Abstract
A key goal of regenerative medicine is an understanding of the genetic factors that define the properties of stem cells. However, stem cell research in mammalian tissue has been hampered by a paucity of stem cell-specific markers. Although increasing evidence suggests that members of the Musashi (Msi) family of RNA-binding proteins play important functions in progenitor cells, it remains unclear whether there is a stem cell-autonomous requirement for Msi because of an inability to distinguish stem cells from early-lineage cells in mammalian tissues. Here, using the Drosophila testis as a model system for the study of stem cell regulation, we show specific evidence for a cell-autonomous requirement for Msi family proteins in regulating stem cell differentiation, leading to the identification of an RNA-binding protein required for spermatogonial stem cell maintenance. We found that loss of Msi function disrupts the balance between germ-line stem cell renewal and differentiation, resulting in the premature differentiation of germ-line stem cells. Moreover, we found that, although Msi is expressed in both somatic and germ cells, Msi function is required intrinsically in stem cells for maintenance of stem cell identity. We also discovered a requirement for Msi function in male meiosis, revealing that Msi has distinct roles at different stages of germ cell differentiation. We describe the complementary expression patterns of the murine Msi paralogues Msi1 and Msi2 during spermatogenesis, which support the idea of distinct, evolutionarily conserved roles of Msi.
Keywords: spermatogenesis, Drosophila, testis, spermatocyte, translation
Recent studies have shown that the Musashi (Msi) family of RNA-binding translational repressors is expressed in proliferative cells of the mammalian central nervous system (1, 2), intestine (3), and stomach (4), leading to the proposal that Msi proteins are stem cell markers (5). Despite these observations, a functional requirement for Msi within stem cells has not been demonstrated, largely because of an inability to distinguish stem cells from their immediate daughter cells.
The Drosophila testis and ovary have become excellent model systems for the study of stem cell regulation because the identity of germ-line stem cells (GSCs) and their position in relation to neighboring cells are well defined (6, 7). Moreover, many of the molecular mechanisms and signaling pathways underpinning Drosophila GSC regulation are conserved in other systems (8, 9). For example, bone morphogenetic protein signaling has emerged as a common pathway for the control of stem cell self-renewal from Drosophila to mammals (8). The Janus kinase–signal transducer and activator of transcription signaling pathway is indispensable for GSC maintenance in the Drosophila testis (10, 11) and for maintenance of mammalian ES cells (12). Interestingly, the RNA-binding proteins and translational repressors Pumilio and Nanos have been shown to be required for GSC maintenance in the Drosophila ovary (13, 14), and two Pumilio-like Fem-3 mRNA-binding factors (FBF-1 and FBF-2) have been shown to control GSC self-renewal and proliferation in Caenorhabditis elegans, presumably by translational repression (15). Although translational regulation appears to be a common conserved mechanism by which stem cell regulation occurs (16), to date no RNA translational repressors have been discovered to function in the Drosophila testis.
In this study we show that Drosophila Msi is both expressed and required in GSCs for maintenance of stem cell fate. We found that cell-autonomous loss of Msi in the Drosophila testis results in the premature differentiation of GSCs, indicating an intrinsic requirement for Msi for regulation of stem cell maintenance. Furthermore, we also identified a requirement for Msi function later in spermatogenesis for correct meiotic segregation and cytokinesis. Interestingly, the distinct functions for Msi in early and late spermatogenesis appear to be evolutionarily conserved because the expression of the two murine Msi paralogues, Msi1 and Msi2, has diverged to encompass these two stages of spermatogenesis.
Results and Discussion
Loss of Msi Function Results in the Loss of Early Germ Cells.
