenic B lymphocytes. We observed that all ILF B lymphocytes were CD5−, CD23+, and IgDhi IgMlo. These findings are consistent with previous observations demonstrating that ILFs do not contain B-1 B lymphocytes and extend these observations to demonstrate that ILFs are composed of predominantly one population of B-2 B lymphocytes, which have the surface phenotype of mature follicular B lymphocytes. This B lymphocyte population is very similar to that seen in the PP. In comparison, the splenic population contains IgMhi IgDlo populations, consistent with the presence of transitional B lymphocytes that have recently emigrated from the bone marrow (6, 28). This observation has several implications. First, ILF B lymphocytes are mature follicular B-2 B lymphocytes, and, as such, they will likely function in a manner similar to follicular B lymphocytes at other sites with respect to requirements for T cell help, activation, and homing. Second, unlike the spleen, ILFs do not contain transitional B lymphocytes, suggesting that ILFs are not sites where recently emigrating B lymphocytes mature. Finally, given that essentially all ILF B lymphocytes fall into a homogenous population, it is unlikely that ILFs are sites where mucosal B lymphocytes are generated from lin− c-kit+ cells, which have been suggested to be putative lymphoid hematopoietic progenitors (21).
To better understand the state of activation of ILF B lymphocytes, and to gain a better understanding of the potential function of ILFs, we chose to compare the expression cell surface markers associated with activation between splenic, PP, and ILF B lymphocytes. Because ILF B lymphocytes are predominantly, if not exclusively, IgDhi IgMlo, we chose to gate on these cells to have a more accurate comparison between the populations. We observed that ILF B lymphocytes had higher level of expression of CD69 and a low to intermediate level of expression of CD62L compared with the spleen and PP. The level of expression of CD44 in ILF B lymphocytes was less than that seen in splenic B lymphocytes and equivalent to that seen in PP B lymphocytes. Although these cell surface molecules can be modulated by stimuli independent of B cell receptor ligation and are more accepted as markers of T lymphocyte activation, their expression is nonetheless modulated in parallel manners upon B lymphocyte activation. Together, these findings suggest that ILF B lymphocytes are more activated compared with splenic and PP B lymphocytes. This observation is consistent with the physical location of ILFs at a site in close proximity to bacterial antigens and stimuli, which could account for the increased activated state of B lymphocytes. The augmented state of activation may appear to be in contrast to our previous observations showing that ILF formation does not require antigenic stimulation of B lymphocytes and that ILFs can contain antigen naïve B lymphocytes (33). Together, these observations are most consistent with naïve B lymphocytes preferentially entering ILFs and quickly becoming activated due to the close proximity of ILFs to multiple stimuli.
To gain a better understanding of the potential of ILF B lymphocytes to function as antigen-presenting cells, we compared the expression of MHC II and B7 family members by splenic, PP, and ILF B lymphocytes. We observed that ILF B lymphocytes expressed high levels of CD80, intermediate to high levels of CD86, and lower levels of MHC II compared with splenic and PP B lymphocytes. Some of the recently identified members of the B7 and CD28 families are expressed by B lymphocytes and function to modulate or dampen immune responses. Programmed death (PD)-1 is a CD28 family member that is upregulated after lymphocyte activation and pairs with B7H1 to modulate/dampen lymphocyte responses (1). B7X (B7H4) binds to a yet-unidentified ligand on activated T lymphocytes and acts as a negative regulator of T lymphocyte responses (49). ILF B lymphocytes expressed high levels of B7X and PD-1 and equivalent levels of B7H1 compared with splenic and PP B lymphocytes. Together, these finding imply that ILF B lymphocytes have an equivalent capacity to function as antigen-presenting cells and are more likely to act as modulators of T lymphocyte responses compared with their counterparts in the PP and spleen. Whether this difference indicates that ILF B lymphocytes are functionally distinct from splenic B lymphocytes or whether this is a result of increased activation of B lymphocytes within ILFs is not known.
