Abstract
In this study we investigated why bloodstream forms of Trypanosoma brucei gambiense cross human brain microvascular endothelial cells (BMECs), a human blood-brain barrier (BBB) model system, at much greater efficiency than do T. b. brucei. After noting that T. b. gambiense displayed higher levels of cathepsin L–like cysteine proteases, we investigated whether these enzymes contribute to parasite crossing. First, we found that T. b. gambiense crossing of human BMECs was abrogated by N-methylpiperazine-urea-Phe-homopheylalanine-vinylsulfone-benzene (K11777), an irreversible inhibitor of cathepsin L–like cysteine proteases. Affinity labeling and immunochemical studies characterized brucipain as the K11777-sensitive cysteine protease expressed at higher levels by T. b. gambiense. K11777-treated T. b. gambiense failed to elicit calcium fluxes in BMECs, suggesting that generation of activation signals for the BBB is critically dependant on brucipain activity. Strikingly, crossing of T. b. brucei across the BBB was enhanced upon incubation with brucipain-rich supernatants derived from T. b. gambiense. The effects of the conditioned medium, which correlated with ability to evoke calcium fluxes, were canceled by K11777, but not by the cathepsin B inhibitor CA074. Collectively, these in vitro studies implicate brucipain as a critical driver of T. b. gambiense transendothelial migration of the human BBB.
Introduction
Human African trypanosomiasis (HAT; commonly known as sleeping sickness) has been claimed to be more deadly than other vector-borne diseases such as malaria because death is inevitable if a patient is not treated. The terminal stages of human sleeping sickness are characterized by neurological signs including seizures, a marked increase in nighttime insomnia and daytime drowsiness (from which the disease gets its name), and coma. Sleeping sickness is caused by 2 subspecies of African trypanosomes: Trypanosoma brucei rhodesiense, which causes East African sleeping sickness, and T. b. gambiense, which causes West African sleeping sickness. In classical human sleeping sickness, the parasites invade the CNS, and the infected individuals suffer white matter encephalitis with concomitant psychiatric and motor disorders (reviewed in ref. 1). The neurological manifestations of HAT appear after the invasion of the CNS by the parasite (1).
In cattle, both T. congolense and T. vivax are known intravascular parasites, while T. b. brucei can leave the blood vessels and invade other tissues in cattle, but not usually the CNS (2). About half of all cattle infected with T. b. rhodesiense develop fatal CNS disease, a rate comparable with that found in humans. Furthermore, T. b. gambiense has been found to cross an in vitro model of the human blood-brain barrier (BBB) more efficiently than does T. b. brucei (3). Collectively, the data show that T. b. gambiense and T. b. rhodesiense are truly CNS tropic organisms, while T. b. brucei is probably not.
In HAT infections, the tight junctions of the brain-endothelial barrier are not disrupted, and damage to the barrier is minimal, making it difficult to correlate CNS invasion with parasite-endothelium interactions (4, 5). Although the process of CNS invasion is still poorly understood (4, 5), a growing body of evidence from studies in animal models of the disease indicates that the parasites may cross the BBB directly. In experimental animals infected with African trypanosomes, the parasites appear early during infection in the choroid plexus and other circumventricular organs (6) that lack a BBB. At later stages, the parasites penetrate the true BBB without apparent disruption of the endothelial tight junctions and enter the brain parenchyma. This was shown by double immunohistochemical labeling of parasites and brain endothelial cells (7). Of further interest, Masocha et al. (8) have shown that T. b. brucei cross the cerebral blood vessels of mice through interactions with endothelial cells that express laminin 8, suggesting that these parasites and leukocytes may traverse the intact BBB through the same or similar sites.
In vitro models of the BBB have become important tools for identifying the cellular and molecular elements that are possible targets for intervention of the transmigration of many different pathogens into the CNS. In order to study the mechanisms underlying human BBB traversal by bloodstream forms of African trypanosomes (e.g., T. b. gambiense), we recently developed an in vitro model composed of the major functional unit of BBB, i.e., brain microvascular endothelial cells (BMECs) (3). Accordingly, bloodstream-form trypanosomes attach firmly to BMECs, whereas procyclic forms (present in the tsetse fly vector) bind poorly to the monolayer, perhaps due to the acidic nature of the procyclin coat (3). Furthermore, procyclic forms do not cross human BMECs, while human-infective bloodstream forms of T. b. gambiense cross human BMECs far more efficiently than those derived from animal-infective T. b. brucei. In our previous work, we observed a transient decay and recovery in transendothelial electric resistance during bloodstream-form parasite passage (3). We proposed that parasite binding at or near intercellular junctions of the BBB precedes crossing via a paracellular route. In addition, we reported that T. b. gambiense induces oscillatory changes in the intracellular calcium ([Ca2+]i) of BMECs and proposed that signaling events triggered by bloodstream-form parasites might render the endothelial cells permissive to T. brucei traversal (3). To date, the molecular players involved in parasite-induced signaling and crossing of BBB are unknown.
Cysteine proteases belonging to the C1 (papain) family are important for the growth and survival of several pathogenic protozoa, including T. brucei (reviewed in ref. 9). In T. brucei, cysteine proteases are represented by 2 members: (a) a cathepsin B–like enzyme, encoded by a single gene; and (b) a family of highly similar cathepsin L–like enzymes, termed brucipain, encoded by 11 gene copies (10). Previous characterization on congopain, the cathepsin L–like cysteine protease of T. congolense, suggested that the enzyme may become refractory to inhibition by natural serum inhibitors upon binding to immunoglobulins; this may lead to extracellular activity of such cysteine proteases in vivo (11, 12). Studies performed with irreversible cysteine protease inhibitors revealed that these compounds can induce parasite death in vitro and protect mice from experimental T. brucei infections (13, 14). However, the role of brucipain in the transendothelial migration of African trypanosomes has not yet been addressed. An interesting precedent linking parasite cysteine protease activity with endothelial activation emerged from analysis of the mechanisms underlying kinin receptor activation by T. cruzi, the etiologic agent of Chagas’ disease (15–17). These studies revealed that T. cruzi trypomastigotes rely on the major cysteine protease cruzipain to release the kinin agonist from their inert precursors, the kininogens (reviewed in ref. 17). More recently, it was shown that cruzipain can activate smooth muscle cells by inducing release of [Ca2+]i via an alternative (i.e., kinin-independent) signaling pathway (18). Considering that the structurally related cruzipain and brucipain share many biochemical and kinetic properties (19), here we sought to determine whether BMEC crossing by T. b. gambiense depends on the activity of parasite cysteine proteases. Our results demonstrate that transendothelial migration of T. b. gambiense depends on their ability to trigger [Ca2+]i fluxes in BMECs by a cysteine protease–dependent mechanism.
