Abstract
During aging and degeneration, many changes occur in the structure and composition of human cartilaginous tissues, which include the accumulation of the AGE (advanced glycation end-product), pentosidine, in long-lived proteins. In the present study, we investigated the accumulation of pentosidine in constituents of the human IVD (intervertebral disc), i.e. collagen, aggrecan-derived PG (proteoglycan) (A1) and its fractions (A1D1–A1D6) in health and pathology. We found that, after maturity, pentosidine accumulates with age. Over the age range studied, a linear 6-fold increase was observed in pentosidine accumulation for A1 and collagen with respective rates of 0.12 and 0.66 nmol·(g of protein)−1·year−1. Using previously reported protein turnover rate constants (kT) obtained from measurements of the D-isomer of aspartic residue in collagen and aggrecan of human IVD, we could calculate the pentosidine formation rate constants (kF) for these constituents [Sivan, Tsitron, Wachtel, Roughley, Sakkee, van der Ham, DeGroot, Roberts and Maroudas (2006) J. Biol. Chem. 281, 13009–13014; Tsitron (2006) MSc Thesis, Technion-Israel Institute of Technology, Haifa, Israel]. In spite of the comparable formation rate constants obtained for A1D1 and collagen [1.81±0.25 compared with 3.71±0.26 μmol of pentosidine·(mol of lysine)−1·year−1 respectively], the higher pentosidine accumulation in collagen is consistent with its slower turnover (0.005 year−1 compared with 0.134 year−1 for A1D1). Pentosidine accumulation increased with decreasing buoyant density and decreasing turnover of the proteins from the most glycosaminoglycan-rich PG components (A1D1) to the least (A1D6), with respective kF values of 1.81±0.25 and 3.18±0.37 μmol of pentosidine·(mol of lysine)−1·year−1. We concluded that protein turnover is an important determinant of pentosidine accumulation in aggrecan and collagen of human IVD, as was found for articular cartilage. Correlation of pentosidine accumulation with protein half-life in both normal and degenerate discs further supports this finding.
Keywords: aggrecan, collagen, degeneration, intervertebral disc, non-enzymatic glycation, turnover
Abbreviations: AF, annulus fibrosus; AGE, advanced glycation end-product; BCA, bicinchoninic acid; DMMB, Dimethylmethylene Blue; GAG, glycosaminoglycan; GdnHCl, guanidinium chloride; IVD, intervertebral disc; NP, nucleus pulposus; PG, proteoglycan
INTRODUCTION
The accumulation of age-related intermolecular cross-links is an accompaniment of aging in long-lived proteins [1–4]. One of the pathways for cross-link formation is via a post-translational non-enzymatic glycation, known as the Maillard reaction, resulting in the formation of AGEs (advanced glycation end-products) such as CML [Nϵ-(carboxymethyl)lysine], CEL [Nϵ-(carboxyethyl)lysine] and pentosidine. The formation of pentosidine is initiated by the non-enzymatic condensation of a sugar molecule with the ϵ-amino group of lysine residue or the ϵ-guanidino group of an arginine residue to form a reversible Schiff base adduct, which undergoes further rearrangement to form an Amadori product. This Amadori product undergoes Maillard reactions that result in the formation of AGEs. One of these AGEs, pentosidine, is considered a reliable and well-characterized measure of non-enzymatic glycation in collagen [1,5,6]. The pathway is not regulated and can be influenced by the type and concentration of sugar, the availability of free amino acids on the protein, and by the protein half-life. The cross-link formation is irreversible; thus long-lived proteins such as lens crystallin and collagen are susceptible to AGE accumulation [1,4,6–9].
Differences in AGE levels have been attributed, among other factors, to differences in protein turnover rates [7]. Thus pentosidine levels differ considerably between collagens of different tissues, e.g. articular cartilage and skin [1,7,10], between different constituents of the same tissue, e.g. aggrecan and collagen of articular cartilage [11] as well as between healthy and osteoarthritic tissues [11]. Pentosidine levels increase with decreasing size and buoyant density of the aggrecan monomers, which is attributable to a decrease in protein turnover rates [11]. The correlation between the increase in pentosidine levels and the D/L-isomerization of aspartic residue in articular cartilage, the latter considered as a probe of protein half-life, was taken to suggest that turnover rate is also a major determinant of pentosidine accumulation in the tissue [7].
Collagen of the IVD (intervertebral disc) may be particularly susceptible to cross-linking by glycation due to low oxygen tension that favours these reactions. Indeed, studies by Hormel and Eyre [12] and Yang et al. [13] showed an age-related increase in collagen-associated fluorescence in the IVD, some of which is probably related to the presence of pentosidine. The physiological consequence of these additional cross-links is unknown, but they are expected to affect the biomechanical properties of the tissue. In particular, in articular cartilage, they cause an increase in stiffness as well as a decrease in resilience of the collagen network [13,14]. Therefore the physicochemical characterization of aging in a load-bearing tissue would, of necessity, include the measurement of the accumulation of AGE-related cross-links. In the present study, we have concentrated on pentosidine, one of the three major AGEs found in cartilaginous tissues.
In the present study, pentosidine levels in collagen, aggrecan-derived PG (proteoglycan) (A1) and aggrecan-derived components of different buoyant density (A1D1–A1D6) were studied in both normal and degenerate human IVD as a function of age. The protein turnover rate constants previously obtained from the racemization of aspartic residue in IVD [15,16] were used in order to calculate the pentosidine formation rates for collagen and the various forms of aggrecan. Comparable values for the rate of pentosidine formation were obtained for collagen and aggrecan. Thus steric factors influencing cross-link formation as well as the availability of sugar molecules appear to be similar for both of these IVD constituents. Since a much higher accumulation of pentosidine is found for collagen, this can be attributed to its lower turnover rate [7,15,17]. We also found that pentosidine levels were higher in normal as compared with degenerate tissue, where increased turnover has been reported [18–20].
