Abstract
Tetraspan vesicle membrane proteins (TVPs) comprise a major portion of synaptic vesicle proteins, yet their contribution to the synaptic vesicle cycle is poorly understood. TVPs are grouped in three mammalian gene families: physins, gyrins, and secretory carrier-associated membrane proteins (SCAMPs). In Caenorhabditis elegans, only a single member of each of these families exists. These three nematode TVPs colocalize to the same vesicular compartment when expressed in mammalian cells, suggesting that they could serve overlapping functions. To examine their function, C. elegans null mutants were isolated for each gene, and a triple mutant was generated. Surprisingly, these animals develop normally and exhibit normal neuronal architecture and synaptic contacts. In addition, functions of the motor and sensory systems are normal as determined by pharmacological, chemotaxis, and thermotaxis assays. Finally, direct electrophysiological analysis of the neuromuscular junction revealed no phenotype in the TVP mutants. We therefore conclude that TVPs are not needed for the basic neuronal machinery and instead may contribute to subtle higher order functions.
Keywords: integral membrane proteins, phylogenesis, vesicle trafficking, synaptophysin, synaptogyrin
Among the different synaptic vesicle proteins, those with four membrane-spanning domains [referred to as tetraspan vesicle membrane proteins (TVPs)] are a particularly abundant group whose function in neurotransmission is not understood (1, 2). These polypeptides belong to the physin, gyrin, and secretory carrier-associated membrane protein (SCAMP) families. Members are present in different combinations in synaptic vesicles and also in other transport vesicles of various mammalian cell types (2). Especially puzzling are the mild or even absent phenotypic defects in neurons of knockout mice lacking synaptophysin, synaptogyrin-1, or SCAMP-1 (3–6). A likely explanation is that other members of the same family or even members of other TVP families compensate for loss of a particular TVP. For example, synaptophysin-deficient mice exhibited no neuronal defects except in the photoreceptors that lack the related neuronal physin isoform synaptoporin (7). Furthermore, combined knockouts of the synaptic TVPs synaptophysin and synaptogyrin lead to changes in synaptic plasticity, whereas the single gene inactivations do not (6).
Numerous publications propose roles for TVPs for practically all aspects of the synaptic vesicle cycle, including vesicle biogenesis, exocytosis, and endocytotic recycling (2). This notion is supported by the multiple interactions of TVPs with lipids, notably cholesterol (8) or various components of the recycling machinery including soluble N-ethylmaleimide-sensitive fusion attachment protein receptors (SNAREs) (9–11), dynamin (12, 13), adaptor proteins (14), and eps15 homology (EH)-domain proteins (15). In addition, it has been postulated that TVPs are directly responsible for microvesicle formation (8, 16, 17) and are involved in fusion pore formation (18–21).
Thus, there is a discrepancy between the manifold functions attributed to TVPs and the lack of phenotypic defects in mutants. One possibility is that the different TVP families can compensate for one another. In the nematode Caenorhabditis elegans, there are only three TVPs (2, 22); thus, complete elimination of TVPs is possible. Here we demonstrate that these triple mutants lack profound nervous system defects.
Results
Evolutionary Conservation of TVPs.
Each of the three mammalian TVP families, SCAMPs, physins, and gyrins, are represented by a single family member in C. elegans: SCM-1, SPH-1, and SNG-1, respectively (2). Interspecies comparisons revealed comparable domain structures except for a predicted hydrophobic amino-terminal extension in SPH-1 that is absent in murine physins. To test the predicted structure, we mapped the sph-1 mRNA 5′ end experimentally. Single reaction products were obtained in PCR amplifications from cDNA by using the transsplice leader SL1 in combination with two alternative downstream primers; thus, the sph-1 transcript is subject to transsplicing (23). No product was detectable by using an SL2 primer with the same downstream primers, suggesting that sph-1 is not part of an operon (23). By sequencing the PCR products, the 5′ end was assigned to position 17,855 in AF038618, which is exactly at position 1 of the previously assigned exon 2. The corrected gene consists of only four exons coding for a 227-aa polypeptide with a calculated molecular mass of 25,464 and a 17-aa-long cytoplasmic amino terminus, much like the other physins. Using the corrected SPH-1 sequence, a phylogenetic tree was determined for murine and C. elegans TVP types, which supports the previously proposed relationships between the nematode and mouse TVPs (ref. 2; Fig. 7, which is published as supporting information on the PNAS web site). The calculations further demonstrate that the monophyletic SCAMP group is not related to the other TVP types, despite their common transmembrane topology. On the other hand, all physins and gyrins share the same evolutionary origin.
