Abstract
In animals, liver and white adipose are the main sites for the de novo fatty acid synthesis. Deletion of fatty acid synthase or acetyl-CoA carboxylase (ACC) 1 in mice resulted in embryonic lethality, indicating that the de novo fatty acid synthesis is essential for embryonic development. To understand the importance of de novo fatty acid synthesis and the role of ACC1-produced malonyl-CoA in adult mouse tissues, we generated liver-specific ACC1 knockout (LACC1KO) mice. LACC1KO mice have no obvious health problem under normal feeding conditions. Total ACC activity and malonyl-CoA levels were ≈70–75% lower in liver of LACC1KO mice compared with that of the WT mice. In addition, the livers of LACC1KO mice accumulated 40–70% less triglycerides. Unexpectedly, when fed fat-free diet for 10 days, there was significant up-regulation of PPARγ and several enzymes in the lipogenic pathway in the liver of LACC1KO mice compared with the WT mice. Despite the significant up-regulation of the lipogenic enzymes, including a >2-fold increase in fatty acid synthase mRNA, protein, and activity, there was significant decrease in the de novo fatty acid synthesis and triglyceride accumulation in the liver. However, there were no significant changes in blood glucose and fasting ketone body levels. Hence, reducing cytosolic malonyl-CoA and, therefore, the de novo fatty acid synthesis in the liver, does not affect fatty acid oxidation and glucose homeostasis under lipogenic conditions.
Keywords: Cre-loxP, fatty acid synthesis, tissue-specific knockout
In eukaryotes, acetyl-CoA carboxylase (ACC) is a biotinylated enzyme that catalyzes the ATP-dependent carboxylation of acetyl-CoA to produce malonyl-CoA. Fatty acid synthase (FAS), the multifunctional enzyme, catalyzes the synthesis of long-chain fatty acid, palmitate, by using acetyl-CoA as a primer, malonyl-CoA as a two-carbon donor for chain elongation, and NADPH for the reduction reactions. The synthesis of malonyl-CoA is the committed step toward the synthesis of fatty acids (1–5). In addition, malonyl-CoA also plays an important role in the regulation of fatty acid oxidation in the mitochondria as an inhibitor of the carnitine palmitoyl transferase 1, which performs the first step in the transfer of long-chain fatty acyl CoA into mitochondria for their oxidation (6, 7). Hence, malonyl-CoA participates in two opposing pathways, a substrate for fatty acid synthesis and a regulator of fatty acid oxidation. Lately, there are also reports that malonyl-CoA regulates orexigenic responses in hypothalamus (8) and insulin secretion by pancreatic β-islets (9, 10). Malonyl-CoA is generated by two isoforms of acetyl-CoA carboxylases, ACC1 and ACC2 of molecular mass 265 kDa and 280 kDa, respectively (11–16). ACC1 is a cytosolic enzyme, and ACC2 is associated with mitochondria (17). Although both isoforms are expressed in various tissues, ACC1 is predominantly expressed in lipogenic tissues such as liver, adipose, and lactating mammary gland, and ACC2 is predominantly expressed in muscle tissues and heart (12, 13). To understand the roles of ACC1 and ACC2, we generated knockout mice. Although ACC1 knockout mice died during embryonic development (18), ACC2 mutant mice live and breed normally, are leaner, have increased fatty acid oxidation, and resist becoming obese and insulin-resistant when fed high-fat/high-carbohydrate diets (19, 20). The studies on ACC knockout mice suggested that ACC2-produced malonyl-CoA is involved in the regulation of fatty acid oxidation and that ACC1 produced malonyl-CoA is dedicated for de novo fatty acid synthesis (18–21).
The ACC1 and FAS knockout studies (18, 22) showed that the de novo fatty acid synthesis is essential during embryonic development. To understand the importance of de novo fatty acid synthesis and the role of ACC1-produced malonyl-CoA in adult mouse tissues, we generated tissue-specific knockout mice of ACC1. Here we describe our studies of the liver-specific ACC1 knockout mice (LACC1KO). When fed a normal chow, the LACC1KO mice do not exhibit any significant physiological differences from WT mice. When fed a fat-free diet, the LACC1KO mice accumulate less triglyceride in the liver compared with the WT mice. Furthermore, LACC1KO mice have normal glucose levels and slightly elevated insulin levels. These results are in contradiction with the studies reported on mice with liver-specific knockout of FAS, which became hypoglycemic when fed a fat-free diet (23). We will discuss our observations, the differences between ACC1 and FAS liver-specific knockout mice, and the role of ACC1 produced malonyl-CoA in animal physiology.
Results
Generation of Liver-Specific ACC1 Knockout Mice.
The generation of targeting construct, ES cells containing floxed ACC1 allele, and chimeras are described in Materials and Methods and Fig. 1 legend. The C57BL/6J lox+/− mice (Fig. 1), were interbred to generate mice homozygous for ACC1 alleles with floxed biotin exon (lox+/+). The lox+/+ mice are normal and breed like WT C57BL/6J mice. Based on the RT-PCR analysis of ACC1 mRNA and ACC1 activity in the liver, the presence of loxP sites in introns 21 and 22 did not affect the expression of functional ACC1. To generate liver-specific ACC1 knockout mice, lox+/+ mice were bred with C57BL/6J mice expressing Cre recombinase under the control of rat albumin promoter to obtain initially heterozygotes (lox+/−Cre+). The lox+/−/Cre+ progeny was interbred to obtain lox+/+Cre+ mice that are expected to contain LACC1KO. When the LACC1KO mice were interbred, the progeny contained lox+/+ Cre+ and the lox+/+ (WT) with the ratios expected from Mendelian segregation of Cre, indicating that there was no embryonic lethality of LACC1KO mice. Moreover, the LACC1KO mice breed normally when interbred, producing lox+/+ Cre+ mice in the expected numbers.