Results
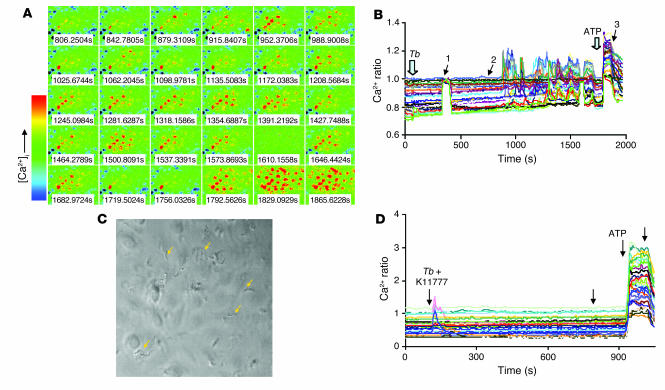
First, we analyzed T. b. gambiense–induced oscillatory changes in the levels of free [Ca2+]i of human BMECs. As previously reported (3), the incubation of human BMEC monolayers with bloodstream forms of T. b. gambiense induced rapid elevations in [Ca2+]i in approximately 30%–50% of the cell population (see time-lapse images in Figure 1A), the response being associated mostly with transient peaks (Figure 1B). The changes of [Ca2+]i were expressed as the 340:380 ratio (see Methods). The physical interaction between responding cells and the motile parasites was confirmed by taking differential interference contrast images (Figure 1C).
Figure 1. Ca2+ oscillations of human BMECs in response to T. b. gambiense are mediated by papain-like cysteine proteases.
Fura-2/AM–loaded BMECs were mounted on a recording chamber, and 25–40 regions of interest representing individual cells were selected. Cells were exposed to bloodstream forms of T. b. gambiense (106 parasites/ml) in HEPES-buffered HBSS, and real-time fluorescent images were captured by alternating excitation at 340 and 380 nm. (A) Time-lapse images of [Ca2+]i changes presented between the time points marked with arrows 2 and 3 in B. Increased [Ca2+]i indicated by color change from blue to red. (B) Kinetics of [Ca2+]i changes. T. b. gambiense (Tb; 106 parasites/ml) was added where indicated. To show functional integrity of human BMECs after T. b. gambiense treatment, 10 μM ATP was added at the end of the experiments at 1770 seconds as indicated. (C) To show the presence of parasites, during Ca2+ measurements a differential interference contrast image was taken at the time point marked by arrow 1 in B. Arrows show some of the motile T. b. gambiense. [Ca2+]i changes were expressed as the 340:380 ratio. (D) K11777 (20 μM) was added to cells in 0.5% DMSO immediately before addition of parasites, and the variations of fluorescence were measured. DMSO alone had no effect on parasite-induced calcium changes. ATP (1 μM) was added at the end of the recording as a positive control of BMEC integrity. Unlabeled arrows indicate when a change to normal medium was made. Results are representative of 2 independent experiments.
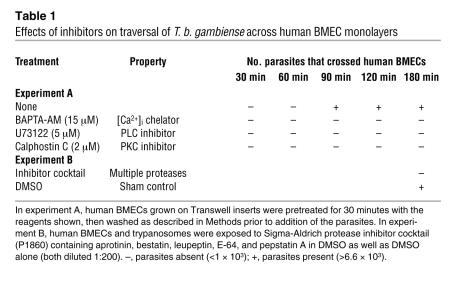
We then determined whether parasite-induced calcium responses in human BMECs are required for parasite transmigration across the endothelial cell barrier. As shown in Table 1, the transmigration of T. b. gambiense across human BMECs grown on Transwell inserts was inhibited when the BMECs were pretreated for 30 minutes with 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester (BAPTA-AM; a [Ca2+]i chelator) prior to washing and addition of the parasites. While trypanosome crossing of human BMECs was observed at 90 minutes, no parasites were detected in the BAPTA-AM–pretreated human BMECs, even at the 3-hour time point. It is well known that activated phospholipase C (PLC) splits phosphatidyl-4,5-biphosphate (PIP2) to yield inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 causes release of Ca2+ from intracellular stores (i.e., ER), which in turn activates PKC, an event that also requires DAG binding. In accordance with the known function of PLC, human BMECs pretreated for 30 minutes with either the PLC-β inhibitor 1-(6-[([17β]-3Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl)-1H-pyrrole-2,5-dione (U73122) or calphostin C, a PKC inhibitor, no longer permitted trypanosome crossing of BMECs. This lends further support for PLC’s role as a downstream effector of BBB transmigration (Table 1).
Table 1 .
Effects of inhibitors on traversal of T. b. gambiense across human BMEC monolayers
We then investigated in further detail the role of parasite proteases in BBB crossing. These studies were motivated by the initial findings that addition of a protease inhibitor cocktail (containing trans-Epoxysuccinyl- l-leucylamido(4-guanidino)butane [E-64], leupeptin, cystatin, and aprotinin) prevented trypanosomes from crossing human BMECs (Table 1). The data above imply that protease activity was somehow required for the Ca2+ signaling responses leading to parasite transmigration across BMECs. In order to characterize the proteases involved in this process, we tested the effects of a more selective irreversible cysteine protease inhibitor, N-methylpiperazine-urea-Phe-homopheylalanine-vinylsulfone-benzene (K11777) (20), added to the cultures at the start of parasite-endothelium interaction. We chose to test this particular inhibitor because it displays at least 30-fold higher affinity for cathepsin L–like cysteine proteases such as the T. cruzi cysteine protease cruzipain (KI, 0.36 μM) than for cathepsin B–like cysteine proteases (KI, 11 μM) (20). This compound also efficiently inhibits (association constant [Kass], 268,200 M/s) the recombinant cathepsin L–like enzyme of T. b. rhodesiense, named rhodesain (19). We found that addition of K11777 completely abolished the parasite-induced calcium oscillations in human BMECs (Figure 1D), indicating that cysteine protease activity was essential for parasite-mediated increases in endothelial cell [Ca2+]i. Importantly, Ca2+ signaling was ATP inducible in BMECs exposed to K11777, indicating that the functional integrity of endothelial cells was preserved (Figure 1D).