B lymphocytes have a unique function in immune responses as the precursors to antibody-producing plasma cells. Mucosal B lymphocytes are further distinguished from their counterparts in the systemic immune system by their preference to become IgA-producing plasma cells as opposed to IgG-producing plasma cells. In B-2 B lymphocytes, such as ILF B lymphocytes, this process is under the direct control of CD4+ T lymphocytes and the local cytokine milieu that they produce. The class switch to specific immunoglobulin isotypes is driven by different profiles of cytokines, with the switch to IgA being driven by the relative abundance of IL-5 and TGFβ and the switch to IgG2a being driven by the local abundance of Th1 cytokines. Therefore, examining the isotype of immunoglobulins produced by ILF B lymphocytes not only yields insight into the potential function of ILF B lymphocytes but also into the phenotype of the T lymphocyte responses occurring within ILFs. Previous observations, including our own, have indicated that ILFs can be the sites for the IgA class switch and production (30, 42); however, these studies did not assess the profile of other immunoglobulin isotypes produced by ILFs, and, therefore, the overall phenotype of the immune response resulting in immunoglobulin production could not be assessed. In this study, we found that, similar to PPs, ILFs preferentially support the production of IgA. Moreover, we observed that, on a per cell basis, ILFs were a larger source for IgA production, and, in contrast to PPs, we were unable to detect IgG production by ILFs. This profile was most similar to that produced by lamina propria cellular populations and least similar to that produced by splenocytes and the profile of serum immunoglobulins. We interpret these findings to indicate that ILFs have a cytokine milieu that supports IgA production as opposed to other immunoglobulin isotypes. These findings are supportive of ILFs as contributing to the pool of mucosal immunoglobulin production as opposed to the pool of systemic immunoglobulin production.
The ability of B lymphocytes to produce cytokines in response to a variety of stimuli has been long appreciated (31, 40). A recent study (16) has indicated that B lymphocytes can be divided into subsets based on the production of IL-4 and IFN-γ and that these B lymphocyte derived cytokines act to polarize T lymphocyte responses in vivo. Further studies (11, 35) have indicated that IL-10 production by B lymphocytes can act to regulate autoimmunity and chronic intestinal inflammation. We therefore examined the ability of ILF B lymphocytes to produce IL-4, IL-10, and IFN-γ. We observed that ILF B lymphocytes produced more IL-4 and IL-10 and less IFN-γ compared with their splenic counterparts. BALB/c mice, compared with some other strains, have been noted to preferentially develop immune responses with a Th2 phenotype (17, 26, 34). Therefore, it is possible that in other mouse strains, ILF B lymphocytes may produce a different profile of cytokines. Despite this caveat, it is important to note that compared with the responses seen in other tissues within the same animal, ILF B lymphocytes displayed a more immunomodulatory phenotype. These findings are most consistent with ILFs mediating “homeostatic” or noninflammatory responses. Mesenteric LN B lymphocytes from SAMP1/YitFc mice, which develop spontaneous intestinal inflammation, were found to inhibit the development of regulatory T lymphocytes, and this function was attributed to increased expression of glucocorticoid-induced tumor necrosis factor-related receptor ligand (GITRL) on these mesenteric LN B lymphocytes (38). Related to this observation and our above findings, we were unable to detect GITRL on ILF B lymphocytes (data not shown).
To gain a better understanding of the origin and complexity of the B lymphocyte population within ILFs, we examined the immunoglobulin repertoire of individual ILFs using a DNA-based, PCR-based approach. We observed that the immunoglobulin repertoire of individual ILFs has a complexity that is comparable to that of the PP and spleen. Moreover, we observed that the spleen and PP and all the individual ILFs we examined preferentially used members of the J558 VH family. The J558 family is the largest VH family and represents ~50% of all the immunoglobulin variable regions expressed in the adult mouse (12). Therefore, the predominant usage of this VH family in all the individual ILFs examined is consistent with the ILF immunoglobulin repertoire, and hence ILF B lymphocytes, arising from the systemic pool, and is less consistent with ILFs developing from the expansion of one or a few B lymphocytes. Our findings would appear to be in contrast to a study by Fargarasan et al. (10), which examined individual ILFs from aged AID−/− mice using an RNA-based PCR approach. Although both our findings demonstrated that ILFs have a diverse immunoglobulin repertoire, the study of Fagarasan et al. (10) demonstrated the preferential expression of different VH family members within individual ILFs. In addition to differences in the age and genotype of the mice used in the different studies, the differences in approaches could also contribute to the differences in the findings. The study by Fagarasan et al. (10) examined RNA isolated from ILF B lymphocytes. Although this approach provides an accurate evaluation of VH gene expression, if utilized to estimate the immunoglobulin repertoire this approach may “overrepresent” highly expressed VH members and consequently “underrepresent” overall diversity; this may be particularly relevant in a population that may contain plasma cells. Here, we used a DNA-based approach. This approach cannot evaluate VH expression but is not subject to the influences of high immunoglobulin expression by a minority of B lymphocytes or plasma cells and, therefore, should give a more accurate evaluation of the diversity of the immunoglobulin repertoire.
