Abstract
The major problem in treating excessive eating is high rates of relapse to maladaptive eating habits during diet treatments; this relapse is often induced by stress or anxiety states. Preclinical studies have not explored this clinical problem. Here, we adapted a reinstatement model (commonly used to study relapse to abused drugs) to examine the role of stress and anxiety in relapse to palatable food seeking during dieting. Rats were placed on restricted diet (75–80% of daily standard food) and for 12 intermittent training days (9 h/day, every other day) lever-pressed for palatable food pellets (25% fat, 48% carbohydrate) under a fixed ratio 1 (20-s timeout) reinforcement schedule. Subsequently, the rats were given 10 daily extinction sessions during which lever presses were not reinforced, and were then injected with yohimbine (an α-2 adrenoceptor antagonist that induces stress and anxiety in humans and non-humans) or given a single food pellet to assess reinstatement of food seeking. The rats rapidly learned to lever press for the palatable pellets and across the training days the ratio of timeout nonreinforced lever presses to reinforced lever presses progressively increased more than three-fold, suggesting the development of compulsive eating behavior. After extinction, yohimbine injections and pellet priming reliably reinstated food seeking. The corticotropin-releasing factor1 (CRF1) receptor antagonist antalarmin attenuated the reinstatement induced by yohimbine, but not pellet priming. Antalarmin also reversed yohimbine’s anxiogenic effects in the social interaction test. These data suggest that CRF is involved in stress-induced relapse to palatable food seeking, and that CRF1 antagonists should be considered for the treatment of maladaptive eating habits.
Keywords: corticotropin-releasing factor, extinction, noradrenaline, palatable food, relapse, reinstatement, stress
INTRODUCTION
The major problem in dietary treatment of excessive eating is high rates of relapse to the maladaptive eating habits (Peterson and Mitchell, 1999). This relapse is often induced by stress and anxiety states (Herman and Polivy, 1975; Crowther et al, 2001). Surprisingly, this issue has not yet been explored in preclinical studies, which have focused instead on the effect of stress on ongoing feeding behavior (Morley et al, 1983; Dallman et al, 2003; Hagan et al, 2003). The neuronal mechanisms underlying drug reward are known to overlap with those of food reward (Colantuoni et al, 2002; Volkow and Wise, 2005). Therefore, a preclinical reinstatement model that has been used to study relapse to abused drugs (Stewart, 2000; See, 2002; Shalev et al, 2002) can also be employed to explore mechanisms underlying relapse to maladaptive food-taking behavior. In the reinstatement model, laboratory animals are trained to self-administer drugs and are then given extinction training during which lever presses are not reinforced. Reinstatement of lever responding (the operational measure of drug seeking) is then determined after exposure to drug or non-drug stimuli (de Wit and Stewart, 1981; Stewart and de Wit, 1987; Shaham et al, 2003).
Recent studies using the reinstatement model showed that the α-2 adrenoceptor antagonist yohimbine reinstates cocaine seeking in monkeys (Lee et al, 2004) as well as alcohol and methamphetamine seeking in rats (Shepard et al, 2004; Le et al, 2005). Yohimbine is a commonly used pharmacological stressor that increases brain noradrenaline cell firing (Aghajanian and VanderMaelen, 1982) and release (Abercrombie et al, 1988), and induces stress- and anxiety-like symptoms in both humans and non-humans (Holmberg and Gershon, 1961; Lang and Gershon, 1963; Davis et al, 1979; Charney et al, 1983; Bremner et al, 1996a, b). An important finding from the studies with the methamphetamine- and alcohol-trained rats (Shepard et al, 2004; Le et al, 2005) was that the effect of yohimbine on reinstatement of drug seeking was generally stronger and more robust than that of intermittent footshock. Footshock is a common laboratory stressor (Van Loon et al, 1989) that over the last decade has been used by several investigators to explore the neuronal mechanisms of stress-induced relapse to drug seeking (Shaham et al, 2000a; Le and Shaham, 2002; Lu et al, 2003; Bossert et al, 2005).
