Abstract
MUC5AC is the main gel-forming mucin expressed by goblet cells of the airways and stomach where it protects the underlying epithelia. We expressed the C-terminal cysteine-rich part of the human MUC5AC mucin in CHO-K1 cells (Chinese-hamster ovary K1 cells) where it formed disulfide-linked dimers in the ER (endoplasmic reticulum). After reducing the disulfide bonds of these dimers, not only the expected monomers were found, but also two smaller fragments, indicating that the protein was partially cleaved. The site of cleavage was located at an Asp–Pro bond situated in a GDPH (Gly-Asp-Pro-His) sequence found in the vWD4 (von Willebrand D4) domain. This sequence is also found in the human MUC2 mucin, previously shown to be cleaved at the same site by a slow, non-enzymatic process triggered by a pH below 6 [Lidell, Johansson and Hansson (2003) J. Biol. Chem. 278, 13944–13951]. In contrast with this, the cleavage of MUC5AC started already in the neutral ER. However, it continued and was slightly accelerated at a pH below 6.5, a pH found in the later parts of the secretory pathway. The cleavage generated a reactive group in the new C-terminus that could link the protein to a primary amine. No cleavage of MUC5AC has so far been reported. By using an antibody reacting with the C-terminal cleavage fragment, we could verify that the cleavage occurs in wild-type MUC5AC produced by HT-29 cells. The cleavage of MUC5AC and the generation of the reactive new C-terminus could contribute to the adherent and viscous mucus found at chronic lung diseases such as asthma and cystic fibrosis, characterized by mucus hypersecretion and lowered pH of the airways.
Keywords: Asp–Pro bond, Chinese-hamster ovary-K1 cell (CHO-K1 cell), cleavage, dimer, MUC5AC, mucin
Abbreviations: AP, alkaline phosphatase; α-His5, anti-His5; α-myc, anti-Myc; B-EDA, biotin ethylenediamine hydrobromide; CF, cystic fibrosis; CHO-K1 cells, Chinese-hamster ovary K1 cells; endo H, endoglycosidase H; ER, endoplasmic reticulum; Ig, immunoglobulin; Goat-α-Mouse-HRP, horseradish peroxidase; H3, heavy chain 3; mAb, monoclonal antibody; NEM, N-ethylmaleimide; SMC, sialomucin complex; vWC, von Willebrand C; vWD, von Willebrand D
INTRODUCTION
Mucins are large glycoproteins found on epithelial surfaces throughout the body. They can be divided into membrane-bound and secreted mucins. Nine membrane-bound (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC16, MUC17 and MUC20) [1–9] and six secreted mucins (MUC2, MUC5B, MUC5AC, MUC6, MUC7 and MUC19) [10–15] have been described so far. The secreted mucins can be further subdivided into gel-forming (MUC2, MUC5B, MUC5AC, MUC6 and MUC19) and non-gel-forming mucins (MUC7). The gel-forming mucins constitute the main structural component of the mucus gel protecting the underlying epithelia. For the mucus gel to exhibit its protective role, it is important for the mucins to have the right properties and alterations in these properties are associated with pathological conditions. An example of this is CF (cystic fibrosis), where the mucus layer, due to dehydration, appears very viscous and sticky [16]. As a consequence, these patients suffer from mucus plugging in their airways, leading to persistent and chronic infections. An event that has been proposed to influence the properties of the mucus gel is proteolytic cleavages of the mucins. For instance, a yet undefined cleavage in the N-terminal region of MUC5B has been proposed to bring the mucin from the gel- into the sol-phase [17]. We have previously studied a cleavage in the GDPH (Gly-Asp-Pro-His) sequence located in the C-terminal cysteine-rich part of the human MUC2 mucin [18]. This cleavage was a relatively slow non-enzymatic process taking place at a pH below 6. Neutralization of the acidic pH of the later parts of the secretory pathway inhibited the cleavage, suggesting that it could occur naturally in this environment. Furthermore, the cleavage resulted in the formation of a reactive anhydride at the newly formed C-terminus, giving the mucin the capacity to covalently link to other molecules. As MUC5AC, the main gel-forming mucin expressed in the goblet cells of the airways and stomach, also contains the GDPH sequence and shows high sequence similarities to MUC2 in the area surrounding this sequence, we hypothesized that the MUC5AC mucin should undergo a similar cleavage. However, no cleavage has previously been reported to occur in the GDPH sequence of MUC5AC. By expressing the C-terminal cysteine-rich part of the human MUC5AC mucin recombinantly in CHO-K1 cells (Chinese-hamster ovary-K1 cells), we show that MUC5AC indeed is partially cleaved in its GDPH sequence. By using an antibody reacting with the C-terminal cleavage fragment, the cleavage could also be verified to occur in wild-type MUC5AC isolated from HT-29 cells. In contrast with the cleavage of MUC2, the MUC5AC cleavage is initiated already in the neutral ER (endoplasmic reticulum). An ER-localized cleavage in the GDPH sequence of the membrane-bound MUC4 mucin was recently suggested to be due to the action of a serine protease since the serine protease inhibitor Pefabloc SC inhibited the cleavage [19]. This inhibitor did not affect the cleavage of MUC5AC, suggesting that no serine protease is involved in the cleavage of this mucin. We also show that the cleavage generates a reactive new C-terminus that has the potential to link the cleaved protein to other molecules.
EXPERIMENTAL
Antisera and antibodies
The α-myc (anti-Myc) mAb (monoclonal antibody) was from spent culture medium of the 1-9E10.2 hybridoma (ATCC CRL-1729). Other antibodies and reagents used were: 62M1 mAb [20], α-His5 (anti-His5) mAb (Qiagen), HRP (horseradish peroxidase)-conjugated goat anti-mouse Igs (Pierce), AP (alkaline phosphatase)-conjugated goat anti-mouse Igs (Southern Biotech), streptavidin–AP (Dako) and the antiserum α-MUC5ACCR (PH1489) raised against the CysD domains of human MUC5AC [21].
Construction and mutagenesis of the plasmid pSM-MUC5AC-CH
L31, a cDNA clone coding for almost the complete C-terminal cysteine-rich part of the human MUC5AC mucin, was obtained from Dr Francisco Real (Universitat Autonoma de Barcelona, Barcelona, Spain) [22]. By using the primer pair, 5′-TATTCTAGAGAAGAGGGCCTGGTGTGCCGGAACCAGGACCAGCAGGGACCCTTCAAG-3′ and 5′-ACGCGCTAGCTCAATGATGATGATGATGGTGCATGGGGGACACTGGGACGCC-3′, the MUC5AC sequence was PCR-amplified and ligated into the XbaI site of the pSM vector [23]. NP3a, a cDNA clone containing a part of the 3′-end of the human MUC5AC, was obtained from Dr Mary Rose (Children's National Medical Center, Washington, DC, U.S.A.) [24]. By using the primer pair, 5′-GCTTCTAGACACGAGAAGACAACCCACTCCC-3′ and 5′-GCGAGGTCTCTGTGGCGGTATATGGTG-3′, the missing 5′-end of the MUC5AC C-terminal cysteine-rich part was amplified and ligated into the previously produced vector by its XbaI/BamHI sites. The final vector named pSM-MUC5AC-CH encodes a signal sequence (S), an Myc tag (M), the complete human MUC5AC C-terminal cysteine-rich part (MUC5AC-C) followed by a His tag (H). In mutational studies, the aspartic residue located in the GDPH sequence of MUC5AC was changed to either an alanine or a glutamic residue by site-directed mutagenesis (QuikChange™; Stratagene) of the pSM-MUC5AC-CH plasmid using the primer pairs: 5′-CAGCGGCTGGGGTGCCCCCCACTACATCAC-3′ and 5′-GTGATGTAGTGGGGGGCACCCCAGCCGCTG-3′, and 5′-CAGCGGCTGGGGTGAGCCCCACTACATCAC-3′ and 5′-GTGATGTAGTGGGGCTCACCCCAGCCGCTG-3′ respectively.
Recombinant expression and tissue culture
The plasmids were transfected into CHO-K1 (ATCC CCL-61) and LS 174T cells (ATCC CL-188), using Lipofectamine™ 2000 (Invitrogen). Permanently expressing CHO-K1 cells were selected by G418 at a final concentration of 250 μg/ml. These cells and the cell line HT-29 (ATCC HTB-38) were cultured as described earlier [25].
Metabolic labelling
Confluent cells were metabolically labelled by pre-incubation in methionine- and cysteine-free Dulbecco's minimal essential medium (BioWhittaker) containing 10% (v/v) fetal bovine serum at 37 °C for 1 h followed by addition of Redivue Pro-mix L-[35S] (Amersham Biosciences) to a final concentration of 72 μCi/ml. After incubation at 37 °C for 6 h, cell lysates and media were prepared. For pulse–chase studies, the labelling mixture was removed after 5 or 15 min and the cells were washed once with PBS and chased with normal cell culture medium with excess of methionine and cysteine (15 and 25 μg/ml respectively).