Colocalization of C. Elegans TVPs in Mammalian Cells.
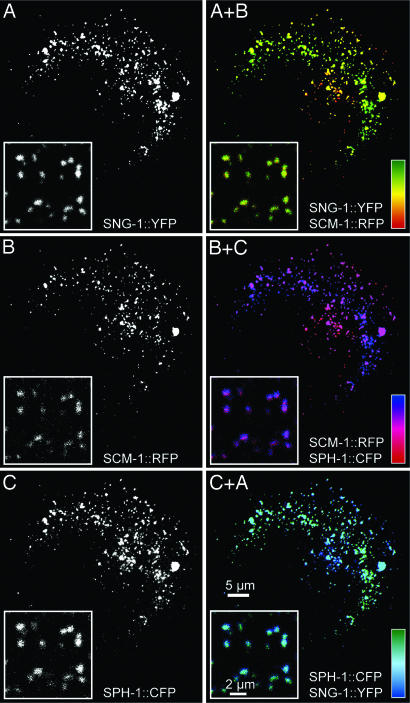
A prerequisite of complementary TVP function is their presence in the same subcellular compartments. To test this property, C. elegans TVPs were expressed in human epithelial cells in which cellular compartments can be easily distinguished. Fluorescence microscopy showed a considerable degree of colocalization of all three TVPs (Fig. 1). Pearson correlation coefficients were 0.65 for SNG-1/SCM-1, 0.62 for SCM-1/SPH-1, and 0.67 for SPH-1/SNG-1, demonstrating that a considerable proportion of all three TVP types are targeted to the same vesicular compartment and that all three TVPs could potentially replace each other.
Fig. 1.
Microscopy of human epithelial PLC cells producing fluorescent C. elegans TVP fusions. Images of triple-transfected cells were recorded by epifluorescence microscopy and the insets were recorded by confocal microscopy. Color calibration bars show the degree of colocalization for each overlay.
Distribution of TVPs in C. Elegans.
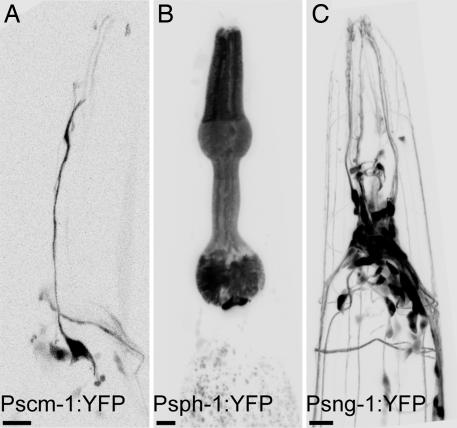
To further determine whether TVPs are coexpressed in the nematode, we examined the expression patterns of the genes by using promoter-GFP fusion constructs. Synaptogyrin is expressed pan-neuronally (22). Synaptophysin is mostly localized to the muscles of the pharynx and anal sphincter. SCAMP is present in an amphidial neuron of the right side, in coelomocytes, and in the spermatheca (Fig. 2). Translational fusions further revealed a punctate distribution, suggesting that these proteins are associated with vesicles (data not shown). Although promoter fusions do not always reflect complete expression patterns, these data argue that the TVPs do not act redundantly within a single cell type.
Fig. 2.
Tissue distribution of TVPs in C. elegans. Yellow fluorescent protein (YFP) expressed under the control of the indicated promoters. An inverted fluorescence micrograph of the head of an adult is shown in each panel; anterior is up. SCAMP is expressed in an amphidial neuron (A), synaptophysin in the pharyngeal muscle cells (B), and synaptogyrin in neurons. (C). (Scale bars: 10 μm.)
Isolation of TVP Mutants.