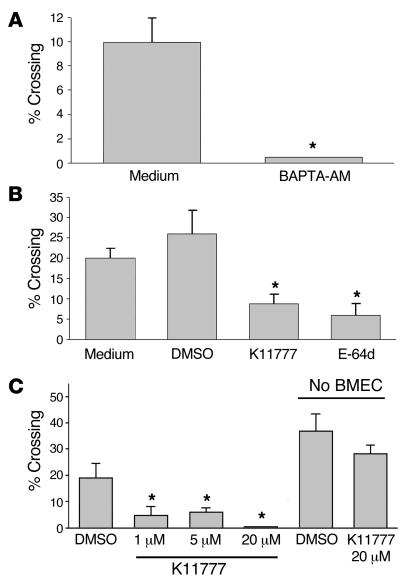
Next we sought to determine whether the [Ca2+]i responses induced by parasite cysteine proteases were required for efficient migration of T. b. gambiense through the BMEC monolayer. First, we observed that the pretreatment of human BMECs with BAPTA-AM completely abolished parasite transmigration (Figure 2A). Similarly, we observed that the membrane-permeable inhibitors of cysteine proteases (2S,3S)-trans-Epoxysuccinyl- l-leucylamido-3-methylbutane ethyl ester (E-64d; a membrane-permeable derivative of E-64) and K11777 reduced human BMEC monolayer crossing by T. b. gambiense by 40%–80% (Figure 2B). The inhibition of human BMEC traversal by K11777 was dose dependent, and the compound was effective at low micromolar concentrations (Figure 2C). Importantly, parasite traversal of human BMECs pretreated with or without K11777 (and subsequent washing) was the same, again demonstrating that the inhibitor was not toxic to the endothelial cells (data not shown). Parasite viability was tested by performing crossing experiments using inserts devoid of the BMEC monolayers (Figure 2C). The logic behind this is that considering the size of a trypanosome (approximately 5 × 20 μm), the parasite has to be alive and motile in order to pass through a 3-μm hole on the Transwell inserts. In these conditions, the live parasites reached the bottom chambers at equal numbers in the absence and presence of K11777, indicating that this compound was not affecting parasite motility and/or viability during the time course and conditions of our experiments (180 minutes; Figure 2C). In contrast, in a control experiment we found that dead trypanosomes did not cross the insert membrane (data not shown). We also found that K11777 did not alter active parasite migration from the bottom wells into the upper Transwells.
Figure 2. The traversal of the human BBB model by T. b. gambiense requires calcium and the activity of papain-like cysteine proteases.
(A) Bloodstream forms of T. b. gambiense parasites (106) were incubated with human BMECs on Transwell inserts for 3 hours. The number of parasites at the lower chamber was subsequently estimated by counting in a hemacytometer. The percentage of parasites ± SEM that crossed relative to the 106 parasites added is shown. Experiments were performed in triplicate. To show the role of [Ca2+]i, the BMECs were incubated with 15 μM BAPTA-AM for 30 minutes and washed twice prior to adding parasites. Controls were performed by adding medium containing 0.5% DMSO. (B) Bloodstream-form parasites (106) were added to the endothelial cells and incubated for 3 hours at 37°C, after which the percentage of parasites that crossed was estimated as in A. DMSO (0.5%), K11777 (20 μM), and E-64d (20 μM) were added to human BMEC monolayers immediately before the addition of parasites. (C) Bloodstream-form parasites (106) were added to inserts containing human BMECs or empty inserts (No BMECs) and incubated for 3 hours at 37°C, after which the percentage of parasites that crossed was estimated as in A. DMSO (0.5%) and K11777 (1–20 μM) were added to insert monolayers immediately before the addition of parasites. *P < 0.05. The groups showing statistical significance against all other groups in the same graph are marked. In B, K11777 and E-64d are significantly different from medium or DMSO; in C, K11777 is significantly different from DMSO. There is no significant difference between DMSO and K11777 in wells with no BMECs.
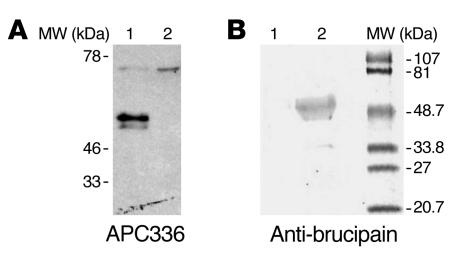
In order to identify the molecular target of K11777 in T. b. gambiense, we analyzed by SDS-PAGE parasite lysates that had been incubated with biotin-methylpiperazine-urea-Phe-homopheylalanine-vinylsulfone-benzene (APC336), a biotinylated analog of K11777. Upon subsequent blotting, we observed labeling of 2 proteins of similar molecular mass (Figure 3A, lane 1). Treatment of the lysates with the soluble irreversible cysteine protease inhibitor E-64 prior to incubation with the biotinylated inhibitor completely prevented labeling, confirming that the biotinylated compound bound to cysteine proteases via the enzyme’s active site (Figure 3A, lane 2). In order to confirm that the target of the biotinylated inhibitor in the parasite was brucipain, we incubated the lysate with APC336 and then subjected it to immunoprecipitation with anti-brucipain antibodies bound to protein G–linked agarose beads and visualized the reactive molecules in blots (Figure 3B). As expected, incubation with control serum did not result in the precipitation of any labeled molecules (Figure 3B, lane 1), while treatment with anti-brucipain (Figure 3B, lane 2) led to the precipitation of 2 proteins, similar to that observed in Figure 3A. Taken together, these results suggest that K11777 prevents T. b. gambiense crossing of the in vitro BBB model by inactivating brucipain.
Figure 3. The main target of K11777 in T. b. gambiense is brucipain.