Preparation of cell lysates and media
Cells washed with ice-cold PBS were lysed in lysis buffer [50 mM Tris/HCl, pH 7.9, 150 mM NaCl and 1% (v/v) Triton X-100] containing protease inhibitors (2× Complete™, EDTA Free; Roche), 5 mM EDTA and 5 mM NEM (N-ethylmaleimide; Sigma–Aldrich), sonicated (intensity 15) for 3×2 s (MSE Soniprep 100 sonifier), and the cell debris was removed by centrifugation (16000 g for 10 min at 4 °C). The medium was supplemented with protease inhibitors, EDTA and NEM (as above) and cleared by centrifugation. When preparing cell lysates from HT-29 cells, the cells were washed and lysed in lysis buffer as above. The lysates were incubated for 1 h at 4 °C before removing cell debris by centrifugation (1000 g for 10 min at 4 °C).
Immunoprecipitation and gel electrophoresis
Immunoprecipitations from the CHO-K1 cells were performed by goat anti-mouse IgG-coupled magnetic beads (Dynabeads; Dynal) precoated with α-myc mAb as described earlier [18]. Immunoprecipitation of wild-type MUC5AC was performed with α-MUC5ACCR antiserum precoated on Protein G–Sepharose beads (Santa Cruz Biotechnology) and the immunocomplexes were washed six times with lysis buffer. Immunoprecipitated material was released in sample buffer [2×; 4% SDS, 125 mM Tris/HCl, pH 6.8, 30% (v/v) glycerol, 5% (v/v) Bromophenol Blue and 200 mM dithiothreitol for reducing gels] with or without 100 mM dithiothreitol for 5 min at 95 °C and analysed by discontinuous SDS/PAGE [26]. After analysis of radiolabelled samples, the gels were fixed and subjected to autoradiography as described earlier [18]. The molecular markers used were the 14C-labelled High-Range Rainbow (Amersham Biosciences) and Precision Protein Standards (Bio-Rad).
Western blotting and immunodetection
The proteins were transferred on to PVDF membranes (Immobilon-PSQ, 0.20 μm; Millipore) as described earlier [18]. After blotting, the membranes were placed in blocking solution and incubated overnight at 4 °C. PBS containing 5% (w/v) milk powder and 0.1% (v/v) Tween 20 was used as blocking solution when using the α-myc mAb and 62 ml of mAb for detection. BSA (2%, w/v) in 10 mM Tris/HCl, 100 mM NaCl and 0.1% (v/v) Tween 20 (pH 7.5) was used as blocking solution when the α-His5 mAb and streptavidin–AP were used for detection. After blocking, the membranes were incubated with α-myc mAb (1 μg/ml), α-His5 mAb (100 ng/ml), 62 ml of mAb (1:1000) or streptavidin–AP (1:1000) in blocking solution for 2 h at room temperature (20 °C). The membranes were washed 3×5 min with PBS-T [PBS containing 0.1% (v/v) Tween 20] and incubated with HRP-conjugated goat anti-mouse Igs (10 ng/ml) or AP-conjugated goat anti-mouse Igs (1:1000) in blocking solution for 1 h at room temperature. The membranes were developed with Supersignal Westpico (Pierce) or NBT (Nitro Blue Tetrazolium)/BCIP (5-bromo-4-chloroindol-3-yl phosphate) (Promega).
Glycosidase and serine protease inhibitor treatments
Immunoprecipitated (using α-myc mAb) cell lysates and media were treated for 15 h at 37 °C, while still attached to the magnetic beads, with endo H (endoglycosidase H; 250 m-units/ml; Roche), O-glycosidase (20 m-units/ml; Roche), neuraminidase (50 m-units/ml; Roche) or a combination of the latter two enzymes, in 90 mM sodium citrate and 4 mM CaCl2 (pH 6.0). For serine protease inhibitor treatment, the cells were starved (1 h) and labelled (4 h) with 200 μM Pefabloc SC (Roche) present.
Neutralization of the secretory pathway
When NH4Cl was used, the cells were pre-incubated (12 h at 37 °C), pretreated (1 h) and radiolabelled (6 h) with 25 mM NH4Cl present in the medium. When bafilomycin A1 (a gift from AstraZeneca, Mölndal, Sweden) was used, it was present at 300 nM during the starvation and labelling steps.
Purification of secreted M-MUC5AC-CH
Spent culture media from CHO-K1-pSM-MUC5AC-CH cells were desalted and the buffer was changed to PBS, using PD-10 columns (Amersham Biosciences). After addition of NaCl to 500 mM, the sample was loaded on to a 1 ml HiTrap Chelating HP column (Amersham Biosciences) charged with Co2+. Following a wash with 20 mM sodium phosphate buffer (pH 7.4) and 500 mM NaCl and another one with 20 mM sodium phosphate buffer (pH 7.4), 500 mM NaCl and 20 mM imidazole, M-MUC5AC-CH was eluted with 20 mM sodium phosphate buffer (pH 7.4), 500 mM NaCl and 200 mM imidazole. The fractions positive for M-MUC5AC-CH were pooled and concentrated and the buffer was changed to PBS by ultrafiltration [Vivaspin concentrator; 30000 MWCO; Vivascience].
Edman sequencing
Purified M-MUC5AC-CH was separated by SDS/PAGE, blotted on to a PVDF membrane and stained with Coomassie Blue [18]. The band corresponding to the C-terminal cleavage fragment was cut out and sequenced on a Procise 492 protein sequencer (Applied Biosystems).
pH-dependence of the cleavage reaction
Aliquots of radiolabelled and α-myc immunoprecipitated material from CHO-K1-pSM-MUC5AC-CH cell lysates and fresh media were treated in citric acid/Na2HPO4 buffers [27] of different pH values for different times at 37 °C before being terminated by the addition of sample buffer.
Linking of a biotinylating agent to the cleavage product
Fresh medium from CHO-K1-pSM-MUC5AC-CH cells was immunoprecipitated with α-myc mAb-coated magnetic beads. Aliquots of the precipitated material were incubated for 1 h at 37 °C in a citric acid/Na2HPO4 buffer (pH 7.2 or 5.4) in the presence of 1.5 μmol of B-EDA (biotin ethylenediamine hydrobromide; Sigma).
RESULTS
Expression of a recombinant MUC5AC C-terminus in CHO-K1 cells
To study the C-terminal cysteine-rich part of the human MUC5AC mucin, a plasmid (pSM-MUC5AC-CH) encoding the last 1041 amino acids of the mucin was constructed. An Myc tag was added to the N-terminal end and a His tag to its C-terminal end and the Igκ-chain signal sequence was used to direct the protein synthesis to the secretory pathway. The construct was transfected into CHO-K1 cells and stable clones were selected for expression of the recombinant protein (M-MUC5AC-CH) (Figure 1A). One of the clones, CHO-K1-pSM-MUC5AC-CH, was used for further studies.