EXPERIMENTAL
Materials
Caesium chloride (CsCl), chondroitinase ABC (EC 4.2.2.4), Streptomyces hyaluronidase (EC 3.2.1.35), trypsin (EC 3.4.21.4), chondroitin sulfate, glucuronolactone, papain, ϵ-amino-hexanoic acid, benzamidine, N-ethylmaleimide, L-cysteine hydrochloride, pyridoxine, DMMB (Dimethylmethylene Blue) and homoarginine were obtained from Sigma (St. Louis, MO, U.S.A.). Pepsin, EDTA, heptafluorobutyric acid, 9-fluorenylmethyl chloroformate and GdnHCl (guanidinium chloride) were from Fluka (Buchs, Switzerland). BCA (bicinchoninic acid) kit for protein determination was from Pierce (Rockford, IL, U.S.A.). Analytical grade chemicals were used when available.
Tissue sampling
Lumbar and thoracic discs from healthy (ages 22–87 years, n=15) and pathological (ages 30–77 years, n=14) specimens were obtained post mortem or during routine surgical procedures for treatment of herniation or IVD degeneration. In the present study, healthy IVD will be referred to as ‘normal’ and pathological IVD as ‘degenerate’. All discs were divided into NP (nucleus pulposus) and AF (annulus fibrosus), diced or cryosectioned into 20 μm slices and stored at −20 °C until analysed.
Extraction and purification of PG (A1)
The extraction of A1 from NP and AF was carried out for 48 h at 4 °C in 10 vol. of 4 M GdnHCl containing proteinase inhibitors in 50 mM Tris buffer [10 mM N-ethylmaleimide, 25 mM EDTA, 25 mM ϵ-amino-hexanoic acid and 5 mM benzamidine (pH 7.4)] [21,22]. The extracts were clarified by centrifugation at 12000 g for 40 min at 4 °C and exhaustively dialysed against the same buffer containing no GdnHCl. The A1 preparations were separated from other tissue proteins by associative CsCl equilibrium density-gradient centrifugation at a starting density of 1.5 g/ml at 50000 rev./min for 48 h at 10 °C (60.2 Ti) [23]. A1 was then recovered from the densest fraction of the gradient (density greater than 1.59 g/ml). This method should eliminate most of the matrix proteins or PGs that do not interact specifically with hyalyronan, as they will sediment in the lower density fractions. Versican may be present in the A1 preparations, but its abundance is much lower than that of aggrecan in the IVD. All A1 fractions were assayed for density, GAG (glycosaminoglycan) [24] and protein content. The A1 fractions contain a mixture of intact aggrecan and its proteolytic degradation products, some of which are in the form of PG aggregate via their interaction with hyaluronan.
Isolation of aggrecan subfractions (A1D1–A1D6)
A1 fractions were further separated by means of dissociative CsCl density-gradient centrifugation in the presence of 4 M GdnHCl (50 mM Tris buffer, pH 7.4) at a starting density of 1.5 g/ml with centrifugation at 50000 rev./min for 48 h at 10 °C (60.2 Ti), and subsequently fractionated into aggrecan components of decreasing buoyant density (A1D1–A1D6) [17]. All fractions were exhaustively dialysed against distilled water, assayed for density, GAG [24] and protein content and freeze-dried. GAG-rich aggrecan components, including intact aggrecan and its GAG-rich fragments, sediment at higher density than small protein-rich fragments; A1D6 fraction, which sediments at the lowest density, includes also link protein.
In addition, aggrecan subfractions (A1D1–A1D4) from individuals aged 24 and 56 years were recovered from the aggregated portion alone of the A1 fractions. Briefly, the A1 fractions (8–12 mg/ml) were fractionated by gel filtration through a Sepharose CL-2B column (100 cm×2.5 cm) at a flow rate of 30 ml/h using 0.2 M sodium acetate (pH 5.5). Fractions were divided into aggregated PGs eluting at the void volume (A1Vo preparation) and non-aggregated PGs eluting in the included volume (A1Vi1 and A1Vi2 preparations). The aggregated PG preparation was further fractionated to yield the aggrecan subfractions (A1D1–A1D4) by means of dissociative CsCl density-gradient centrifugation in the presence of 4 M GdnHCl at a starting density of 1.5 g/ml. The resulting fractions were assayed for GAG and protein contents.
Determination of GAG content
For determination of sulfated GAG content derived predominantly from aggrecan, 100 μl samples were analysed using the DMMB dye-binding assay [24], with chondroitin sulfate as a standard.
Determination of protein content
The BCA™ Protein Assay (Pierce) based on BCA for the colorimetric detection and quantification of total protein was used. BSA was used as a common standard.
Purification of collagen
Collagen was purified by depleting the tissue of all the PGs and non-collagenous protein using sequential enzymatic treatment with chondroitinase ABC (0.125 unit/ml in 0.05 M Tris buffer/0.06 M sodium acetate, pH 8.0, for 24 h at 37 °C), Streptomyces hyaluronidase (1 unit/ml in 0.05 M Tris buffer/0.15 M NaCl, pH 6.0, for 24 h at 37 °C) and trypsin (1 mg/ml in 0.05 M NaHPO4/0.15 M NaCl, pH 7.2, for 16 h at 37 °C) as previously described by Schmidt et al. [25]. Using this method, over 97% of the non-collagenous molecules are removed from the tissue [6]. Collagen content on a dry tissue basis was determined by hydroxyproline analysis [26] of papain-digested tissue samples by using a conversion factor of 7.6 [27].