(A) Bloodstream forms of T. b. gambiense Triton X-100 lysates were prepared, and equal aliquots of the soluble fractions (equivalent to 3 × 105 parasites) were incubated with 5 mM DTT (lane 1) or with 5 mM DTT and 20 μM E-64 (lane 2) for 15 minutes at room temperature, followed by incubation with 20 μM APC336, a biotinylated cysteine protease inhibitor related to K11777, for 30 minutes. The samples were diluted in SDS-PAGE sample buffer under reducing conditions and boiled for 5 minutes prior to separation by electrophoresis and transfer to nitrocellulose. The membranes were incubated with anti-biotin antibodies, and the reactive bands were visualized by chemiluminescence. (B) Immunoprecipitation of inhibitor-bound brucipain. Lysate (165 μg) of bloodstream forms of T. b. gambiense was incubated with 20 μM APC336 for 30 minutes and mixed with protein G agarose beads coated with nonimmune mouse serum (lane 1) or anti-brucipain antiserum (lane 2) for 2 hours. The beads were washed, then boiled for 5 minutes in SDS-PAGE sample buffer under reducing conditions prior to separation by electrophoresis and transfer to nitrocellulose. The membranes were incubated with avidin-alkaline phosphatase. The prestained molecular weight markers are shown at the right.
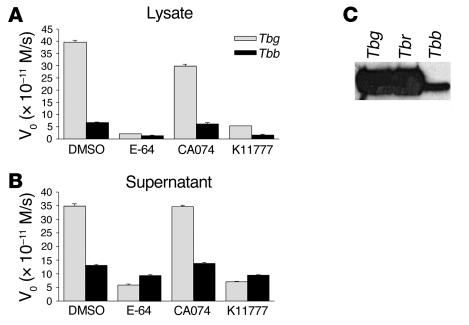
Considering that cysteine protease activity is required for efficient BBB crossing by trypanosomes in vitro, we next analyzed the relative amount of active cysteine proteases in different T. brucei subspecies. Peptidase activity assays using the synthetic fluorogenic substrate Z-Phe-Arg-AMC revealed that lysates obtained from human infective subspecies T. b. gambiense possessed approximately 8-fold more active cysteine proteases than did those from T. b. brucei (Figure 4A). The cysteine protease inhibitors E-64 and K11777 decreased peptidase activity by 94%, showing that we were detecting mainly the activity of papain-like cysteine proteases in our assay. In contrast, ( l-3-trans-(propylcarbamyl)oxirane-2-carbonyl)- l-isoleucyl- l-proline (CA074), an inhibitor of cathepsin B–like cysteine proteases, led only to a 25% decrease in the overall activity of bloodstream-form lysates, suggesting that this cysteine protease is a minor component in these lysates. Analysis of bloodstream-form secretion products (supernatants) showed that T. b. gambiense secreted 10-fold more active cysteine proteases (85% inhibition with E-64) than did T. b. brucei (30% inhibition with E-64; Figure 4B). No inhibition was observed using CA074, suggesting that the bloodstream-form trypanosomes secrete brucipain but not the cathepsin B–like enzyme. The fact that the cathepsin B–like enzyme was absent from supernatants also argues against the possibility that the extracellular peptidase activity resulted from parasite lysis. To further characterize cysteine protease expressed by T. b. gambiense, T. b. rhodesiense, and T. b. brucei, we labeled the parasite lysates with APC336. The analysis of the blotting profiles confirmed that T. b. gambiense (and T. b. rhodesiense) expressed higher levels of brucipain than did T. b. brucei (Figure 4C).
Figure 4. Cysteine protease activity in T. brucei subspecies.
(A) Lysates of T. b. gambiense (t) or T. b. brucei (Tbb) bloodstream-form parasites were prepared, and the protease activity of equal aliquots (equivalent to 3.3 μg/ml) was determined using Z-Phe-Arg-AMC as a substrate. Substrate hydrolysis was followed by continuous recording of increase in fluorescence, and the initial velocities (V0) were calculated by linear regression of the hydrolysis curves. To discriminate the activity of papain-like cysteine proteases from other proteases (e.g., serine proteases), samples were incubated with 2 μM E-64 and the residual activity was measured. Cathepsin B– or cathepsin L–like activities were discriminated from each other by incubating the samples with 2 μM CA074 and 2 μM K11777 (to inhibit cathepsin B– and cathepsin L–like enzymes, respectively). (B) Conditioned medium of bloodstream-form trypanosomes. Both T. brucei subspecies described in A were washed in serum-free HMI-9 medium and incubated (2 × 107 parasites/ml) for 1 hour at 37°C. Parasite-conditioned medium was prepared, and the protease activities of 200-μl aliquots were determined as described in A. (C) Lysates of bloodstream forms of T. b. gambiense, T. b. rhodesiense (Tbr), and T. b. brucei (equivalent to 2 × 106 parasites) were incubated with 5 mM DTT and 20 μM APC336 for 30 minutes at room temperature, separated by SDS-PAGE, and transferred to nitrocellulose. The reactive bands were visualized after incubation with anti-biotin antibodies followed by addition of the substrates for chemiluminescence.
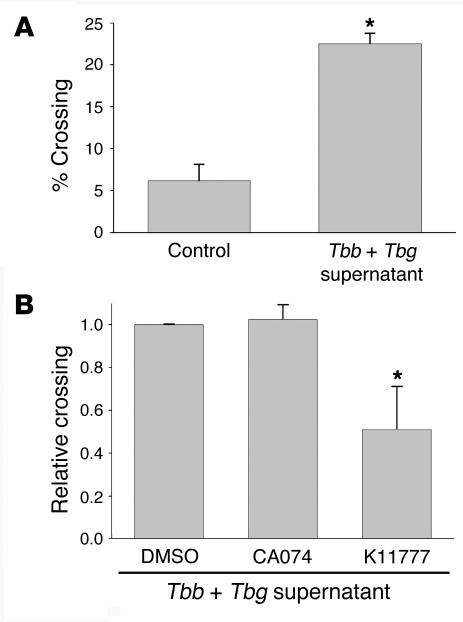
We previously reported that bloodstream forms of T. b. brucei are capable of crossing the human BBB, albeit less efficiently than T. b. gambiense (3). Taking advantage of these discrepant phenotypes, we sought to test the premise that cysteine proteases liberated by the parasites were required for efficient BMEC signaling and subsequent BBB traversal. To test this, we evaluated whether the parasite-free conditioned medium (supernatants) obtained from T. b. gambiense was able to enhance T. b. brucei traversal across the monolayers (Figure 5). Strikingly, we found that T. b. gambiense supernatants increased the crossing efficiency of T. b. brucei by approximately 5-fold. Importantly, the observed enhancement in T. b. brucei crossing was negated when the T. b. gambiense–conditioned medium was treated with the cathepsin L inhibitor K11777, but no effect was observed after treatment with the cathepsin B inhibitor CA074 (Figure 5B). This result indicated that the effect of T. b. gambiense–conditioned medium in BBB traversal is critically dependent on cysteine protease activity and that cathepsin L–like brucipain, not the cathepsin B–like enzyme, is the parasite cysteine protease responsible for the enhanced crossing.