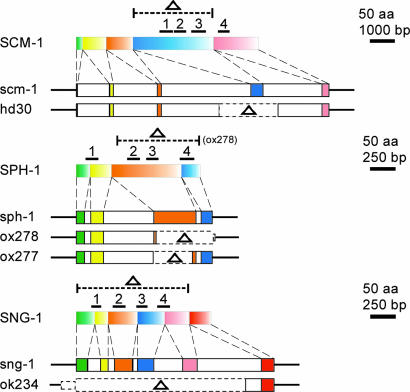
To determine the function of the TVPs, we generated mutations in each of these genes. Chemically mutagenized C. elegans libraries were screened for the presence of deletions, and mutant alleles were identified for scm-1 and sph-1. scm-1 allele hd30 (Fig. 3) deletes 1,729 bp, including all of exon 4. This deletion removes transmembrane domains 1 through 3 (amino acids N104–N253). Two sph-1 alleles were identified (Fig. 3): sph-1(ox277) contains a 347-bp deletion that removes most of exon 3, thereby removing coding regions for the first and second transmembrane domains, and sph-1(ox278) contains a 539-bp deletion that removes most of exons 3 and 4, thus deleting all but the first transmembrane domain. Strain RB503 carrying sng-1 allele ok234 was obtained from Caenorhabditis Genetics Center (Minneapolis) (Fig. 3). The 1,702-bp deletion is nearly a complete deletion of the gene and only leaves exon 6 coding for the terminal 38 amino acid. We assume that all four TVP alleles result in functional null mutants given the absence of transmembrane domains that are known to be crucial for protein stability and function (2). Moreover, these mutations were not associated with duplications of these genes elsewhere in the genome (Fig. 8, which is published as supporting information on the PNAS web site).
Fig. 3.
Schematic representation of the TVP genes scm-1, sph-1, and sng-1 and their corresponding polypeptides, SCM-1, SPH-1, and SNG-1, together with the mutated alleles hd30 (deletion from 28,951–30,679 in AF003739), ox278 (deletion from16,583–17,122 in AF038618), ox277 (deletion from 16,805–17,151 in AF038618), and ok234 (deletion from15,736–17,437 in U40417). For each, top portion shows the protein structure, exons are colored boxes, bars denote the transmembrane domains, and dotted lines indicate deleted regions (Δ).
Development and Neuronal Circuits in TVP Mutants.
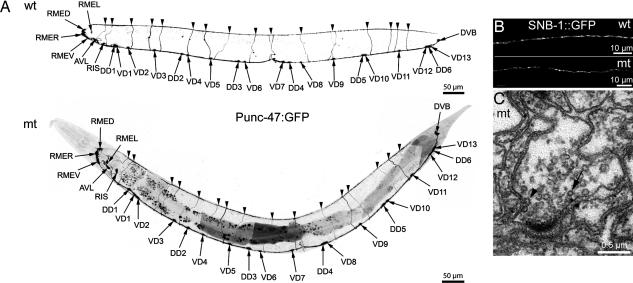
The morphologies and gross behaviors of all mutant worms were remarkably unaffected. To determine whether TVP genes might be acting redundantly, we constructed all combinations of double mutants and two strains of triple mutants. The brood sizes and developmental rates of mutant strains were not significantly different from the wild type (Fig. 9, which is published as supporting information on the PNAS web site). Given the reported interaction of the TVP synaptophysin with cholesterol (8), we examined the influence of cholesterol depletion on brood size but could not detect any abnormalities (Fig. 9). To assess the development of the nervous system, we analyzed the architecture of the GABA neurons by using a GFP-expression construct (24). Crosses with the triple mutants revealed proper positioning and formation of these neurons and their extensions (Fig. 4A). To evaluate synapse numbers and positioning, we analyzed synaptic vesicle clustering by using a synaptobrevin-GFP construct (22). The gross density of synaptic varicosities in the dorsal nerve cord of wild-type and mutant worms was similar (35.5 ± 3.4 vs. 34.7 ± 2.2 per 100 μm); these data also suggest that synaptobrevin, a potential TVP binding partner, is correctly targeted to synaptic vesicles (Fig. 4B). Electron microscopy was performed to find out whether ultrastructural changes occur in synapses of TVP triple mutants. Synaptic vesicles are clustered in association with a presynaptic specialization in presynaptic areas, which also contain clathrin-coated vesicles and endocytotic figures (Fig. 4C). A statistically significant increase in the number of clathrin-coated vesicles was observed in the mutant [1.93 ± 0.35% (21 terminals) vs. 0.74 ± 0.15% (16 terminals; P = 0.006)]. The mean synaptic vesicle diameter was almost identical in the wild type (27.82 ± 0.2 nm; n = 556) and triple mutant (26.66 ± 0.19 nm; n = 553) with comparable size distribution patterns (Fig. 10, which is published as supporting information on the PNAS web site). Synaptic vesicle density measured in ten neuromuscular synapses each was similar in the wild type (152 ± 8 vesicles per μm2) and mutant (163 ± 13 vesicles per μm2; P = 0.463). The total vesicle pool determined in single GABA synapses was 1,247 in the wild type and 1,518 in the mutant. Similar results were obtained in worms preserved by high-pressure freezing. In addition, the percentage of synaptic vesicles contacting the presynaptic specialization, and those within 30 nm of it, was the same for all worms (Fig. 11, which is published as supporting information on the PNAS web site).