Based on the findings of Le et al 2005 and Shepard et al 2004, we used yohimbine as a stressor and adapted the reinstatement model to study stress-induced relapse to palatable food seeking. Rats were placed on a restricted diet (75–80% of their regular standard food) and were trained to lever press for palatable food pellets (25% fat, 48% carbohydrate) for 9 h/day every other day. We chose this training schedule and diet conditions because previous nonoperant food-consumption studies have shown that rats placed on a restricted diet and given intermittent access to palatable food develop binge-like eating behavior (Colantuoni et al, 2002; Corwin and Buda-Levin, 2004) and become hypersensitive to the effect of stress on palatable food intake (Hagan et al, 2002, 2003). After self-administration training, the rats were subjected to extinction training during which lever presses were not reinforced. Reinstatement of extinguished lever responding was then determined after injections of yohimbine or noncontingent exposure to a food pellet, a condition known to reinstate extinguished food responding (de Wit, 1996). We tested our rats under a restricted-diet condition because clinical studies indicate that humans are particularly vulnerable to the effect of stress on feeding behavior when dieting (Herman and Polivy, 1975; Elfhag and Rossner, 2005).
We also explored the role of the stress neurohormone corticotropin-releasing factor (CRF) in yohimbine-induced reinstatement of palatable food seeking by using a selective CRF1 receptor antagonist, antalarmin (Webster et al, 1996). Previous studies reported that systemic injections of a related CRF1 receptor antagonist (CP-154,526) attenuate footshock-stress-induced reinstatement of heroin, cocaine, and alcohol seeking (Shaham et al, 1998; Le et al, 2000). Finally, we used a social-interaction test (File, 1980) to independently verify the stress- and anxiety-like effects of yohimbine and the impact of antalarmin on these effects.
MATERIALS AND METHODS
Subjects and Apparatus
Male Long–Evans rats (Charles River, Raleigh, NC; 300–380 g) were housed in the self-administration chambers for the duration of the experiment under a reverse 12:12-h light–dark cycle (light off at 0930 hours). Rats were kept on a restricted diet of 20 g/day (about 75–80% of their regular daily Purina Rat Chow). The rats’ body weight was taken daily 1–2 h prior to the start of the dark cycle (on two occasions, body weight was not measured due to snowstorms; for these days, body weights were estimated from those of the two preceding and subsequent days). The procedures followed the ‘Principles of laboratory animal care’ (NIH publication no. 85–23). Experiments were conducted in standard self-administration chambers (Med Associates, Georgia, VT). Each chamber had two levers 9 cm above the floor, but only one lever (‘active’, retractable lever) activated the pellet dispenser, which delivered 45-mg food pellets containing 25% fat and 48% carbohydrate (Bioserv, Frenchtown, NJ).
Drugs
Yohimbine HCl (RBI) was dissolved in distilled water and was injected in a volume of 0.5 ml/kg. The yohimbine dose used (2 mg/kg, i.p.) was based on previous work (Shepard et al, 2004; Le et al, 2005) and on a pilot experiment (n = 10) with a longer training period (22 sessions). During the reinstatement tests of this experiment, the number of active lever responses per 3 h were 20 ± 2 (mean ± SEM), 32 ± 5, 53 ± 5, and 64 ± 11, for vehicle and yohimbine doses of 0.5, 1.0 and 2.0 mg/kg, respectively. Antalarmin was synthesized by one of the authors (KCR) and was dissolved just prior to drug injections in sterile saline containing 10% emulphor (pH = 6.0; solutions were heated to 70–80°C) and injected at a volume of 1 ml/kg. The doses of antalarmin (20 and 40 mg/kg, i.p.) are based on published reports (Briscoe et al, 2000; Zorrilla et al, 2002) and on previous studies using the CRF1 antagonist CP-154,526 (Shaham et al, 1998; Le et al, 2000).
Procedures
Experiment 1: reinstatement.
The experiment included three phases: pellet self-administration training (24 days), extinction (10 days), and reinstatement tests (6 days).
Self-administration.
Rats were given one 6-h daily session of ‘autoshaping’ during which pellets were administered noncontingently every 5 min into a receptacle located near the active lever. Pellet delivery was accompanied by a 5-s tone-light cue. Subsequently, over a period of 24 days, the rats were trained every other day for 9 h/day (three 3-h sessions separated by 1 h) on a fixed ratio 1 (20-s timeout) schedule to self-administer the pellets. At the start of each session, the houselight was turned on and the active lever was extended. Following each pellet delivery, the tone-light cue was turned on for 5 s. At the end of each session, the houselight was turned off and the active lever retracted. During the training days, regular food (20 g) was given immediately after the first 3-h daily session, which started at 1000 hours; during the off days, the 20 g regular food was given at the start of the dark cycle. Owing to experimenter error, on the 5th training day, the regular food was given after the end of the second 3-h training session; the data for the second and third 3-h sessions of that day were estimated from the corresponding values of the previous and subsequent training days.
Extinction.