We examined Msi function in the testis proliferation center of Drosophila, which includes both GSCs and somatic stem cells along with the mitotically amplifying GSC daughter cells (Fig. 1A). Normally, five to nine GSCs are attached to a cluster of somatic hub cells in the apex of the testis (Fig. 1A) (17). Hub cells provide a microenvironment, or “niche,” for the GSCs (10, 11), which divide radially to ensure that one daughter cell remains at the hub and retains stem cell identity, whereas the displaced daughter cell differentiates into a gonialblast (Fig. 1A) (18). We discovered that loss-of-function mutations in msi resulted in a loss of early germ cells in the Drosophila adult testis (Fig. 1 C–E). In wild-type pharate adult testes, the nuclear stain DAPI brightly labels both GSCs and mitotically amplifying spermatogonia in the apex of the testis, whereas spermatocytes and spermatids weakly fluoresce distal to the apex (Fig. 1B). Testes from the msi1-null mutant (19), the hypomorphic msi2 mutant (19), and the msi1/msi2 transheterozygote exhibited fewer brightly stained cells at the apex of the testis, indicative of a loss of early germ cells (Fig. 1 C–E). The null mutant testis also exhibited a swelling of the apical region in 90% of testes analyzed (n = 52) (Fig. 1C). This phenotype was also observed, although variably (19% of testes; n = 21), in the weaker msi2 mutant and could be attributed to defects in the development of the outer muscle sheath of the testis (data not shown). Wild-type control testes rarely exhibited a swelling of the apical region (2%; n = 55).
Fig. 1.
Msi function is required for maintenance of early germ cells. (A) Schematic of spermatogenesis in the testis apex. GSCs (red) and CPCs (yellow) are anchored around somatic hub cells (light blue). Gonialblasts (pink) and mitotically active spermatogonia (dark blue) outside of the stem cell niche are encapsulated by differentiated cyst cells (green). These cells constitute the apical proliferation center and stain intensely with DAPI in a wild-type testis (B; arrowhead shows hub). In msi1-null mutants (C), hypomorphic msi2 mutants (E), and transheterozygotes (msi½) (D), fewer DAPI-labeled cells can be detected. (Scale bars: 50 μm.)
Msi Function Is Required for the Maintenance of GSCs.
Further characterization of msi mutant testes was undertaken to determine the nature of the cell types lost from the apical proliferation center (Fig. 2A–J). msi mutants exhibited fewer than normal Vasa-positive GSCs adjacent to the hub (Fig. 2 B–D). Furthermore, individual cells not labeled by the germ cell marker Vasa but expressing Discs large (Dlg), an immunohistochemical marker that outlines hub, cyst, and germ cells, were frequently observed adjacent to the hub in the gaps where stem cells should lie (Fig. 2 B–D). These results demonstrate that GSCs are lost from msi mutants and also suggest that somatic cells may be occupying the empty spaces caused by the loss of GSCs.
Fig. 2.
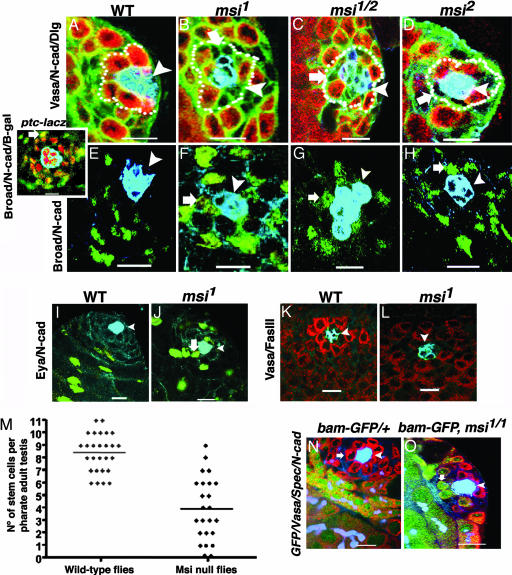
GSCs are not maintained in Msi mutants. (A) Vasa-expressing GSCs (red, dotted line) are anchored around hub (N-cadherin, blue). Dlg (green) outlines all cells. msi1 (B), msi½ (C), and msi2 (D) have fewer GSCs but have Vasa-negative cells (thick arrows) abutting the hub. (E) Wild-type Broad expression (green) is observed in CPCs near the hub (N-cadherin, blue) and in differentiated cyst cells. (Inset) ptc-lacZ (β-galactosidase, red) marks hub and CPCs. In most cases differentiated cyst cells show increased Broad expression (green, arrow) compared with that observed in CPCs. msi1 (F), msi½ (G), and msi2 (H) show Broad-expressing cyst cells abutting the hub (thick arrows). (I) Wild-type Eya expression (green) is observed in differentiated cyst cells but is absent from CPCs. (J) In msi1 mutants, differentiated cyst cells expressing Eya abut the hub. (K) Wild-type third-instar gonad GSCs express high levels of Vasa (red, hub; blue, Fasciclin III). (L) msi1 has fewer germ cells adjacent to the hub. (M) Scatter plot of GSC distribution in msi-null mutant and control pharate adults. Line represents the mean. (N) bam-GFP is excluded from wild-type GSCs (arrow), Vasa (red), bam-GFP (green), and spectrin/N-cadherin (blue), but msi1 testes exhibit bam-GFP-positive germ cells abutting the hub (O, arrow). Arrowheads, somatic hub. (Scale bars: 10 μm.)