Fig. 1.
Generation of tissue-specific knockout mice. (A) The strategy to delete, in a tissue-specific manner, the biotin binding site (Met-Lys-Met) containing exon is shown. The targeting vector contained a floxed Neo gene inserted in intron 21 and a loxP site inserted in intron 22, and, in addition, the TK gene for negative selection of nonhomologous events. Homologous recombination in ES cells generated targeted allele (ACC1neo) allele. Chimeras generated by using the ES cells were bred with C57BL/6J mice to generate mice containing an ACC1neo allele. The generation of mice containing ACC1lox allele and tissue-specific deletion of exon 22 (ACC1del) are described in Materials and Methods. LoxP sites are indicated by arrowheads. Primers for PCR analysis are indicated by arrows. (B) A typical Southern blot analysis of ES cell DNA by using the probes indicated in A. (C) PCR-based genotyping of tail DNA to distinguish WT (lox−/−, lane 1), heterozygote (lox+/−, lane 2), and homozygote (lox+/+, lane 3) by using the primers a and b and c and d, indicated in A. (D) PCR-based genotyping of liver DNA by using the primers a and d. (E) RT-PCR analysis for liver mRNA by using primers derived from the exons 21 and 24. In D and E, + and − refer to the presence and absence of Cre.
Liver-Specific Inactivation of ACC1 Leads to Lower Malonyl-CoA Level and Decreased Hepatic Lipid Accumulation.
Based on the PCR and RT-PCR analyses of the DNA and the RNA isolated from the livers of LACC1KO mice, deletion of exon 22 coding for biotin carboxyl carrier protein was >95% (Fig. 1 D and E). To confirm these results further, we have analyzed extracts of the livers of these mice by SDS/PAGE, followed by Western blotting and detection of ACCs with avidin-peroxidase. As shown by SDS/PAGE analysis, deletion of exon 22 generated an in-frame splicing of exons 21 and 23, resulting in a mutant form of ACC1 protein, which is ≈10 kDa shorter than the WT ACC1 (Fig. 2A). Surprisingly, even though biotin carboxyl carrier protein coding exon is deleted, the mutated ACC1 reacted well with avidin-peroxidase, suggesting that there is an alternative biotinylation site in this protein, which is under investigation. Next, we fed a fat-free diet for 2 days after 2 days of fasting to induce the de novo fatty acid synthesis and measured the ACC activities and the levels of malonyl-CoA in the liver extracts of the mice. In crude extracts of the livers, the ACC activity in the WT was 4.9 ± 0.3 nmol·min−1·mg−1 protein, and in avidin-Sepharose-purified samples, the activity was 1,614 ± 93 nmol·min−1·mg−1 (Fig. 2 B and C). In LACC1KO mice, ACC activity in the crude extracts of the livers and affinity-purified samples were 1.4 ± 0.3 and 408 ± 52 nmol·min−1·mg−1 protein, respectively (Fig. 2 B and C). Hence, the ACC activity levels in LACC1KO livers were reduced by ≈75% of the levels observed in the livers of WT mice. Malonyl-CoA levels in the livers of WT mice were 6.53 ± 0.76 nmol/g of wet weight and that of LACC1KO mice were 1.99 ± 0.54 nmol/g of wet weight, indicating a 70% reduction of malonyl-CoA levels in the livers of LACC1KO mice (Fig. 2D). The low ACC activity and malonyl-CoA levels in the livers and the high efficiency (>95%) of Cre-mediated deletion of exon 22 (Fig. 1 D and E) suggested that the mutated ACC1 protein, although biotinylated, does not seem to have carboxylase activity. Hence, the residual ACC activity could be attributed mainly to that of the ACC2 isoform, which might contribute to the levels of malonyl-CoA observed in the livers of LACC1KO mice.
Fig. 2.
Analysis of ACC1 expression in the WT and LACC1KO mice. The preparation of liver extracts, affinity purification of biotinylated proteins, and determination of ACC activities were performed as described in Materials and Methods. (A) Avidin-affinity purified proteins from liver homogenates were fractioned by 6% Tris-glycine SDS/PAGE. The gel was stained with Coomassie (Left, lanes 1–4). A similar gel was electroblotted to nitrocellulose sheet and probed with avidin–horseradish peroxidase (Right, lanes 7–10). The mutated ACC1 protein is 10 kDa less than the WT ACC1. Lanes 5 and 6 show the 250-kDa molecular mass standard. (B and C) ACC activities in the liver extracts and avidin-affinity purified proteins, respectively, of mice (n = 4–5) after 2 days of fasting, followed by 2 days of refeeding with fat-free diet. Open bars, WT; filled bars, LACC1KO. (D) Malonyl-CoA content in the livers from mice (n = 5) fed fat-free diet as described in B and C. (E) Liver histology of WT and LACC1KO of mice fed fat-free diet for 28 days. Liver sections were stained with Oil Red O as described in ref. 19.
General Characterization of LACC1KO Mice.