After training, the rats were given 10 daily extinction sessions. The experimental conditions were identical to those in training, except that responses did not lead to pellet delivery. Initially, the rats were given three 3-h sessions (separated by 1 h) each day for 6 days. Subsequently, 3-h extinction sessions were given daily for 4 days, during which the rats were given daily injections of the yohimbine vehicle and the antalarmin vehicle in order to habituate them to the injection procedure. During the extinction phase, the regular food (20 g) was given immediately after the first 3-h daily session, which started at 1000 hours.
Tests for reinstatement.
Initially, we tested the effect of antalarmin on yohimbine-induced reinstatement in two tests that were conducted 48-h apart under extinction conditions. On the intervening day, a regular extinction session was conducted. We used a factorial design, with the between-subjects factor of antalarmin dose (0, 20, or 40 mg/kg) and the within-subjects factor of yohimbine dose (0 and 2.0 mg/kg). The rats were randomly assigned to the antalarmin dose groups. Yohimbine or its vehicle was injected 30 min before the test sessions, and antalarmin or its vehicle was injected 30 min before yohimbine. The order of yohimbine and its vehicle was counterbalanced. Subsequently, the rats were given an additional extinction session, and 1 day after that, we examined the effect of antalarmin on pellet-induced reinstatement. A pellet was delivered noncontingently at the beginning of this 3-h test session. This reinstatement test was also conducted under extinction conditions, and antalarmin or its vehicle was injected 30–60 min before the start of the session. During the testing phase, regular food (20 g) was given immediately after the 3-h daily sessions, which started at 1000 hours.
Experiment 2: social interaction.
The social-interaction test is sensitive to the anxiogenic and anxiolytic effects of drugs (File and Seth, 2003). Exposure to stressors decreases social interaction in rats (Morilak et al, 2003), and this effect is reversed by CRF1 receptor antagonists (Gehlert et al, 2005). We used the social-interaction test to independently verify that under our experimental conditions, yohimbine induces stress- and anxiety-like responses that are reversed by antalarmin. A total of 16 rats previously used in Exp. 1 were placed in locomotor-activity chambers in eight weight-matched pairs. During the 10-min sessions, we scored the time spent in social interaction (grooming, sniffing, following, crawling over/under) and in ‘antisocial’/aggressive interaction (wrestling, distress vocalization, confrontation), using measures derived from previous reports (see Morley and McGregor, 2000). The scorer was blind to the experimental conditions. In the first three baseline sessions, each rat was injected with the vehicles for antalarmin and yohimbine 60 and 30 min, respectively, prior to being placed in the chambers. We used a nested factorial design with the within-subjects factors of antalarmin dose (0 and 20 mg/kg) and yohimbine dose (0 and 2.0 mg/kg) to evaluate the effect of antalarmin on the behavioral effects of yohimbine. Over four 10-min social-interaction sessions, one rat in each pair received antalarmin or its vehicle, as well as yohimbine or its vehicle. The other rat in each pair was not injected. Yohimbine was injected 30 min prior to these sessions, and antalarmin was injected 30 min prior to the injections of yohimbine. The order of the different combinations of the drug doses was counterbalanced.
RESULTS
Experiment 1: Yohimbine- and Pellet-Priming-Induced Reinstatement
Self-administration training and extinction.
During the training phase, the rats (total n = 35) were given 9-h access to the food pellets every other day. They gained weight when pellets were available and lost weight when they were not (Figure 1b and c). A nested repeated-measures ANOVA using pellet availability and training day as the factors and body weight as the dependent measure revealed a significant interaction between these factors (F11,374 = 25.1, P<0.01). This weight fluctuation was not observed during the extinction and reinstatement phases when the pellets were not available (Figure 1b and c). During training, the rats demonstrated reliable pellet self-administration (Figure 2a) with a 3.8-fold increase in pellets earned (F11,374 = 67.0, P<0.01) and a 7.2-fold increase in active lever presses for pellets (F11,374 = 40.4, P<0.01); inactive-lever presses were very low and did not change over time (P>0.1, Figure 2a). As training progressed, the number of nonreinforced lever presses during the 20-s timeout also substantially increased (10.3-fold) over time (F11,374 = 34.5, P<0.01). Thus, the ratio of timeout nonreinforced lever presses to reinforced lever presses (ie pellet earned) increased from 0.8 (day 1) to 2.7 (day 12) (F11,374 = 28.3, P<0.01) in a roughly monotonic fashion (Figure 2a). Lever pressing was extinguished over six 9-h sessions (Figure 2b). A repeated-measures ANOVA revealed that active lever presses decreased significantly over those days (F5,170 = 144.0, P<0.01) and remained low over the subsequent four 3-h sessions (P>0.1).