Pentosidine and amino acid analysis
Pentosidine and amino acid content were determined by HPLC as previously described [28,29]. Samples were hydrolysed in 6 M HCl at 100 °C for 20–24 h and dried. Samples were then dissolved in an internal standard solution containing 10 μM pyridoxine and 2.4 mM homoarginine. For pentosidine analysis, samples were diluted 5-fold with 0.5% (v/v) heptafluorobutyric acid in 10% (v/v) acetonitrile and analysed by HPLC. An aliquot of the samples used for pentosidine analysis was diluted 50-fold with 1 M sodium borate buffer (pH 11.4) and derivatized with 9-fluorenylmethyl chloroformate for HPLC amino acid analysis. The pentosidine content of the collagen and aggrecan samples is expressed either as nmol/g of protein {assuming a molecular mass of 300 kDa for a triple helical collagen molecule [29], 264 kDa for the aggrecan core protein, and 36 kDa for the G1-domain of aggrecan (the hyaluronan-binding domain in the core protein, through which aggrecan monomers are attached to hyaluronan; this interaction is being stabilized by the presence of a link protein} [30] or as mol of pentosidine/mol of lysine.
Calculation of the rate constant of pentosidine formation
The rate of pentosidine accumulation is controlled by two competing processes: the formation of the pentosidine cross-link on the one hand, and the protein turnover on the other. The equation used to determine the pentosidine formation rate constant is:
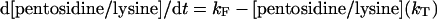 |
(1) |
where [pentosidine/lysine] is the molar ratio of pentosidine to lysine; kF is the rate constant of formation of pentosidine with units year−1; kT is the protein turnover rate constant with units year−1. It is clear that the rate of pentosidine accumulation will increase with increase in the formation rate, and will decrease with increase in the protein turnover rate. Pentosidine formation is influenced by the availability of sugar as well as steric factors that may hinder cross-linking. If two samples have similar pentosidine formation rates, then differences in accumulation will be determined by differences in the protein turnover rate. Details concerning the use of this equation are given in the Supplemental data (at http://www.BiochemJ.org/bj/399/bj3990029add.htm). The values of kT were obtained for collagen [16] and aggrecan [15], using the racemization of aspartic residue as a marker.
Statistical analysis
Linear regression analysis of the age-related pentosidine increase in NP and AF (normal and degenerate) obtained from aggrecan-derived PG (A1) or collagen was performed. The statistical significance of the differences in pentosidine accumulation (i) between normal young and elderly aggrecan subfractions, A1D1–A1D6 and (ii) between aggregated and non-aggregated PGs was analysed using two-factor ANOVA including the Tukey post hoc test and interactions. In all cases studied, P<0.05 was considered to represent a statistically significant difference.
RESULTS
Age-related changes in pentosidine levels in aggrecan and collagen from IVD
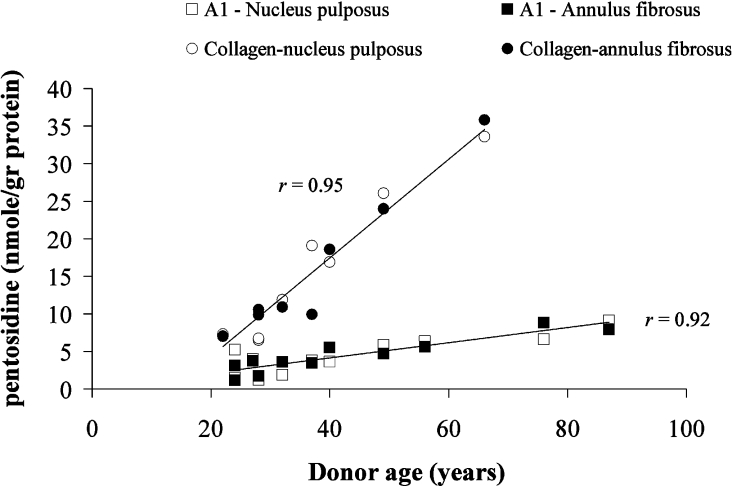
After maturity (>20 years), pentosidine accumulation [expressed as nmol·(g of protein)−1] increases linearly with age in collagen and aggrecan obtained from normal human IVD (Figure 1), a behaviour that is similar to that found for articular cartilage [11]. Over the age range from 22 to 66 years (the value of pentosidine accumulation for aggrecan at the age of 66 years is interpolated from the linear fit equation: Y=0.1X+0.086), a 6-fold increase was observed in pentosidine accumulation for both collagen and aggrecan with respective rates of 0.66 and 0.12 nmol·(g of protein)−1·year−1. Linear regression analysis showed similarity (P>0.05) in pentosidine accumulation with age between NP and AF in both aggrecan and collagen; therefore the linear best-fit lines presented in Figure 1 are based on pooled data from both NP and AF (r=0.92 for aggrecan and r=0.95 for collagen). However, a significant difference in pentosidine accumulation was observed between aggrecan (A1 fraction) and collagen (linear regression analysis, P<0.002).
Figure 1. Pentosidine accumulation in aggrecan (A1) and collagen from human IVD.
Pentosidine accumulation in aggrecan (A1) from NP (□) and AF (■) and in collagen from AF (●) and NP (○) were obtained from normal human IVD as a function of donor age. Data for NP and AF are statistically similar (P>0.05) for both collagen and aggrecan. Linear regression analysis revealed significant difference (P<0.002) in the accumulation of pentosidine in aggrecan as compared with collagen. Since no difference was noted between NP and AF data for collagen or aggrecan (P>0.05), mean correlation coefficients are presented.