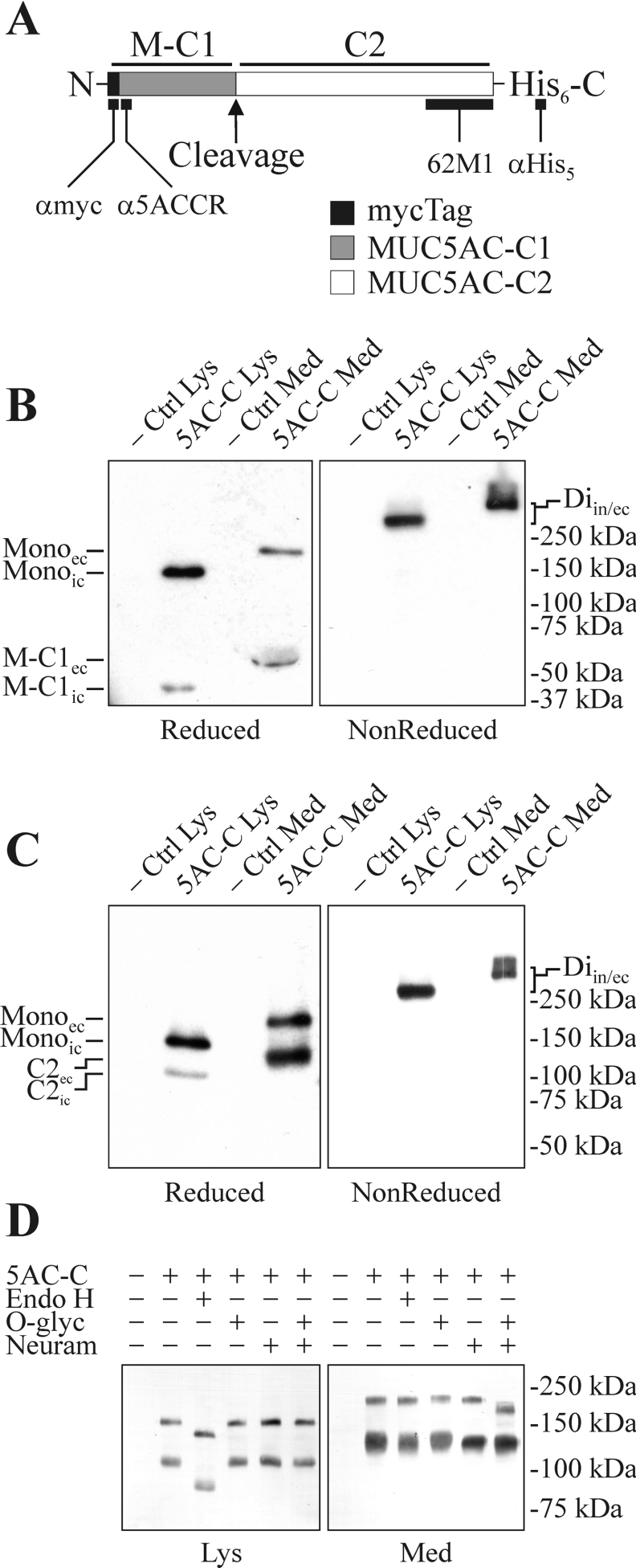
Figure 1. Expression of a recombinant human MUC5AC C-terminus in CHO-K1 cells.
(A) Schematic picture of the recombinant protein containing the C-terminal cysteine-rich part of the human MUC5AC mucin fused to an Igκ-chain signal sequence and a Myc tag in its N-terminus and a His tag in its C-terminus. α-myc, α-5ACCR, 62M1 and α-His5 indicate the localization of the epitopes for the antibodies with the same names. M-C1 and C2 represent the N- and C-terminal cleavage fragments respectively. Cell lysates and spent culture media from CHO-K1-pSM-MUC5AC-CH, a clone of CHO-K1 cells stably expressing the recombinant fusion protein, were analysed by SDS/PAGE (3–10% gels; both reducing and non-reducing conditions) and Western blotting using α-myc mAb (B) and α-His5 mAb (C) for detection. −Ctrl, non-transfected CHO-K1 cells; Di, position of the dimer of the MUC5AC C-terminus; Mono, position of the monomer of the MUC5AC C-terminus. The subscripts ‘ic’ and ‘ec’ indicate the intra- and extra-cellular forms of the protein respectively. In ‘Diin/ec’, the subscript ‘in’ refers to the lanes from cell lysates (Lys) and the subscript ‘ec’ refers to the lanes from the media (Med). (D) Cell lysates and spent cell-culture media from CHO-K1 and CHO-K1-pSM-MUC5AC-CH cells were immunoprecipitated by α-myc mAb-coated magnetic beads and treated with glycosidases or buffer alone for 15 h at 37 °C. The samples were analysed by SDS/PAGE (6% gel; reducing conditions) followed by Western blotting and immunodetection with the α-His5 mAb. O-glyc, O-glycosidase; Neuram, neuraminidase; Lys, material from cell lysates; Med, material from spent cell-culture media. Positions of molecular-mass standards are indicated to the right.
Cell lysates and spent media from the CHO-K1-pSM-MUC5AC-CH cells were analysed by SDS/PAGE and Western blotting using either α-myc mAb (Figure 1B) or α-His5 mAb (Figure 1C) for detection. When the cell lysate was analysed under non-reducing conditions, a single band migrating with an estimated apparent molecular mass of approx. 270 kDa was detected with both antibodies (Diic, Figures 1B and 1C). If the same cell lysate was analysed under reducing conditions, three bands were observed. One band, detected by both antibodies, migrated with an estimated molecular mass of approx. 150 kDa, a size compatible with the monomeric M-MUC5AC-CH (Monoic, Figures 1B and 1C). In addition to this band, the α-myc mAb detected a band migrating at approx. 40 kDa (M-C1ic, Figure 1B), and the α-His5 mAb detected another band at approx. 105 kDa (C2ic, Figure 1C), indicating that the protein was cleaved at one position. The analyses of the spent cell-culture media under non-reducing conditions showed a single band, detected with both antibodies, migrating at approx. 330 kDa (Diec, Figures 1B and 1C). Separation of the media under reducing conditions showed three bands. One band migrating at approx. 190 kDa was detected with both antibodies (Monoec, Figures 1B and 1C), while the other bands migrating at 55 and 130 kDa were detected with the α-myc mAb (M-C1ec, Figure 1B) or α-His5 mAb (C2ec, Figure 1C) respectively. Taken together, these results indicate that the protein forms disulfide-linked dimers and that the protein is partially cleaved at one position. That the cleavage is only observed after reduction shows that the fragments are still held together by disulfide bonds after the cleavage. That the secreted protein migrates with a higher apparent molecular mass compared with the intracellular form is probably due to differences in glycosylation. This has been shown for MUC2, where the intracellular form carried N-glycans of high-mannose type as for ER-localized proteins, while the secreted form contained O-glycans added in the Golgi apparatus [18]. To verify this, M-MUC5AC-CH was immunoprecipitated from cell lysates and spent cell-culture media of the CHO-K1-pSM-MUC5AC-CH cells. The precipitated protein was then treated with glycosidases or buffer alone followed by analysis by SDS/PAGE and Western blotting using the α-His5 mAb for detection (Figure 1D). The results confirm that the intracellular M-MUC5AC-CH contains N-glycans of high-mannose type as the treatment with endo H causes both the intact monomer and C-terminal cleavage fragment to migrate faster. The secreted M-MUC5AC-CH is on the other hand resistant to the endo H treatment. Instead, the secreted form is sensitive to the combined treatment with neuraminidase and O-glycosidase, while the intracellular form is resistant to this treatment. Hence the intracellular form is likely to represent an ER-localized form, while the secreted form has passed this compartment and attained its O-glycans in the Golgi apparatus before secretion. The fact that O-glycosidase alone could not remove the O-glycans is in agreement with the observation that CHO-K1 produces mainly mono- and di-sialylated Gal-GalNAc-O-glycans where the sialic acids make the glycan chain resistant to O-glycosidase treatment [28]. The results thus indicate that the protein, in contrast with the C-terminus of MUC2, can be cleaved early, in the non-acidic parts of the secretory pathway, presumably already in the ER. They also indicate that the disulfide-linked dimers that are formed between wild-type MUC5AC in the ER [21] are formed via their C-terminal cysteine-rich parts.