The body weight and food intake of LACC1KO mice that were fed normal diet were not significantly different from WT mice (Table 1). To determine whether the loss of ACC1 function in the livers of LACC1KO mice affected the glucose and lipid metabolism of the animal, we measured the levels of various blood constituents of mice that were fed a normal diet. Under nonfasting conditions, the levels of blood glucose, insulin, cholesterol, triglyceride, ketone bodies (β-hydroxybutyrate) of the WT and LACC1KO mice were similar, however, the nonesterified fatty acid (NEFA) was lower in the LACC1KO mice (Table 1). In addition, the weights of livers and the epididymal fat pads were not significantly different in either of these two groups of mice (Table 1). Interestingly, the liver triglyceride levels in LACC1KO mice were ≈40% lower than those of WT mice (Table 1). These analyses showed that the reduced de novo fatty acid synthesis in the liver leads to lower accumulation of the triglyceride in the liver. When the mice fasted for 24 h, there were no significant differences between the LACC1KO and WT mice in the parameters analyzed (Table 1). However, as expected, during fasting, there was a significant increase in liver triglyceride levels in both LACC1KO and WT mice compared to the fed state because of mobilization of extrahepatic TG to liver (Table 1).
Table 1.
Comparison of WT and LACC1KO mice fed with normal chow diet or fat-free diet
| Parameter | Nonfasted |
Fasted |
Fat-free diet |
|||
|---|---|---|---|---|---|---|
| WT | LACC1KO | WT | LACC1KO | WT | LACC1KO | |
| Body weight, g | 28.2 ± 0.8 | 28.2 ± 0.4 | 25.2 ± 0.4 | 24.9 ± 0.3 | 28.8 ± 0.4 | 28.2 ± 0.4 |
| Liver weight, g | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 |
| Fat pad weight, g | 0.44 ± 0.03 | 0.38 ± 0.04 | 0.35 ± 0.03 | 0.26 ± 0.02* | 0.38 ± 0.03 | 0.45 ± 0.01 |
| Liver triglyceride, mg/g | 7.8 ± 0.8 | 4.8 ± 0.4** | 38.6 ± 4.3 | 35.9 ± 3.2 | 12.1 ± 1.3 | 4.2 ± 0.1** |
| Liver cholesterol, mg/g | 2.7 ± 0.1 | 2.2 ± 0.2* | 3.5 ± 0.3 | 3.6 ± 0.4 | 2.5 ± 0.2 | 1.7 ± 0.1** |
| Blood glucose, mg/dl | 187 ± 7 | 173 ± 8 | 92 ± 3 | 102 ± 7 | 186 ± 10 | 188 ± 13 |
| Serum ALT, U/liter | 31 ± 1 | 33 ± 1 | 32 ± 3 | 28 ± 2 | ND | ND |
| Serum insulin, ng/ml | 0.88 ± 0.12 | 1.03 ± 0.16 | 0.20 ± 0.02 | 0.21 ± 0.02 | 0.97 ± 0.15 | 1.39 ± 0.13 |
| Serum cholesterol, mg/dl | 77 ± 4 | 73 ± 1 | 76 ± 1 | 79 ± 3 | ND | ND |
| Serum triglyceride, mg/dl | 81 ± 3 | 81 ± 5 | 76 ± 4 | 71 ± 6 | ND | ND |
| Blood ketone, mmol/liter | 0.2 | 0.2 | 1.13 ± 0.11 | 0.93 ± 0.08 | ND | ND |
| Serum NEFA, mEq/liter | 0.83 ± 0.07 | 0.61 ± 0.05* | 1.06 ± 0.03 | 0.91 ± 0.05 | 0.59 ± 0.02 | 0.43 ± 0.03* |
The blood samples were taken from 5- to 6-month-old male mice (n = 5–7). The nonfasted groups were fed a normal chow diet ad libitum before the study. The fasted groups of mice were fasted 24 h before the study. The fat-free diet groups of mice were fed with fat-free diet for 10 days. Each value represents the mean ± SEM.
∗, P < 0.05 and
∗∗, P < 0.005. ND, not determined.
To examine the differences between the WT and LACC1KO under conditions where the de novo fatty acid synthesis in the liver would be enhanced, we fed the mice with a fat-free diet for 10 days. Analyses of blood parameters and other parameters (Table 1) were performed after fasting for 4 h. The lipid accumulations in the liver of LACC1KO were significantly lower (66%) than that in the WT cohorts (Table 1). The NEFA levels in LACC1KO mice were ≈30% lower than that in WT mice. There was no significant difference between the fat pad weights of both WT and LACC1KO mice. The levels of blood glucose were not significantly different under these lipogenic conditions; however, the insulin levels were elevated. When the mice were fed with a fat-free diet for 28 days, all of the blood parameters are essentially similar with those mice fed with a fat-free diet for 10 days. The Oil Red O staining of the liver sections showed that the livers of LACC1KO mice accumulated much less lipid than that of WT mice (Fig. 2E). The liver glycogen contents in LACC1KO mice were not significantly different compared with that in WT mice, as estimated by periodic acid schiff staining of the liver sections (data not shown). The levels of blood glucose and ketone bodies also were not significantly different. Prolonged feeding with fat-free diet until 28 days did not significantly change the ALT levels (32 ± 2.6 U/liter in WT vs. 27.8 ± 2.8 U/liter in LACC1KO), indicating that the liver function in those mice were not impaired. Hence, feeding a lipogenic diet (fat-free diet) reduced the lipid accumulation in the livers of LACC1KO but did not alter the glucose homeostasis.