Figure 5. Secretion products of T. b. gambiense enhance transendothelial migration of bloodstream forms of T. b. brucei.
(A) Bloodstream forms of T. b. brucei parasites (106) were incubated for 3 hours at 37°C with human BMECs on Transwell inserts in medium or T. b. gambiense conditioned medium with 10% FBS. The percentage of parasites ± SEM that crossed in control medium or T. b. gambiense–conditioned medium relative to the 106 parasites added is shown. *P < 0.05. (B) T. b. gambiense–conditioned medium was also incubated with DMSO (0.25 %) and 10 μM CA074 or K11777 for 15 minutes at 37°C prior to addition to BMEC monolayers. Bloodstream forms of T. b. brucei parasites (2 × 106) were incubated for 3 hours at 37°C with BMECs on Transwell inserts in T. b. gambiense–conditioned medium with 10% FBS. The relative percentage of parasites ± SEM that crossed in the presence of inhibitor relative to DMSO controls is shown. *P < 0.05.
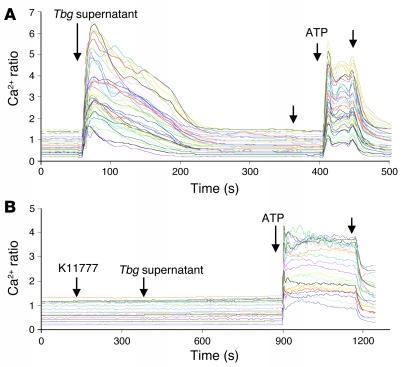
Finally, we evaluated whether the cysteine protease activity present in the parasite-free supernatant was also responsible for the induction of [Ca2+]i rises in human BMECs. Indeed, we detected a sustained increase in the free [Ca2+]i immediately after addition of T. b. gambiense–conditioned medium (Figure 6A). Similar to the data described earlier with the living bloodstream forms of T. b. gambiense (Figure 1), the [Ca2+]i responses evoked by parasite supernatants were abolished by incubation with K11777 (Figure 6B). Collectively, these results suggest that variable BMEC crossing efficiency by T. brucei subspecies reflects differences in the activity levels of brucipain, a cysteine protease implicated in parasite-induced [Ca2+]i in human BMECs.
Figure 6. Cysteine proteases secreted by T. b. gambiense induce Ca2+ oscillations in human BMECs.
(A) Fura-2/AM–loaded BMECs were mounted on a microscope, and 25–40 regions of interest representing individual cells were selected. T. b. gambiense–conditioned medium (200 μl) was added to cells, and real-time fluorescent images were captured by alternating excitation at 340 and 380 nm. [Ca2+]i changes were expressed as the 340:380 ratio. (B) K11777 (20 μM) was added to the Fura-2/AM–loaded endothelial cells prior to addition of conditioned medium (Tbg supernatant). ATP (1 μM) was added at the end of the recording as a positive control for human BMEC integrity. Unlabeled arrows indicate when a change to normal medium was made. Results are representative of 2 independent experiments.
Discussion
We have recently reported that T. b. gambiense provokes transient decreases in transendothelial electrical resistance and oscillatory increases in [Ca2+]i in human BMECs, indicating that these parasites are able to modulate the dynamics and integrity of the endothelial cell monolayers (3). Based on previous findings that mechanical stimulation of a single cortical capillary endothelial cell can trigger calcium waves (21), we initially proposed that actively swimming trypanosomes might be inducing changes in [Ca2+]i by mechanical stimulation of BMECs (3). We also suggested that bloodstream-form trypanosome proteases might subsequently process or degrade proteins at the intercellular junctions, enabling parasites to cross through the barrier between the cells (3).
We now demonstrate that transient increases in [Ca2+]i can be elicited either by living parasites or by their secretion products. In both cases, the calcium transients were completely abolished by the irreversible cathepsin L cysteine protease inhibitor K11777, suggesting that parasite-derived cysteine proteases are mediating the calcium signaling response in BMECs. Importantly, by using 2 membrane-permeable cysteine protease inhibitors, K11777 and E-64d, or the calcium chelator BAPTA-AM, we were able to correlate cysteine protease–induced calcium signaling with the ability of parasites to cross the BBB model. Previous studies on the role of [Ca2+]i responses in the mechanisms underlying cellular invasion by T. cruzi revealed that this intracellular pathogen relies on its major cysteine protease, cruzipain, to activate human umbilical vein endothelial cells or human smooth muscle cells (13, 18). Although we did not address the nature of the calcium pools mobilized during the interaction between T. b. gambiense and human BMECs, it is possible that, like T. cruzi, T. b. gambiense cysteine proteases may induce the recruitment of calcium from intracellular stores. T. b. gambiense–mediated calcium responses and transmigration of BMECs were both abolished when the endothelial cells were pretreated with BAPTA-AM or PLC inhibitors. At present, there is no information on the signals and receptors responsible for the calcium responses. Future studies may determine whether cysteine proteases trigger such responses by generating agonists for G protein–coupled receptors: e.g., bradykinin receptors (15) or protease-activated receptors (22). Alternatively, crossing of BMECs may depend on proteolytic generation of ligands for receptor tyrosine kinases (via activation of PLC-γ). It should be noted that we have not yet ruled out possible additive effects of the mechanical stimulation of the cells by the parasites and biochemical stimulation by secreted cysteine protease.
In a previous study, we reported that the number of bloodstream forms of T. b. gambiense that migrate through human BMEC monolayers is much higher than that of T. b. brucei (3). Interestingly, we found that the ability of T. b. gambiense and T. b. brucei to cross our BBB model roughly correlated with their levels of cysteine protease activity. Procyclic forms of T. b. brucei, which are markedly deficient in cysteine protease activity (23), fail to cross human BMEC monolayers (3). Furthermore, we consistently observed greater amounts of active cysteine proteases in T. b. gambiense than in T. b. brucei regardless of the methodology used for detection namely fluorogenic substrates or active-site labeling with biotinylated inhibitors. The protease assays also indicated that bloodstream forms of T. b. gambiense secrete more enzymatically active cysteine proteases than do bloodstream forms of T. b. brucei. Importantly, the use of cysteine protease inhibitors with distinct specificities allowed us to rule out the participation of cathepsin B–like cysteine protease in the crossing by bloodstream-form parasites. Consistent with the notion that parasite cysteine proteases contribute to BBB traversal, BMEC crossing by bloodstream forms of T. b. brucei was dramatically increased upon addition of T. b. gambiense cysteine protease–rich supernatants.