Pulse–chase studies of cells expressing the recombinant MUC5AC C-terminus
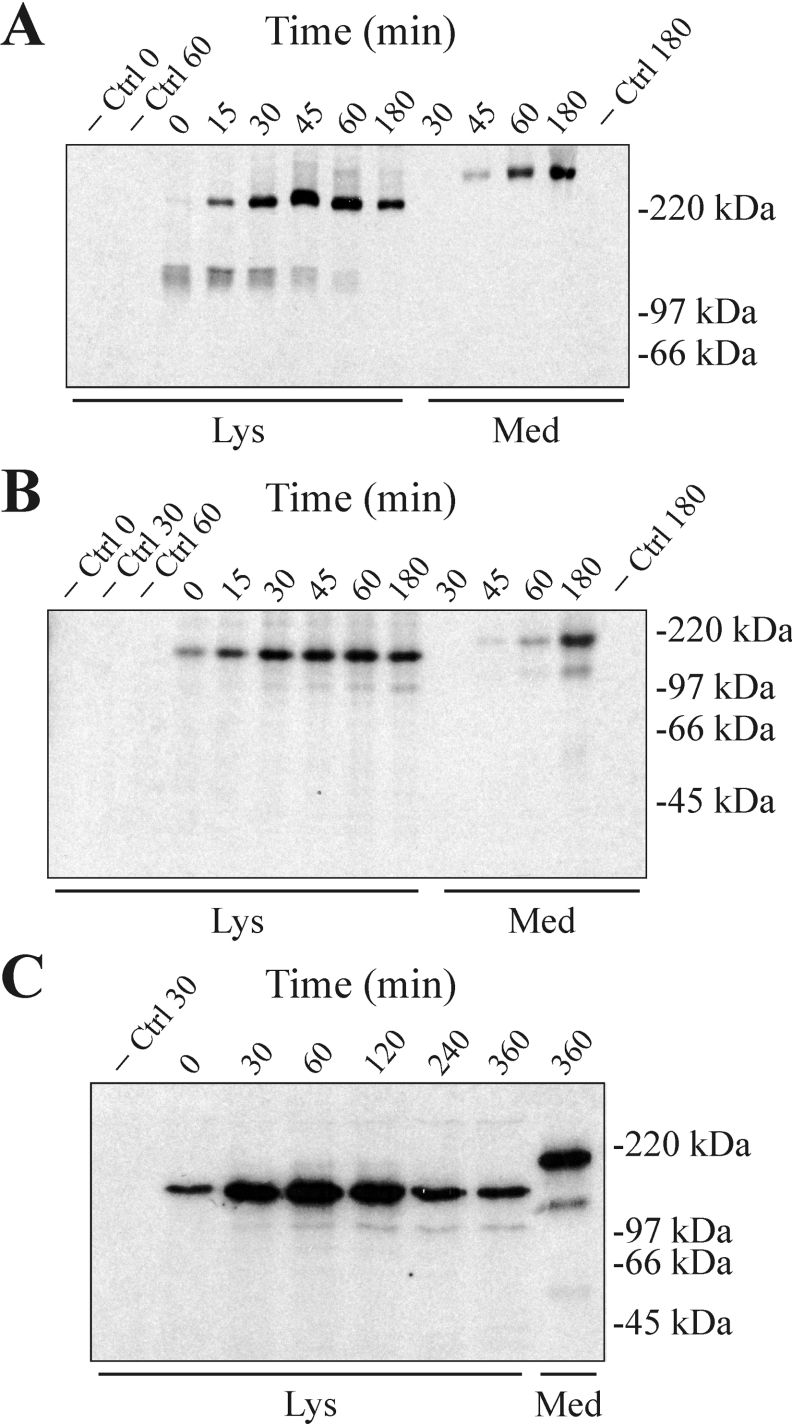
To analyse the assembly of the recombinant MUC5AC C-terminus, pulse–chase studies were performed. When the cell lysates were analysed under non-reducing conditions, the monomer was observed already after the 15 min pulse (Figure 2A). The monomer was rapidly converted into an intracellular dimer (appearing after 15 min) and after 45 min no monomers were detected. The dimer reached its peak between 45 and 60 min when also a smear appeared above it, likely representing more fully glycosylated forms. A band migrating as the fully glycosylated form appeared in the cell-culture media after 45 min. When the analyses were performed under reducing conditions (Figure 2B), the monomer was present in the cell lysate already after the 15 min pulse, and reached a maximum level between 45 and 60 min. After 30 min, a band migrating at approx. 105 kDa (representing the C2ic fragment) appeared in the cell lysate. The reduced monomer emerged in the media after 45 min accompanied by a band migrating as the C2ec fragment (at ∼130 kDa). After longer exposure, the M-C1ec fragment could also be detected in the media (results not shown). The recombinant protein seems to be slow in passing the ER quality control system as ER-localized material is still seen after a 3 h chase. To further investigate this, a pulse–chase study with a shorter pulse (5 min) and longer chase times was performed (Figure 2C). Analysis under reducing conditions indicated that even after 6 h some intracellular MUC5AC C-terminus was detected. While the intracellular monomer reached its highest level after 60 min and then diminished, the C2ic fragment appeared after 30 min and remained at the same level through all the chase times. This suggests that the cleavage occurs late in ER or in a compartment just after the ER, but before trimming of the high-mannose-type glycans, and that the intensity of the C2ic fragment reflects the steady-state level of the protein in this compartment before a more rapid transportation out into the media. It is also noticeable that the amount of cleaved protein seems to be relatively higher in the medium compared with intracellularly, suggesting that further cleavage might occur after the protein has passed the ER.
Figure 2. Biosynthesis of the human MUC5AC C-terminus in CHO-K1 cells.
The CHO-K1 cell line CHO-K1-pSM-MUC5AC-CH, expressing the C-terminal cysteine-rich part of the human MUC5AC was metabolically labelled with Redivue Pro-mix L-[35S] in vitro labelling mixture for 15 min (A, B) or 5 min (C) and chased in non-radioactive medium with excess of methionine and cysteine for different times as indicated above each Figure. Cell lysates and media were prepared and immunoprecipitations, using α-myc mAb-coated magnetic beads, were performed. Samples were analysed by SDS/PAGE (A, C: 3–10% gels; B: 8% gel) under non-reducing (A) or reducing (B, C) conditions followed by autoradiography. −Ctrl, non-transfected CHO-K1 cells; Lys, material from cell lysates; Med, material from spent cell culture media. Positions of molecular-mass standards are indicated to the right.
Localization of the cleavage site by Edman sequencing
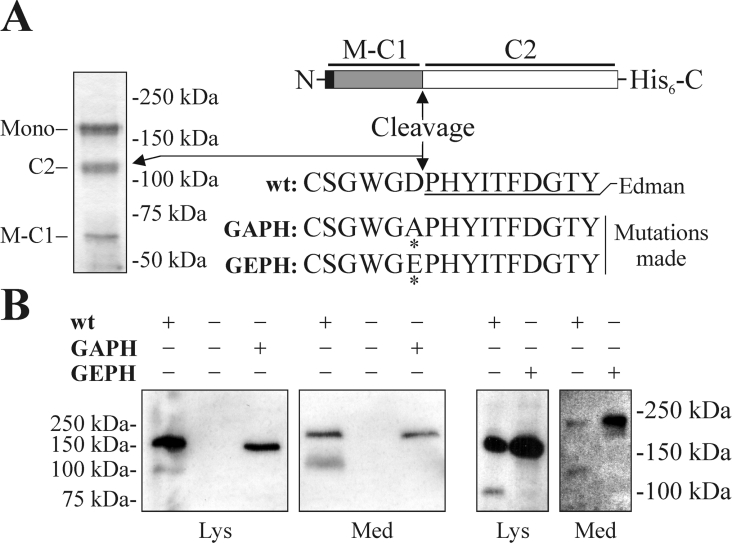
To localize the cleavage site, secreted M-MUC5AC-CH was purified from spent cell-culture media, separated by SDS/PAGE, blotted on to a PVDF membrane and stained with Coomassie (Figure 3A). The band corresponding to the C-terminal cleavage fragment (C2ec) was excised and subjected to Edman sequencing. The results from this analysis gave the N-terminal sequence PHYITFDGTY, thereby confirming that the cleavage had occurred between the aspartic residue and the proline residue in the GDPH sequence.
Figure 3. The human MUC5AC mucin is cleaved in its GDPH sequence.
(A) Secreted MUC5AC C-terminus from CHO-K1-pSM-MUC5AC-CH cells was purified on a HiTrap Chelating HP column. The purified material was separated by SDS/PAGE (3–10% gel; reducing conditions), blotted on to a PVDF membrane and visualized by Coomassie Blue staining. In the schematic picture of the recombinant MUC5AC C-terminus, an arrow indicates the cleavage site, and the underlined sequence shows the sequence obtained after Edman sequencing of the C-terminal cleavage product (C2). The N-terminal cleavage product is indicated by M-C1 and the intact monomer as Mono. The cleavage site was mutated as indicated (changed amino acids are highlighted by asterisks) and the plasmids were expressed in CHO-K1 cells. Cell lysates and spent cell culture media were analysed by SDS/PAGE (GAPH mutation: 8% gel; GEPH mutation: 3–10% gel; both under reducing conditions), Western blotting and immunodetection using the α-His5 mAb (B). Lys, material from cell lysates; Med, material from spent cell culture media.
Site-directed mutagenesis in the GDPH sequence
The importance of an intact GDPH sequence for the cleavage was studied by mutagenesis altering the GDPH sequence to GAPH and GEPH respectively. The mutated plasmids were transfected into CHO-K1 cells and the cell lysates and spent cell culture media were analysed by SDS/PAGE under reducing conditions, followed by Western blotting and detection with the α-His5 mAb (Figure 3B). The results suggest that the aspartic residue in the GDPH sequence is essential for the cleavage as both mutations abolished the cleavage observed in both the cell lysates and media. It is also obvious that the cleavage is not critical for the protein to be properly handled by the secretory machinery, as no alteration in the transit time was observed at pulse–chase (results not shown), and that also mutated M-MUC5AC-CH was secreted to the media (Figure 3B). Thus the results suggest that the aspartic residue in the GDPH sequence is essential for the cleavage and that this event does not influence the transport of the protein through the secretory pathway.
