Abstract
Chemokines play a key role in inflammation. They are expressed not only in neuroinflammatory conditions, but also constitutively by different cell types, including neurons in the normal brain, suggesting that they may act as modulators of neuronal functions. Here, we investigated a possible neuroendocrine role of the chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. We demonstrated the colocalization of SDF-1 and its receptor CXCR4 with arginine vasopressin (AVP) in the magnocellular neurons of the supraoptic nucleus (SON) and the paraventricular hypothalamic nucleus and on AVP projections to the neurohypophysis. Electrophysiological recordings of SON neurons demonstrated that SDF-1 affects the electrical activity of AVP neurons through CXCR4, resulting in changes in AVP release. We observed that SDF-1 can blunt the autoregulation of AVP release in vitro and counteract angiotensin II-induced plasma AVP release in vivo. Furthermore, a short-term physiological increase in AVP release induced by enhanced plasma osmolarity, which was produced by the administration of 1 M NaCl i.p., was similarly blocked by central injection of SDF-1 through CXCR4. A change in water balance by long-term salt loading induced a decrease in both SDF-1 and CXCR4 parallel to that of AVP immunostaining in SON. From these data, we demonstrate that chemokine actions in the brain are not restricted to inflammatory processes. We propose to add to the known autoregulation of AVP on its own neurons, a second autocrine system induced by SDF-1 able to modulate central AVP neuronal activity and release.
Keywords: dehydration, hypothalamic magnocellular neurons, neuroendocrine, neuromodulation, posterior pituitary
Arginine vasopressin (AVP) and oxytocin (OT) are synthesized in the magnocellular neurosecretory neurons of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN) and then transported through the median eminence to the posterior lobe of the pituitary gland, where they are secreted into the general blood circulation. AVP is known to be primarily involved in water absorption in the distal nephron of the kidney, thus regulating drinking behavior, whereas the functions of OT during parturition to increase uterine contraction and during suckling have been well described (1).
Chemokines are small, secreted molecules (7–14 kDa) with chemoattractant properties whose main accepted role is leukocyte recruitment in inflammatory sites (2, 3). Recent investigations into the possible involvement of chemokines in a number of neurological disorders associated with neuroinflammation have led to the possibility that many chemokines and their respective receptors can be expressed in the brain (4). However, recent data demonstrated that they not only are observed in neuroinflammatory conditions but also are constitutively expressed by different cell types, including neurons in the normal brain (5–7). These data suggest that chemokines may act as modulators of neuronal functions.
Among chemokines and chemokine receptors with possible neuromodulatory functions, the CXC chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12 and its cognate receptor CXCR4 have attracted much attention. SDF-1 can increase intracellular calcium, modulates ionic current in CXCR4-expressing neurons (8–11), and plays an important role in neurogenesis (12, 13).
We recently demonstrated that SDF-1 and the receptor CXCR4 exhibit a highly regionalized neuronal distribution in the rat brain. In particular, we reported the presence of neuronal CXCR4 immunoreactivity in both SON and PVN in the normal rat hypothalamus (14) as well as a constitutive expression of its ligand SDF-1 (15). Interestingly, we reported that SDF-1 is selectively colocalized with AVP-expressing neurons and not with OT neurons in both SON and PVN (ref. 15 and see Fig. 5).
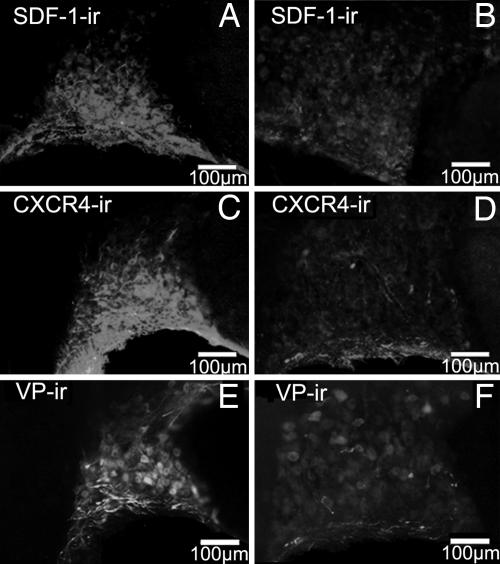
Fig. 5.
Effect of salt loading on SDF-1, CXCR4, and AVP (VP) immunoreactivity (ir) in the rat SON. SDF-1 colocalized with AVP in the control SON (15) dramatically decreases after salt loading (A and B). CXCR4 protein also decreases after 3% NaCl for 12 days (C and D) as does AVP (E and F). Both control (Left) and salt-loading (Right) pictures were taken at the same intensity. Similar data were obtained with the other animals tested. (Scale bars, 100 mm.)
These neuroanatomical findings suggested the possible involvement of SDF-1 and CXCR4 in the regulation of AVP release. The aim of the present study was thus to determine whether a chemokine such as SDF-1 may have neuroendocrine functions in the AVP system. We first anatomically identified that not only SDF-1 but also its receptor CXCR4 can be expressed in the cell bodies expressing AVP in both SON and PVN. Furthermore, we showed that both were colocalized with AVP in the projecting areas of AVP neurons, such as the median eminence and the posterior pituitary. Then, we demonstrated in vitro that SDF-1 can impair the specific patterns of AVP neurons, such as their electrical activity and their autoregulation mechanisms. By means of physiological experiments with intracerebral injections of SDF-1 into the third ventricle, we determined in normal rats that SDF-1 in vivo was able to inhibit, by CXCR4, plasma AVP release induced by angiotensin II (AII) and 1 M NaCl. Finally, we refined our understanding of the involvement of SDF-1 with AVP release, showing a decrease in SDF-1 and CXCR4 immunostaining in both SON and PVN in response to long-term salt loading similar to that observed with AVP (16).