A pair of somatic stem cells called cyst progenitor cells (CPCs) flank each GSC and also abut the hub (Fig. 1A). As with GSCs, CPCs divide asymmetrically to self-renew and produce a daughter cyst cell. Two cyst cells remain in intimate contact with the gonialblast and its progeny throughout spermatogenesis and provide extrinsic signals that are necessary for the regulation of germ cell behavior (20–22). We analyzed somatic cyst cell distribution in msi mutants using an antibody raised against the transcriptional regulator Broad (23), which we determined to normally be expressed in CPC nuclei surrounding the hub and in the nuclei of daughter cyst cells distal to the hub (Fig. 2 E and Inset). In msi mutant testes, large Broad-expressing cyst cell nuclei were often observed abutting the hub (Fig. 2 F–H). To determine whether these cyst cells were somatic stem cells or differentiated cyst cells, null mutants were labeled with anti-Eyes absent (Eya), a marker that normally specifically labels only differentiated cyst cells (Fig. 2I). In msi-null mutant testes, Eya-expressing cyst cells were commonly observed near to or abutting the hub (Fig. 2J), suggesting that differentiated cyst cells may have migrated into the niche normally occupied by GSCs. Alternatively, loss of Msi function could result in premature differentiation of CPCs into cyst cells, thus resulting in the appearance of cyst cells expressing Eya next to the hub. Characterization of msi mutant testes by using anti-N-cadherin also revealed hub cell defects in 83% of pharate adults (n = 46) (Fig. 2 B–D and F–H). In ≈50% of cases, msi mutants appeared to have a smaller than normal hub with few or no hub cells (Fig. 2 B and F), whereas in the other cases the hub appeared to be loosely compacted and degenerating (Fig. 2G).
To determine whether GSC loss in msi mutants occurred before the pharate adult developmental stage, the msi mutant phenotype was characterized in third-instar larvae, a stage when the hub was present in 97% of testes analyzed (n = 37), and exhibited milder morphological defects than those observed in pharate adults. The germ cell marker Vasa is normally highly expressed in GSCs adjacent to the hub and is reduced in germ cells distal to the hub (Fig. 2K). In null mutants, there were fewer than normal Vasa-positive germ cells adjacent to the hub and no evidence of differential expression of Vasa in any germ cells (Fig. 2L). Furthermore, GSCs did not form a tight rosette pattern around the hub (Fig. 2L). These results suggest that GSC loss in null mutants is not subsequent to loss of somatic hub cells.
Loss of Msi Function Causes Premature Differentiation of GSCs.