Neutralization of the secretory pathway and treatment with a protease inhibitor
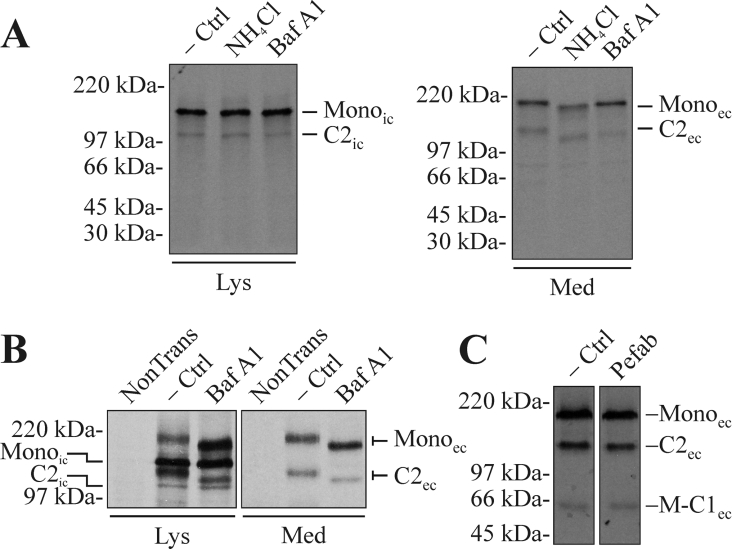
To decipher if the cleavage was influenced by the slightly acidic pH of the late secretory pathway, the CHO-K1-pSM-MUC5AC-CH cells were treated with either ammonium chloride or the proton pump inhibitor bafilomycin A1. Radiolabelled cells and media were immunoprecipitated using α-myc mAb and analysed by SDS/PAGE and autoradiography (Figure 4A). As expected, the cleavage in the ER was not inhibited by either of the treatments as no change in the extent of cleavage was observed in the cell lysates. Neither did the treatments affect the extent of cleavage observed in the media, indicating that the level of cleavage was not affected by the fast passage through the later, more acidic parts of the secretory pathway. From our studies of MUC2, we know that the level of MUC2 cleavage, dependent on the low pH of the late secretory pathway, was higher in the human colon adenocarcinoma cell line LS 174T [18]. To investigate how the recombinant MUC5AC C-terminus is handled by this cell line, these cells where transfected with pSM-MUC5AC-CH, treated with bafilomycin A1 and analysed as above. The results show that neutralization reduces the level of cleavage in the secreted protein (Figure 4B). In this cell line, the fully glycosylated form can also be detected in the cell lysates and the analyses of these not only show that the level of cleavage of the fully glycosylated protein is lower in the treated cells, but also that the ER cleavage is not inhibited (Figure 4B). In both cell lines, one can observe that the mature M-MUC5AC-CH acquires a slightly higher mobility after the treatments. This is in agreement with earlier studies showing that neutralization of the secretory pathway leads to a redistribution of glycosyltransferases and a decrease in O-glycan chain length [29]. Taken together, these studies show that after the initial cleavage in the ER, the extent of cleavage can increase in the later, more acidic parts of the secretory pathway. The fact that the fully glycosylated form can be seen in the LS 174T cells but not in the CHO-K1 cells is likely due to a longer transit time through the post-ER compartments in the LS 174T cells compared with the CHO-K1 cells. This was previously suggested by pulse–chase studies in the two cell lines expressing a C-terminal cysteine-rich domain of MUC2 [18]. The reason for the faster transport through the post-ER compartments in the CHO-K1 cells is not known, but probably reflects cell-specific differences. Due to the sequence similarities between gel-forming mucins and the vWF (von Willebrand factor), it is believed to be the N-terminal parts of the wild-type mucins that direct their transport into the regulated secretory pathway. Hence, one would expect the recombinant C-terminal cysteine-rich domain of MUC5AC to be continuously secreted also from cells with a regulated secretory pathway.
Figure 4. The cleavage in the MUC5AC C-terminus starts early in the secretory pathway, continues in the acidic parts of the late secretory pathway and does not involve serine protease activity.
(A) CHO-K1-pSM-MUC5AC-CH cells were pre-incubated with media with or without 25 mM ammonium chloride for 12 h. After starving the cells for 1 h, they were metabolically labelled with Redivue Pro-mix L-[35S] in vitro labelling mixture for 6 h. Both the starving and labelling steps were performed in the presence of the same concentration of ammonium chloride as during the pre-incubation. In the case where bafilomycin A1 was used as the neutralizing agent, 300 nM bafilomycin A1 was present during the starvation and labelling steps. Cell lysates and media were prepared, immunoprecipitated with α-myc mAb-coated magnetic beads and analysed by SDS/PAGE (3–10%, reducing conditions) and autoradiography. −Ctrl, non-treated cells; NH4Cl, ammonium chloride-treated cells; Baf A1, bafilomycin A1-treated cells. (B) LS 174T cells were transfected with the plasmid encoding the recombinant MUC5AC C-terminus. After 40 h, the cells were treated with bafilomycin A1 and cell lysates and media were immunoprecipitated and analysed as above. NonTrans, non-transfected cells. (C) CHO-K1-pSM-MUC5AC-CH cells were starved (1 h) and labelled (4 h) as above, in the presence of the serine protease inhibitor Pefabloc SC (200 μM). The spent cell-culture media was immunoprecipitated and analysed as above. Pefab, Pefabloc SC-treated cells. Lys, material from cell lysates; Med, material from spent cell-culture media. Mono represents the intact monomer, while M-C1 represents the N-terminal cleavage fragment and C2 the C-terminal one. The subscripts ‘ic’ and ‘ec’ indicate the intra- and extra-cellular forms of the protein respectively. Positions of molecular-mass standards are indicated to the left.
The human MUC4 is a membrane-bound mucin that also contains a GDPH sequence located in a vWD (von Willebrand D) domain. This mucin is also cleaved at the Asp–Pro bond in the ER and it has recently been proposed that the cleavage is mediated by a serine protease as the serine protease inhibitor Pefabloc SC reduced the level of cleavage [19]. To decipher if this was the case for the MUC5AC C-terminus, the CHO-K1-pSM-MUC5AC-CH cells were treated with Pefabloc SC. After radiolabelling of the cells in the presence of the inhibitor, the spent culture medium was immunoprecipitated using α-myc mAb-coated beads and analysed by SDS/PAGE and autoradiography (Figure 4C). This inhibitor did not have any effect on the cleavage, indicating that the MUC5AC cleavage is not mediated by a serine protease.
In vitro treatment of the recombinant MUC5AC C-terminus
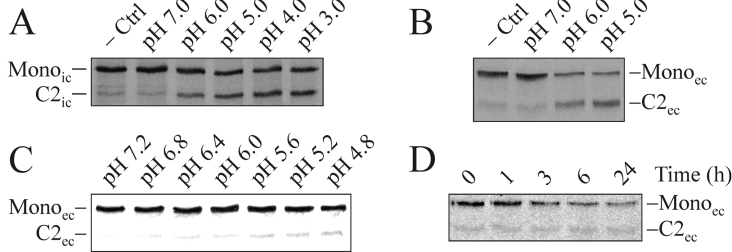
To test the hypothesis of a low pH-dependent cleavage, in addition to the initial ER-localized cleavage, radiolabelled material from CHO-K1-pSM-MUC5AC-CH cell lysates and fresh media was immunoprecipitated using the mAb α-myc. While still attached to the beads, aliquots of the material were treated in buffers with pH from 7.0 to 3.0 for 18 h at 37 °C and analysed by SDS/PAGE and autoradiography (Figures 5A and 5B). An increased level of cleavage was observed at pH 6.0 and below for both the intracellular and extracellular forms of M-MUC5AC-CH. To further pinpoint at what pH the cleavage is triggered, material from fresh media was treated in buffers with pH from 7.2 to 4.8 for 1 h at 37 °C (Figure 5C). The results indicate that the cleavage is triggered already at a pH around 6.4 and that an even lower pH promotes the cleavage. To investigate the time-dependence of the cleavage reaction, aliquots of material from fresh media were incubated at pH 6.5 and 37 °C for different times (0–24 h) (Figure 5D). Although the cleavage increased with time, it was not complete even after 24 h. Together, these results reveal that the cleavage is slow and that it is accelerated by a low pH.
Figure 5. The cleavage of the MUC5AC C-terminus can occur at an acidic pH.