Fig. 4.
Neuronal architecture is normal in the TVP triple mutant. (A) Fluorescence micrographs (negative image) of the distribution of GABA neurons expressing GFP under the control of the unc-47 promoter in wild-type (wt) and mutant (mt) backgrounds (strains EG1285 and BJ1, respectively). (B) Fluorescence micrographs of SNB::GFP in the dorsal nerve cord in wild-type and mutant backgrounds (strains BJ22 and BJ28, respectively). (C) Electron microscopy of a synapse in the ventral nerve cord of a triple TVP mutant worm (strain EG2960). Note the presence of clustered synaptic vesicles close to a typical presynaptic specialization, the occurence of clathrin-coated vesicles (arrow), and the detection of an endocytotic figure (arrowhead).
Neuronal Functions in TVP Mutants.
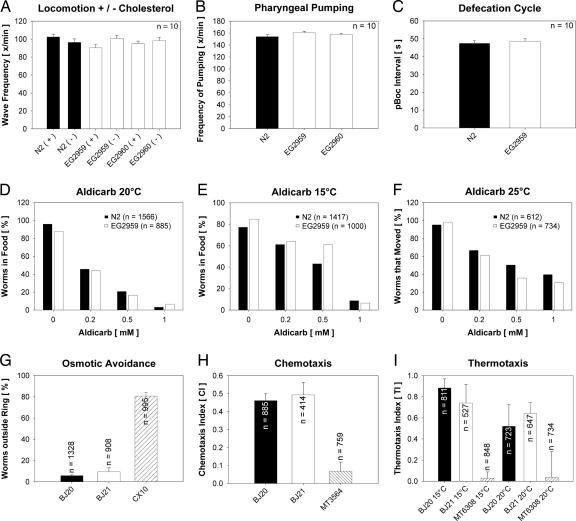
Overall motor activity was normal in mutant animals. Body bend frequencies in the wild-type and mutant strains did not differ even in the absence of cholesterol (Fig. 5A). In addition, thrashing in these strains after 90 min and 150 min was identical, suggesting that the mutant strains did not suffer premature fatigue of synaptic transmission. Similarly, frequency of pharyngeal pumping was the same in the wild-type and triple mutant animals (Fig. 5B). Examination of the defecation cycle revealed that all motor steps of this behavior (posterior body, anterior body, and enteric muscle contractions) were normal in the triple mutant. Furthermore, the cycle length was almost identical in wild-type and mutant strains (Fig. 5C). To detect minor defects in neurosecretion, graded radial aldicarb-resistance assays were performed. Similar sensitivity to this inhibitor of acetylcholinesterase were observed in wild-type and mutant worms (Fig. 5D). Some synaptic vesicle proteins are required to maintain normal transmission in response to extreme temperatures (25, 26). To examine whether there was an underlying temperature sensitivity, worms were grown for several generations either at 15°C or at 25°C and then subjected to aldicarb testing at the respective temperatures. Again, no significant differences were noted (Fig. 5 E and F).