Using different cysteine protease inhibitors (K11777 and CA074), we obtained evidence that cathepsin L–like brucipain is probably the cysteine protease responsible for BBB crossing. To identify the molecular target(s) of K11777, we used the biotin-coupled K11777 inhibitor APC336 to label T. b. gambiense lysates. We detected exclusive binding of this compound to 2 proteins of approximately 50 kDa, both of which were immunoprecipitated by antibodies raised to recombinant brucipain. Taken together, our results indicate that the main target of K11777 in T. b. gambiense is the cathepsin L–like enzyme brucipain, not the parasite’s 35-kDa cathepsin B–like cysteine protease. This was expected, since K11777 shows a 30-fold preference for cathepsin L–like enzymes compared with cathepsin B–like enzymes (21). Furthermore, these results are in agreement with a recent report showing that antibodies against recombinant rhodesain recognize 2 bands of 45 kDa and 47 kDa in the lysates of T. b. brucei (24). Although we cannot rule out the participation of the cathepsin B–like enzyme in BBB crossing, the findings that K11777 reduced T. b. gambiense traversal by 80% and that the biotinylated form of K11777 (APC336) bound exclusively to brucipain at the tested concentrations argue against a major role for cathepsin B in BBB traversal. Furthermore, the lack of detectable cathepsin B–like activity in the supernatants of bloodstream-form T. b. gambiense combined with the observation that the cathepsin L inhibitor K11777, but not the cathepsin B inhibitor CA074, abolished the effect of T. b. gambiense–conditioned medium in the traversal of the BBB by T. b. brucei strongly support a role of brucipain in mediating parasite migration through the BMEC monolayers.
During natural infections, it is possible that cysteine proteases liberated by the parasites in the proximity of the brain endothelium may help to promote efficient parasite crossing of the BBB. The importance of proximity, or necessity of the parasites to form a cellular synapse with BMECs, gets credence from our observations that procyclic forms of T. b. brucei parasites fail to cross human BMECs even when cocultured with cysteine protease–rich bloodstream forms of T. b. gambiense, since procyclic forms (which express low contents of cysteine proteases) poorly bind to the brain endothelium (3). These findings suggest that procyclics are unable to take advantage of the cysteine proteases that are released by bloodstream forms of T. b. gambiense to facilitate their crossing, probably because they cannot firmly attach to human BMECs (3).
It has previously been reported (25) that the bloodstream forms of T. b. gambiense are capable of directly promoting the activation of human bone marrow endothelial cells in vitro, leading to synthesis of proinflammatory cytokines and nitric oxide as well as the expression of several adhesion molecules. In addition, parasite secretion products were found to increase dextran permeability of the endothelial cell monolayer. The authors provided evidence that soluble forms of the so-called variant surface glycoproteins present in the parasite culture supernatant may be responsible for activation of those processes of human bone marrow endothelial cells by inducing NF-κB translocation into the nucleus (25). Our data indicate that cysteine proteases (particularly brucipain) secreted by T. b. gambiense induce Ca2+ signaling by the endothelial cells, this being presumably required for subsequent parasite traversal of the endothelial cell monolayer. Furthermore, considering the critical role of Ca2+ in NF-κB activation (26, 27), it is possible that the parasite cysteine proteases also induce NF-κB translocation from the cytoplasm into the nucleus and mediate inflammatory processes in human BMECs.
On a final note, we have recently found that some bloodstream forms of T. b. gambiense have the capacity to enter into human BMECs (28). The intracellular location of the trypanosomes was demonstrated in relation to the endothelial cell plasma membrane and to the actin cytoskeleton. It is possible that these parasites are destroyed within a lysosomal compartment, but they may also be viable trypanosomes with the capacity to exit the endothelial cell. Given the finding that T. cruzi invasion of endothelial cells is increased at the expense of G protein–coupled receptor triggering (15, 29), the possibility that T. b. gambiense may use similar strategies to penetrate into endothelial cells that comprise the human BBB remains an intriguing possibility.
In summary, we have examined the role of parasite proteases in the interaction of T. b. gambiense with human BMECs. Collectively, our findings suggest that T. b. gambiense crosses the human BBB by generating Ca2+ activation signals in BMECs through the activity of parasite cysteine proteases.
Methods
Materials and biochemicals.
K11777 was kindly provided by J.H. McKerrow (UCSF, San Francisco, California, USA), and APC336 was from J. Palmer (Axys, San Francisco, California, USA). E-64, E-64d, BAPTA-AM, calphostin C, U73122, and protease inhibitor cocktail — for use in tissue culture, containing aprotinin, bestatin, leupeptin, E-64, and pepstatin A (catalog no. P 1860) — were from Sigma-Aldrich. CA074 was acquired from Calbiochem. Iscove’s medium (used to make HMI-9) and M199 medium were from Invitrogen. Heat-inactivated FBS was from Hyclone or Omega Scientific Inc.
Human BMECs.
The human BMEC cultures have been previously described (3, 30). Human BMECs take up acetylated low-density lipoprotein and are positive for factor VIII–Rag, carbonic anhydrase IV, Ulex europaeus agglutinin I, the drug-transporting (or mdr1-type) P-glycoprotein, γ-glutamyl transpeptidase, protease-activated receptors PAR1–PAR4, and tight junctional proteins (3, 22, 30, 31). The cells were cultivated in M199 medium supplemented with 10% FBS and cultured up to passage 13 without losing their specific properties.
Trypanosomes and parasite-conditioned medium.