CHO-K1-pSM-MUC5AC-CH cells were metabolically labelled for 6 h and cell lysates and spent cell-culture media were immunoprecipitated with α-myc mAb-coated magnetic beads. Aliquots of the precipitated material from cell lysates (A) and cell-culture media (B–D) were treated in citric acid/Na2HPO4 buffers of different pH for either 18 h (A, B) or 1 h (C) or at pH 6.5 for different times (0–24 h) (D). All incubations were performed at 37 °C with the material still attached to the beads. The treatments were terminated by addition of SDS/PAGE sample buffer before analysis by SDS/PAGE (7% gels; reducing conditions) and autoradiography. −Ctrl, non-treated cells. Mono represents the intact monomer, whereas C2 represents the C-terminal cleavage fragment. The subscripts ‘ic’ and ‘ec’ indicate the intra- and extra-cellular forms of the protein respectively.
A primary amine reacts with the cleaved MUC5AC C-terminus
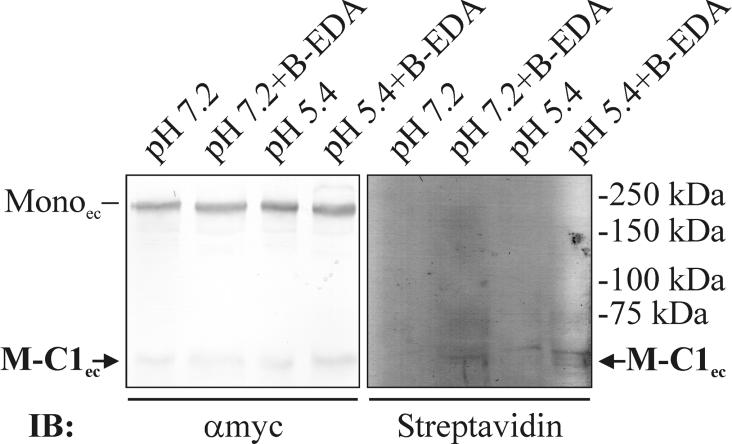
The mechanism for cleavages at Asp–Pro bonds has been proposed to involve the formation of an internal anhydride between the two carboxy groups of the generated C-terminal aspartic residue [30]. Such anhydrides are relatively reactive and can react with, for instance, primary amines or hydroxy groups. In an attempt to show the presence of an anhydride in the new C-terminus generated after MUC5AC cleavage, immunoprecipitated M-MUC5AC-CH from fresh spent cell-culture media of the CHO-K1-pSM-MUC5AC-CH cells was incubated at pH 7.2 or 5.4 (37 °C) with or without the primary amine B-EDA present. Analysis by SDS/PAGE, Western blotting and detection with streptavidin revealed that only one band was specifically stained (Figure 6). It was observed as a weak band in the sample treated at pH 5.4 in the presence of the amine and as a fainter band in the sample treated at pH 7.2 in the presence of the amine. This band migrated at the same position as expected for the N-terminal cleavage fragment (MG-C1ec) as determined by the α-myc mAb staining. That the non-cleaved MUC5AC and the C-terminal fragment were not labelled indicated that the reaction with B-EDA was specific for the N-terminal fragment harbouring the new C-terminus. This suggests that the MUC5AC mucin has a possibility to covalently link to other molecules or itself. An interesting observation is that MG-C1ec linked to the amine also at pH 7.2. This means that M-MUC5AC-CH is further cleaved at pH 7.2 or that an intracellularly cleaved protein maintains its reactive anhydride.
Figure 6. Biotin with a primary amine reacts with the MUC5AC C-terminus cleavage product.
Spent cell-culture medium from CHO-K1-pSM-MUC5AC-CH cells was immunoprecipitated with α-myc mAb-coated magnetic beads. Aliquots of the precipitated material were incubated for 1 h at 37 °C in a citric acid/Na2HPO4 (pH 7.2) buffer, a citric acid/Na2HPO4 (pH 5.4) buffer or in the buffers containing 1.5 μmol of B-EDA. After SDS/PAGE (7% gel; reducing conditions), the proteins were blotted and detected by either the α-myc mAb or streptavidin. Monoec, extracellular monomer of the MUC2 C terminus; M-C1ec, N-terminal cleavage fragment. IB indicates the antibody used for detection. Positions of molecular-mass standards are indicated to the right.
Cleavage of wild-type MUC5AC in HT-29 cells
HT-29 is a human colon adenocarcinoma cell line widely used for studies of the MUC5AC assembly [31,32]. This cell line was used as a source for wild-type MUC5AC to analyse if the cleavage also occurs in vivo. An antiserum raised against the CysD domains of the human MUC5AC mucin was used for immunoprecipitations from cell lysates. After SDS/PAGE under reducing conditions and Western blotting, the mAb 62M1 was used for detection (Figure 7). The epitope of this antibody has been mapped to the far C-terminus of the human MUC5AC mucin including the vWC and cysteine-knot domains [20], and should therefore detect the C2 fragment. The results showed a band migrating as the C2ic fragment from the cell lysates of the CHO-K1-pSM-MUC5AC-CH cells at approx. 100 kDa. Another band, in the interface between the stacking gel and the separation gel, is likely to represent the full-length, non-cleaved MUC5AC mucin. From these results, it is obvious that the wild-type MUC5AC, like the recombinant MUC5AC C-terminus, is partially cleaved and that this cleavage most likely can occur already in the ER.
Figure 7. The cleavage occurs in the wild-type MUC5AC mucin.
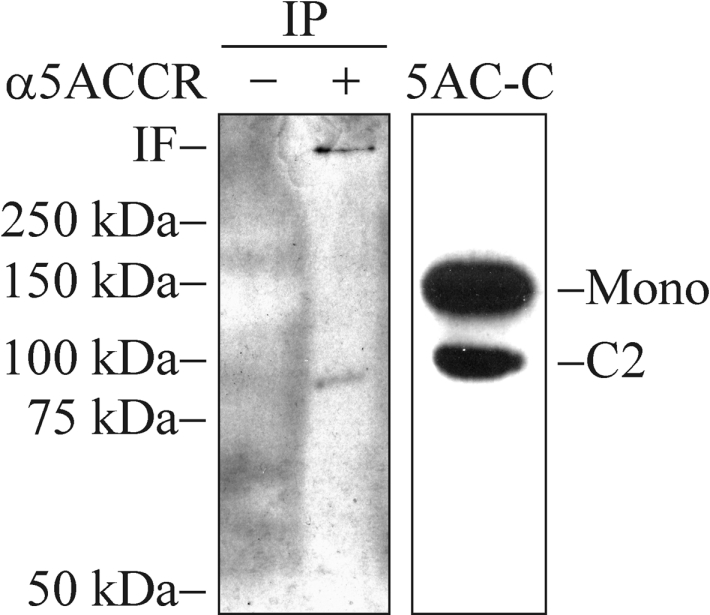
Cell lysates prepared from confluent HT-29 cells were immunoprecipitated with Protein G–Sepharose beads coated with α-MUC5ACCR (+) or beads alone (−). The samples were analysed by SDS/PAGE (6% gel; reducing conditions) and Western blotting using the 62M1 mAb for detection. IF, interface between stacking gel and separation gel; IP, immunoprecipitations; 5AC-C, cell lysate from CHO-K1-pSM-MUC5AC-CH used as a positive control. Positions of molecular mass standards are indicated to the left.
DISCUSSION
The human MUC2 mucin was previously shown to be partially cleaved between the aspartic and proline residues in the GDPH sequence located in the N-terminal end of its vWD4 domain [18]. A GDPH sequence surrounded by similar sequences is found also in the MUC5AC mucin, but not in the other human gel-forming mucins (MUC5B, MUC6 and MUC19). As the MUC5AC mucin is an important structural component in the mucus layer protecting the epithelia of the airways and stomach, we considered it important to address if also MUC5AC is cleaved at the GDPH sequence. Indeed, MUC5AC was also cleaved here, but in contrast with the cleavage of MUC2 that is triggered by a pH below 6.0 [18], the cleavage of MUC5AC was initiated already in the neutral ER. This suggests that these two mucins have different pH set points for cleavage in the GDPH sequence. However, the cleavage of the MUC5AC mucin was accelerated at pH 6.4 or below. The conservative replacement of the aspartic residue with glutamic residue in the GDPH sequence, thus only extending the side chain of the aspartic residue with one carbon, eliminated the cleavage. Interestingly, the mechanism proposed for autocatalytic cleavages at Asp–Pro bonds at low pH involves the formation of an unstable five-membered ring [33] and the aspartic residue to glutamic residue replacement would prevent the formation of such an intermediate.
An interesting observation is that a higher proportion of M-MUC5AC-CH seems to be cleaved at the GDPH site if the protein is purified by affinity chromatography at neutral pH from spent culture media compared with protein directly immunoprecipitated from newly harvested media (results not shown). This suggests that the protein can be further cleaved after prolonged time at a neutral pH. Incubation of the affinity-purified material at low pH does not result in any obvious further cleavage and no linking to B-EDA is observed. This implies that the cleavage reaction can come to completion before the protein has been fully cleaved. Taken together, our results suggest that the GDPH cleavage of MUC5AC is a slow process that occurs at a neutral pH, but is accelerated by a lower pH.