Fig. 5.
Analysis of neuronal functions in wild-type (N2 and BJ20) and triple TVP mutant worms (EG2959, EG2960, and BJ21). Strains CX10 osm-9(ky10), MT3564 osm-7(n1515), and MT6308 eat-4(ky5) were used as controls in assays for osmotic avoidance, chemotaxis, and thermotaxis. Note that none of the assays revealed significant differences between wild-type and mutant animals. Scale bars depict standard error of the mean, when applicable.
Several sensory responses were tested in the triple mutant. Mechanosensation was normal; mutant animals responded normally to touch to the nose or tail with a hair. A slight defect in osmotic avoidance behavior was observed in initial trials with the triple mutant. However, this phenotype appears to be caused by unrelated background mutations. We outcrossed the three alleles of the triple mutant for an additional 12 generations against the wild type. The resulting homozygous triple mutant strain BJ21 no longer exhibited a defect in response to high osmotic gradients in comparison to wild-type controls from the same outcross (BJ20; Fig. 5G). The triple mutants exhibited normal chemotaxis to the attractant diacetyl (Fig. 5H) and to isoamyl alcohol and adaptation to isoamyl alcohol (Fig. 12, which is published as supporting information on the PNAS web site). In addition, dye-filling of the sensory neurons showed that position and morphology of amphids was completely normal (data not shown). Finally, wild-type and mutant strains behaved similarly in thermotaxis memory assays (Fig. 5I). Taken together, the motor system and all sensory functions tested were within the normal range in the triple TVP mutant worms.
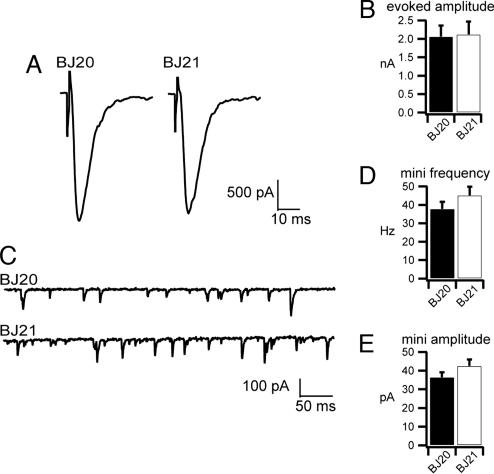
To directly assay neuronal function, evoked postsynaptic currents and endogenous miniature postsynaptic currents were measured from voltage-clamped muscles at the neuromuscular junction. Evoked responses did not differ between TVP triple mutants (2.12 ± 0.35 nA) and wild-type controls (2.06 ± 0.30 nA; P = 0.324; Fig. 6A and B). We also detected no difference in the frequency of spontaneous fusion events in the TVP triple mutants (45.2 ± 4.73 fusions per second) compared with controls (37.9 ± 3.79 fusions per second; P = 0.321; Fig. 6 C and D). Finally, the amplitude of endogenous fusion events was comparable in mutants (42.5 ± 3.51 pA) and controls (36.4 ± 2.69 pA; P = 0.287; Fig. 6E). In sum, the direct assays for synaptic function at the neuromuscular junction revealed no changes in loading and fusion of synaptic vesicles. In addition, because evoked transmission requires tight coupling of the calcium signal to the release machinery, the lack of a difference in the evoked amplitude suggests that TVPs are unlikely involved in calcium sensing.
Fig. 6.
Electrophysiological analysis of outcrossed triple TVP mutant worms (BJ21) and wild-type control worms (BJ20) derived from the same outcross. (A and B) Representative evoked responses (A) and mean amplitudes of evoked responses (B) (n = 5 for the wild type; n = 6 for mutant) reveal no differences (P = 0.91). (C) Representative traces of endogenous fusion events [miniature postsynaptic currents (mPSC)] in 5 mM Ca2+. (D and E) Mean frequencies of mPSCs (n = 6 each) (D), and mean amplitudes of individual miniature currents (n = 6 each) (E) were unaffected in TVP mutants (P = 0.26 and P = 0.20, respectively).