Figure 1.
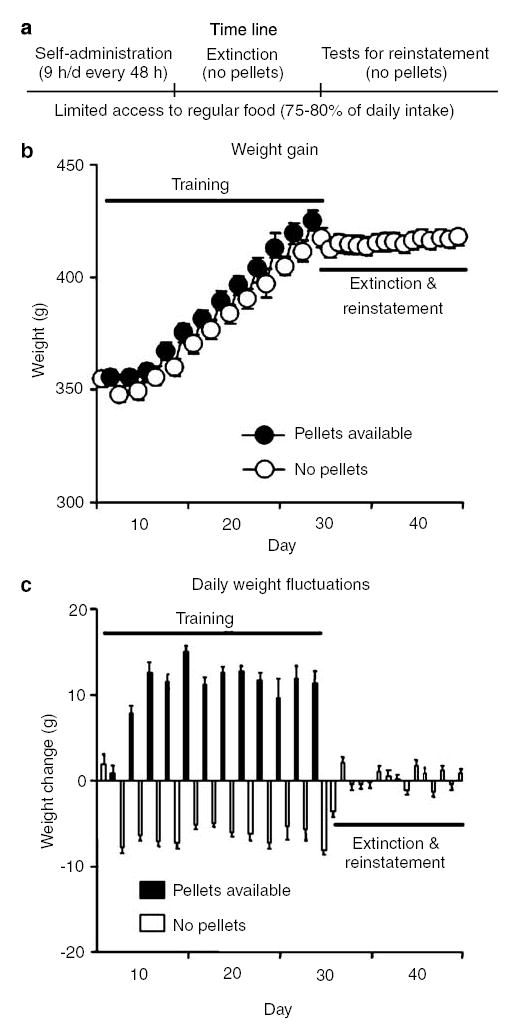
Weight gain and daily weight fluctuations. (a) Timeline of the experiment. (b) Mean ± SEM weight (g) and (c) daily weight fluctuations during the experiment. During training, the palatable food pellets (25% fat, 48% carbohydrate) were available for 9-h/day every other day; the food pellets were not available during the extinction and reinstatement phases (n = 35).
Figure 2.
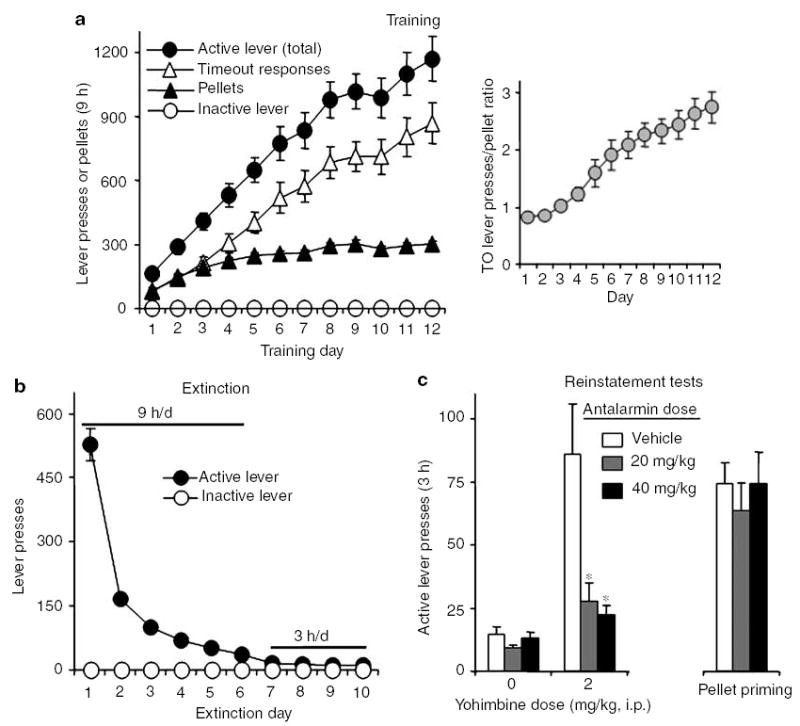
Training of palatable pellet self-administration, extinction, and reinstatement of food seeking. (a) Training: Mean ± SEM number of pellets, total active lever presses (pellet earned + timeout responses), nonreinforced active lever presses during the 20-s timeout after pellet delivery, and inactive lever presses (n = 35). Right column: ratio of timeout nonreinforced lever presses to reinforced lever presses (ie pellets earned) during training (see text). (b) Extinction: Mean ± SEM number of presses on the previously active lever and on the inactive lever during the extinction phase. The session duration was 9 h for sessions 1–6, and 3 h for sessions 7–10 (n = 35). (c) Tests for reinstatement: Mean ± SEM number of nonreinforced active lever presses during testing. Antalarmin or its vehicle was given 60 min before the test sessions and yohimbine or its vehicle was given 30 min before the test sessions. Antalarmin attenuated the reinstatement of lever responding induced by yohimbine, but not the pellet priming (n = 11–12 per antalarmin dose condition). *Different from the antalarmin vehicle condition, P<0.01, post hoc Bonferroni test.