Results
Colocalization of SDF-1 and CXCR4 with AVP-Expressing Neurons in Rat Hypothalamus and Posterior Pituitary.
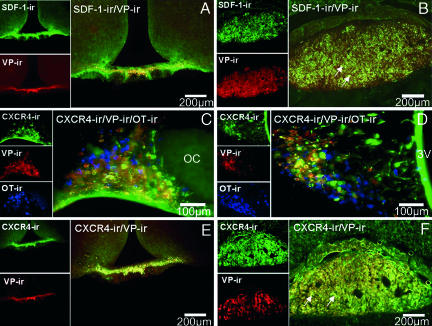
Using the same tested specific antibodies as in the present study, we described in ref. 15 that SDF-1 colocalized exclusively with AVP-expressing neurons in both the SON and the magnocellular PVN and not with OT neurons (see Fig. 5). Fig. 1 shows that SDF-1 also colocalizes all along the AVP pathway to the neural lobe of the pituitary. Such colocalization can be observed in the main AVP projection to the internal palisade zone of the median eminence (Fig. 1A) and to its nerve terminals in the posterior pituitary, in particular in the Herring bodies, often in association with capillaries (Fig. 1B).
Fig. 1.
Colocalization of SDF-1 and CXCR4 receptor immunoreactivity (ir) with AVP (VP) in the hypothalamus and posterior pituitary in the rat. (A and B) Double immunohistochemistry of SDF-1 (stained in green) with AVP (stained in red) in the median eminence (A) and in the posterior pituitary (B). Simple stainings are presented in the small pictures of each panel. In the median eminence, SDF-1 strictly colocalizes with the AVP processes in the internal zone. In the pituitary, a strong SDF-1 immunostaining is observed in the posterior lobe of the pituitary, which is similar to the localization of AVP nerve terminals in this region, in particular in the probable Herring bodies (arrows). (Scale bars, 200 μm.) (C and D) Triple immunohistochemistry of CXCR4 (stained in green) with AVP (stained in red) and OT-expressing neurons (stained in blue) in the SON (C) and PVN (D). Eighty-five percent and 95% of the AVP neurons colocalize with CXCR4 immunoreactivity in the SON and PVN, respectively. No colocalization is observed between OT-positive neurons and CXCR4 immunostaining. Simple stainings are presented in the small pictures of each panel. (Scale bars, 100 μm.) (E and F) Double immunohistochemistry of CXCR4 (stained in green) with AVP (stained in red) in the median eminence (E) and in the posterior pituitary (F). Simple stainings are presented in the small pictures of each panel. (Scale bars, 200 μm.) In the median eminence, CXCR4 strictly colocalizes with the AVP processes in the internal zone. In the pituitary, a strong CXCR4 immunostaining is observed in the posterior lobe of the pituitary, which is similar to the localization of AVP nerve terminals in this region, in particular in the probable Herring bodies (arrows). All sections were observed with a fluorescence microscope (BX61; Olympus, Melville, NY), and images were computed for quantitative analysis.
Fig. 1 C and D shows that not only SDF-1 but also CXCR4 is present in both SON and PVN of normal rats in association with AVP-expressing neurons. As demonstrated by triple immunostaining of CXCR4, AVP, and OT, a large proportion of AVP-expressing neurons (stained in red) colocalize with CXCR4 immunoreactivity (stained in green). In contrast, no OT-positive neurons (stained in blue) coexpressed CXCR4. In both SON and magnocellular PVN, CXCR4 colocalization can be found in >85% and 95% of AVP neurons, respectively (Fig. 1 C and D).
As for SDF-1, the receptor CXCR4 can be found in the internal zone of the median eminence and posterior pituitary in close association with AVP-labeled nerve terminals (Fig. 1 E and F). Furthermore, we noted that a strong CXCR4 receptor immunoreactivity can be observed in the lamina terminalis, which consists of the median preoptic nucleus and two circumventricular organs (CVOs), the subfornical organ and the organum vasculosum of the lamina terminalis, structures known to be the prime brain targets for circulating AII to influence central pathways regulating body fluid homeostasis (17) (data not shown). Altogether, these anatomical data strongly suggested that CXCR4 could be involved in the possible effects of SDF-1 on AVP neurons.
Electrical Activity of SON AVP Neurons After SDF-1.
To test the hypothesis that SDF-1 can affect AVP neuronal activity, the electrical activity of AVP neurons was recorded on ventral hypothalamic slices after local perfusion of SDF-1. Previous studies performed in the same preparation (18) have shown that irregularly spiking neurons selectively respond to AVP, indicating that they are putative AVP-secreting neurons. These irregular neurons were thus tested in the present study.