Pentosidine levels in aggrecan subfractions (A1D1–A1D6)
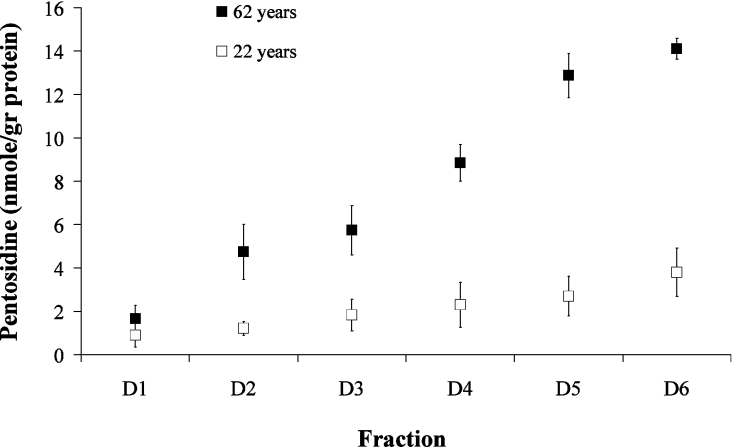
Pentosidine levels in aggrecan subfractions obtained from normal young (22 years) and elderly (62 years) tissue are presented in Figure 2 (results are averages between NP and AF). For the elderly samples, pentosidine levels increase monotonically with decreasing buoyant density of the aggrecan, i.e. from A1D1 to A1D6, suggesting different protein half-lives of these fractions in the tissue; for the young sample, the increase is much more moderate. Using two-factor ANOVA, a statistically significant difference (P<0.02) was noted between young and elderly subfractions, except for A1D1. A comparison of means between the different subfractions (independent of age) revealed significant differences in pentosidine accumulation (P<0.05) only between A1D1 and A1D5–A1D6 and also between A1D2 and A1D6.
Figure 2. Pentosidine accumulation in aggrecan subfractions (A1D1–A1D6).
Pentosidine accumulation was monitored in aggrecan subfractions obtained from young normal (22 years) and elderly normal (62 years) human IVDs. Pentosidine values are the means between NP and AF. Using two-factor ANOVA, a statistically significant difference (P<0.05) was shown between subfractions of young and elderly discs, except for A1D1.
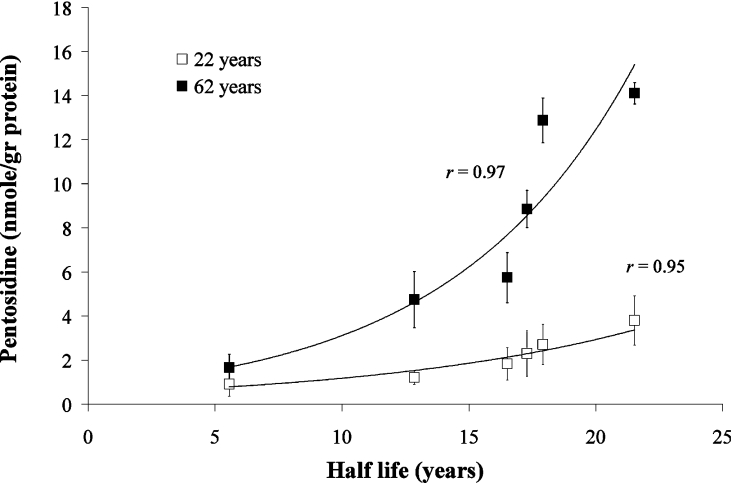
The protein-rich fragments of aggrecan bearing the G1-domain (A1D6) are relatively rich in arginine and lysine residues as compared with more intact aggrecan core proteins [30]. Thus the increase in pentosidine accumulation for the elderly samples could be due to the higher concentration of lysine and arginine, as the proportion of free G1-containing components increases with age. However, correction of pentosidine levels for the lysine content showed that pentosidine levels increase from A1D1 to A1D6 regardless of lysine content [e.g. from 12.5 to 49 μmol of pentosidine·(mol of lysine)−1 for the 62-year-old individual]. The increase in pentosidine could be correlated with the corresponding protein half-life (as determined from the accumulation of D-aspartic residue [15] (Figure 3), suggesting that pentosidine accumulation may be a reliable measure of protein turnover.
Figure 3. Relationship of pentosidine levels in aggrecan subfractions to protein half-life.
Pentosidine levels were correlated with values of protein half-life obtained from measurements of aspartic residue racemization [15] in aggrecan subfractions (A1D1–A1D6) of normal young (22 years) and elderly (62 years) discs.
Pentosidine levels in aggrecan from normal and degenerate IVDs
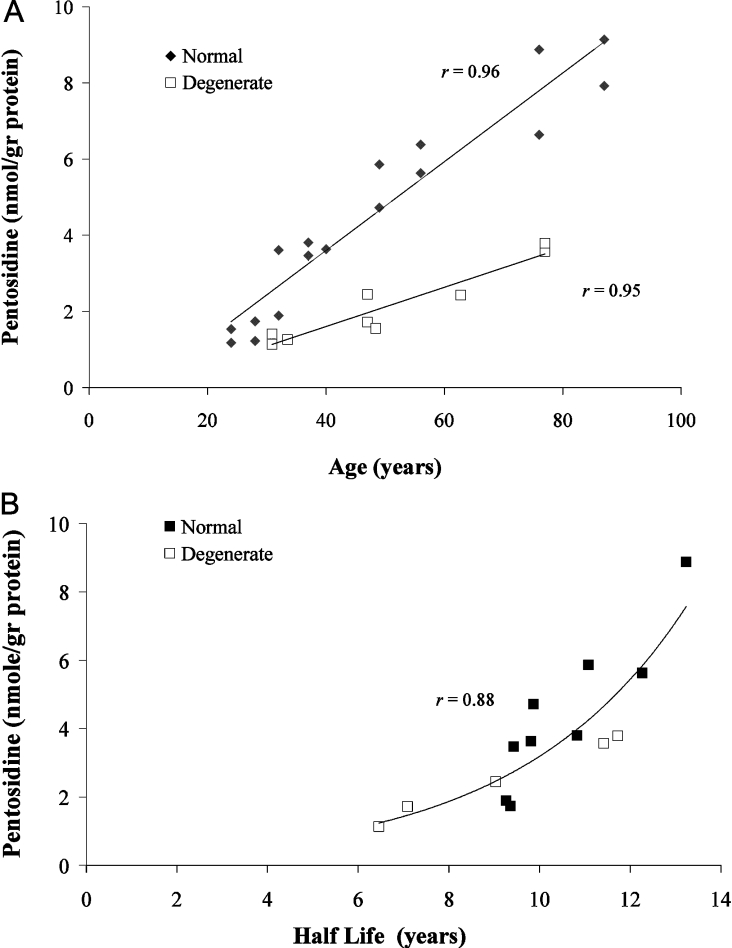
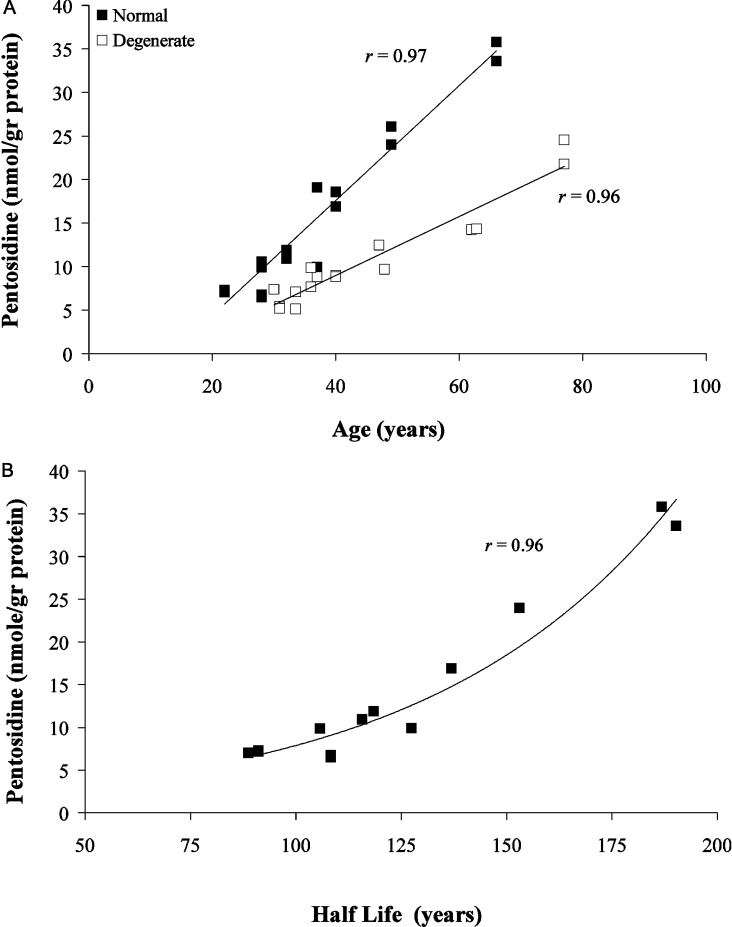
