Abstract
We have prepared and characterized several lanthanide ion complexes of multidentate ligands or chelates in an effort to develop new upconverting luminescent labels that can be immune to autofluorescence and photobleaching. This study has involved the characterization of various chelates of Nd, Er, and Tm with respect to relative luminescent efficiency and excited-state lifetimes and explored various two-photon stepwise excitation mechanisms. Using peak laser powers near 100 kW, the upconversion emissions of Nd in Nd(EDTA)2 5− at 386 nm, Er in Er(DPA)3 3− at 550 nm, and Tm in Tm(DPA)3 3− at 480 nm, at levels of ~10−12 moles can be detected.
Upconversion refers to conversion of excitation light to shorter wavelengths or higher energy. Efficient upconversion has been obtained in crystalline materials containing trivalent lanthanide ions in the form of phosphor particles,1–4 displays,5 or fibers for upconversion lasers.6,7 Upconversion in Er has been obtained also in low-phonon-energy liquids.8 To date, upconversion has not been achieved in aqueous solutions.9 We demonstrate upconversion in aqueous phase lanthanide chelates using stepwise infrared excitation. This work is a step in the direction of realizing molecular scale upconverting reporters for biomedical diagnostics.
Lanthanides have been applied to biomedical assays in two forms previously: as chelated ligands in a downconverting method or as upconverting phosphor particles. Downconverting lanthanide chelates have been studied for immunoassay10 or labeling cells.11 Autofluorescent backgrounds for these materials can be reduced by time-gated detection.10 Upconverting phosphors have also been applied as reporters for biomedical assays.12,13 Advantages of these materials include freedom from autofluorescent backgrounds, no photobleaching, narrow emission profiles (which is beneficial for multiplexing), and excitation with diode lasers. Upconversion in lanthanide chelates combines attributes of these two research directions: the small size of the downconverting chelates with the low backgrounds and infrared excitation of the upconverting phosphors.
The chemistry of the upconverting chelate is similar to downconverting chelates: a lanthanide ion is complexed with a multidentate ligand or chelating agent that can be covalently bound to a biological probe molecule such as an antibody or DNA oligomer. In the work reported here the light is absorbed and emitted by lanthanide ions, which cannot photobleach. We prefer excitation schemes using near-infrared excitation and visible emission. There are several reasons for using such transitions. With near-infrared excitation, compact and inexpensive laser diodes may be used as excitation sources and the phototoxicity for living cells is low. Visible emission can be detected with high efficiency by using silicon detectors or photocathode materials.
In this Letter we report direct pumping of a lanthanide ion by use of both one- and two-wavelength upconverting pathways for Nd, Er, and Th ions complexed with the familiar chelating agents, ethylene-diaminetetraacetic acid (EDTA), diethylenetriamine-pentaacetic acid (DTPA), 1,4,7,10-tetraaza-cyclododecane-1,4,7,10-tetraacetic acid (DOTA), and dipicolinic acid (DPA). Using coincident 10 ns laser pulses with peak laser powers near 100 kW, we can detect the upconversion emissions of Nd in Nd(EDTA)2 5− at 386 nm, Er in Er(DPA)33− at 550 nm, and Tm in Tm(DPA)33− at 480 nm. Power dependence measurements confirmed that these were two-photon processes. Based on the signal-to-noise ratio, chelate concentration, and detection volume we estimate a detection limit of ~10−12 moles for these chelates. To our knowledge, this work is the first demonstration of upconversion from aqueous phase lanthanide chelates.
Sequential two-photon absorption involves ground-state absorption to an intermediate state followed by excited-state absorption to an upper excited state in a single ion. Figure 1 shows one such pathway observed for Er chelates. The first absorption step requires a pump wavelength near 980 nm to excite Er3+ to the 4 I11/2 level. Nonradiative relaxation to the 4I13/2 is then followed by the second absorption step, which utilizes a pump wavelength near 850 nm to populate the 4 S3/2 energy level, producing upconversion emission at ~550 nm. We have observed a number of upconversion mechanisms for these chelates. The most efficient pathways for Er, Tm, and Nd are listed in Table 1. We have also observed upconversion using a single wavelength: in Er, using either ~800 or,~980 nm, and in Nd using,~590 nm.
Fig. 1.
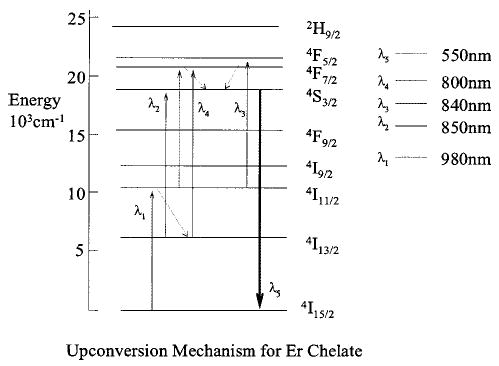
Energy-level diagram of Er3+ and its stepwise excitation upconversion mechanism (not all energy levels are shown).
Table 1.