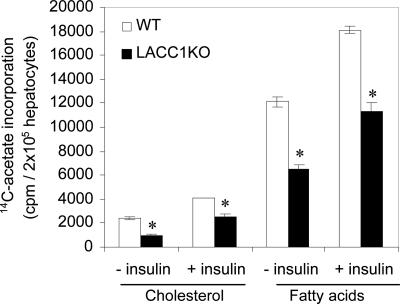
These analyses, although indicating that there is less triglyceride accumulation in the livers of LACC1KO mice, did not help us to determine whether these lipids were synthesized de novo or transferred from the adipose tissues. Hence, we measured the de novo fatty acid synthesis in the primary hepatocytes isolated from WT and LACC1KO mice that were fed a fat-free diet for 2 days by using 14C-acetate as a precursor. The rate of fatty acid synthesis in LACC1KO hepatocytes, as measured by the incorporation 14C-acetate into the saponifiable fraction, which contains mainly fatty acids, was ≈50% less than those isolated from WT mice (Fig. 3). Similarly acetate incorporation into the nonsaponifiable fraction, which contains mainly cholesterol, was reduced in LACC1KO hepatocytes to ≈40% of that of the WT hepatocytes as shown in Fig. 3. Analysis of fatty acids synthesized from radioactive acetate by reverse-phase thin layer chromatography revealed that most of the incorporation of 14C-acetate was into palmitate and significantly less was into the other fatty acids (data not shown). These results were confirmed by determining the levels of various fatty acids of the liver lipids by gas chromatography. As shown in Table 2, the levels of saturated fatty acids and the monounsaturated fatty acids in LACC1KO liver all were significantly lower than in those of the livers of WT mice when fed a fat-free diet for 10 days. However, based on the composition (mol%) of various fatty acids, both WT and LACC1KO livers have similar lipid composition (data not shown). Interestingly, the total fatty acid analysis of total lipids (Table 2) indicated that oleate is the predominant constituent of liver lipids. Thus, it appears that the most of the lipids accumulating in the livers of LACC1KO mice after feeding fat-free diet for 10 days contain both the de novo synthesized fatty acids and the fatty acids mobilized from extrahepatic tissues. However, the rates of fatty acid oxidation measured by using 14C-palmitate in primary hepatocytes isolated from WT and LACC1KO were very similar (data not shown). Further, as shown in Table 1, the levels of ketone bodies in the blood under fasting conditions were essentially identical in both LACC1KO and WT mice. The observations that the rate of fatty acid oxidation in LACC1KO hepatocytes was not affected by the loss of ACC1-produced malonyl-CoA is consistent with our previous observations (19), which indicated that only the ACC2-produced malonyl-CoA regulates carnitine palmitoyl transferase 1 activity.
Fig. 3.
De novo lipogenesis in the isolated primary hepatocytes. Hepatocytes isolated from the perfused livers of WT and LACC1KO mice were incubated with 14C-acetate for 2 h, and the radioactivity in the nonsaponifiable (cholesterol) and saponifiable (fatty acids) fractions was measured. Where indicated, insulin was added to the medium at 0.2 μM. ∗, P < 0.001 (LACC1KO vs. WT mice).
Table 2.
Gas chromatography analyses of fatty acids in the livers of 5-month-old male mice fed with fat-free diet for 10 days
| Fatty acid | mmol/g of wet weight |
|
|---|---|---|
| WT | LACC1KO | |
| C14:0 | 2.02 ± 0.17 | 1.30 ± 0.07** |
| C16:0 | 47.67 ± 2.78 | 27.69 ± 0.98** |
| C18:0 | 13.53 ± 0.78 | 9.91 ± 0.52** |
| C20:0 | 0.30 ± 0.02 | 0.15 ± 0.01** |
| C22:0 | 0.41 ± 0.17 | 0.14 ± 0.01** |
| C24:0 | 0.26 ± 0.02 | 0.25 ± 0.01 |
| C16:1 | 22.80 ± 1.06 | 12.67 ± 0.65** |
| C18:1 | 119.96 ± 14.45 | 54.15 ± 5.31** |
| C20:1 | 3.41 ± 0.62 | 1.33 ± 0.20* |
| C22:1 | 0.67 ± 0.14 | 0.87 ± 0.29 |
| C24:1 | 0.70 ± 0.04 | 0.73 ± 0.03 |
Each value represents the mean ± SEM (n = 5).
∗, P < 0.05;
∗∗, P < 0.005.
High Fat, High Carbohydrate Diet Could Not Prevent Obesity and Insulin Resistance in LACC1KO Mice.
Because loss of ACC1 activity leads to decreased lipid accumulation in the livers, feeding high fat, high carbohydrate (HF/HC) diet might not induce the LACC1KO mice to become obese or diabetic. To test this possibility, we fed the 14-week-old mice with a HF/HC diet for 5 months; surprisingly, both WT and LACC1KO mice became equally obese (body weight of 46.3 ± 2.2 g vs. 47.4 ± 1.5 g; n = 5) and developed fatty livers with intensive triglyceride accumulation in the livers (88.69 ± 5.73 mg/g vs. 77.06 ± 4.68 mg/g). Both WT and LACC1KO mice developed glucose intolerance and insulin resistance (data not shown) as determined by a glucose tolerance test and an insulin tolerance test by i.p. injection of 1 g/kg d-glucose and 0.75 units/kg insulin, respectively. The LACC1KO mice developed hyperglycermia with elevated fasting glucose level of 185 ± 7 mg/dl compared with 155 ± 8 mg/dl of WT mice. The NEFA levels in LACC1KO mice were ≈30% (P < 0.001) higher than that in WT mice (1.01 ± 0.06 mEq/liter vs. 0.73 ± 0.01 mEq/liter). The levels of fasting ketone bodies are lower in LACC1KO mice compared with WT mice (0.50 ± 0.08 mmol/liter vs. 1.42 ± 0.13 mmol/liter).