Bloodstream-form T. b. gambiense IL 1852, a cerebrospinal fluid isolate, was obtained from the International Livestock Research Institute. Bloodstream forms of T. b. brucei TREU 927 (32) were from P. Englund (Johns Hopkins University, Baltimore, Maryland, USA), and bloodstream forms of T. b. rhodesiense LouTAT 1.0 (33) were provided by J. Mansfield (University of Wisconsin, Madison, Wisconsin, USA). These parasites were grown in HMI-9 medium (Invitrogen) supplemented with 10% inactivated FBS at 37°C in a 5% CO2 humidified atmosphere. The cultures were split every 2 days or when they reached a density of 5 × 106 parasites/ml. Parasite-conditioned medium was obtained as follows: bloodstream-form parasites were washed twice in serum-free HMI-9 and incubated in this solution at a concentration of 2 × 107 parasites/ml for 1 hour at 37°C. The parasites were centrifuged at 3,000 g for 15 minutes, and parasite-free conditioned medium was filtered with 0.22-μm–pore size filters (Millipore). The conditioned media were kept at –20°C.
Measurement of African trypanosome traversal across human BMECs.
Assays to assess the ability of African trypanosomes to cross human BMECs were performed as described previously (3). Briefly, the endothelial cells were seeded on top of collagen type I–coated Transwell inserts (Costar; Corning Inc.) containing 3-μm pores and cultured for approximately 7 days or until the transendothelial electric resistance reached ≥25 ohm × cm2 (3, 28). Parasites were collected by centrifugation at 3,000 g for 10 minutes at 4°C and resuspended at a density of 107 parasites/ml in serum-supplemented HMI-9, and 106 parasites were immediately added to the top of the human BMEC-containing inserts. The cultures were incubated at 37°C for 3 hours, and the number of parasites present at the bottom chamber was determined by counting aliquots in the Neubauer chamber. All experiments were performed in duplicate on 3 independent occasions. E-64d or K11777 was added at 20 μM final concentrations at the top chamber immediately prior to the addition of parasites. In controls, 0.5% of the DMSO diluent was used. In some experiments, the human BMECs were incubated for 30 minutes with BAPTA-AM, U73122, or calphostin C, then washed free of the inhibitors prior to parasite addition.
[Ca2+]i measurements.
Human BMECs were grown in M199 medium supplemented with 10% FBS and 10% Nu-serum on 22-mm collagen-coated square glass coverslips until at least 80% confluence. Cells were washed with serum-free medium and cultured overnight in low-serum (0.5% FBS) M199 medium. Before starting the experiments, the monolayers were washed with phenol red–free HEPES-buffered HBSS (137 mM NaCl, 4.2 mM NaHCO3, 0.4 mM Na2HPO4, 5.4 mM KCl, 0.4 mM KH2PO4, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, 5.6 mM d-glucose, 2 mM Na pyruvate, and 15 mM HEPES), pH 7.4, and incubated for 40 minutes with 3 μM Fura-2/AM and 0.04% Pluronic-123 in the dark at room temperature. After loading, the cells were washed to remove extracellular Fura-2/AM and incubated in phenol red–free HEPES-buffered HBSS for an additional 20 minutes. Fura-2–loaded cells were mounted into the recording chamber (Warner Instruments) on the microscope stage and fluorescence images were captured using an Olympus fluorescence microscopy system equipped with an inverted Olympus microscope IX70, a cooled OlymPix CCD camera (model TE3/A/S) used at a magnification of ×40, a 1.3–numerical aperture oil-immersion objective and a computer-controlled Sutter Filter Wheel (Sutter Instrument). Before starting fluorescence measurements, 25–40 regions of interest representing individual cells were selected on the field of view. Fura-2 fluorescent images were captured at 2-second intervals by alternating excitation of cells at 340- and 380-nm wavelengths, reflected off a dichroic mirror with a cutoff wavelength at 510 nm and bandpass emission filtering centered at 530 nm. The real-time fluorescent images were displayed on a monitor and stored on a hard drive for subsequent detailed analysis using UltraView software (version 4.0; PerkinElmer). The changes of [Ca2+]i were expressed as the 340:380 ratio, where F340 and F380 are Fura-2 fluorescence intensities obtained at 340- and 380-nm excitation wavelengths, respectively (34). Ca2+-free solutions were made using HEPES-buffered Ca2+-free HBSS with an additional 0.1 mM EGTA. For the interaction with T. b. gambiense, the parasites were washed twice in phenol red–free HEPES-buffered HBSS and resuspended in the same solution. Approximately 106 parasites were added to each monolayer, and the variation of fluorescence was measured as described above. K11777 inhibitor (20 μM) and 0.5% DMSO were added prior to addition of parasites to show that these agents did not cause [Ca2+]i changes in human BMECs. In the experiments using T. b. gambiense supernatants, 200 μl of parasite-free supernatants collected as described above were added to the coverslips.
Preparation of trypanosome lysates for enzyme assay.
Parasites were washed twice with HBSS containing 1 mM glucose, collected by centrifugation at 3,000 g, and lysed at a density of 5 × 105/μl in 50 mM sodium acetate; 200 mM NaCl; 5 mM EDTA, pH 5.5; and 1% Triton X-100. After incubation for 10 minutes on ice, the samples were cleared by centrifugation at 13,000 g for 15 minutes. Protein concentration was determined using the Dc-Protein kit (Bio-Rad), and equal amounts of protein were used to measure enzyme activity.
Assays of protease activity.
The protease activities of lysates was determined by the hydrolysis of 5 μM Z-Phe-Arg-AMC (Sigma-Aldrich) in 50 mM sodium phosphate; 200 mM NaCl; 5 mM EDTA, pH 6.0; 5 mM DTT; and 0.5% DMSO. Substrate hydrolysis was monitored in a Hitachi-F4500 Spectrofluorimeter at 380 nm excitation and 440 nm emission in continuous assays. The amount of activity attributable to cysteine proteases was verified by inhibition with E-64, CA074, or K11777, incubated separately at 2 μM with the samples for 15 minutes at room temperature prior to the addition of substrate. The initial velocities were calculated by linear regression of the hydrolysis curves.
Immunoprecipitation and active site affinity labeling blots.