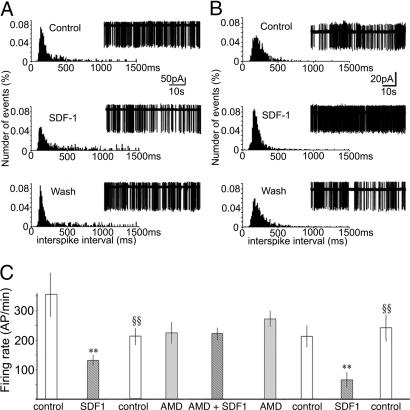
The action of SDF-1 was first tested in the whole-cell configuration, but no SDF-1-induced current was recorded, probably as a result of dialysis of crucial intracellular components. The electrical activity was then recorded extracellularly in the cell-attached configuration, and the effect of SDF-1 perfusion (25 nM) on the pattern of action potential firing was studied. Cells were recorded in the ventro–posterior part of the SON. Fig. 2 shows the normalized distribution histograms of the interspike intervals (left drawings) of action potentials (right drawings) recorded before (Control), during SDF-1, and after (Wash) perfusion of SDF-1. Of the 30 neurons successfully recorded, SDF-1 decreased activity in 9 neurons (Fig. 2A), with an average number of spikes per min decreased by 44 ± 7%; 7 responded (Fig. 2B) with an increase in activity by 94 ± 50%, and SDF-1 had no significant effect on 14 neurons (3 ± 4%). Altogether, SDF-1 did not affect the maximum firing frequency, but rather it inhibited the firing pattern of AVP neurons by decreasing either the duration of active periods or the duration of silences, possibly resulting in changes in AVP release. Five minutes after washing, the firing of AVP neurons returned to a normal pattern (Fig. 2 A and B). To test that these effects of SDF-1 were mediated by CXCR4 receptors, their sensitivity to 10 μM AMD was determined in inhibited neurons. As shown in Fig. 2C, the inhibitory effect of SDF-1 on electrical activity was totally blocked by the CXCR4 antagonist AMD, which had no effect by itself on the firing pattern. Similar to what was observed on inhibited neurons, AMD blocked the excitatory effects of SDF-1 (not shown). Furthermore, AMD had no effect on SDF-1 on cells insensitive to the chemokine (data not shown).
Fig. 2.
Electrophysiological characterization of the effect of SDF-1 on the action-potential firing pattern of AVP neurons in SON. Modulation by SDF-1 of action-potential firing in magnocellular neurosecretory cells is shown, as are typical normalized distribution histograms of the interspike intervals (ISIs) (left sides) of action potentials (right sides) recorded before, during, and after perfusion of 25 nM SDF-1 in different neurons. (A) SDF-1 inhibits the activity of AVP and decreases the duration of active periods. (B) SDF-1 stimulates the activity of AVP neurons and blunts the silent periods. (C) Sequence of a typical experimental protocol used to study the effect of SDF-1 and the implication of CXCR4. The number of action potentials per minute (AP/min) was recorded during 5-min periods under the conditions indicated. Note that the inhibitory effect of SDF-1 on action potentials is reproducible and reversibly inhibited by 10 μM AMD. Results are expressed as the means ± SEM. ∗∗, P < 0.01 vs. respective controls; §§, P < 0.01 vs. SDF-1.
To test whether the electrophysiological data with SDF-1 could be linked to changes in AVP release, in vitro and in vivo experiments were carried out.
Neuromodulatory Effect of SDF-1 on Vasotocin-Induced AVP Release.
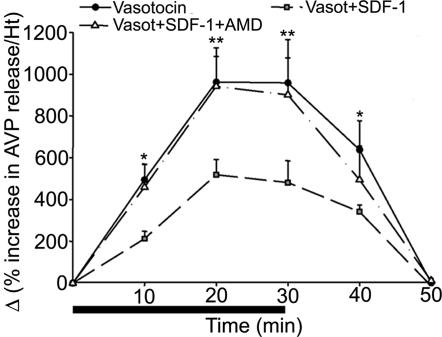
Mature AVP hypothalamic magnocellular neurons are known to autocontrol their electrical activity and release by somatodendritic release of the hormone (18–21). We have used this specific property of AVP neurons to set up an in vitro perifusion model to induce AVP release physiologically. To measure AVP release, we used vasotocin as an analog of AVP that does not crossreact with our ELISA. Under these conditions, 100 nM vasotocin strongly and reproducibly induced a 10-fold increase in AVP release (Fig. 3). The effect is already significant after a 10-min perifusion with vasotocin, and it is maximal at 20 min. When vasotocin administration is stopped, the AVP release returns to basal levels at 20 min. Under these conditions, 50 nM SDF-1 dramatically inhibits vasotocin-induced AVP release by 50%, whereas it has no effect by itself on basal AVP secretion (data not shown). CXCR4 receptor is involved in the effect of SDF-1 because 10 μM AMD completely reversed the inhibitory effect of SDF-1. In this in vitro model, AII has no direct effect on AVP release (data not shown).
Fig. 3.
Effect of SDF-1 on vasotocin-induced AVP release. Perifusion of hypothalamic tissues with 100 nM vasotocin for 30 min induces a 10-fold increase in AVP release. The effect is already observed after 10-min infusion with the peptide. SDF-1 (50 nM) significantly blunts vasotocin-induced AVP release. The inhibitory effect of SDF-1 is totally blocked by the CXCR4 antagonist AMD (10 μM). Results are expressed as Δ (% increase in AVP release per hypothalamus), mean ± SEM of three experiments. ∗, P < 0.05; and ∗∗, P < 0.01 vs. basal level.
In Vivo Effect of SDF-1 on Basal and Induced Plasma AVP Secretion.