Because the number of GSCs adjacent to the hub appeared to vary in msi mutants, we quantified the average number of stem cells in testes from msi-null mutant pharate adults compared with wild-type control testes dissected at the same developmental stage. GSCs and daughter gonial cells in the testis contain characteristic spectrin-rich spherical spectrosomes, whereas interconnected spermatogonial and spermatocyte cysts contain branched fusomes (24). Cells were counted as GSCs if they were Vasa-positive, were adjacent to and contacting the hub, and contained spherical spectrosomes. A bag-of-marbles–GFP transgene (25) (bam-GFP) was also used to distinguish GSCs from differentiated germ cells around the hub because Bam is normally expressed only in spermatogonial cells and is excluded from GSCs and daughter gonialblasts (26). In wild-type flies, the average number of GSCs per testis was 8.4 ± 0.29 (±SEM; n = 27), which was significantly different from the average number of these cells in msi-null mutants [3.9 ± 0.52 (±SEM; n = 24; P < 0.0001, two-tailed unpaired t test)] (Fig. 2M). Furthermore, the differentiation marker bam-GFP was observed in cells contacting the hub in the msi-null mutant (Fig. 2O), suggesting that GSCs may prematurely differentiate in msi-null mutants.
Msi Is Expressed in both Somatic and Germ Cells in the Drosophila Testis.
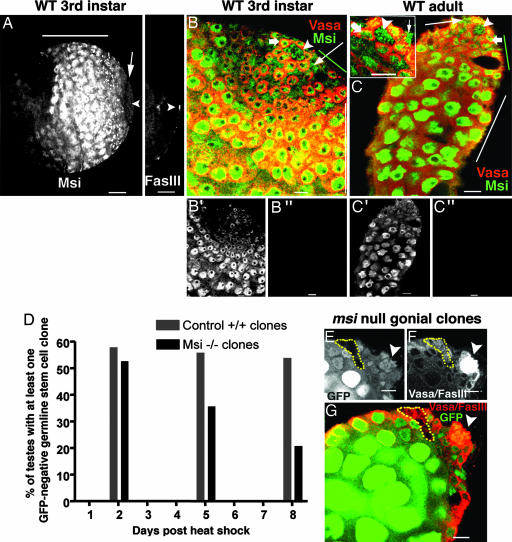
The phenotype of msi mutants suggested that Msi should be expressed in GSCs. We therefore examined Msi protein distribution using an antibody previously shown to be specific to Msi (27). Msi expression was observed in GSCs but also in somatic hub cells, cyst cells, proliferating spermatogonial cells, and differentiating spermatocytes at both third-instar larval and adult stages of development (Fig. 3A–C). Msi expression was observed at lower levels in the apical region of the testis, where spermatogonial amplification takes place, but at higher levels in maturing spermatocytes (Fig. 3 A, B′, and C′). Immunohistochemistry with the Msi antibody did not detect any signal in msi1-null mutant larval or adult testes (Fig. 3 B″ and C″). A line of Msi down-regulation at the transition between proliferating spermatogonia and G2-arrested spermatocytes was often observed (Fig. 3A). This result suggests that at least one protein may need to be released from Msi translational suppression to allow progression from spermatogonia to spermatocytes.
Fig. 3.
Msi is expressed in somatic and germ cells but is required intrinsically in GSCs for stem cell maintenance. (A) Low-magnification image of a wild-type third-instar testis stained with Msi (Left) and Fasciclin III (Right). Msi is expressed at low levels in the apical region, where gonial amplification normally occurs, but at higher levels in spermatocytes (horizontal line). A region of Msi down-regulation (arrow) before spermatocyte differentiation is observed. (Scale bar: 50 μm.) Arrowhead, hub. (B) Wild-type third-instar testis stained with Msi (green) and Vasa (red). (Scale bar: 10 μm.) Msi labels hub (arrowhead), GSCs (thick arrow), cyst cells (thin arrow), early germ cells (green line), and spermatocytes (white line). (B′) Grayscale image of B (Msi expression only) shows the differential expression pattern of Msi, with low levels observed in the apical region and high levels observed in spermatocytes. (B″) No Msi expression can be detected in msi1-null third-instar gonads. (C) Msi expression persists in hub, cyst, and germ cells in adult testes. (C Inset) High-magnification image of apex of adult testis. (C′) Grayscale image of C (Msi expression only) shows differential expression pattern of Msi in adults. (C″) No Msi expression can be detected in msi1 mutant adult testes. (D) Comparison of wild-type and msi1 GSC clone maintenance over time. (E–G) msi1 gonial clone observed 8 days after heat shock in the absence of a stem cell clone. (Scale bars: 10 μm.) (E and F) msi1 germ cell clones are GFP-negative (E, dotted line) and Vasa-positive (F, dotted line). (G) Merged image; dotted line shows msi1 gonial cell clones. No msi1 GSC clones are observed because all GSCs abutting the hub (arrowhead, Fasciclin III) express GFP.