A number of other proteins are cleaved in Asp-Pro sequences. Another mucin that contains a GDPH sequence in a vWD domain is the membrane-bound MUC4 mucin as well as its rat orthologue, the SMC (sialomucin complex). SMC is encoded as a single protein, but is found as a heterodimeric glycoprotein complex of one extracellular and one transmembrane subunit [34,35]. These subunits are formed by an intracellular cleavage at the Asp–Pro bond of its GDPH sequence early during biosynthesis, most likely in the ER [36,37]. In this context, it should just be noticed that also the other transmembrane mucins that have an SEA (sea-urchin sperm protein, enterokinase and agrin) domain instead of a vWD domain are cleaved by an autocatalytic mechanism in the ER [38]. The current observation that MUC5AC is cleaved in the GDPH sequence in the ER could suggest a similar mechanism as for MUC4. It was recently proposed that the cleavage of MUC4 is due to an unidentified serine protease, as the serine protease inhibitor Pefabloc SC inhibited the cleavage [19]. This inhibitor had no effect on the MUC5AC cleavage. This observation together with the fact that MUC4 is completely cleaved suggests that the two proteins are cleaved by different mechanisms. It has also been proposed that the cleavage in MUC4 is essential for the maturation of the protein and that non-cleaved MUC4 is degraded [39]. For MUC5AC, complete inhibition of the cleavage by site-directed mutagenesis of the aspartic residue in the GDPH sequence did not affect its transit time through the secretory pathway. Other proteins known to be cleaved in their GDPH sequences are H3 (heavy chain 3) [33], haemojuvelin [40] and zonadhesin [41], where all these cleavages are triggered by a low pH. In addition, the FrpC protein, a toxin protein from Neisseria meningitidis is also cleaved at an Asp–Pro bond, but in this case calcium triggers the reaction [42].
The suggested mechanism for cleaving Asp–Pro bonds will generate an internal aspartic residue anhydride. This group can react with a hydroxy group to form an ester, with a primary amine to form an amide or just become hydrolysed. The pre-α-inhibitor is a serum protein secreted from the liver as a heterodimer of H3 and bikunin. H3 is synthesized as a precursor that is cleaved at the Asp–Pro in its GDPH sequence when it reaches the acidic compartments of the late secretory pathway [33]. After the autocatalytic cleavage, H3 becomes esterified, via its C-terminal aspartic residue, to C-6 of an internal N-acetylgalactosamine of a glycosaminoglycan chain present on bikunin [43]. The FrpC toxin secreted from N. meningitidis undergoes a calcium-dependent autocatalytic cleavage at an Asp–Pro bond producing a new reactive C-terminus that links to the γ-amino group of a lysine residue on another FrpC molecule [42]. In this way, the FrpC toxin is cross-linked to form high-molecular-mass oligomeric species. So far, MUC5AC has not been proved to be linked to other molecules or itself. However, Sheehan et al. [31] have previously observed a small amount of a reduction-insensitive MUC5AC dimer/oligomer in secretions from HT-29 cells that could be hypothesized to be due to a GDPH-dependent cross-link to itself. Our study also suggests that MUC5AC can link to other molecules after its cleavage as the generated new C-terminus could react with a biotinylated primary amine. The hypothesis that the cleavage at the Asp–Pro bond in MUC5AC gives the mucin the possibility to covalently link to other components is further strengthened by the fact that cleavages at such bonds in other proteins result in adduct formation. However, the size of the mucin, its high number of cysteine residues and especially the glycan heterogeneity all make studies aimed at deciphering the nature of mucin cross-linking or attached molecule very difficult.
When studying the literature, there has so far not been any reports claiming that the human MUC5AC mucin is subjected to cleavage in its C-terminus. Removal of the C-terminal cleavage fragment of wild-type MUC2 does not lead to detectable differences in electrophoretic mobility or chromatographic behaviour [44]. This is likely to be the case also for MUC5AC, suggesting that such a cleavage should be difficult to detect without an antibody detecting the C-terminal cleavage fragment. Such an antibody has so far not been used, but the 62M1 mAb made by the group of Bara [20] and used here will be a good tool in further studies concerning the cleavage of MUC5AC. This mAb was raised against gastric mucin and its epitope has been localized to a part containing the vWC and cysteine-knot domains found in the far C-terminus of the human MUC5AC mucin [20]. The fact that the removal of the 120 kDa C-terminal cleavage fragment of MUC2 does not lead to a detectable difference in electrophoretic mobility or chromatographic behaviour also implies that it is unlikely that one can determine whether, even a large protein, has attached the new C-termini of mucins by these methods.
Apart from the airways, MUC5AC is mainly expressed in the stomach, where it is subjected to a very acidic environment. One could therefore hypothesize that the cleavage would be more pronounced here compared with the airways. It has been shown that there is a pH gradient across the mucus layer of the stomach, the pH in the lumen is very acidic and the pH at the epithelial surface is almost neutral [45]. Hence one can expect that a potential cross-linking would be more pronounced on the luminal side of the mucus gel compared with the epithelial cell surface. An increased cross-linking could contribute to the protection of the underlying epithelial cells, but further studies are necessary to test this hypothesis.
What physiological consequences can a cleavage of MUC5AC have in the airways? Under normal conditions, the MUC5AC mucin is stored in the granulae of the goblet cells for appreciable time. This will not only allow the mucin to be cleaved already in the ER, but also further in the acidic conditions of the secretory granulae. Such prolonged storage should give the anhydride sufficient time to be linked with components found in this compartment or to be hydrolysed. However, in disease states characterized by a faster release of mucins, it can be assumed that the secreted MUC5AC mucins are less cleaved inside the cells. This could allow further extracellular cleavage as we observed for freshly secreted M-MUC5AC-CH. If also the pH is lower in the secretions, the extracellular mucin cleavage will be faster and could have the potential to cross-link the mucin to itself or other components found in the mucus gel or on the cell surface. One such disease is CF where the patients suffer from highly viscous mucus in the airways. Studies indicate that the pH of the mucus is reduced from 7.2 in normal airways down to 6.5 in CF [16]. Advanced asthma and status asthmaticus is also characterized by a decreased pH down to 5.2 compared with 7.6 for healthy individuals [46]. Very condensed mucus and mucus plugging characterize such advanced asthma. It could thus be speculated that such a decrease in pH could lead to an increased cleavage of MUC5AC and cross-links within the mucus gel or to the epithelial surface adding to the mucus phenotype seen in these diseases. However, such a hypothesis has to be evaluated further.
Acknowledgments
We are indebted to Mr Per-Ingvar Olsson (Umeå University, Umeå, Sweden) for help with the Edman sequencing, Drs Francisco Real and Mary Rose for partial cDNA clones and Dr Jacques Bara (Hôpital Saint-Antoine, Paris, France) for the mAb 62M1. This work was supported by the Swedish Research Council (no. 7461) and by IngaBritt and Arne Lundberg's Foundation.