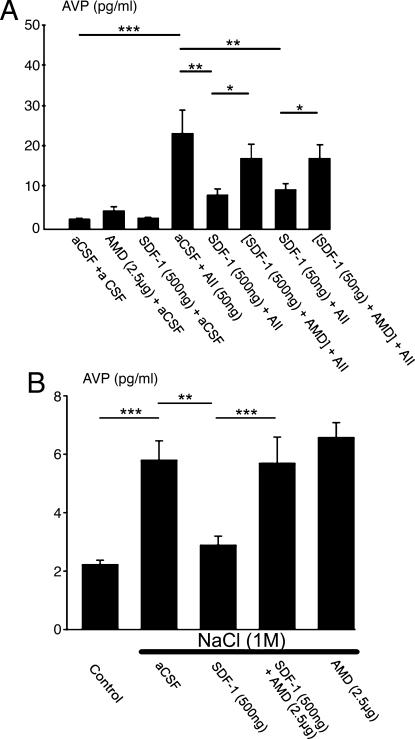
To confirm the hypothesis of a neuromodulatory role of SDF-1 through CXCR4 on AVP secretion, we examined whether this chemokine could physiologically modulate the release of AVP in vivo. For this purpose, we carried out two different sets of experiments by means of injecting SDF-1 into the third ventricle. Fig. 4A shows that, similarly to the in vitro models tested above, SDF-1 (50 and 500 ng) has no effect by itself on basal AVP. In contrast, at both concentrations, SDF-1 significantly counteracts AII-induced plasma AVP release. AMD, which has no effect per se, is able to abolish the effect of SDF-1, indicating that the in vivo inhibitory effect of SDF-1 on AII action is mediated by CXCR4.
Fig. 4.
Effect of third-ventricle injection of SDF-1 on plasma AVP release induced by AII and NaCl. (A) Rats were injected in the third ventricle with artificial cerebrospinal fluid (aCSF) (control), AII, SDF-1, or AMD for 5 min. In the experimental groups in which two drugs were administered (or aCSF), the second was injected 7 min after the first. Plasma was collected 10 min after the final injection. Control rats received vehicle under similar experimental conditions. (B) Rats were injected with the various drugs in the third ventricle as described in A, and 4 min later they received an i.p. injection of 1 M NaCl. Control rats did not receive the NaCl injection. Plasma was collected 15 min after the final injection. SDF-1 blocks AVP release induced by AII or hypertonic solution, an effect counteracted by AMD. Results are expressed as the means ± SEM of at least six to eight animals per group. ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 vs. respective groups.
As shown in Fig. 4B, hyperosmolarity induced by i.p. injection of 1 M NaCl produced a 3-fold increase in plasma AVP. As for the injection of AII, 500 ng of SDF-1 significantly blocks AVP release. This effect is abolished by cotreatment with 2.5 μg of AMD. In this paradigm, AMD alone has no significant effect.
Salt-Loading Experiments.
Finally, to demonstrate the possible modifications of SDF-1 levels in physiological conditions in which AVP release was increased, long-term salt-loading experiments were performed. Three percent NaCl in drinking water for 12 days induced a strong, sustained release in plasma AVP, resulting in a decrease in AVP protein content in both SON and PVN (ref. 20 and see Fig. 5 E and F). Under these conditions, a similar decrease in SDF-1 immunostaining can be observed in the SON (Fig. 5 Aand B) and PVN (data not shown). Under the same conditions, a strong decrease in the level of CXCR4 protein was also found in the SON (Fig. 5 C and D) and PVN (data not shown), suggesting a decreased responsiveness of the SDF-1/CXCR4 system.
Discussion
The present data show that (i) the chemokine SDF-1 and its receptor CXCR4 colocalize with AVP in magnocellular neurons of the PVN and SON as well as in the AVP pathway and the AVP-positive nerve terminals in the posterior pituitary; (ii) SDF-1 modulates the firing pattern of AVP neurons as well as its specific autoregulatory mechanisms; (iii) the chemokine also inhibits, by CXCR4 interaction, plasma AVP release induced by AII and 1 M NaCl in vivo, suggesting a neuromodulatory role of SDF-1 in neuroendocrine functions; and (iv) physiological conditions that modify hypothalamic AVP protein content also affect SDF-1.
We recently provided evidence for the presence of SDF-1 and CXCR4 immunoreactivity in the magnocellular neurons of the SON and PVN (14, 15). Moreover, with respect to SDF-1, we observed that this localization strictly corresponded to that of AVP neurons, with no colocalization with OT neurons (ref. 15 and Fig. 5). Here, we demonstrate a close relationship between SDF-1 and the whole AVP system because such colocalization between AVP and SDF-1 can be observed not only in the AVP cell bodies, but also all along the AVP tract through the internal palisade zone of the median eminence and in the nerve terminal projections into the posterior pituitary. Interestingly, we show here that not only the ligand but also the receptor for SDF-1, CXCR4, is colocalized with AVP in the SON and PVN, median eminence, and in the AVP nerve terminals in the posterior pituitary. The possibility that chemokines can be transported in vitro in neuronal processes (22) corroborates the present in vivo data. Because >85% and 95% of AVP-positive neurons also expressed CXCR4 in the SON and PVN, respectively, a significant proportion of AVP neurons should express both SDF-1 and CXCR4, thus suggesting that the chemokine could act as an autocrine modulator of neuronal activity and, in particular, of AVP release.