Pentosidine accumulation in aggrecan (A1) obtained from degenerate discs was significantly lower (linear regression, P<0.002) than that obtained from normal ones. In both cases, pentosidine accumulation, expressed as nmol/g of protein, increased linearly with age (r=0.92 for normal aggrecan; r=0.90 for degenerate aggrecan) (Figure 4A). Over the age range 31–77 years, an increase of more than 3-fold was observed in the amount of pentosidine in aggrecan from normal and degenerate tissues, with respective rates of 0.12 and 0.05 nmol·(g of protein)−1·year−1. This suggests that turnover is more rapid in pathological tissue. Correlation of pentosidine accumulation with protein half-life for different ages, both normal and degenerate, supports the idea that turnover is a controlling factor in pentosidine accumulation (Figure 4B). The fact that lower accumulation correlates with shorter half-life suggests that aggrecan from degenerate tissue is ‘younger’. This could be due either to increased synthesis of the intact monomer in degenerate tissue, similar to the case of osteoarthritis in articular cartilage [18,32], or to an increased rate of removal of small aggrecan fragments and link protein from the tissue [20].
Figure 4. Relationship of pentosidine levels in normal and degenerate aggrecan (A1) to (A) donor age and (B) protein half-life.
Values for protein half-life were obtained from the measurements of aspartic residue racemization [15] of aggrecan from normal (◆; ages 28–87 years) and degenerate (□; ages 30–77 years) IVDs.
Pentosidine levels in aggregated and non-aggregated PGs from normal IVD
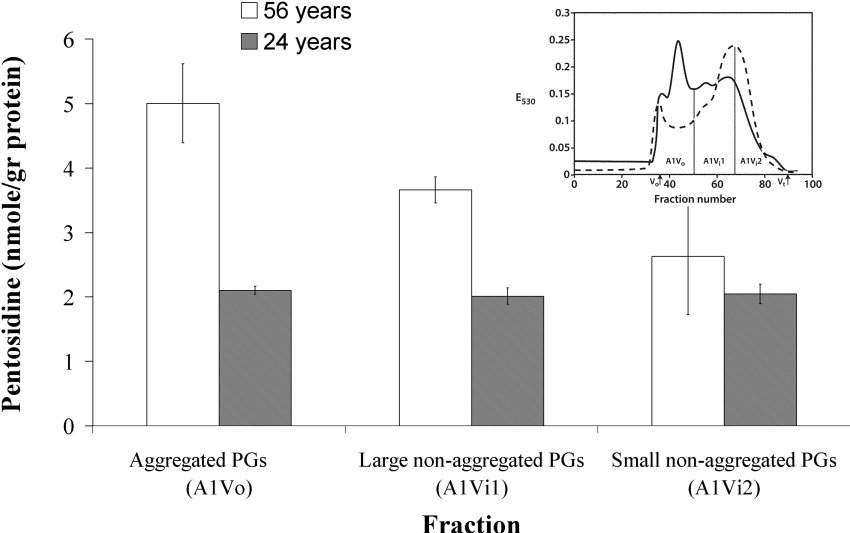
In addition to the dissociative CsCl preparation method, non-denaturing gel permeation chromatography (Sepharose CL-2B) was also employed to separate normal A1 preparations into aggregated and non-aggregated components. Figure 5 (inset) shows a typical elution profile (A1 prepared from a 24-year-old donor) of three fractions representing aggregated PGs (Peak A1Vo) and large and small non-aggregated PGs (peaks A1Vi1 and A1Vi2 respectively). The above fractions were characterized with respect to their pentosidine content. The fact that no significant difference (P>0.05) was observed between pentosidine content of aggregated (A1Vo) and non-aggregated fractions (A1Vi1 and A1Vi2), from the 24-year-old individual (Figure 5), suggests that the non-aggregated PGs were synthesized at the same time as the aggregated PGs and that both PG types exhibit similar retention times in the IVD, as previously found using racemization of aspartic residue as a marker [15]. The fact that the pentosidine content of the non-aggregated PGs is significantly lower (P<0.016) than that of aggregated PGs in the 56-year-old individual implies that some of the non-aggregating PGs are preferentially lost, and it is most likely that these are the smaller fragments with higher amounts of pentosidine. Throughout life, there is probably continual degradation of the non-aggregated PGs and once they reach a critically small size, they will be lost from the disc by diffusion. This implies that the smallest non-aggregated molecules will be derived from the oldest aggrecan molecules and hence will have the highest pentosidine content.