References
- 1.Gendler S., Lancaster C., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 2.Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem. Biophys. Res. Commun. 1990;171:407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- 3.Pratt W. S., Crawley S., Hicks J., Ho J., Nash M., Kim Y. S., Gum J. R., Swallow D. M. Multiple transcripts of MUC3: evidence for two genes, MUC3A and MUC3B. Biochem. Biophys. Res. Commun. 2000;275:916–923. doi: 10.1006/bbrc.2000.3406. [DOI] [PubMed] [Google Scholar]
- 4.Williams S. J., McGuckin M. A., Gotley D. C., Eyre H. J., Sutherland G. R., Antalis T. M. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- 5.Moniaux N., Nollet S., Porchet N., Degand P., Laine A., Aubert J. P. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem. J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- 6.Williams S. J., Wreschner D. H., Tran M., Eyre H. J., Sutherland G. R., McGuckin M. A. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 7.Yin B. W. T., Lloyd K. O. Molecular cloning of the CA125 ovarian cancer antigen. J. Biol. Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 8.Gum J. R., Jr, Crawley S. C., Hicks J. W., Szymkowski D. E., Kim Y. S. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi T., Orita T., Nakanishi S., Katsuya K., Watanabe H., Yamasaki Y., Waga I., Nanayama T., Yamamoto Y., Munger W., et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J. Biol. Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 10.Gum J., Jr, Hicks J., Toribara N., Siddiki B., Kim Y. Molecular cloning of human intestinal mucin (MUC2) cDNA: identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 11.Desseyn J.-L., Buisine M.-P., Porchet N., Aubert J.-P., Laine A. Genomic organization of the human mucin gene MUC5B. J. Biol. Chem. 1998;273:30157–30164. doi: 10.1074/jbc.273.46.30157. [DOI] [PubMed] [Google Scholar]
- 12.Li D., Gallup M., Fan N., Szymkowski D. E., Basbaum C. B. Cloning of the amino-terminal and 5′-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J. Biol. Chem. 1998;273:6812–6820. doi: 10.1074/jbc.273.12.6812. [DOI] [PubMed] [Google Scholar]
- 13.Toribara N., Roberton A., Ho S., Kuo W., Gum E., Hicks J., Gum J., Jr, Byrd J., Siddiki B., Kim Y. Human gastric mucin. Identification of a unique species by expression cloning. J. Biol. Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- 14.Bobek L., Tsai H., Biesbrock A., Levine M. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7) J. Biol. Chem. 1993;268:20563–20569. [PubMed] [Google Scholar]
- 15.Chen Y., Zhao Y. H., Kalaslavadi T. B., Hamati E., Nehrke K., Le A. D., Ann D. K., Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am. J. Respir. Cell Mol. Biol. 2004;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Vilar J., Boucher R. C. Reevaluating gel-forming mucins' roles in cystic fibrosis lung disease. Free Radical Biol. Med. 2004;37:1564–1577. doi: 10.1016/j.freeradbiomed.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Wickstrom C., Carlstedt I. N-terminal cleavage of the salivary MUC5B mucin. J. Biol. Chem. 2001;276:47116–47121. doi: 10.1074/jbc.M106593200. [DOI] [PubMed] [Google Scholar]
- 18.Lidell M. E., Johansson M. E. V., Hansson G. C. An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J. Biol. Chem. 2003;278:13944–13951. doi: 10.1074/jbc.M210069200. [DOI] [PubMed] [Google Scholar]
- 19.Soto P., Zhang J., Carraway K. L. Enzymatic cleavage as a processing step in the maturation of Muc4/sialomucin complex. J. Cell. Biochem. 2005;97:1267–1274. doi: 10.1002/jcb.20718. [DOI] [PubMed] [Google Scholar]
- 20.Nollet S., Forgue-Lafitte M. E., Kirkham P., Bara J. Mapping of two new epitopes on the apomucin encoded by MUC5AC gene: expression in normal GI tract and colon tumors. Int. J. Cancer. 2002;99:336–343. doi: 10.1002/ijc.10335. [DOI] [PubMed] [Google Scholar]
- 21.Asker N., Axelsson M. A., Olofsson S. O., Hansson G. C. Human MUC5AC mucin dimerizes in the rough endoplasmic reticulum, similarly to the MUC2 mucin. Biochem. J. 1998;335:381–387. doi: 10.1042/bj3350381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesuffleur T., Roche F., Hill A. S., Lacasa M., Fox M., Swallow D. M., Zweibaum A., Real F. X. Characterization of a mucin cDNA clone isolated from HT-29 mucus-secreting cells. J. Biol. Chem. 1995;270:13665–13673. doi: 10.1074/jbc.270.23.13665. [DOI] [PubMed] [Google Scholar]
- 23.Lidell M. E., Johansson M. E., Morgelin M., Asker N., Gum J. R., Jr, Kim Y. S., Hansson G. C. The recombinant C-terminus of the human MUC2 mucin forms dimers in Chinese-hamster ovary cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem. J. 2003;372:335–345. doi: 10.1042/BJ20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meezaman D., Charles P., Daskal E., Polymeropoulos M., Martin B., Rose M. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5) J. Biol. Chem. 1994;269:12932–12939. [PubMed] [Google Scholar]
- 25.Asker N., Baeckstrom D., Axelsson M. A., Carlstedt I., Hansson G. C. The human MUC2 mucin apoprotein appears to dimerize before O-glycosylation and shares epitopes with the ‘insoluble’ mucin of rat small intestine. Biochem. J. 1995;308:873–880. doi: 10.1042/bj3080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.McIlvaine T. C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921;49:183–186. [Google Scholar]
- 28.Backstrom M., Link T., Olson F. J., Karlsson H., Graham R., Picco G., Burchell J., Taylor-Papadimitriou J., Noll T., Hansson G. C. Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster ovary cells. Biochem. J. 2003;376:677–686. doi: 10.1042/BJ20031130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axelsson M. A., Karlsson N. G., Steel D. M., Ouwendijk J., Nilsson T., Hansson G. C. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 2001;11:633–644. doi: 10.1093/glycob/11.8.633. [DOI] [PubMed] [Google Scholar]
- 30.Piszkiewicz D., Landon M., Smith E. L. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem. Biophys. Res. Commun. 1970;40:1173–1178. doi: 10.1016/0006-291x(70)90918-6. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan J. K., Brazeau C., Kutay S., Pigeon H., Kirkham S., Howard M., Thornton D. J. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem. J. 2000;347:37–44. [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan J. K., Kirkham S., Howard M., Woodman P., Kutay S., Brazeau C., Buckley J., Thornton D. J. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem. 2004;279:15698–15705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- 33.Thuveson M., Fries E. The low pH in trans-Golgi triggers autocatalytic cleavage of pre-α-inhibitor heavy chain precursor. J. Biol. Chem. 2000;275:30996–31000. doi: 10.1074/jbc.M002399200. [DOI] [PubMed] [Google Scholar]
- 34.Sherblom A. P., Buck R. L., Carraway K. L. Purification of the major sialoglycoproteins of 13762 MAT-B1 and MAT-C1 rat ascites mammary adenocarcinoma cells by density gradient centrifugation in cesium chloride and guanidine hydrochloride. J. Biol. Chem. 1980;255:783–790. [PubMed] [Google Scholar]
- 35.Helm R. M., Carraway K. L. Evidence for the association of two cell surface glycoproteins of 13762 mammary ascites tumor cells: concanavalin A-induced redistribution of peanut agglutinin-binding proteins. Exp. Cell Res. 1981;135:418–424. doi: 10.1016/0014-4827(81)90181-6. [DOI] [PubMed] [Google Scholar]
- 36.Sheng Z. Q., Hull S. R., Carraway K. L. Biosynthesis of the cell surface sialomucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high molecular weight precursor. J. Biol. Chem. 1990;265:8505–8510. [PubMed] [Google Scholar]
- 37.Sheng Z., Wu K., Carraway K. L., Fregien N. Molecular cloning of the transmembrane component of the 13762 mammary adenocarcinoma sialomucin complex: a new member of the epidermal growth factor superfamily. J. Biol. Chem. 1992;267:16341–16346. [PubMed] [Google Scholar]
- 38.Macao B., Johansson D. G. A., Hansson G. C., Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 2006;13:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 39.Price-Schiavi S. A., Zhu X., Aquinin R., Carraway K. L. Sialomucin complex (rat Muc4) is regulated by transforming growth factor beta in mammary gland by a novel post-translational mechanism. J. Biol. Chem. 2000;275:17800–17807. doi: 10.1074/jbc.275.23.17800. [DOI] [PubMed] [Google Scholar]
- 40.Zhang A.-S., West A. P., Jr, Wyman A. E., Bjorkman P. J., Enns C. A. Interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. J. Biol. Chem. 2005;280:33885–33894. doi: 10.1074/jbc.M506207200. [DOI] [PubMed] [Google Scholar]
- 41.Bi M., Hickox J. R., Winfrey V. P., Olson G. E., Hardy D. M. Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem. J. 2003;375:477–488. doi: 10.1042/BJ20030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osicka R., Prochazkova K., Sulc M., Linhartova I., Havlicek V., Sebo P. A novel ‘clip-and-link’ activity of repeat in toxin (RTX) proteins from gram-negative pathogens: covalent protein cross-linking by an Asp–Lys isopeptide bond upon calcium-dependent processing at an Asp–Pro bond. J. Biol. Chem. 2004;279:24944–24956. doi: 10.1074/jbc.M314013200. [DOI] [PubMed] [Google Scholar]
- 43.Enghild J. J., Salvesen G., Hefta S. A., Thogersen I. B., Rutherfurd S., Pizzo S. V. Chondroitin 4-sulfate covalently cross-links the chains of the human blood protein pre-α-inhibitor. J. Biol. Chem. 1991;266:747–751. [PubMed] [Google Scholar]
- 44.Herrmann A., Davies J. R., Lindell G., Martensson S., Packer N. H., Swallow D. M., Carlstedt I. Studies on the ‘insoluble’ glycoprotein complex from human colon: identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J. Biol. Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 45.Schade C., Flemstrom G., Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994;107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 46.Hunt J. F., Fang K., Malik R., Snyder A., Malhotra N., Platts-Mills T. A., Gaston B. Endogenous airway acidification: implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]