Indeed, such autocrine regulation may be related to the modulatory action of SDF-1 observed here on both electrical activity and AVP autoregulation. AVP neurons display in vitro an electrical activity almost comparable with that observed in vivo, characterized by a succession of shorter periods of activity and silence than in vivo (23). Electrical stimulations of isolated neurohypophysial terminals led to the demonstration that the silent and active periods in the firing activity of AVP neurons are equally necessary for optimal release (24, 25). SDF-1, as shown here, has various effects on the electrical activity of SON neurons, resulting probably from the heterogeneity of the population recorded or from a variety of actions on the AVP neuronal population (26). Thus, SDF-1 can inhibit or stimulate AVP neuronal electrical activity. Both excitatory and inhibitory actions of SDF-1 are mediated by the same receptor, CXCR4, because both responses are blocked by the CXCR4 antagonist AMD. The fact that AMD alone has no effect suggests that CXCR4 is not activated by endogenous SDF-1 under basal conditions. The present electrophysiological data suggested that the alteration of the firing pattern by SDF-1 could modify the firing activity of AVP neurons and, consequently, AVP release (23).
To ascertain that SDF-1 is able to affect AVP release, the autoregulation of AVP release by AVP observed in vivo (20, 21) has been reproduced in the in vitro perifusion system described here. Thus, we have used as an autocrine stimulus vasotocin, a structural analog of AVP, because it did not crossreact with the anti-AVP antibody used in the ELISA. Vasotocin reproducibly and potently stimulates AVP release from hypothalamic tissues, and this effect is markedly antagonized by SDF-1. Furthermore, SDF-1 inhibition is mediated by CXCR4 because it is blocked, like the electrophysiological response, by AMD.
The in vivo experiments using intraventricular injection of SDF-1 clearly demonstrate that the chemokine is able to blunt plasma AVP-induced release physiologically. AII has been used as a well known potent stimulatory substance of AVP release (27). The in vivo inhibitory effect of SDF-1 on AII-induced AVP secretion probably takes place in brain structures other than SON and PVN because no direct effect of AII has been observed on hypothalamic explants. Indeed, in the course of the present study (data not shown), we also observed that a strong CXCR4 immunoreactivity can be found in the median preoptic nucleus and in two CVOs, the subfornical organ and the organum vasculosum of the lamina terminalis. These CVOs, located in the walls of the brain ventricular system and forming the lamina terminalis, are highly vascularized structures lacking the blood–brain barrier (28). Not only do neurons of the lamina terminalis detect changes in plasma tonicity and send this information to the AVP-secreting neurons in SON and PVN, they also are the prime cerebral targets for AII to mediate AVP secretion (29, 30). Thus, CXCR4 receptors may play an essential role in the neuroendocrine interconnection between circulating hormones and central brain structures, and they can be added to the numerous peptidergic G protein-coupled receptors found in the CVOs (31).
It is well known that osmolarity plays an essential role in maintaining the bodily fluid balance. Several studies have demonstrated that injection of hypertonic NaCl solution induces a rapid AVP release (32, 33). In such an acute physiological situation, SDF-1 exerts an inhibitory effect on induced AVP secretion. Similar to what is observed with AII, the effect of SDF-1 is mediated by the CXCR4 receptor because it is inhibited by the antagonist AMD.
The importance of SDF-1 in physiological situations in which AVP release is dramatically modified can be illustrated in the present work, showing that dehydration induced by long-term salt loading produces a decrease in SDF-1 immunostaining in the magnocellular nuclei, a decrease that correlates with that of AVP. However, the decrease in both AVP and SDF-1 after dehydration is not contradictory with the inhibitory effect of SDF-1 on AVP release. Indeed, both types of regulation can be observed according to the situation and time scale. On the one hand, a short-time effect, such as minutes, for the inhibitory effect on acutely induced AVP release is observed versus, on the other hand, a long-term effect associated with a 12-day dehydration. Moreover, the fact that under the same conditions of dehydration CXCR4 is, as is SDF-1, dramatically decreased in magnocellular neurons suggests a reduced responsiveness of SDF-1. Thus, in physiological situations such as long-term dehydration, in which a strong AVP release is necessary, a blockade of the inhibitory effect of SDF-1 resulting from a down-regulation of CXCR4 can be observed. Future studies will have to focus on the question of how the SDF-1/CXCR4 system is involved in various situations in which AVP is released.
In conclusion, in this work we demonstrate that in normal rat, the chemokine SDF-1 and its receptor CXCR4 colocalize with AVP in magnocellular neurosecretory neurons, modulating the firing pattern of these neurons and the somatodendritic AVP release, thereby resulting in an inhibition of AVP-induced release. Although several neuropeptides have been found to be expressed in magnocellular AVP neurons (31), the SDF-1/CXCR4 system seems unique and original in the sense that not only the synthesizing AVP neurons in SON and PVN, but also AVP projections to the posterior pituitary, coexpressed the chemokine and its receptor. Thus, we propose that the SDF-1/CXCR4 system can be added to the well described autoregulation of AVP on its own neurons, thus becoming a second autocrine system by chemokines able to induce in physiological conditions a fine modulatory tuning of AVP neurons. The present findings show evidence that chemokine actions in the brain are not restricted to neuroinflammatory processes, but that they may also represent targets able to modulate brain and neuroendocrine functions.
Materials and Methods
Drugs and Antibodies.