Figure 5. Rates of pentosidine accumulation in aggregated and non-aggregated PGs from human IVD.
Pentosidine accumulation in aggregated (A1Vo) and non-aggregated PGs (A1Vi1 and A1Vi2) obtained from normal young (24 years) and elderly (56 years) human IVDs after gel filtration chromatography through Sepharose CL-2B. The inset in the upper right corner depicts typical elution profiles of A1 preparations from AF (solid line) and NP (dashed line). PG elution was assessed by the DMMB dye-binding assay and monitored by absorbance at 530 nm (E530). The void (Vo) and total (Vt) volumes of the column are indicated, together with the fractions that were pooled to yield A1Vo, A1Vi1 and A1Vi2 preparations. A significant difference (P<0.016) in pentosidine accumulation was observed between aggregated and non-aggregated PGs of 56 years, using Student's t test, whereas no differences were observed for the young tissue.
Pentosidine levels in collagen from normal and degenerate IVDs
The rate of accumulation of pentosidine in collagen was measured to be between 5 and 6 times higher than that in aggrecan (A1) (Figure 1). However, as was also found for aggrecan, levels in degenerate tissue were significantly lower (linear regression, P<0.002) than those measured in normal tissue (Figure 6A). Here also, pentosidine content increases linearly with age (r=0.95 for normal collagen; r=0.92 for degenerate collagen), with over a 5-fold increase for normal collagen as compared with a 3-fold increase in the degenerate collagen over the entire age range. The corresponding rates are 0.7 and 0.3 nmol·(g of protein)−1·year−1, suggesting that, as in the case of aggrecan, turnover is more rapid in pathological tissue. No statistical difference (P>0.05) was found between pentosidine accumulation in NP as compared with AF in both normal and degenerate tissues, in agreement with previous findings for degenerate and scoliotic discs [31]. The relationship between pentosidine accumulation and protein half-life obtained for collagen from normal discs is similar to that obtained for aggrecan (Figure 6B).
Figure 6. Relationship of pentosidine levels in collagen from IVD to (A) donor age and (B) protein half-life.
Values for protein half-life were obtained from the measurements of aspartic residue racemization [16] of collagen obtained from normal (■; ages 22–66 years) and degenerate (□; ages 30–77 years) tissues.
Formation rate of pentosidine in aggrecan and collagen of normal tissue
Pentosidine formation rates (kF) in aggrecan (A1D1), its G1-domain (A1D6) and collagen from normal tissue were calculated using rate constants of protein turnover (kT) obtained previously from measurements of aspartic residue racemization [15,16], as described in the Supplementary data. The pentosidine formation rate constants calculated for aggrecan (A1D1), its G1-domain (A1D6) and collagen, and averaged over all ages, are 1.81±0.25, 3.18±0.37 and 3.71±0.26 μmol of pentosidine·(mol of lysine)−1·year−1 respectively (Table 1). These kF values are comparable with those obtained for aggrecan (A1D1) and collagen from articular cartilage [2.22 and 1.99 μmol of pentosidine·(mol of lysine)−1·year−1 respectively] [11,17], suggesting similar kinetics of non-enzymatic glycation in these tissues.
Table 1. Pentosidine formation and turnover rate constants for collagen, aggrecan (A1D1) and G1-domain (A1D6) obtained from normal human IVD.
The kF values presented here are averages of different sample ages, as explained in the Supplementary data.
| Tissue zone | Fraction | kT (year−1)* | kF [μmol of pentosidine·(mol of lysine) −1·year−1] |
|---|---|---|---|
| NP (normal) | Collagen | 0.0052±0.01 | 3.72±0.26 |
| AF (normal) | 0.0056±0.01 | 3.7±0.27 | |
| NP+AF (normal) | Aggrecan (A1D1) | 0.134±0.042 | 1.81±0.25 |
| G1-domain (A1D6) | 0.033±0.0012 | 3.18±0.37 |
DISCUSSION
Both aging and pathology of the IVD are characterized by changes in composition and metabolism of the tissue constituents. Thus changes occur both in the amounts of aggrecan and collagen protein present as well as in the number of intermolecular cross-links.
Pentosidine is an AGE that accumulates with age [1] in both normal and degenerate discs, as demonstrated here and in previous work [31,33]. Its accumulation is governed primarily by two opposing linear processes, i.e. the rate of pentosidine formation on one hand, and the rate of protein turnover on the other (eqn 1). The availability of sugar molecules as well as steric factors that may hinder cross-linking is implicitly included in the pentosidine formation rate. After maturity, pentosidine accumulates at a lower rate in components with a faster turnover, e.g. aggrecan (A1) and collagen have accumulation rates of 0.12 and 0.66 nmol·(g of protein)−1·year−1 respectively. Similar formation rate constants are calculated here for collagen and aggrecan (A1D1) [3.71±0.26 compared with 1.81±0.25 μmol of pentosidine·(mol of lysine)−1·year−1 respectively]. The higher pentosidine accumulation in collagen is consistent with its lower turnover rate constant [0.005 year−1 as compared with 0.134 year−1 for aggrecan (A1D1)], obtained previously from the racemization of aspartic residue in the same tissue [15,34].