Reinstatement tests.
Antalarmin attenuated reinstatement of lever responding induced by yohimbine, but not pellet priming (Figure 2c). For yohimbine, an ANOVA with antalarmin dose (0, 20, 40 mg/kg) as the between-subjects factor and yohimbine dose (0, 2 mg/kg) as the within-subjects factor revealed a significant interaction between these factors for active (F2,32 = 9.4, P<0.01) but not inactive (P>0.05) lever responses. For pellet priming, the analysis included the between-subjects factor of antalarmin dose and the within-subjects factor of pellet priming (priming, no priming); the data for the no-priming condition were derived from the session in which the three groups were pretreated with antalarmin or its vehicle and were injected with the yohimbine vehicle. This analysis revealed a main effect of pellet priming (F1,32 = 99.7, P<0.01); the effect of antalarmin dose and the priming × dose interaction were not significant. Finally, responding on the inactive lever was very low (less than three presses per 3 h) and was not altered by the experimental manipulations (data not shown).
Experiment 2: Social Interaction
Yohimbine decreased social behavior and profoundly increased ‘antisocial’/aggressive behavior; these effects were reversed by the low dose of antalarmin (Figure 3). For social behavior, repeated-measures ANOVA with yohimbine dose and antalarmin dose as the within-subject factors revealed significant effects of yohimbine dose (F1,7 = 18.0, P<0.01) and antalarmin dose (F1,7 = 8.6, P<0.05). For ‘antisocial’/aggressive behavior, the analysis revealed significant effects of yohimbine dose (F1,7 = 30.8, P<0.01) and antalarmin dose (F1,7 = 19.1, P<0.01), and a significant interaction (F 1,7 = 21.6, P<0.01).
Figure 3.
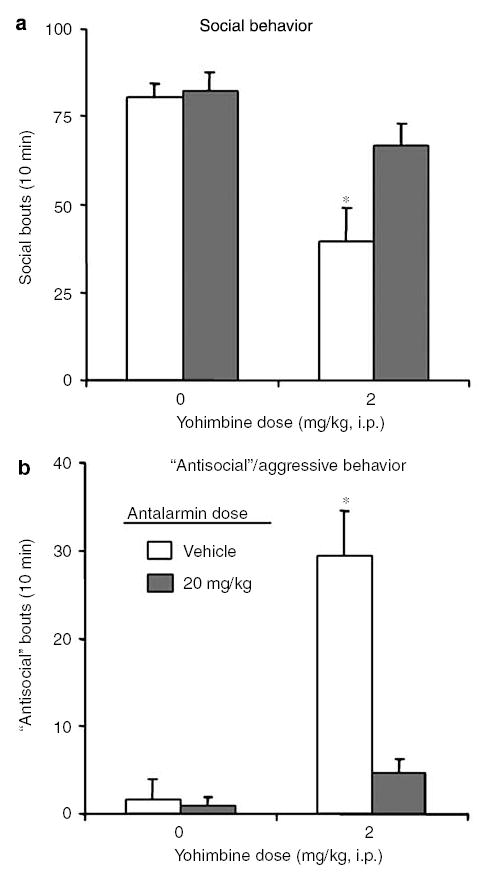
Effects of antalarmin and yohimbine on social interaction. (a) Social behavior: Mean ± SEM occurrences of social behavior (grooming, sniffing, or crawling together) per 10 min. (b) ‘Antisocial’/aggressive behavior: Mean ± SEM occurrences of aggressive/’antisocial’ behavior (confrontation, distress vocalizations, or wrestling) per 10 min. Yohimbine decreased social behavior (a) and increased ‘antisocial’/aggressive behavior (b); these effects were reversed by antalarmin (n = 8 per condition). *Different from all other conditions, P<0.01, post hoc Bonferroni test.