The polyclonal goat antibody against the SDF-1 was obtained from Santa Cruz Biotechnology (catalog no. sc-6193). This affinity-purified antibody, raised against a peptide mapping at the carboxyl terminus (C-19) of human SDF-1, crossreacts totally with murine and rat SDF-1; its specificity for immunohistochemistry on rat brain tissues was tested as described in ref. 15. The polyclonal goat antibody against CXCR4 was obtained from Santa Cruz Biotechnology (catalog no. sc-6190). This affinity-purified antibody, raised against a peptide mapping at the carboxyl terminus (C-20) of human CXCR4, crossreacts totally with murine and rat CXCR4; its specificity for immunohistochemistry on rat brain tissues was tested as described in ref. 14. Murine anti-AVP antibody was a gift from A. Burlet (Centre National de la Recherche Scientifique, Nancy, France), and rabbit anti-OT antibody was a gift from H. Hardin-Pouzet (Université Pierre et Marie Curie, Paris). Human recombinant SDF-1 was purchased from PeproTech (Le Perray en Yvelines, France). AII was from Sigma. Vasotocin was a gift from G. Guillon (Centre National de la Recherche Scientifique, Montpellier, France). The CXCR4 antagonist bicyclam AMD was synthesized by Orga-Link (Gif-sur-Yvette, France).
Animals.
Male Wistar rats were bred in the local animal facilities and maintained on a 12-h dark/light cycle (7 a.m./7 p.m.) with food and water ad libitum except in salt-loading experiments. All experiments except the electrophysiological studies were performed on adult male rats (250–300 g). All of the protocols were carried out in accordance with French standard ethical guidelines for laboratory animals (agreement 75-178, 5/16/2000).
Tissue Preparation and Immunohistochemistry.
Rats were anesthetized with sodium pentobarbital (70 mg/kg, i.p.; Sanofi, Libourne, France) and perfused transcardially with 50 ml of heparin (1,000 units/ml) in physiological saline, followed by 400–500 ml of a freshly prepared solution of 4% (wt/vol) paraformaldehyde in PBS, pH 7.4. The brains and pituitaries were rapidly removed and postfixed overnight in 4% paraformaldehyde at 4°C. Pituitaries were then embedded in 30% (wt/vol) gelatin. Forty-micrometer-thick coronal sections were cut with a Vibratome (VT 1000S; Leica, Nussloch, Germany); pituitary sections were mounted on glass slices, and brain sections were collected in cold PBS. To characterize SDF-1- or CXCR4-expressing neurons in the hypothalamus, double and triple immunohistochemistry was performed as described in refs. 14 and 15. To estimate relative densities of AVPergic neurons expressing CXCR4 at both the SON and PVN levels, pictures of double-immunostained sections (CXCR4/AVP) were taken (three to five fields per region) (magnification, ×20). The percentage of AVPergic neurons that express CXCR4 in a given field was calculated as the ratio of the number of double-labeled CXCR4/AVP-positive cells.
Patch Clamp Recordings of Hypothalamic Slices.
Horizontal slices (250 μm) were prepared from 12- to 20-day-old male rat ventral hypothalamus according to published methods (18). Before use, slices were maintained for at least 1 h at room temperature in the recording medium containing 110 mM NaCl, 1.2 mM KCl, 26 mM NaHCO3, 2 mM CaCl2, 10 mM glucose, 1.2 mM KH2PO4, and 2 mM MgCl2 at pH 7.4 maintained with constant bubbling of 95% O2/5% CO2 (300 mosmol·liter−1). A hemislice containing one SON was then transferred into an immersion-type recording chamber, under the objective (×40) of a conventional microscope equipped with infrared interference optics, and activity was recorded at 35°C with conventional patch clamp techniques (18). Pipettes (4–6 MΩ) were filled with 135 mM KMeSO3, 9 mM KCl, 1 mM CaCl2, 5 mM Na-EGTA, 1 mM MgATP, and 10 mM Na-Hepes at pH 7.2 (290 mosmol·liter−1). Spontaneous electrical activity was recorded extracellularly with the loose-patch configuration. At the end of the experiment, the membrane patch was disrupted, and the electrophysiological characteristics of the cell were measured to confirm that the recorded neuron expressed a notch-delaying action-potential firing during step depolarization, the hallmark of magnocellular neurosecretory cells (34). Currents were filtered at 1 kHz, digitized, acquired, and stored in a PC-type computer by using the pclamp software set (Axon Instruments, Union City, CA).
Analysis of the Action-Potential Firing Pattern.
The electrical activity was recorded for 5 min before, during, and after local perfusion of 25 nM SDF-1. The chemokine was applied locally with a gravity-fed pipette positioned on the surface of the slice, within 200 μm of the recorded cell. In neurons in which the CXCR4 antagonist AMD was tested (10 μM), SDF-1 was perfused alone and before and after its perfusion with AMD, which allowed control of the reproducibility and stability of the SDF-1 effect after repetitive applications. To test the effect of SDF-1 on the spiking activity, a first indication was given by changes in the average frequency (number of action potentials per min), and this value is given in the present paper for simplification purposes. However, the firing of magnocellular neurosecretory cells is not regular, and the distribution of the interval between action potentials [interspike interval (ISI)] does not follow a Gaussian distribution, as described in ref. 35. Thus, an additional analysis was performed in which ISI durations were measured for each action potential, and distribution histograms were constructed. The number of events in each interval class was then multiplied by the class duration and divided by the total recording time. This commonly used procedure provides a value of the percent time spent in each interval class, more sensitive to pattern changes than the simple distribution of ISI durations (35).