Pentosidine levels in the nucleus and the annulus (inner and outer sections) of both normal and degenerate discs were found to be similar, suggesting that protein turnover rates in the two regions [15,16], as well as availability of the components required for cross-linking, are also similar; this is true for both collagen and aggrecan. In a study carried out on degenerate and scoliotic discs, Duance et al. [31] also found little difference between the levels of pentosidine in collagen of the nucleus and the annulus from human IVD. In all cases, accumulation of pentosidine in degenerate discs was lower as compared with normal tissue [0.05 and 0.12 nmol·(g of protein)−1·year−1 respectively], suggesting that degenerate discs are actually ‘younger’, similar to the findings for osteoarthritic articular cartilage [7]. The 2–3-fold increase in pentosidine accumulation in collagen of degenerate discs between ages 30 and 60 years as measured here is comparable with that found for scoliotic discs in the same age range [31].
An increase in pentosidine accumulation with decreasing buoyant density of aggrecan subfractions (particularly for the elderly samples) suggests differential and decreasing protein turnover rates in these fractions as confirmed by previous studies [7,15]. The difference in age-related pentosidine accumulation between A1 and A1D1 is consistent with the data on aspartic residue racemization, where a clear age-related increase in D-aspartic residue is observed for A1, while no age-related change is observed for A1D1 [15,17]. While the age-averaged rate constant of pentosidine formation (kF) was only calculated to increase from 1.81±0.25 μmol of pentosidine·(mol of lysine)−1·year−1 for A1D1 to 3.18±0.37 μmol of pentosidine·(mol of lysine)−1·year−1 for A1D6, the corresponding protein turnover rate (kT) decreased from 0.134±0.042 to 0.033±0.0012 year−1 from A1D1 to A1D6, thereby explaining the significantly higher pentosidine accumulation in the latter.
It has been suggested that age-related changes in AGEs may alter the physical properties of connective tissues, thus contributing to the disease process itself. However, it is unclear whether the pentosidine itself has any direct influence on the tissue properties, as it is present in minute amounts. There are other AGEs that are present in more substantial amounts and are thought to have a greater influence on the properties of the tissue [35–37].
Pentosidine accumulation, which can be assayed accurately, correlates with protein half-life, suggesting that it may be considered as a reliable, although non-linear, measure for turnover of long-lived proteins in both healthy and degenerate human IVDs. Of course, the accessibility of lysine groups for cross-linking and the concentration of sugar molecules are determinants of the pentosidine formation rate. However, the fact that in the present study we found the rate to be approximately the same, within a factor of two, for both collagen and aggrecan of normal IVD and for all sample ages indicates that there is little variability of these parameters in the tissue. In a previous study [15], an attempt was made to address the question regarding the origin of the small aggregated PGs as well as the non-aggregated PGs in human IVD, using the racemization of aspartic residue as a tool for determining in vivo turnover rates of different PG populations, in both normal and pathological tissues. Our current finding that pentosidine accumulation in the non-aggregated PGs of a normal 24-year-old specimen is similar to that measured for aggregated PGs (P>0.05, Figure 6) and hence may be considered to be of similar molecular age suggests that the non-aggregated PGs were synthesized at the same time as aggregated PGs. This is in accordance with data obtained for the racemization of aspartic residue [15]. As there is no evidence to support the existence of unique gene products with structures similar to the non-aggregated PGs, the data are compatible with the non-aggregated PGs being synthesized as aggregating PGs that were later cleaved by proteolysis. The PG degradation products that were no longer able to interact with hyaluronan then formed the non-aggregated PGs [27]. Retention of such non-aggregating fragments of aggrecan within the extracellular matrix appears to be unique to the IVD and does not occur in articular cartilage. This probably reflects the large size and avascular nature of the disc, which prevents ready loss of large macromolecules by diffusion.
Online data
Acknowledgments
This study was supported by the European Commission, grant no. QLK6-CT-2002-02582 (EURODISC). We acknowledge the enormous contribution of the late Professor Michael Bayliss. His input in scientific as well as human terms will be sadly missed.