Msi Is Required Intrinsically in GSCs to Maintain Stem Cell Identity.
Next, we examined whether Msi was autonomously required in GSCs for stem cell maintenance. The widespread expression of Msi in the apical proliferation center suggested that it may function in multiple cell types, so we used the Flp-mediated FRT mitotic recombination system to generate msi loss-of-function GSC clones marked by the absence of GFP. Because all known loss-of-function msi mutations result in the same testicular defects (see Figs. 1 and 2 B–D and F–H), we generated clones using the null allele msi1. GFP-negative GSC clones homozygous for the msi1 mutation were observed at approximately the same frequency (53%; n = 34) as control wild-type GSC clones (58%; n = 38) 2 days after clone induction from a pulse of heat shock (Fig. 3D). Although wild-type GSC clones were maintained at similar levels over time [5 days after heat shock, 56% GSC clones observed (n = 39); 8 days after heat shock, 54% GSC clones observed (n = 26)], msi1 stem cell clones could not be maintained at the same frequencies [5 days after heat shock, 36% GSC clones observed (n = 36); 8 days after heat shock, 21% GSC clones observed (n = 33)]. By 8 days after heat shock, msi-null mutant GSC clones were observed significantly less frequently than control wild-type GSC clones of the same age, indicating that msi−/− stem cells were lost over time and that Msi is intrinsically required by GSCs for their maintenance in the niche (Fig. 3D).
Several lines of evidence suggest that GSC loss in msi mutants is not due to cell death. First, we tested whether msi-null clones could be chased into spermatogonia. Because gonialblasts become spermatocytes after ≈2 days, negatively marked spermatogonia present 5 days after heat-shock treatment must have originated from a negatively marked GSC. msi-null spermatogonia were found in 47% (n = 36) testes 5 days after heat-shock treatment, a similar number to that detected in control testes (54%; n = 39). Second, we had already observed the presence of the differentiation marker bam-GFP in cells contacting the hub in msi-null mutants (Fig. 2O), suggesting that GSCs prematurely differentiate in the absence of Msi protein. Third, acridine orange staining did not reveal the presence of any excess dying cells in msi-null mutants (data not shown). In 15% of cases (n = 33), we observed msi-null spermatogonial clones 8 days after heat shock in the absence of msi-null GSC clones (Fig. 3 E–G), indicating that msi-null GSCs had differentiated into spermatogonial cells without regeneration of GSCs. These results demonstrate that loss of Msi function disrupts the balance between GSC self-renewal and differentiation, leading to the premature differentiation of GSCs.
Msi Maintains Stem Cell Fate Through a Novel Mechanism.
Our results clearly show that Msi is required intrinsically in GSCs for stem cell maintenance. Tramtrack69 (Ttk69) protein repression in developing neuronal lineages is the only target of Msi-mediated translational repression to be identified so far in Drosophila (28). We examined Ttk69 expression in testes, and, although we could detect Ttk69 protein in GSCs, we observed no difference in expression levels in msi mutants (data not shown). Two targets of murine Msi1 have been identified to be translationally repressed in multipotential neural progenitors, m-Numb (29) and p21WAF-1 (30). We examined expression of Drosophila orthologues of these proteins, Numb and Dacapo, respectively, but we also observed no alteration of expression in msi mutants (data not shown). Repression of Bam expression by bone morphogenetic protein family signaling in GSCs is also required for GSC maintenance; therefore, Bam could be considered a candidate for regulation by Msi (31). However, Bam expression has been extensively studied, and the gene is known to be transcriptionally repressed in GSCs but not subject to translational regulation (25). Our results indicate that Msi is regulating GSC maintenance by means of a novel mechanism and that Msi may have distinct targets in different progenitor or stem cell populations.