Most Efficient Pathways for Er3+, Nd3+, and Tm3+ Chelates
| Chelate Compound | λ1 (nm) | λ2 (nm) | λemission (nm) |
|---|---|---|---|
| Er3+(DPA)33− | 986 | 800 | ~550 |
| Tm3+(DPA )33− | 797 | 1134 | ~480 |
| Nd3+(EDTA )25− | 799 | 593 | ~380 |
Solutions of lanthanide chelate complexes (0.02 M) were prepared in water or D2O by combining the proper number of equivalents of lanthanide ion (in the form of hydrated nitrate or chloride salts) and chelate. The solutions were adjusted to pH 7–9 with NaOH or NaOD. The lanthanide EDTA complexes were prepared using the tetrasodium salt of EDTA and therefore did not require pH adjustment. Preparation of lanthanide DPA complexes required heating for all components to be brought into solution. It is expected that three equivalents of DPA are combined with one equivalent of lanthanide (Ln) ion, the predominant species in solution is Ln(DPA)33−.14–16 In the case of Er and Tm, stable EDTA complexes were obtained only when two equivalents of EDTA per lanthanide ion were used. Nd formed a stable complex with one equivalent of EDTA but when two equivalents were combined with a Nd ion the upconversion emission was considerably increased. The excited-state lifetime was likewise increased, indicating superior protection of the Nd ion from the solvent when two equivalents were present. We use the notation Ln(EDTA 2−5 to tentatively describe these complexes.
We use two Nd:YAG-pumped dye laser systems to provide laser light over a wide range of near-infrared wavelengths. Longer wavelengths (e.g., 980 nm) are produced by Raman shifting the output of a dye laser). The two laser beams are focused to a spot size of ~100 μm in diameter and overlapped in the sample cell in a counterpropagating geometry. Upconverted emission is then collected and analyzed in a spectrometer. The emission is detected by a photomultiplier tube, amplified, and averaged using a boxcar integrator. A function generator provides for scanning of the temporal delay between the two laser pulses, allowing the measurement of the lifetime of the first excited state. Data acquisition and processing are performed using a computer with Labview software.
The solutions did not precipitate and demonstrated no significant laser scattering, indicating that they are molecular solutions. Ocean Optics spectrometers were used to measure the absorption spectra of the lanthanide chelates, and the characteristic absorption spectra confirmed that complexation had taken place. The absorbance at the excitation wavelength ranged from ~0.02 for Er to ~0.5 Nd for a 1 cm path length. These measurements show different relative absorption efficiencies for each transition when the chelating ligand is changed because of the ligand properties.16 This suggests that optimum excitation wavelengths for upconversion will be dependent on the chelate composition. An upconversion excitation study of the different Nd3+, Er3+, and Tm3+ chelates confirmed this. Table 2 lists the two optimum excitation wavelengths for first- and second-step absorption for Nd, Er, and Tm chelates. Emission profiles from Nd, Tm, and Er are shown in Fig. 2. A few nanometers difference in optimum excitation wavelength is found for the various chelates of the same lanthanide, demonstrating the influence of the ligand on the lanthanide ion.
Table 2.
Excited-State Lifetime for Different Er3+, Nd3+, and Tm3+ Chelate Compounds
First excited-state lifetime.
Second excited-state lifetime.
Fig. 2.
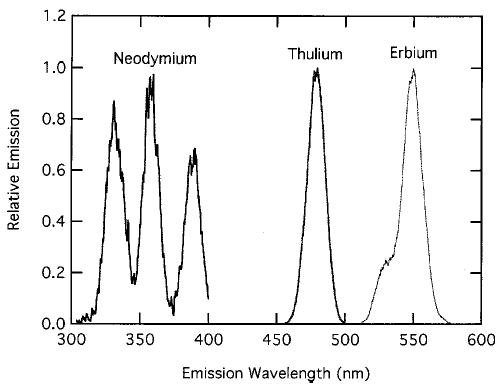
Upconversion emission spectra for Nd3+, Tm3+, and Er3+ chelates.
The upconversion efficiencies for Er3+, Nd3+, and Tm3+ vary significantly depending on the chelating ligand used. In addition, solutions in H2O exhibit lower efficiency than those in D2O. This is mainly because certain chelates protect the lanthanide ion from solvent molecules, water being particularly detrimental as it promotes nonradiative decay of the excited state of the lanthanide ion through high-frequency OH vibrations. Three DPA ligands are particularly effective in protecting lanthanide ions from the solvent,17 as evidenced by significantly longer excited-state lifetimes for Ln(DPA )33− chelates.