DISCUSSION
Stress and anxiety states can provoke relapse to maladaptive eating habits, and humans are particularly vulnerable to this effect when dieting (Herman and Polivy, 1975; Elfhag and Rossner, 2005). Surprisingly, this significant clinical phenomenon had not been studied in preclinical models. In the present report, we found that injections of yohimbine (which induces stress- and anxiety-like responses in humans and nonhumans) reinstate food seeking in food-restricted rats with a history of intermittent access to palatable food that led to escalation of lever responding for the food. The effect of yohimbine on reinstatement is similar in magnitude to that of acute priming exposure to the palatable food and is not likely due to the induction of hunger, as yohimbine does not increase food consumption in nonoperant procedures (Wellman, 2000). In our initial pharmacological characterization, we found that the CRF1 receptor antagonist antalarmin attenuates yohimbine-induced reinstatement of food seeking and also reverses the stress- and anxiety-like effects of yohimbine on social interaction in an ethologically valid model (File and Seth, 2003). Antalarmin had no effect on pellet-priming-induced reinstatement of food seeking, suggesting that its effect on yohimbine-induced reinstatement is not due to nonselective effects on lever responding or satiety. The selective effect of antalarmin on yohimbine-induced reinstatement of food seeking suggests that different neuronal mechanisms underlie relapse induced by stress vs food stimuli.
Role of Noradrenaline and CRF in Stress-Induced Reinstatement of Food Seeking
We used yohimbine, which induces stress- and anxiety-like symptoms in humans and non-humans (Redmond and Huang, 1979), to examine the role of stress in reinstatement of palatable food seeking. Yohimbine increases central release of noradrenaline, which previous studies have shown to play a critical role in footshock stress-induced reinstatement of drug seeking (Erb et al, 2000; Shaham et al, 2000b; Leri et al, 2002). Thus, we interpret the present data to indicate that brain noradrenaline is involved in yohimbine-induced reinstatement of palatable food seeking. Yohimbine also binds to D2, α-1, 5-HT1a, and benzodiazepine receptors, but, as argued elsewhere (Shepard et al, 2004; Le et al, 2005), it is unlikely that the effect of yohimbine on reinstatement is mediated by these other receptors. In addition, Lee et al 2004 found that the effect of yohimbine on reinstatement of cocaine seeking is blocked by the α-2 adrenoceptor agonist clonidine and mimicked by the selective α-2 adrenoceptor antagonist RS-799948-197.
The main finding in this report is that the CRF1 receptor antagonist antalarmin attenuated the effect of yohimbine on reinstatement of palatable food seeking. In contrast, antalarmin had no effect on pellet-priming-induced reinstatement. This selective effect of antalarmin parallels results from previous studies with rats with a history of heroin or cocaine self-administration, in which ventricular injections of nonselective CRF receptor antagonists attenuated stress-induced but not drug-priming-induced reinstatement of drug seeking (Shaham et al, 1997; Erb et al, 1998). We do not yet know which brain sites mediate the effect of yohimbine on reinstatement of palatable food seeking or the blockade of this effect by antalarmin. One potential site is the bed nucleus of the stria terminalis (BNST). In this brain area, antagonism of either CRF receptors or β adrenoceptors attenuates footshock-stress-induced reinstatement of cocaine seeking (Erb and Stewart, 1999; Erb et al, 2001; Leri et al, 2002). In addition, 6-OHDA lesions of the main input to the BNST, the ventral noradrenergic bundle originating from the lateral tegmental noradrenergic nuclei (Aston-Jones et al, 1999), attenuate footshock-stress-induced reinstatement of heroin seeking (Shaham et al, 2000b); see also Wang et al 2001 for a similar finding using a morphine conditioned place preference model. Another potential brain site is the ventral tegmental area (VTA). Wang et al 2005 recently reported that antagonism of VTA CRF receptors attenuates footshock-stress-induced reinstatement of cocaine seeking. Like the BNST, the VTA receives its main noradrenergic input from the lateral tegmental nuclei (Mejias-Aponte et al, 2004).
Alternatively, antalarmin may attenuate yohimbine-induced reinstatement of palatable food seeking by acting on CRF receptors in the paraventricular nucleus (PVN) of the hypothalamus to prevent release of the stress hormone corticosterone. Previous studies have shown that yohimbine modestly increases corticosterone release (Suemaru et al, 1989), presumably via activation of CRF neurons in the PVN (Plotsky, 1987). Other studies have shown that increased levels of corticosterone can promote palatable food intake in nonoperant procedures (Dallman et al, 2003). However, it is somewhat unlikely that yohimbine’s hypothalamic effects are required for its effects on reinstatement of palatable food seeking. Previous studies have shown that footshock stress can reinstate heroin, cocaine, or alcohol seeking even when footshock-induced increase in corticosterone secretion is prevented by pharmacological or surgical (adrenalectomy) inhibition of corticosterone secretion (Shaham et al, 1997; Erb et al, 1998; Le et al, 2000). The data from these studies suggest that the effect of the CRF receptor antagonists on footshock-induced reinstatement of drug seeking is independent of their effect on the hypothalamic–pituitary–adrenal axis (Shaham et al, 2000a).