Discussion
We show that, in the absence of TVPs, neuronal functions appear to be normal in the nematode C. elegans. These assays include behaviors using all muscle groups: pharyngeal, enteric, vulval, and body muscles. These assays include sensory detection of mechanical, osmotic, odorant, and thermal stimuli. These behaviors employ many different excitatory, inhibitory, and modulatory neurotransmitters. Taken together, our observations lead to the almost inescapable conclusion that TVPs are not essential for synaptic vesicle biogenesis, exocytosis, or endocytosis. This conclusion is quite remarkable, considering the evolutionary conservation of TVPs and their abundance in mammalian synaptic vesicles. Several possibilities should be considered:
Stress: TVPs might be essential to neurotransmission in stressful circumstances. However, absence of cholesterol, elevated temperatures, and drugs that elevate neurotransmission did not reveal differences between the triple mutant and the wild-type animals.
Integration: TVPs might be required for higher order neuronal functions. However, in the chemotaxis and osmotic avoidance assays, the mutants appropriately engaged the correct motor program in response to environmental stimuli. Moreover, the mutants performed well in the thermotaxis assay; this test requires the animal to recall the specific temperature with the presence of food.
Development: TVPs might act as modulators of the synaptic vesicle cycle during development. The interaction between the vesicle-associated soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (vSNARE) synaptobrevin and synaptophysin, for instance, has been shown to be developmentally regulated (27–29). Although this hypothesis cannot be completely ruled out, we view it as unlikely because synaptic architecture, ultrastructure, and function appear to be normal in the adult nerve cords.
Redundancy: The TVPs might be redundant with an unrelated protein. It has been proposed, for example, that endocytosis can occur through both a clathrin-dependent mechanism after full fusion and a clathrin-independent mechanism after kiss-and-run fusion events. Interestingly, we do find a small increase in clathrin-coated vesicles in the TVP mutants, suggesting that a compensatory nonclathrin pathway might be compromised in TVP mutants. This observation is in agreement with data from the photoreceptors of synaptophysin-deficient mice, which also display an increase in clathrin-coated vesicles (7).
In brief, it is remarkable that this protein family, which is abundantly expressed and has been highly conserved in animals, lacks a profound mutant phenotype.
Materials and Methods
DNA Cloning, Transcriptional Start Site Determination, and Synthetic Oligonucleotides.
Standard techniques were used and specific details are given in Table 1 and Supporting Materials and Methods, which are published as supporting information on the PNAS web site.
Transgenesis.
Eukaryotic expression constructs were transfected into hepatocellular carcinoma-derived PLC cells by using the lipofectamine method and fluorescence was analyzed after 2 days in methanol/acetone-fixed cells (as described in ref. 30). Promoter-driven fluorescent protein constructs were injected into worms by using standard techniques. The resulting fluorescence was analyzed by confocal laser scanning microscopy (TCS SP2; Leica).
Isolation of Mutants.
Deletion alleles were isolated from frozen libraries of ethyl methanesulfonate (EMS) mutagenized worms by using a poison primer PCR approach (31). External primers 02-01 and 02-02 together with poison primer 02-03, and internal primers 02-04 and 02-05 were used for identification of scm-1 mutations, external primers 02-06, and 02-07 together with poison primer 02-08 and internal primers 02-09 and 02-10 for detection of sph-1 mutations. After two rounds of PCR screening, worms were thawed to isolate mutant strains. Duplex PCR was performed to distinguish heterozygous from homozygous mutants. Primers 02-01, 02-02, and 02-03 generate a 614-bp wild- type and a 709-bp mutant fragment (allele hd30) from scm-1; primers 02-11, 02-07, and 02-08 generate a 860-bp wild-type and 1011-bp mutant fragment for allele ox278 or a 347-bp mutant fragment for allele ox277 from sph-1; primers 04-81, 04-84, and 04-86 generate a 684-bp wild-type and a 880-bp mutant fragment (allele ok234) from sng-1. All mutants were outcrossed at least four times with the wild-type strain resulting in strains VH690 (hd30), EG2948 (ox278), EG2961 (ox277), and VH619 (ok234). Subsequently, all combinations of double mutants and two lines of triple mutants were established by using the more extensive sph-1 deletion allele ox278 (strains EG2959 and EG2960).