Perifusion Experiments.
Hypothalamic explants from adult male rats (coordinates bregma −0.26 mm to −3.60 mm) according to ref. 36 were individually placed in temperature-controlled 1-ml plastic chambers (37°C) under 5% CO2/95% O2 at a constant flow (150 μl/min) of minimum essential medium with Earle’s salts (Invitrogen) containing 20 μM bacitracin (Sigma), 500 kallikrein-inhibiting units per ml of Trasylol (Bayer, Puteaux, France), and 0.1% BSA (Sigma). Equilibrium was reached after a 2-h perfusion, and then samples were taken every 10 min for 2 h, kept in the cold, and frozen. The sampling procedure consisted of a 30-min control basal period, a 30-min period during which substances to be tested were then added independently or in combination (50 nM SDF-1, 100 nM vasotocin, 0.1 μM AII, 10 μM AMD), and a 60-min wash with the medium. AVP was measured in the samples by means of an [Arg-8]AVP ELISA kit (R & D Systems) with a sensitivity of 3 pg/ml and a crossreactivity of 7% with [Lys-8]AVP only, but not with vasotocin or OT. At least three experiments were carried out for each substance tested (four chambers per group in each experiment).
In Vivo Intraventricular Injections.
Cannula guide implantation.
Wistar adult male rats were anesthetized by an i.p. injection of chloral hydrate (400 mg/kg) and implanted with a stainless-steel cannula guide (catalog no. C313) (Plastics One, Roanoke, VA) 1 mm above the third ventricle. The stereotaxic coordinates from Paxinos and Watson (36) were as follows: anteriority, −0.92 mm; laterality, 0 mm from bregma; depth, −6.4 mm from the skull surface. Eight days after implantation of the guide cannula, third-ventricle injections were made with stainless-steel needles connected to a 10-μl microsyringe (Hamilton) by polyethylene tubes. Animals were placed in a cage and were able to move freely during the injection.
Drug administration.
SDF-1 (50 or 500 ng/5 μl), AMD (2.5 μg/5 μl), SDF-1 + AMD, or vehicle [artificial cerebrospinal fluid (aCSF); Harvard Apparatus] was administered by an infusion pump (Harvard Apparatus) at a constant flow rate (1 μl/min) for 5 min. The needle was then left in place for 1 min after the end of the injection to allow the diffusion of the liquid. Seven minutes later, animals received a second injection [aCSF or AII (50 ng/5 μl)]. Ten minutes after the end of the second injection, animals were decapitated, and trunk blood was collected into chilled tubes containing EDTA and Trasylol.
Hypertonic stimulation.
Wistar adult rat previously implanted with a cannula guide as above were injected in the third ventricle with SDF-1 (500 ng/5 μl), AMD (2.5 μg/5 μl), SDF-1 + AMD, or with vehicle (aCSF) by an infusion pump at a constant flow rate (1 μl/min) for 5 min. Four minutes later, the animals received an i.p. injection of 1 M NaCl (4 ml/kg). Fifteen minutes after the i.p. injection, rats were decapitated, and trunk blood was collected into chilled tubes containing EDTA and Trasylol.
Plasma AVP Determination.
Blood samples were centrifuged at 4°C, and plasma was collected. Plasma AVP was extracted through a Sep-Pak C18 cartridge (Waters) and measured with the [Arg-8]AVP ELISA kit reported above.
Salt-Loading Experiments.
Rats were divided into two groups: controls with free access to water (n = 9), and rats subjected for 12 days to a 3% NaCl intake in the drinking water (n = 9) (20). SDF-1 and CXCR4 immunostaining was carried out in the SON and PVN as described above.
Statistical Analysis.
Data are shown as the means ± SEM. For electrophysiological experiments, standardized histograms were compared by using the Kolmogorov–Smirnoff test, and they were considered different if P < 0.05. For the perifusion or in vivo experiments, a two-way ANOVA test of repeated measures or one-way ANOVA was used, respectively, to analyze the differences between groups, followed by a Student–Newman–Keuls post hoc test with a threshold of significance of P < 0.05, P < 0.01, and P < 0.001 using a statistical software package (sigmastat 2.03; Jandel, San Rafael, CA).
Acknowledgments
We thank Drs. Gilles Guillon, Catherine Llorens-Cortes, and Michael J. McKinley for helpful discussions and Laurent Parent for technical help. This work was supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Institut Fédératif de Recherche 65, and Université Pierre et Marie Curie. C.C. is the recipient of a French government (Ministère de la Recherche et de la Technologie) fellowship. G.B. was supported by a fellowship from the Fondation pour la Recherche Médicale.