Msi also Functions Late in Spermatogenesis for Correct Meiosis and Cytokinesis, and Distinct Roles for Msi May Be Evolutionarily Conserved in the Murine Testis.
The widespread expression pattern of Msi in the testis (Fig. 3 A–C) suggests that Msi may have multiple targets or biological functions associated with germ cell differentiation. In support of the latter, we found that Msi is also required in spermatocytes for correct meiotic chromosome segregation and cytokinesis. In wild-type early round spermatids, the nucleus and the mitochondrial derivative in each haploid cell appear to be approximately the same diameter when viewed by phase-contrast microscopy (Fig. 4A). In msi mutants, early round spermatids commonly contained two or four nuclei and a large mitochondrial derivative (Fig. 4B), indicating that one or both meiotic cytokinetic divisions had failed. Additionally, haploid nuclei were often of different sizes, resulting from errors in chromosome segregation during meiosis (Fig. 4C). The separate roles for Msi protein in early and late spermatogenesis appear to have been evolutionarily conserved as the expression patterns of the two murine Msi paralogues, Msi1 and Msi2, have diverged to encompass these two stages of spermatogenesis (32, 33). Msi1 is expressed in spermatogonial stem cells, spermatogonia, and Sertoli cells (Fig. 4D) whereas Msi2 is expressed in spermatocytes and round spermatids (Fig. 4E).
Fig. 4.
Msi is required for meiotic chromosome segregation and cytokinesis. (A–C) Phase-contrast micrographs of postmeiotic spermatids in wild type (A) and msi1 (B–C). Arrowheads, mitochondrial derivatives; arrows, nuclei. (Scale bar: 10 μm.) (A) Onion-stage spermatids show one haploid nucleus and one mitochondrial derivative, each approximately the same diameter. (B) In msi1 mutants, two or four nuclei per mitochondrial derivative are often detected. (C) Nuclei in msi1 mutants are often different sizes (arrow). (D) Msi1 expressed in both adult murine spermatogonia (arrowhead) and Sertoli cells (arrow). (E) Msi2 expressed in both adult murine pachytene spermatocytes (arrowhead) and round spermatids (arrow). (Scale bars: 50 μm.)
In this study we have shown evidence for a cell-autonomous role of Msi proteins in regulating stem cell differentiation. Maintenance of stem cells relies not only on signals from the niche but also on Msi activity intrinsic to the stem cells. The balance between stem cell maintenance and differentiation must be tightly regulated and yet capable of plasticity to maintain tissue homeostasis under varied environmental conditions, e.g., nutrition, wounding, and other demands for variations in differentiated progeny. Msi proteins provide an additional level of regulation within the complex set of signals that control stem cell behavior within the niche. The functional requirement for Drosophila Msi within different stages of stem cell differentiation supports the hypothesis of an ancestral function that has diverged in vertebrates to generate a stem cell-specific Msi1 and a role for Msi2 in differentiating tissues. The wide variety of stem cell populations that express Msi proteins (1–4) also suggests that Msi proteins are part of a mechanism that plays a general role in regulating stem cell maintenance and differentiation.
Methods
Cytology.
Whole testes were viewed under phase-contrast microscopy or stained with 10 μg/ml DAPI (Sigma), 1:10 rabbit anti-Msi (H. Okano, Keio University, Japan), 1:100 goat anti-Vasa (Santa Cruz Biotechnology), 1:500 rabbit anti-GFP (Molecular Probes), 1:20 rat anti-N-cadherin (Developmental Studies Hybridoma Bank), 1:100 mouse anti-Dlg (Developmental Studies Hybridoma Bank), 1:100 mouse anti-Broad core domain (Developmental Studies Hybridoma Bank), 1:3,000 rabbit anti-β-galactosidase (Cappel), 1:20 mouse anti-Fasciclin III (Developmental Studies Hybridoma Bank), 1:100 mouse anti-α-spectrin (Developmental Studies Hybridoma Bank), 1:20 mouse anti-Dacapo (L. Quinn, Peter MacCallum Cancer Institute, Melbourne, Australia), 1:200 rabbit anti-Ttk69 (P. Badenhorst, University of Birmingham, Birmingham, U.K.), and 1:2,000 rabbit anti-Numb [F. Matsuzaki, The Institute of Physical and Chemical Research (RIKEN), Kobe, Japan]. Immunofluorescence was conducted as per ref. 34.