Worm Strains.
Strains Bristol N2, RB503 sng-1(ok234) X, EG1285 lin-15(n765); oxIs12 X, NM440 unc-104(e1265) II; jsIs, CX10 osm-9(ky10) IV, MT3564 osm-7(n1515) III, MT6308 eat-4(ky5) III, and RB503 sng-1(ok234) X were obtained from Caenorhabditis Genetics Center (Minneapolis). Strain EG1285 lin-15(n765); oxIs12 X has been described (24). Mutant strains EG2947 scm-1(hd30) I, EG2948 sph-1(ox278) IV, and VH619 sng-1(ok234) X, each of which was obtained after at least four outcrosses of the original mutant strain with N2, were combined to triple mutants EG2959 and EG2960. Subsequently, each allele was outcrossed for 12 generations with N2. From the resulting breeding, triple mutant strain BJ21 and wild-type control strain BJ20 were established. BJ22 jsIs was obtained by crossing BJ20 with NM440, and the jsIs allele was introduced into the triple mutant background by breeding BJ21 with BJ22 resulting in strain BJ28. Allele oxIs12 was introduced into the triple mutant background by mating BJ21 with EG1285, thereby producing strain BJ1.
Assays of Neuronal Function.
All assays were done, employing standard procedures, with synchronized adult worms 1 day after molting from the L4 larval stage (further details in Supporting Materials and Methods).
Electron Microscopy.
Electron microscopy was done on conventionally fixed worms and animals frozen under high pressure (32, 33).
Electrophysiology.
Details of the methods described in ref. 34 are given in Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. A. Beg and K. Schuske for introducing us to C. elegans and L. Bai, Y. Wang, K. Hübner, K. Hüsken, U. Wilhelm, V. Gladkova, and I. von Graevenitz for help at various stages of the project. This work was supported by the VolkswagenStiftung, German Research Council Grant LE566/8, and the Forschungsförderungsprogramm of the Johannes Gutenberg University Mainz (all to R.E.L.).
Abbreviations
- SCAMP
secretory carrier-associated membrane protein
- TVP
tetraspan vesicle membrane protein.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Südhof T. C. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Hübner K., Windoffer R., Hutter H., Leube R. E. Int. Rev. Cytol. 2002;214:103–159. doi: 10.1016/s0074-7696(02)14004-6. [DOI] [PubMed] [Google Scholar]
- 3.Eshkind L. G., Leube R. E. Cell Tissue Res. 1995;282:423–433. doi: 10.1007/BF00318874. [DOI] [PubMed] [Google Scholar]
- 4.McMahon H. T., Bolshakov V. Y., Janz R., Hammer R. E., Siegelbaum S. A., Südhof T. C. Proc. Natl. Acad. Sci. USA. 1996;93:4760–4764. doi: 10.1073/pnas.93.10.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Chacon R., Alvarez de Toledo G., Hammer R. E., Südhof T. C. J. Biol. Chem. 1999;274:32551–32554. doi: 10.1074/jbc.274.46.32551. [DOI] [PubMed] [Google Scholar]
- 6.Janz R., Südhof T. C., Hammer R. E., Unni V., Siegelbaum S. A., Bolshakov V. Y. Neuron. 1999;24:687–700. doi: 10.1016/s0896-6273(00)81122-8. [DOI] [PubMed] [Google Scholar]
- 7.Spiwoks-Becker I., Vollrath L., Seeliger M. W., Jaissle G., Eshkind L. G., Leube R. E. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 8.Thiele C., Hannah M. J., Fahrenholz F., Huttner W. B. Nat. Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 9.Washbourne P., Schiavo G., Montecucco C. Biochem. J. 1995;305:721–724. doi: 10.1042/bj3050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelmann L., Hanson P. I., Chapman E. R., Jahn R. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calakos N., Scheller R. H. J. Biol. Chem. 1994;269:24534–24537. [PubMed] [Google Scholar]