Abbreviations
- AII
angiotensin II
- aCSF
artificial cerebrospinal fluid
- AMD
AMD3100 (bicyclam)
- AVP
arginine vasopressin
- CVO
circumventricular organ
- ISI
interspike interval
- OT
oxytocin
- PVN
hypothalamic paraventricular nucleus
- SDF-1
stromal cell-derived factor 1
- SON
hypothalamic supraoptic nucleus.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Brownstein M. J., Russell J. T., Gainer H. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- 2.Rossi D., Zlotnik A. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Proudfoot A. E. I. Nat. Rev. Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White F. A., Bhangoo S. K., Miller R. J. Nat. Rev. Drug Discov. 2005;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banisadr G., Rostène W., Kitabgi P., Mélik Parsadaniantz S. Curr. Drug Targets Inflamm. Allergy. 2005;4:387–399. doi: 10.2174/1568010054022097. [DOI] [PubMed] [Google Scholar]
- 6.Bajetto A., Bonavia R., Barbero S., Schettini G. J. Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 7.Tham T. N., Lazarini F., Franceschini I. A., Lachapelle F., Amara A., Dubois-Dalcq M. Eur. J. Neurosci. 2001;13:845–856. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- 8.Limatola C., Giovannelli A., Maggi L., Ragozzino D., Castellani L., Ciotti M. T., Vacca F., Mercanti D., Santoni A., Eusebi F. Eur. J. Neurosci. 2000;12:2497–2504. doi: 10.1046/j.1460-9568.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 9.Ragozzino D., Renzi M., Giovannelli A., Eusebi F. J. Neuroimmunol. 2002;127:30–36. doi: 10.1016/s0165-5728(02)00093-0. [DOI] [PubMed] [Google Scholar]
- 10.Oh S. B., Endoh T., Simen A. A., Ren D., Miller R. J. J. Neuroimmunol. 2002;123:66–75. doi: 10.1016/s0165-5728(01)00485-4. [DOI] [PubMed] [Google Scholar]
- 11.Guyon A., Skrzydelski D., Rovère C., Rostène W., Mélik Parsadaniantz S., Nahon J. L. J. Neurochem. 2006;96:1540–1550. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q., Jones D., Borghesani P. R., Segal R. A., Nagasawa T., Kishimoto T., Bronson R. T., Springer T. A. Proc. Natl. Acad. Sci. USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu M., Grove E. A., Miller R. J. Proc. Natl. Acad. Sci. USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banisadr G., Fontanges P., Haour F., Kitabgi P., Rostène W., Mélik Parsadaniantz S. Eur. J. Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- 15.Banisadr G., Skrzydelski D., Kitabgi P., Rostène W., Mélik Parsadaniantz S. Eur. J. Neurosci. 2003;18:1593–1606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig M., Williams K., Callahan M. F., Morris M. Neurosci. Lett. 1996;215:1–4. doi: 10.1016/s0304-3940(96)12956-6. [DOI] [PubMed] [Google Scholar]
- 17.McKinley M. J., Gerstberger R., Mathai M. L., Oldfield B. J., Schmid H. J. Clin. Neurosci. 1999;6:289–301. doi: 10.1054/jocn.1998.0056. [DOI] [PubMed] [Google Scholar]
- 18.Chevaleyre V., Dayanithi G., Moos F. C., Desarménien M. G. J. Neurosci. 2000;20:5813–5819. doi: 10.1523/JNEUROSCI.20-15-05813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig M., Pittman Q. J. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 20.Morris J. F., Ludwig M. J. Neuroendocrinol. 2004;16:403–408. doi: 10.1111/j.0953-8194.2004.01182.x. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig M., Bull P. M., Tobin V. A., Sabatier N., Landgraf R., Dayanithi G., Leng G. J. Physiol. (London) 2005;564:515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jong E. K., Dijkstra I. M., Hensens M., Brouwer N., Van Amerongen M., Liem R. S. B., Boddeke H. W. G. M., Biber K. J. Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulain D. A., Wakerley J. B. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 24.Cazalis M., Dayanithi G., Nordmann J. J. J. Physiol. (London) 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouzenes L., Desarmenien M. G., Hussy N., Richard P., Moos F. C. J. Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kombian S. B., Hirasawa M., Mouginot D., Pittman Q. J. Prog. Brain Res. 2002;139:235–246. doi: 10.1016/s0079-6123(02)39020-4. [DOI] [PubMed] [Google Scholar]
- 27.Lenkei Z., Palkovits M., Corvol P., Llorens-Cortes C. Front. Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 28.McKinley M. J., McAllen R. M., Mendelsohn F. A. O., Allen A. M., Chai S. Y., Oldfield B. J. Front. Neuroendocrinol. 1990;11:91–127. [Google Scholar]
- 29.McKinley M. J., Mathai M. L., McAllen R. M., McClear R. C., Miselis R. R., Pennington G. L., Vivas L., Wade J. D., Oldfield B. J. J. Neuroendocrinol. 2004;16:340–347. doi: 10.1111/j.0953-8194.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- 30.Stricker E. M., Sved A. F. Physiol. Behav. 2002;77:731–736. doi: 10.1016/s0031-9384(02)00926-5. [DOI] [PubMed] [Google Scholar]
- 31.Meister B., Cortés R., Villar M. J., Schalling M., Hökfelt T. Cell Tissue Res. 1990;260:279–297. doi: 10.1007/BF00318631. [DOI] [PubMed] [Google Scholar]
- 32.Brimble M. J., Dyball R. E. J. J. Physiol. (London) 1977;271:253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson A. K., Thunhorst R. L. Front. Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong W. E., Stern J. E. Prog. Brain Res. 1998;119:101–113. doi: 10.1016/s0079-6123(08)61564-2. [DOI] [PubMed] [Google Scholar]
- 35.Bhumbra G. S., Inyushkin A. N., Dyball R. E. J. Neuroendocrinol. 2004;16:390–397. doi: 10.1111/j.0953-8194.2004.01166.x. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]