A potential caveat in generalizing from previous studies with cocaine, heroin, or alcohol-trained rats to the present work is that those studies used intermittent footshock, a stressor that does not reliably reinstate food seeking (Shaham et al, 2000a). Studies evaluating the effect of footshock on reinstatement of food seeking have been done either with nonpalatable food in deprived rats (Ahmed and Koob, 1997; Mantsch and Goeders, 1999) or with sucrose pellets or solutions in nondeprived rats (Le et al, 1998; Buczek et al, 1999). In both cases, rats were trained for 1–2 h/day, unlike our study where rats were trained 9 h/day every other day. Thus, methodological differences related to the type of food, limited vs extended access, and food-deprivation levels might account for differences between these studies and our study. For example, the effect of yohimbine on reinstatement in food-sated rats is weaker and less reliable than in food-deprived rats (unpublished data).
Progressive Increase in Food-Taking Behavior during Training
During the training phase, food-restricted rats given intermittent access to palatable food, which led to significant fluctuations in body weight (Figure 1), progressively increased their lever responding. It is unlikely that this increase is related to learning per se because early in training the rats clearly discriminated between the active and inactive levers (Figure 2). To our knowledge, this is the first demonstration of progressive escalation of food-taking behavior in an operant model. Our data extend results from nonoperant studies in which food-restricted rats given intermittent access to palatable food progressively increased their food intake, an effect interpreted to indicate the development of binge-eating habits (Colantuoni et al, 2001; Hagan et al, 2002, 2003; Corwin and Buda-Levin, 2004).
An interesting observation in the present study is that while pellet intake increased by less than four-fold over the first 5 days of training and then stabilized, the total number of nonreinforced lever presses during the 20-s timeout period after pellet delivery progressively increased by more than 10-fold over the 12-day training period (Figure 2a). This finding may parallel the results of Deroche-Gamonet et al 2004, who reported that cocaine-trained rats that developed an ‘addictive-like phenotype’ lever-pressed at much higher rates during timeout periods than rats that did not develop this phenotype. These authors argued that the persistence of nonreinforced operant responding, which developed following prolonged exposure to cocaine, reflects the development of compulsive drug-taking behavior, a defining characteristic of drug addiction. Thus, one interpretation of the progressive increases in timeout lever responding during training in our experiment is that the rats developed compulsive food-taking habits under conditions of restricted feeding and intermittent access to the palatable food.
It is also possible that the progressive increase in timeout lever responding was due to time-dependent increases in the incentive value of the cues associated with the intermittent access to the palatable food in hungry rats. During training, a discrete tone-light cue was present for 5 s during the timeout period after each pellet delivery. Such a cue can become a conditioned reinforcer that is likely able to maintain responding in the absence of the primary reinforcer (Catania, 1992). We are not familiar with studies that have systematically evaluated the motivational value of reward cues as a function of the duration of training. However, results from several recent studies indicate that reward seeking induced by cues progressively increases after withdrawal from heroin (Shalev et al, 2001; Di Ciano and Everitt, 2004), cocaine (Neisewander et al, 2000; Grimm et al, 2001; Lu et al, 2005), or sucrose (Grimm et al, 2002; Di Ciano and Everitt, 2004; Lu et al, 2004). Perhaps most relevant here, as we have found progressive within-subject increases in timeout lever responses, is the recent finding of Di Ciano and Everitt (2004). These authors used a within-subject design and reported that over a period of 60 days, the conditioned reinforcing effects of cues paired with sucrose or heroin self-administration progressively increased when the rats were exposed to the cues intermittently. Interestingly, in that study, the rats were maintained on restricted feeding conditions similar to those employed in our study.