Mosaic Analysis.
Negatively marked GSC clones were induced in males by Flp-mediated recombination at FRT sites. hs-FLP/Y; FRT82B msi1/FRT82B Ubi-GFP or hs-FLP/Y; FRT82B/FRT82B Ubi-GFP adult males were heat-shocked at 38°C for 1 h and raised at 25°C after heat shock for 2, 5, or 8 days. Testes isolated from males at each time point were stained with anti-Fasciclin III, anti-VASA, and anti-GFP antibodies. Testes were then serially imaged by overlapping confocal optical sections on an MRC1024 Laser Scanning Confocal Microscope (Bio-Rad). The percentage of testes containing one (or more) GFP-negative but Vasa-positive GSC clone(s) abutting the hub was determined by counting 26–39 testes in control or experimental groups at each of the time points. The percentage of testes containing spermatogonial or spermatocyte clones was also determined at each of the time points.
Murine Immunohistochemistry.
Adult murine testes were fixed in ice-cold 4% paraformaldehyde before embedding and sectioning. Tissue sections were incubated with either anti-Msi1 (1:750) or anti-Msi2 (1:750) (H. Okano) as described previously (35) and visualized by using horseradish peroxidase-conjugated secondary antibodies (DAKO). Tissue sections were counterstained with methyl green. Sections stained with horseradish peroxidase-conjugated antibody alone showed no immunoreactivity.
Acknowledgments
We thank H. Okano, P. Badenhorst, and F. Matsuzaki of the Bloomington Drosophila Stock Center, H. Richardson of the Peter MacCallum Cancer Institute, D. McKearin of the University of Texas Southwestern Medical Center, Dallas, and the Developmental Studies Hybridoma Bank (University of Iowa) for Drosophila strains and antibodies and H. Abud, P. Koopman, and S. Bellingham for critical reading of the text. This work was supported by the Australian Research Council Centre of Excellence in Biotechnology and Development.
Abbreviations
- GSC
germ-line stem cell
- CPC
cyst progenitor cell
- Msi
Musashi.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kaneko Y., Sakakibara S., Imai T., Suzuki A., Nakamura Y., Sawamoto K., Ogawa Y., Toyama Y., Miyata T., Okano H. Dev. Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara S., Nakamura Y., Satoh H., Okano H. J. Neurosci. 2001;21:8091–8107. doi: 10.1523/JNEUROSCI.21-20-08091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potten C. S., Booth C., Tudor G. L., Booth D., Brady G., Hurley P., Ashton G., Clarke R., Sakakibara S., Okano H. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 4.Akasaka Y., Saikawa Y., Fujita K., Kubota T., Ishikawa Y., Fujimoto A., Ishii T., Okano H., Kitajima M. Histopathology. 2005;47:348–356. doi: 10.1111/j.1365-2559.2005.02223.x. [DOI] [PubMed] [Google Scholar]
- 5.Sakakibara S., Nakamura Y., Yoshida T., Shibata S., Koike M., Takano H., Ueda S., Uchiyama Y., Noda T., Okano H. Proc. Natl. Acad. Sci. USA. 2002;99:15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie T., Spradling A. In: Stem Cell Biology. Marshak D. R., Gardner R. L., Gottlieb D., editors. Woodbury, NY: Cold Spring Harbor Lab. Press; 2001. pp. 129–148. [Google Scholar]
- 7.Fuller M. T. In: The Development of Drosophila melanogaster. Bate M., Martinez Arias A., editors. Vol. I. Woodbury, NY: Cold Spring Harbor Lab. Press; 1993. pp. 71–147. [Google Scholar]