The excited-state lifetimes of Er3+, Nd3+, and Tm3+ chelates were measured experimentally. The first excited-state lifetime was obtained by scanning the relative time delay between the two pulsed lasers. The time decay constants are given by 1/e decay times as the observed profiles are exponential. The second excited-state lifetime was determined by recording the upconversion emission signal profile directly on the oscilloscope. The results indicate that (1) the second excited-state lifetimes are much shorter than the first excited-state lifetimes for all the Er and Nd chelates; in fact, they were too short to analyze and compare quantitatively because of the finite laser pulse width. The converse is true for Tm; the first excited-state lifetime is much shorter than the second excited-state lifetime for all the Tm compounds. We believe the difference in decay rate for the first and second excited states is largely due to the relative energy gap between the excited states and the lower neighboring states. Nonradiative relaxation is less efficient when the gap is larger. Different ligands affect the decay lifetime by protecting the lanthanide from the solvent to a lesser or greater extent.
It would be expected that longer excited-state lifetimes would be accompanied by better upconversion efficiency, and this appears to be the case for Er and Tm chelates. However, in the case of Nd, EDTA chelates are the strongest upconverting emitters in spite of shorter lifetimes. Nd chelates appear to be less sensitive to solvent effects also, D2O solutions being only marginally more efficient than H2O solutions. This may be due to faster radiative rates in Nd chelates. Our measurements show that the second excited-state lifetime for all Nd chelates is shorter than our laser pulse. Faster radiative rates allow the emission process to compete more effectively with nonradiative decay processes.
We use pulsed lasers for these studies to allow measurements of lifetimes. With continuous-wave excitation, the upconverting chelate efficiency is expected to be reduced because of the finite first excited-state lifetime. For this reason, the best cw performance is expected for Er chelates. Performance of upconverting chelates with cw excitation will be best in microscopic formats where the smaller spot sizes can compensate for the lower peak intensities.
We have performed a systematic study of upconversion emission for Er, Nd, and Tm ions chelated by different ligands. By use of peak laser powers near 100 kW, the upconversion emissions of Nd in Nd(EDTA )25− at 386 nm, Er in Er(DPA )35− at 550 nm, and Tm in Tm(DPA )35− at 480 nm at levels of ~10−12 moles can be detected. The most efficient upconversion occurs for Er and Tm ions chelated with three DPA ligands. This is in contrast with Nd, for which EDTA is the superior ligand. D2O solutions can reduce the nonradiative transfer of the excited-state energy of rare-earth ions to the high-frequency O–H bond vibrations that exist in the H2O solution, resulting in longer excited-state lifetimes and better efficiency. The absorption spectrum study for various compounds shows that the different ligand fields and symmetries lead to different multiplet splittings and line strengths.
References
- 1.Auzel FE. Proc IEEE. 1973;61:758. [Google Scholar]
- 2.Wright JC. Radiationless Processes in Molecules and Condensed Phases. In: Fong FK, editor. Springer-Verlag; 1976. pp. 239–295. [Google Scholar]
- 3.Mita Y. Phosphor Handbook. In: Shionoya S, Yen WM, editors. CRC Press; 1999. pp. 643–650. [Google Scholar]
- 4.Auzel F. Chem Rev (Washington, DC) 2004;104:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 5.Downing E, Hesselink L, Ralston J, Macfarlane R. Science. 1996;273:1185. [Google Scholar]
- 6.Scheps R. Prog Quantum Electron. 1996;20:271. [Google Scholar]
- 7.Smart RG, Hanna DC, Tropper AC, Davey ST, Carter SF, Szebesta D. Electron Lett. 1991;27:1307. [Google Scholar]
- 8.Koeppen C, Jiang G, Zheng G, Garito AF. OptLett. 1996;21:653. doi: 10.1364/ol.21.000653. [DOI] [PubMed] [Google Scholar]
- 9.Reinhard C, Gudel HU. Inorg Chem. 2002;41:1048. doi: 10.1021/ic0108484. [DOI] [PubMed] [Google Scholar]
- 10.Hemmila I, Dakubu S, Mukkala VM, Siitari H, Lovgren T. Anal Biochem. 1984;137:335. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- 11.Leif RC, Thomas RA, Yopp TA, Watson BD, Guarino VR, Hindman DH, Lefkove N, Vallarino LM. Clin Chem. 1977;23:1492. [PubMed] [Google Scholar]
- 12.Faris GW, Wright WH, Pati S, Schneider LV, Zarling DA. Optical Society of America; 1996. Biomedical Optical Spectroscopy and Diagnostics, Vol. 3 of OSA Trends in Optics and Photonics Series; pp. 225–228. [Google Scholar]
- 13.Hampl J, Hall MP, Mufti NA, Yao Y-MM, MacQueen DB, Wright WH, Cooper DE. Anal Biochem. 2001;288:176. doi: 10.1006/abio.2000.4902. [DOI] [PubMed] [Google Scholar]
- 14.Binnemans K, Herck KV, Görller-Walrand C. Chem Phys Lett. 1997;266:297. [Google Scholar]
- 15.Stephens EM, Schoene K, Richardson FS. Inorg Chem. 1984;23:1641. [Google Scholar]
- 16.Devlin MT, Stephens EM, Richardson FS, Cott TCV, Davis SA. Inorg Chem. 1987;26:1204. [Google Scholar]
- 17.Horrocks WD, Jr, Sudnick DR. J Am ChemSoc. 1979;101:334. [Google Scholar]