Tertiary lymphoid structures have been observed in a number of chronic inflammatory and autoimmune conditions including rheumatoid arthritis, Sjo gren’s syndrome, primary sclerosing cholangitis, chronic hepatitis C infection, myasthenia gravis, multiple sclerosis, Hashimoto’s thyroiditis, and in a number of inflammatory conditions involving the gastrointestinal tract, including inflammatory bowel disease and gastritis and animal models of intestinal inflammation (14, 18). Although the roles that ILFs and other tertiary lymphoid structures play in the immune response is unclear, it has been suggested that tertiary lymphoid structures may contribute to inappropriate immune responses by facilitating interactions of antigens, antigen-presenting cells, and lymphocytes in an environment that lacks the “normal” regulatory environment of secondary lymphoid structures (18). The findings presented here extend our understanding of ILFs and potentially of tertiary lymphoid structures in general.
Tertiary lymphoid structures in other tissues have been suggested to form at the sites of local inflammatory responses, and, in general, these responses are felt to be aberrant or pathogenic, whereas ILFs are noted to form in the intestines of “normal” animals. This apparent discrepancy can be resolved by the understanding that in the normal animal, the intestinal immune system is continually exposed to bacteria and bacterial products and hence continually experiencing low levels of inflammation. The apparently simple observations presented here have profound implications for the function of ILFs. These findings suggest that in response to normal levels of inflammation, the intestine can induce the formation of immune inductive sites that can act to mediate homeostatic responses and abort potentially injurious responses. Whether these findings can be extended to ILFs in “challenged” animals or to tertiary lymphoid structures formed in other tissues and other conditions remains to be investigated.
Footnotes
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grant DK-64798, the Crohn’s and Colitis Foundation of America, The Nicholas V. Costrini Award in Inflammatory Bowel Disease Research, and the Washington University School of Medicine NIH Digestive Diseases Research Core Center Grant P30-DK-52574. The work cited in this publication was performed in a facility supported by NIH Grant C06-RR-012466.
References
- 1.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 2.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 3.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 4.Cerwenka A, Carter LL, Reome JB, Swain SL, Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 5.Conrad DH, Waldschmidt TJ, Lee WT, Rao M, Keegan AD, Noelle RJ, Lynch RG, Kehry MR. Effect of B cell stimulatory factor-1 (interleukin 4) on Fc epsilon and Fc gamma receptor expression on murine B lymphocytes and B cell lines. J Immunol. 1987;139:2290–2296. [PubMed] [Google Scholar]
- 6.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 7.Delassus S, Gey A, Darche S, Cumano A, Roth C, Kourilsky P. PCR-based analysis of the murine immunoglobulin heavy-chain repertoire. J Immunol Methods. 1995;184:219–229. doi: 10.1016/0022-1759(95)00091-n. [DOI] [PubMed] [Google Scholar]
- 8.Eidt S, Stolte M. Prevalence of lymphoid follicles and aggregates in Helicobacter pylori gastritis in antral and body mucosa. J Clin Pathol. 1993;46:832–835. doi: 10.1136/jcp.46.9.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elewaut D, Brossay L, Santee SM, Naidenko OV, Burdin N, De Winter H, Matsuda J, Ware CF, Cheroutre H, Kronenberg M. Membrane lymphotoxin is required for the development of different subpopulations of NK T cells. J Immunol. 2000;165:671–679. doi: 10.4049/jimmunol.165.2.671. [DOI] [PubMed] [Google Scholar]
- 10.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 11.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immun. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 12.Freitas AA, Andrade L, Lembezat MP, Coutinho A. Selection of VH gene repertoires: differentiating B cells of adult bone marrow mimic fetal development. Int Immunol. 1990;2:15–23. doi: 10.1093/intimm/2.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, Wu L, Hubscher SG, Adams DH. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immun. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- 19.Kabel PJ, Voorbij HA, de Haan-Meulman M, Pals ST, Drexhage HA. High endothelial venules present in lymphoid cell accumulations in thyroids affected by autoimmune disease: a study in men and BB rats of functional activity and development. J Clin Endocrinol Metab. 1989;68:744–751. doi: 10.1210/jcem-68-4-744. [DOI] [PubMed] [Google Scholar]