References
- 1.Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. J. Biol. Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- 2.Dunn J. A., McCane D. R., Thorpe S. R., Lyons T. J., Baynes J. W. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethylhydroxy)lysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 3.Bank R. A., Bayliss M. T., Lafeber F. P. J. G., Maroudas A., TeKoppele J. M. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biogeochemical properties of cartilage. Biochem. J. 1998;330:345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeGroot J., Verzijl N., Bank R. A., Lafeber F. P. J. G., Bijlsma J. W. J., TeKoppele J. M. Age-related decrease in proteoglycans synthesis of human articular chondrocytes: the role of non-enzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during non-enzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J. Biol. Chem. 1991;266:11654–11660. [PubMed] [Google Scholar]
- 6.Verzijl N., DeGroot J., Oldehinkel E., Bank R. A., Thorpe S. R., Baynes J. W., Bayliss M. T., Bijlsma J. W., Lafeber F. P., Tekoppele J. M. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J. 2000;350:381–387. [PMC free article] [PubMed] [Google Scholar]
- 7.Verzijl N., DeGroot J., Thorpe S. R., Bank R. A., Shaw J. N., Lyons T. J., Bijlsma J. W. J., Lafeber F. P. J. G., Baynes J. W., TeKoppele J. M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 8.Dunn J. A., Patrick J. S., Thorpe S. A., Baynes J. W. Oxidation of glycated proteins: age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989;28:9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- 9.Uchiyama A., Ohishi T., Takahashi M., Kushida K., Inoue T., Fujie M., Horiuchi K. Fluorophores from aging human articular cartilage. J. Biochem. (Tokyo) 1991;110:714–718. doi: 10.1093/oxfordjournals.jbchem.a123646. [DOI] [PubMed] [Google Scholar]
- 10.Monnier V. M., Sell D. R., Nagaraj R. H., Miyata S., Granshee S., Odetti P., Ibrahim S. A. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes. 1992;41:36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 11.Verzijl N., DeGroot J., Bank R. A., Shaw J. N., Bayliss M. T., Bijlsma J. W. J., Lafeber F. P. J. G., Maroudas A., TeKoppele J. M. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 12.Hormel S. E., Eyre D. R. Collagen in the aging intervertebral disc: an increase in covalently bound fluorophores and chromophores. Biochim. Biophys. Acta. 1991;1078:243–250. doi: 10.1016/0167-4838(91)90565-h. [DOI] [PubMed] [Google Scholar]
- 13.Yang C., Mosler S., Rui H., Baetge B., Notbohm H., Muller P. Structural and functional implications of age-related abnormal modifications in collagen II from intervertebral disc. Matrix Biol. 1994;4:643–551. doi: 10.1016/s0945-053x(05)80028-9. [DOI] [PubMed] [Google Scholar]
- 14.Verzijl N., DeGroot J., Ben Zaken C., Braun-Benjamin O., Maroudas A., Bank R. A., Mizrahi J., Schalkwiji C. G., Thorpe S. R., Baynes J. W., et al. Cross-linking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage. A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Sivan S., Tsitron E., Wachtel E., Roughley J., Sakkee N., van der Ham F., DeGroot J., Roberts S., Maroudas A. Aggrecan turnover in human intervertebral disc as determined by the racemization of aspartic acid. J. Biol. Chem. 2006;281:13009–13014. doi: 10.1074/jbc.M600296200. [DOI] [PubMed] [Google Scholar]
- 16.Tsitron E. MSc Thesis. Haifa, Israel: Technion-Israel Institute of Technology; 2006. Molecular age and turnover of proteoglycans and collagen in human intervertebral disc. [Google Scholar]
- 17.Maroudas A., Bayliss M. T., Uchitel-Kaushansky N., Schneiderman R., Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch. Biochem. Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 18.Mankin H. J., Dorfman H., Lipiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 19.Thompson R. C. J., Oegema T. R. J. Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J. Bone Joint Surg. Am. 1979;61:407–416. [PubMed] [Google Scholar]
- 20.Lafeber F. P., van Roy H., Wilbrink B., Huber-Bruning O., Bijlsma J. W. Human osteoarthritic cartilage is synthetically more active but in culture less vital than normal cartilage. J. Rheumatol. 1992;19:123–129. [PubMed] [Google Scholar]
- 21.Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J. Biol. Chem. 1969;244:77–78. [PubMed] [Google Scholar]
- 22.Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem. J. 1978;176:683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayliss M. T., Roughley P. J. The properties of proteoglycan prepared from human articular cartilage by using associative caesium chloride gradients of high and low starting densities. Biochem. J. 1985;232:111–117. doi: 10.1042/bj2320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Chim. Biophys. Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M. B., Mow V. C., Chun L. E., Eyre D. R. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J. Orthop. Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 26.Stageman H., Stadler K. Determination of hydroxyproline. Clin. Chim. Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 27.Adams P., Muir H. Qualitative changes with age of proteoglycans of human lumbar intervertebral discs. Ann. Rheum. Dis. 1976;35:289–296. doi: 10.1136/ard.35.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bank R. A., Jansen E. J., Beekman B., TeKoppele J. M. Amino acid analysis by reverse-phase high-performance liquid chromatography: improved derivatization and detection conditions with 9-fluorenylmethyl chloroformate. Anal. Biochem. 1996;240:167–176. doi: 10.1006/abio.1996.0346. [DOI] [PubMed] [Google Scholar]
- 29.Bank R. A., Beekman B., Vezijl N., de Roose J. A., Sakkee A. N., TeKoppele J. M. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J. Chromatogr. B Biomed. Sci. Appl. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 30.Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. J. Biol. Chem. 1991;266:894–902. [PubMed] [Google Scholar]
- 31.Duance V. C., Crean J. K. G., Sims T. J., Avery N., Smith S., Menage J., Eisentein S. M., Roberts S. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine. 1998;23:2545–2551. doi: 10.1097/00007632-199812010-00009. [DOI] [PubMed] [Google Scholar]
- 32.Bayliss M. T. Metabolism of animal and human osteoarthritic cartilage. In: Kuettner K. E., Schleyerbach R., Peyon J. G., Hascall V. C., editors. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1991. pp. 487–500. [Google Scholar]
- 33.Pokharna H. K., Phillips F. M. Collagen crosslinks in human lumbar intervertebral disc aging. Spine. 1998;23:1645–1648. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 34.Maroudas A., Palla G., Gilav E. Racemization of aspartic acid in human articular cartilage. Connect. Tissue Res. 1992;28:161–169. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- 35.Hanson D. A., Eyre D. R. Molecular site-specificity of pyridinoline and pyrrole cross-links in I type-I collagen of human bone. J. Biol. Chem. 1996;271:26508–26516. doi: 10.1074/jbc.271.43.26508. [DOI] [PubMed] [Google Scholar]
- 36.Kuyper R., Tyler M., Kurth L. B., Jenkins I. D., Horgan D. J. Identification of the loci of the collagen-associated Ehrlich chromogen in type-I collagen confirms its role as a trivalent cross-link. Biochem. J. 1992;283:129–136. doi: 10.1042/bj2830129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul R. G., Bailey A. J. Glycation of collagen: the basis of its central role in late complications of ageing and diabetes. Int. J. Biochem. Cell Biol. 1996;28:1297–1310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.