Up-Regulation of Lipogenic Enzymes in the Livers of LACC1KO Mice.
To understand the affect of chronic reduction in the de novo fatty acid synthesis in the livers of LACC1KO mice on the expression of various genes involved in lipogenesis, we performed real-time PCR analyses. For these analyses, the mice were fed a fat-free diet for 10 days. As was observed by RT-PCR analysis (Fig. 1), the real-time PCR analysis also revealed that the albumin-Cre mediated deletion of exon 22 of ACC1 gene in the liver resulted in a 99% loss of the ACC1 mRNA containing biotin-binding domain. Unexpectedly, we found that the levels of mRNA of genes involved in fatty acid synthesis (total ACC1, 3.8-fold, ACC2, 1.8-fold; FAS, 2.9-fold; ATP-citrate lyase, 3.0-fold, and long-chain fatty acid elongase, 3.5-fold) were significantly higher in LACC1KO livers compared with that of the WT mice (Fig. 4A). Western blot analysis of FAS protein showed the increased gene expression was correlated to increased protein levels (Fig. 4 B and C). Further FAS activity in LACC1KO mice was 2.2 times higher than that of the WT cohort mice (49.7 ± 2.9 vs. 22.4 ± 1.0 nmol·min−1·mg−1; P < 0.01). However, the mRNA levels of ACC1, ACC2, FAS, and ATP-citrate lyase essentially were similar in the epididymal white adipose tissues of the WT and the mutant (data not shown). These results suggest that chronic reduction in fatty acid synthesis in the liver triggers a sensor that up-regulates the expression of the lipogenic genes. The 3.5-fold increases in the expression of each of HNF-4α and PPARγ (Fig. 4A) suggests that these nuclear factors might play a role in this regulation.
Fig. 4.
Analysis of gene expression in the livers of LACC1KO mice. (A) Relative mRNA levels of genes expressed in the livers of LACC1KO mice over that of the WT mice as determined by real-time PCR. ACL, ATP-citrate lyase; LCE, long-chain fatty acid elongase; SCD-1, stearoyl-CoA desaturase-1; HNF4α, hepatic nuclear factor 4α. (B) Coomassie staining of bands obtained by gel electrophoresis (4–12% NuPAGE gel with Mes buffer) of 30 μg of protein samples from livers of WT mice (lanes 1–3) and from livers of LACC1KO mice (lanes 4–6). (C) Western blot analysis by using anti-FAS antibody. Arrows indicate the position of FAS protein, and M denotes standard protein, 188 kDa.
Discussion
The aim of the current studies was to determine the role of liver ACC1 produced malonyl-CoA, and the de novo fatty acid synthesis in lipid metabolism and glucose homeostasis. Liver is a major lipogenic and ketogenic tissue, which plays essential roles in lipid and glucose homeostasis. Lipid accumulation in the liver caused by dysregulation of glucose and lipid metabolism in this tissue is a major cause for hepatic steatosis that is associated with insulin resistance and type 2 diabetes (24). To understand the role of ACC1 in the liver we generated liver-specific ACC1 knockout mice. LACC1KO mice are apparently normal and breed like WT. Based on the genotyping of the liver DNA and RT-PCR analyses of the liver RNA, the deletion of exon 22 coding for biotin binding site was essentially complete (>95%) in LACC1KO mice. After starving the mice for 2 days and refeeding fat-free diet for 2 days the levels of ACC activities in LACC1KO livers were reduced by 75% compared with that of the WT cohorts. Correspondingly, the levels of malonyl-CoA in the livers of mutant mice were ≈70% less than those of WT. The incorporation of 14C-acetate into the fatty acids and cholesterol in mutant hepatocytes was reduced ≈50% compared with that of the WT hepatocytes. Interestingly, the levels of triglyceride accumulation in the livers of LACC1KO mice also was reduced by ≈40% compared with that of WT. These observations suggest that ACC2 activity and the malonyl-CoA it produces could be contributing to the overall ACC activity and malonyl-CoA levels observed in LACC1KO mice. Our studies with ACC2 knockout mice showed that the ACC activity and malonyl-CoA levels of the liver were not significantly different from those of the WT livers (19). Hence, it appears that the ACC2 expression/activity was up-regulated by the loss of functional ACC1 in LACC1KO livers. The analyses of the liver lipid fatty acid composition (Table 2) and the de novo synthesized fatty acids indicates that the lipids that accumulate in the livers of LACC1KO mice were derived from both de novo synthesized and the import of fatty acids synthesized from extrahepatic tissues.