- 12.Daly C., Sugimori M., Moreira J. E., Ziff E. B., Llinas R. Proc. Natl. Acad. Sci. USA. 2000;97:6120–6125. doi: 10.1073/pnas.97.11.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly C., Ziff E. B. J. Biol. Chem. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- 14.Horikawa H. P., Kneussel M., El Far O., Betz H. Mol. Cell. Neurosci. 2002;21:454–462. doi: 10.1006/mcne.2002.1191. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Chacon R., Achiriloaie M., Janz R., Albanesi J. P., Südhof T. C. J. Biol. Chem. 2000;275:12752–12756. doi: 10.1074/jbc.275.17.12752. [DOI] [PubMed] [Google Scholar]
- 16.Leube R. E., Wiedenmann B., Franke W. W. Cell. 1989;59:433–446. doi: 10.1016/0092-8674(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 17.Belfort G. M., Bakirtzi K., Kandror K. V. J. Biol. Chem. 2005;280:7262–7272. doi: 10.1074/jbc.M404851200. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Liao H., Castle A., Zhang J., Casanova J., Szabo G., Castle D. Mol. Biol. Cell. 2005;16:4463–4472. doi: 10.1091/mbc.E05-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas L., Hartung K., Langosch D., Rehm H., Bamberg E., Franke W. W., Betz H. Science. 1988;242:1050–1053. doi: 10.1126/science.2461586. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z., Liu L., Cafiso D., Castle D. J. Biol. Chem. 2002;277:35357–35363. doi: 10.1074/jbc.M202259200. [DOI] [PubMed] [Google Scholar]
- 21.Liu L., Guo Z., Tieu Q., Castle A., Castle D. Mol. Biol. Cell. 2002;13:4266–4278. doi: 10.1091/mbc.E02-03-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nonet M. L. J. Neurosci. Methods. 1999;89:33–40. doi: 10.1016/s0165-0270(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal T. Trends Genet. 1995;11:132–136. doi: 10.1016/s0168-9525(00)89026-5. [DOI] [PubMed] [Google Scholar]
- 24.McIntire S. L., Reimer R. J., Schuske K., Edwards R. H., Jorgensen E. M. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 25.Clark S. G., Shurland D. L., Meyerowitz E. M., Bargmann C. I., van der Bliek A. M. Proc. Natl. Acad. Sci. USA. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salcini A. E., Hilliard M. A., Croce A., Arbucci S., Luzzi P., Tacchetti C., Daniell L., De Camilli P., Pelicci P. G., Di Fiore P. P., Bazzicalupo P. Nat. Cell Biol. 2001;3:755–760. doi: 10.1038/35087075. [DOI] [PubMed] [Google Scholar]
- 27.Becher A., Drenckhahn A., Pahner I., Ahnert-Hilger G. Eur. J. Cell Biol. 1999;78:650–656. doi: 10.1016/S0171-9335(99)80050-8. [DOI] [PubMed] [Google Scholar]
- 28.Becher A., Drenckhahn A., Pahner I., Margittai M., Jahn R., Ahnert-Hilger G. J. Neurosci. 1999;19:1922–1931. doi: 10.1523/JNEUROSCI.19-06-01922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinz B., Becher A., Mitter D., Schulze K., Heinemann U., Draguhn A., Ahnert-Hilger G. Eur. J. Cell Biol. 2001;80:615–619. doi: 10.1078/0171-9335-00196. [DOI] [PubMed] [Google Scholar]
- 30.Windoffer R., Leube R. E. Methods Cell Biol. 2004;78:321–352. doi: 10.1016/s0091-679x(04)78012-7. [DOI] [PubMed] [Google Scholar]
- 31.Edgley M., D’Souza A., Moulder G., McKay S., Shen B., Gilchrist E., Moerman D., Barstead R. Nucleic Acids Res. 2002;30:e52. doi: 10.1093/nar/gnf051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuske K. R., Richmond J. E., Matthies D. S., Davis W. S., Runz S., Rube D. A., van der Bliek A. M., Jorgensen E. M. Neuron. 2003;40:749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- 33.Rostaing P., Weimer R. M., Jorgensen E. M., Triller A., Bessereau J. L. J. Histochem. Cytochem. 2004;52:1–12. doi: 10.1177/002215540405200101. [DOI] [PubMed] [Google Scholar]
- 34.Richmond J. E., Jorgensen E. M. Nat. Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.