Concluding Remarks
We found that the pharmacological stressor yohimbine reinstates palatable food seeking in a preclinical model of relapse. These findings extend the recent observations of Hagan et al 2002, 2003, Dallman and co-workers (Pecoraro et al, 2004), and the earlier reports of Morley et al 1983 on the role of stress in palatable food-taking behavior. We also found that hungry rats that are given intermittent access to palatable food during training escalate their lever responding during nonreinforced timeout periods, suggesting the development of compulsive food-taking behavior and (or) increases in the motivational impact of the food-associated cues. The present findings and experimental procedures may have implications for the understanding of the neurobiological substrates of stress- or anxiety-associated relapse to maladaptive eating in humans. Major progress has been made over the last decade in the understanding of the neuronal and cellular substrates of ongoing feeding behavior in laboratory animals (Kelley and Berridge, 2002; Figlewicz, 2003; Horvath, 2005) and the role of stress in this behavior (Dallman et al, 2003). In contrast, the neuronal and cellular substrates of relapse to palatable-food seeking during periods of abstinence are unknown, despite the fact that relapse is the major clinical issue in the treatment of excessive and unhealthy eating. We hope that the relatively simple experimental procedure described here will facilitate research that advances the understanding of the mechanisms underlying relapse to maladaptive eating and leads to the development of treatment to prevent this relapse. In this regard, our data suggest that CRF1 receptor antagonists, currently in clinical development for the treatment of anxiety, depression, and drug addiction (Kehne and De Lombaert, 2002; Holsboer, 2003; Heidbreder and Hagan, 2005), should also be considered as pharmacological adjuncts in dietary treatments of maladaptive or excessive eating.
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the NIH, the National Institute on Drug Abuse. We thank Dr Diane Lattemann for helpful comments on the manuscript.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. Alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y (2005). Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol (in press). [DOI] [PubMed]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Briscoe RJ, Cabrera CL, Baird TJ, Rice KC, Woods JH. Antalarmin blockade of corticotropin releasing hormone-induced hypertension in rats. Brain Res. 2000;881:204–247. doi: 10.1016/s0006-8993(00)02742-6. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Catania CA (1992). Learning Prentice-Hall: Englewood Cliffs.
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and muopioid receptors in the brain. NeuroReport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Crowther JH, Sanftner J, Bonifazi DZ, Shepherd KL. The role of daily hassles in binge eating. Int J Eat Disord. 2001;29:449–454. doi: 10.1002/eat.1041. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of ‘comfort food’. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4:289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882–R892. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, et al. Stress and central urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of the cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Hagan JJ. Novel pharmacotherapeutic approaches for the treatment of drug addiction and craving. Curr Opin Pharmacol. 2005;5:107–118. doi: 10.1016/j.coph.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84:66–72. [PubMed] [Google Scholar]
- Holmberg G, Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia. 1961;2:93–106. doi: 10.1007/BF00592678. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Corticotropin-releasing hormone modulators and depression. Curr Opin Investig Drugs. 2003;4:46–50. [PubMed] [Google Scholar]
- Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Kehne J, De Lombaert S. Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Targets CNS Neurol Disord. 2002;1:467–493. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch Int Pharmacodyn Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Watchus W, Juzytsch W, Shalev U, Shaham Y. The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzystch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced, but not cocaine-induced reinstatement, by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology. 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Zhu Y, Aston-Jones G. Noradrenegic innervatoin of midbrain dopamine neurons: prominent inputs from A1 and A2 cell groups. Soc Neurosci Abstr. 2004;(No 4654) [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sci. 2003;73:715–726. doi: 10.1016/s0024-3205(03)00392-8. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Rowland NE. Minireview. Stress induced eating. Life Sci. 1983;32:2169–2182. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- Morley KC, McGregor IS. (±)-3,4-methylenedioxymetham-phetamine (MDMA, ‘Ecstasy’) increases social interaction in rats. Eur J Pharmacol. 2000;408:41–49. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Peterson CB, Mitchell JE. Psychosocial and pharmacological treatment of eating disorders: a review of research findings. J Clin Psychol. 1999;55:685–697. doi: 10.1002/(sici)1097-4679(199906)55:6<685::aid-jclp3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Redmond DEJ, Huang YH. Locus coeruleus and anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non peptide antagonist of the corticotropin-releasing factor type 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug-and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H (1987). Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA (ed). Methods of Assessing the Reinforcing Properties of Abused Drugs Springer-Verlag: New York. pp 211–227.
- Suemaru S, Dallman MF, Darlington DN, Cascio CS, Shinsako J. Role of alpha-adrenergic mechanism in effects of morphine on the hypothalamo-pituitary-adrenocortical and cardiovascular systems in the rat. Neuroendocrinology. 1989;49:181–190. doi: 10.1159/000125112. [DOI] [PubMed] [Google Scholar]
- Van Loon GR, Kvetnansky R, McCarty R, Axelrod J (1989). Stress: Neurochemical and Humoral Mechanisms Gordon & Breach Science Publishers S.A.: New York.
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by cortictotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16:837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]