In addition to reduced triglyceride accumulation in the liver, LACC1KO mice had slightly higher insulin levels in the blood, and the glucose levels were unchanged compared with that of WT (Table 1) when fed a fat-free diet. Although decreased lipid synthesis in the liver was observed in liver-specific SREBP cleavage that was perturbed (25–27), there was no effect on blood glucose and insulin levels. However, SREBP is a transcription factor that not only affects the levels of enzymes involved in fatty acid synthesis (ACCs and FAS), it also affects the expression levels of enzymes involved in lipid synthesis.
To study the effect of reduction in cytosolic malonyl-CoA levels in rat livers, An et al. (28) used adenoviral vectors to express a cytosolic form of malonyl-CoA decarboxylase (MCD), which converts malonyl-CoA to acetyl-CoA. These studies showed that the reduction of malonyl-CoA levels in the livers resulted in a significant reduction in the plasma levels of NEFAs and insulin when fed a high-fat diet (28). Further, the expression of malonyl-CoA decarboxylase in the liver resulted in a reversal of insulin resistance in the muscle and liver of mice fed a high-fat diet due to lower-circulating, free fatty acids and ketone bodies (β-hydroxybutyrate) in the muscle leading to insulin sensitivity (28). Consistent with our current studies, An et al. (28) reported that a 7-fold increase in MCD activity in liver only had a modest effect on fatty acid oxidation and did not change the plasma keton bodies. The observation that fatty acid oxidation was not affected in LACC1KO hepatocytes confirmed our previous results that the cytosolic malonyl-CoA produced by ACC1 is not involved in the regulation of fatty acid oxidation in the liver (18). When LACC1KO mice were fed a HF/HC diet, the blood glucose, insulin, and NEFAs all are increased significantly compared with their WT cohorts, and the mice had a fatty liver and were insulin resistant. The difference between what we observed by using LACC1KO mice and the studies by using recombinant adenovirus expressing malonyl-CoA decarboxylase could be due to the way malonyl-CoA levels are lowered or due to the HF/HC diet we used and a high-fat diet their studies used. In addition, MCD is also localized in the peroxisomes, which may affect the oxidation of very long-chain fatty acids. Interestingly, a recent study using i.p. injection of antisense oligonucleotide inhibitors of ACC1 and ACC2 reversed diet-induced hepatic steatosis and hepatic insulin resistance (29). In these studies, it was shown that antisense oligonucleotide inhibitors of ACC1 significantly reduced mRNA level of this enzyme without significantly altering liver malonyl-CoA levels. Based on the lack of significant reduction in the malonyl-CoA levels, it appears that their antisense oligonucleotides were most effective in reducing ACC2 mRNA and ACC2-produced malonyl-CoA levels and not the ACC1-produced malonyl-CoA levels. These results are expected based on our earlier observation that in ACC1+/− heterozygotes, the malonyl-CoA levels were essentially unchanged (18). Furthermore, LACC1KO mice fed HF/HC diet did not prevent the hepatic steatosis or insulin resistance as discussed in Results. Based on our ACC2 knockout studies (19), the reversal of hepatic steatosis and insulin resistance observed by using antisense oligonucletides (29) appears to be mostly due to their affect on reducing ACC2 mRNA levels and ACC2-produced malonyl-CoA.
Recently, Chakravarthy et al. (23) showed that a FAS knockout in liver (FASKOL) resulted in mice mutants that are essentially similar to WT when fed normal chow. However, when FASKOL mice were fed a zero-fat diet for 28 days, they develop fatty liver, hypoglycemia, and hypoinsulinemia, and the levels of blood ketone bodies were decreased. These phenotypes are similar to fasted PPARα-deficient mice and could be rectified by using a PPARα agonist. Hence, these authors conclude that synthesis of “new fat” in the liver is needed for activation of PPARα (23). Unlike FASKOL mice, LACC1KO mice accumulated less triglycerides in the liver when fed a fat-free diet for 28 days. One major difference between the liver-FAS knockout mice and the liver-ACC1 knockout mice is that in FASKOL mice, the malonyl-CoA levels were 3-times higher than those of the WT; however, in LACC1KO mice, the malonyl-CoA levels were reduced to ≈25% of those of the WT. One might speculate that in FASKOL mice, the elevated levels of malonyl-CoA promote fatty acid elongation in the absence of fatty acid synthesis by FAS, which might deplete the levels of long-chain fatty acids needed for PPARα activation. In LACC1KO mice, there was significant up-regulation of PPARγ and several enzymes in the lipogenic pathway, such as FAS, which was up-regulated ≈3-fold at the mRNA and protein and activity levels (Fig. 4A). On the other hand, there was significant decrease of the mRNA of the liver carnitine palmitoyl transferase 1, which together with the increase in ACC2 suggest down-regulation of fatty acid oxidation in the livers of LACC1KO mice (Fig. 4A). These results may explain why triglyceride levels in the livers were decreased only by ≈50% in LACC1KO mice when fed a fat-free diet but remained the same as in WT livers after fasting or when fed a HF/HC diet.
These results suggest that lowering cytosolic malonyl-CoA and, hence, the de novo fatty acid synthesis through the committed step catalyzed by ACC1, signals to the cells that fatty acid synthesis is needed that, in turn, respond by up-regulating the fatty acid synthesis pathway and down-regulating the fatty acid oxidation pathway. Hence, it appears that malonyl-CoA might play a role as a sensor that activates unidentified transcription factor as suggested by Dowell et al. (30). In addition, we have shown that in the liver, malonyl-CoA produced by the cytosolic ACC1 is a regulator of de novo fatty acid synthesis. In contrast to previous reports, lowering cytosolic malonyl-CoA in liver did not affect fatty acid oxidation or glucose homeostasis.