- 8.Li L., Xie T. Annu. Rev. Cell Dev. Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 9.Ohlstein B., Kai T., Decotto E., Spradling A. Curr. Opin. Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Kiger A. A., Jones D. L., Schulz C., Rogers M. B., Fuller M. T. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 11.Tulina N., Matunis E. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda T., Nakamura T., Nakao K., Arai T., Katsuki M., Heike T., Yokota T. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H., Spradling A. C. Development (Cambridge, U.K.) 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z., Lin H. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- 15.Crittenden S. L., Bernstein D. S., Bachorik J. L., Thompson B. E., Gallegos M., Petcherski A. G., Moulder G., Barstead R., Wickens M., Kimble J. Nature. 2002;417:660–663. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- 16.Wong M. D., Jin Z., Xie T. Annu. Rev. Genet. 2005;39:173–195. doi: 10.1146/annurev.genet.39.073003.105855. [DOI] [PubMed] [Google Scholar]
- 17.Hardy R. W., Tokuyasu K. T., Lindsley D. L., Garavito M. J. Ultrastruct. Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita Y. M., Jones D. L., Fuller M. T. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M., Okano H., Blendy J. A., Montell C. Neuron. 1994;13:67–81. doi: 10.1016/0896-6273(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 20.Kiger A. A., White-Cooper H., Fuller M. T. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 21.Tran J., Brenner T. J., DiNardo S. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- 22.Matunis E., Tran J., Gonczy P., Caldwell K., DiNardo S. Development (Cambridge, U.K.) 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- 23.Emery I. F., Bedian V., Guild G. M. Development (Cambridge, U.K.) 1994;120:3275–3287. doi: 10.1242/dev.120.11.3275. [DOI] [PubMed] [Google Scholar]
- 24.Lin H., Yue L., Spradling A. C. Development (Cambridge, U.K.) 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 25.Chen D., McKearin D. M. Development (Cambridge, U.K.) 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- 26.McKearin D., Ohlstein B. Development (Cambridge, U.K.) 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 27.Hirota Y., Okabe M., Imai T., Kurusu M., Yamamoto A., Miyao S., Nakamura M., Sawamoto K., Okano H. Mech. Dev. 1999;87:93–101. doi: 10.1016/s0925-4773(99)00143-4. [DOI] [PubMed] [Google Scholar]
- 28.Okabe M., Imai T., Kurusu M., Hiromi Y., Okano H. Nature. 2001;411:94–98. doi: 10.1038/35075094. [DOI] [PubMed] [Google Scholar]
- 29.Imai T., Tokunaga A., Yoshida T., Hashimoto M., Mikoshiba K., Weinmaster G., Nakafuku M., Okano H. Mol. Cell. Biol. 2001;21:3888–3900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battelli C., Nikopoulos G. N., Mitchell J. G., Verdi J. M. Mol. Cell. Neurosci. 2006;31:85–96. doi: 10.1016/j.mcn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Kawase E., Wong M. D., Ding B. C., Xie T. Development (Cambridge, U.K.) 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 32.McCarrey J. R. In: Cell and Molecular Biology of the Testis. Desjardins C., Ewing L., editors. New York: Oxford Univ. Press; 1993. pp. 53–89. [Google Scholar]
- 33.Prabhu S. M., Meistrich M. L., McLaughlin E. A., Roman S. D., Warne S., Mendis S., Itman C., Loveland K. L. Reproduction. 2006;131:489–499. doi: 10.1530/rep.1.00968. [DOI] [PubMed] [Google Scholar]
- 34.Bunt S. M., Hime G. R. Genesis. 2004;39:84–93. doi: 10.1002/gene.20030. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin E. A., Frayne J., Barker H. L., Jury J. A., Jones R., Ford W. C., Hall L. Mol. Hum. Reprod. 1997;3:801–809. doi: 10.1093/molehr/3.9.801. [DOI] [PubMed] [Google Scholar]