- 20.Kaiserling E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology. 2001;34:22–29. [PubMed] [Google Scholar]
- 21.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasaian MT, Casali P. Autoimmunity-prone B-1 (CD5 B) cells, natural antibodies and self recognition. Autoimmunity. 1993;15:315–329. doi: 10.3109/08916939309115755. [DOI] [PubMed] [Google Scholar]
- 23.Knecht H, Saremaslani P, Hedinger C. Immunohistological findings in Hashimoto’s thyroiditis, focal lymphocytic thyroiditis and thyroiditis de Quervain. Comparative study. Virchows Arch. 1981;393:215–231. doi: 10.1007/BF00431078. [DOI] [PubMed] [Google Scholar]
- 24.Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, Hiroi T, Nanno M, Kiyono H. Prenatal blockage of lymphotoxin beta receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174:4365–4372. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- 25.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 26.Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1−. Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 27.Lockhart-Mummery HE, Morson BC. Crohn’s disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut. 1960;1:87–105. doi: 10.1136/gut.1.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz RG, Newberry RD. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann NY Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- 31.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald HR, Budd RC, Cerottini JC. Pgp-1 (Ly 24) as a marker of murine memory T lymphocytes. Curr Top Microbiol Immunol. 1990;159:97–109. doi: 10.1007/978-3-642-75244-5_6. [DOI] [PubMed] [Google Scholar]
- 33.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigennaive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–5728. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell GF, Handman E, Moll H, McConville MJ, Spithill TW, Kidane GZ, Samaras N, Elhay MJ. Resistance and susceptibility of mice to Leishmania major: a view from Melbourne. Ann Inst Pasteur Immunol. 1987;138:738–743. doi: 10.1016/s0769-2625(87)80029-6. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 36.Newberry RD, McDonough JS, McDonald KG, Lorenz RG. Postgestational lymphotoxin/lymphotoxin beta receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168:4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nakui M, Sekimoto M, Koda T. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol. 2000;12:987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 38.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, Morris MA, Pizarro TT, Ernst PB, Cominelli F, Ley K. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114:389–398. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 40.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 41.Rabin E, Cong YZ, Wortis HH. Loss of CD23 is a consequence of B cell activation. Implications for the analysis of B cell lineages. Ann NY Acad Sci. 1992;651:130–142. doi: 10.1111/j.1749-6632.1992.tb24602.x. [DOI] [PubMed] [Google Scholar]
- 42.Shikina T, Hiroi T, Iwatani K, Jang MH, Fukuyama S, Tamura M, Kubo T, Ishikawa H, Kiyono H. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol. 2004;172:6259–6264. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- 43.Soderstrom N, Biorklund A. Organization of the invading lymphoid tissue in human lymphoid thyroiditis. Scand J Immunol. 1974;3:295–301. doi: 10.1111/j.1365-3083.1974.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 44.Sprent J, Tough DF. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 45.Ueki T, Mizuno M, Uesu T, Kiso T, Tsuji T. Expression of ICAM-I on M cells covering isolated lymphoid follicles of the human colon. Acta Med Okayama. 1995;49:145–151. doi: 10.18926/AMO/30412. [DOI] [PubMed] [Google Scholar]
- 46.Waldschmidt T, Snapp K, Foy T, Tygrett L, Carpenter C. B cell subsets defined by the Fc epsilon R. Ann NY Acad Sci. 1992;651:84–98. doi: 10.1111/j.1749-6632.1992.tb24599.x. [DOI] [PubMed] [Google Scholar]
- 47.Wortis HH, Berland R. Cutting edge commentary: origins of B-1 cells. J Immunol. 2001;166:2163–2166. doi: 10.4049/jimmunol.166.4.2163. [DOI] [PubMed] [Google Scholar]
- 48.Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarstrom S, Hammarstrom ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]