Materials and Methods
Animals.
C57BL/6J mice and the transgenic mice expressing Cre recombinase under control of rat albumin promoter (Alb-Cre mice) or adenovirus EIIa promoter (EIIa-Cre mice) were purchased from The Jackson Laboratory. The mice were maintained on a 12-h/12-h light/dark cycle and provided with ad libitum access to water and standard rodent chow diet (PicoLab 5053). Some groups of mice were also fed a fat-free diet (catalog no. 901683; MP Biomedicals, Aurora, OH) or HF/HC diet (catalog no. F3282; Bioserv, Frenchtown, NJ). All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Generation of ACC1lox mice.
The genomic DNA (5′ arm) we used for the targeting vector has been described in ref. 18. That DNA contains ACC1 gene sequences spanning from exon 19 to 23. We amplified 3′ arm DNA (3′ flanking sequences including exon 23 until exon 25) by PCR with ES cell DNA and combined this DNA fragment to the 5′ arm DNA through the unique EcoRI site located in intron 22 (Fig. 1A). As shown in Fig. 1, we generated “floxed” targeting vector by inserting floxed-neo in intron 21 and a loxP site in intron 22, aiming to eventually delete the floxed exon 22 by Cre recombinase. Deletion of the exon 22 of mouse ACC1, coding for part of biotin carboxyl carrier protein containing biotin-binding site (Met-Lys-Met) would inactivate ACC1 (18). The generation of transgenic mice was performed as described in refs. 18 and 22. Male chimeras (A32 and A33) were bred with C57BL/6J mice, and the progeny from one of the chimeras (A32) contained ACC1 “floxed-neo allele,” as assessed by tail DNA genotyping by Southern blot analysis and PCR. Mice having ACC1neo allele were mated with EIIa-Cre mice, and the progeny were genotyped for deletion of neo gene, leaving one loxP site in intron 21 and another in inron 22 (lox+/−; Fig. 1C, lane 2). These mice were backcrossed with C57BL/6J for 8–10 generations to generate a relatively “pure” line of lox+/− C57BL/6J mice. These mice can be bred with any tissue-specific Cre recombinase expressing mice to eventually generate tissue specific ACC1 knockout mice. PCR genotyping was carried out by using the following primers: for Cre transgene, 5′-CGGTCGATGCAACGAGTGAT-3′ and 5′-CCACCGTCAGTACGTGAGAT-3′; for the upstream loxP site, 5′-AAGTCCTCAAGGAGCTGGACA-3′ and 5′-GCTCACTCTGAAGTTGAGGATG-3′; for downstream loxP site, 5′-ACCTGGAGCTAG GCTGGTAG-3′ and 5′-CCACTGCAATTCAGTCACCATC-3′.
Analyses of ACC1 and FAS Expression in Livers.
Livers resected from mice were snap frozen and ground to powder in liquid nitrogen. The powdered tissues were suspended in 10 ml of PBS containing 0.1 mM PMSF, 5 mM benzamidine, and 5 μg/ml each of protease inhibitors (antitrypsin, leupeptin, aprotinin, and pepstatin), homogenized by using Polytron (3 × 30 sec at high speed), and sonicated briefly to degrade DNA. The extracts were clarified by centrifugation at 16,000 × g for 20 min. The supernatant fluid (crude extract) was subjected to ammonium sulfate (35%) precipitation. The precipitate was collected by centrifugation (23,000 × g for 30 min), dissolved in 1.5 ml of Tris buffer [100 mM Tris (pH 7.5) containing 0.5 M NaCl, 1 mM EDTA, 0.2 mM DTT, and the protease inhibitors described in the extraction buffer above], and the mixture was clarified by centrifugation at 23,000 × g for 30 min. For affinity purification of ACCs, the solution then was incubated with 50 μl of avidin–Sepharose beads for 90 min, and the beads were collected by centrifugation briefly (≈5 sec) at 200 × g and washed three times with Tris buffer described above. The biotin-containing proteins were eluted from the beads by using 50 μl of 50 mM Hepes, pH 7.5/1 mM EDTA/10% glycerol. The beads were washed two times with 50 μl of the same buffer, and the washes were pooled with the eluate. The determination of ACC activity, SDS/PAGE, and Western blot analysis were performed as described in ref. 18. FAS expression levels and activity were analyzed in the crude extracts as described in ref. 22.
Blood Metabolite Assays.
Whole blood glucose and β-hydroxybutyrate were measured with a Glucometer Precision Xtra (Abbott). Insulin was measured by a mouse insulin ELISA kit (Mercodia, Winston Salem, NC) with mouse insulin as the standard. Serum alanine aminotransferase, triglyceride and cholesterol measurements were done by the Comparative Pathology Laboratory (Baylor College of Medicine). Serum NEFAs were measured by using NEFA C kit (Wako Chemicals, Richmond, VA).
Tissue Triglyceride and Cholesterol Contents.
Liver triglyceride and cholesterol contents were carried out as described in ref. 31 by using Cholesterol E Kit (Wako Chemicals) and Infinity Triglyceride Kit (Thermo Electron), adapted for colorimetric analysis in 96-well plate format.
Quantitative Real-Time PCR.
Total RNA was prepared from mouse tissues by using TRIzol reagent (Invitrogen). Equal amounts of RNA from five mice were pooled and treated with DNase I (Turbo DNA-free; Ambion). The real-time PCR was performed as described in ref. 21. Primer sequences are available upon request.
Acknowledgments
We thank Janet DeMayo and Vijayalakshmi Nannegari for their technical help. This work was supported by National Institutes of Health Grant GM-63115 (to S.J.W.).
Abbreviations
- ACC
acetyl-CoA carboxylase
- FAS
fatty acid synthase
- FASKOL
FAS knockout in liver
- HF/HC
high fat/high carbohydrate
- LACC1KO
liver-specific ACC1 knockout
- NEFA
nonesterified fatty acid.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Wakil S. J. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 2.Smith S. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 3.Hillgartner F. B., Salati L. M., Goodridge A. G. Physiol. Rev. 1995;75:47–75. doi: 10.1152/physrev.1995.75.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Girard J., Ferre P., Foufelle F. Annu. Rev. Nutr. 1997;17:325–352. doi: 10.1146/annurev.nutr.17.1.325. [DOI] [PubMed] [Google Scholar]
- 5.Wakil S. J., Stoops J. K., Joshi V. C. Annu. Rev. Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- 6.McGarry J. D., Mannaerts G. P., Foster D. W. J. Clin. Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarry J. D., Brown N. Eur. J. Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 8.Loftus T. M., Jaworsky D. E., Frehywot G. L., Townsend G. L., Ronnett G. V., Lane M. D., Kuhajda F. P. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S., Kim K.-H. Biochem. Biophys. Res. Commun. 1996;229:701–705. doi: 10.1006/bbrc.1996.1868. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Kim K.-H. Cell Signal. 1998;10:35–42. doi: 10.1016/s0898-6568(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 11.Thampy K. G. J. Biol. Chem. 1989;264:17631–17634. [PubMed] [Google Scholar]
- 12.Abu-Elheiga L., Jayakumar A., Baldini A., Chirala S. S., Wakil S. J. Proc. Natl. Acad. Sci. USA. 1995;92:4011–4015. doi: 10.1073/pnas.92.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Elheiga L., Almarza-Ortega D. B., Baldini A., Wakil S. J. J. Biol. Chem. 1997;272:10669–10677. doi: 10.1074/jbc.272.16.10669. [DOI] [PubMed] [Google Scholar]
- 14.Ha H., Lee J.-K., Kim K.-S., Witters L. A., Kim K.-H. Proc. Natl. Acad. Sci. USA. 1996;93:11466–11470. doi: 10.1073/pnas.93.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Casillas F., Bai D.-H., Luo X., Kong I.-S., Hermodson M. A., Kim K. H. Proc. Natl. Acad. Sci. USA. 1988;85:5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takai T., Yokoyama C., Wada K., Tanabe T. J. Biol. Chem. 1988;263:2651–2657. [PubMed] [Google Scholar]
- 17.Abu-Elheiga L., Brinkley W. R., Zhong L., Chirala S. S., Woldegiorgis G., Wakil S. J. Proc. Natl. Acad. Sci. USA. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Elheiga L., Matzuk M. M., Kordari P., Oh W., Shaikenov T., Gu Z., Wakil S. J. Proc. Natl. Acad. Sci. USA. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Elheiga L., Matzuk M. M., Abo-Hashema K. A. H., Wakil S. J. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Elheiga L., Oh W., Kordari P., Wakil S. J. Proc. Natl. Acad. Sci. USA. 2003;100:10207–10212. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh W., Abu-Elheiga L., Kordari P., Shaikenov T., Chirala S. S., Wakil S. J. Proc. Natl. Acad. Sci. USA. 2005;102:1384–1389. doi: 10.1073/pnas.0409451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirala S. S., Chang H., Matzuk M., Abu-Elheiga L., Mao J., Mahon K., Finegold M., Wakil S. J. Proc. Natl. Acad. Sci. USA. 2003;100:6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarthy M. V., Pan Z., Zhu Y., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Browning J. D., Horton J. D. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J., Goldstein J. L., Hammer R. E., Moon Y.-A., Brown M. S., Horton J. D. Proc. Natl. Acad. Sci. USA. 2001;98:13607–13612. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda M., Korn B. S., Hammer R. E., Moon Y.-A., Komuro R., Horton J. D., Goldstein J. L., Brown M. S., Shimomura I. Genes Dev. 2001;15:1206–1216. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuriyama H., Liang G., Engelking L. J., Horton J. D., Goldstein J. L., Brown M. S. Cell Metab. 2005;1:41–51. doi: 10.1016/j.cmet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 28.An J., Muoio D. M., Shiota M., Fujimoto Y., Cline G. W., Shulman G. I., Koves T. R., Stevens R., Millington D., Newgard C. B. Nat. Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 29.Savage D. B., Choi C. S., Samuel V. T., Liu Z. X., Zhang D., Wang A., Zhang X. M., Cline G. W., Yu X. X., Geisler J. G., et al. J. Clin. Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowell P., Hu Z., Lane M. D. Annu. Rev. Biochem. 2005;74:515–534. doi: 10.1146/annurev.biochem.73.011303.074027. [DOI] [PubMed] [Google Scholar]
- 31.Chandler C. E., Wilder D. E., Pettini J. L., Savoy Y. E., Petras S. F., Chang G, Vincent J, Harwood H. J., Jr. J. Lipid Res. 2003;44:1887–1901. doi: 10.1194/jlr.M300094-JLR200. [DOI] [PubMed] [Google Scholar]