For immunoprecipitation, samples containing 165 μg T. b. gambiense lysates were first incubated with 20 μM APC336 (see below). In parallel, 20 μl protein G–agarose beads (Sigma-Aldrich) were incubated either with 100 μl anti-brucipain antiserum (1:2 dilution) or with serum from nonimmunized mice for 2 hours at room temperature. The beads were washed 6 times with PBS before addition of lysate. The APC336-treated lysate was added to the beads and further incubated for 2 hours at room temperature. The beads were washed twice with PBS and diluted in SDS-PAGE sample buffer (62.5 mM Tris-HCl, pH 6.8; 2% SDS; 10% glycerol; 5% β-mercaptoethanol; and 0.012% bromophenol blue) and the mixtures boiled for 5 minutes. The supernatant was resolved on 11% SDS-PAGE gels, transferred to nitrocellulose, and blocked with 9% nonfat milk in PBS containing 0.05% Tween 20 and incubated with avidin–alkaline phosphatase (diluted 1:1,000). The reactive bands were visualized using NBT/BCIP (Promega) according to manufacturer’s instructions. Anti-brucipain antiserum obtained by immunization of mice with 200 μg recombinant brucipain lacking the C-terminal extension, followed by 2 boosts in 3 week intervals (Costa et al., unpublished observations). For active site labeling of cysteine proteases, 50-μg lysate aliquots were incubated for 30 minutes at room temperature with 5 mM DTT to activate the cysteine proteases, since these enzymes need to maintain the active-site cysteine in its reduced state to ensure activity. Then the samples were supplemented with 10 μM APC336 and incubated for 60 minutes at 37°C. Reactions were stopped by addition of SDS-PAGE sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.012% bromophenol blue), and the mixtures were subsequently boiled for 3 minutes. Samples were loaded into 11% SDS-PAGE gels, transferred to nitrocellulose membranes, blocked with nonfat milk, and incubated for 60 minutes with alkaline phosphatase–conjugated anti-biotin antibodies (NEB).
Statistics.
Statistical analyses throughout the study were performed by 1-way ANOVA using Prism software (version 4.0; GraphPad). A P value less than 0.05 was considered to be significant.
Acknowledgments
We wish to thank James H. McKerrow (UCSF, San Francisco, California, USA) for the donation of K11777, Jim Palmer (Axys Pharmaceuticals, San Francisco, California, USA) for the donation of APC336, Paul Englund (Department of Biological Chemistry, Johns Hopkins University, Baltimore, Maryland, USA) for T. b. brucei TREU 927, and John Mansfield (University of Wisconsin, Madison, Wisconsin, USA) for T. b. rhodesiense LouTAT 1.0. We would also like to thank J. Stephen Dumler (Johns Hopkins University, Baltimore, Maryland, USA), Peter G. Kennedy (Glasgow University, Glasgow, United Kingdom), and Masahiro Inoue (Kurume University, Fukuoka, Japan) for critical reading of the manuscript prior to submission. This work was supported by NIH grant 1 RO AI1464-01 and Fogarty International Research Collaboration Award 1 R03 TW006961-01 (to D.J. Grab); by American Heart Association grant 0435177N (to Y.V. Kim); by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) — Universal grant no. 471063/2004-5 (to A.P.C.A. Lima); by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and by funding to class 1A investigators of the CNPq (to J. Scharfstein). D.J. Grab dedicates this work to Maria Lucia Cardoso de Almeida (Universidade Federal de São Paulo, Escola Paulista de Medicina, São Paulo, Brazil) for her role in helping us establish the foundation for this collaborative study.
Footnotes
Nonstandard abbreviations used: APC336, biotin-methylpiperazine-urea-Phe-homopheylalanine-vinylsulfone-benzene; BAPTA-AM, 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester; BBB, blood-brain barrier; BMEC, brain microvascular endothelial cell; [Ca2+]i, intracellular calcium; CA074, ( l-3-trans-(propylcarbamyl)oxirane-2-carbonyl)- l-isoleucyl- l-proline; E-64, trans-Epoxysuccinyl- l-leucylamido(4-guanidino)butane; E-64d, (2S,3S)-trans-Epoxysuccinyl- l-leucylamido-3-methylbutane ethyl ester; K11777, N-methylpiperazine-urea-Phe-homopheylalanine-vinylsulfone-benzene; PLC, phospholipase C; U73122, 1-(6-[([17β]-3Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl)-1H-pyrrole-2,5-dione.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:2739–2747 (2006). doi:10.1172/JCI27798
Olga V. Nikolskaia and Ana Paula C. de A. Lima contributed equally to this work.
John D. Lonsdale-Eccles is retired.
References
- 1.Kennedy P.G.E. Human African trypanosomiasis of the CNS: current issues and challenges. J. Clin. Invest. 2004;113:496–504. doi: 10.1172/JCI200421052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naessens J. Bovine trypanotolerance: a natural ability to prevent severe anaemia and haemophagocytic syndrome? Int. J. Parasitol. 2006;36:521–528. doi: 10.1016/j.ijpara.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Grab D.J, et al. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J. Parasitol. 2004;90:970–979. doi: 10.1645/GE-287R. [DOI] [PubMed] [Google Scholar]
- 4.Dumas M., Bouteille B. Current status of trypanosomiasis. Med. Trop. (Mars). 1997;57:65–69. [PubMed] [Google Scholar]
- 5.Lonsdale-Eccles J.D., Grab D.J. Trypanosome hydrolases and the blood-brain barrier. Trends Parasitol. 2002;18:17–19. doi: 10.1016/s1471-4922(01)02120-1. [DOI] [PubMed] [Google Scholar]
- 6.Schultzberg M., Ambatsis M., Samuelsson E.B., Kristensson K., van Meirvenne N. Spread of Trypanosoma brucei to the nervous system: early attack on circumventricular organs and sensory ganglia. . J. Neurosci. Res. 1988;21:56–61. doi: 10.1002/jnr.490210109. [DOI] [PubMed] [Google Scholar]
- 7.Mulenga C., Mhlanga J.D., Kristensson K., Robertson B. Trypanosoma brucei brucei crosses the blood-brain barrier while tight junction proteins are preserved in a rat chronic disease model. . Neuropathol. Appl. Neurobiol. 2001;27:77–85. doi: 10.1046/j.0305-1846.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 8.Masocha W., et al. Cerebral vessel laminins and IFN-γ define Trypanosoma brucei brucei penetration of the blood-brain barrier. . J. Clin. Invest. 2004;114:689–694. doi: 10.1172/JCI200422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sajid M., McKerrow J.H. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 2002;120:1–21. doi: 10.1016/s0166-6851(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 10.Berriman M., et al. The genome of the African trypanosome Trypanosoma brucei. . Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 11.Troeberg L., Pike R.N., Lonsdale-Eccles J.D., Coetzer T.H.T. Production of anti-peptide antibodies against trypanopain-Tb from Trypanosoma brucei brucei: effects of antibodies on enzyme activity against Z-Phe-Arg-AMC. . Immunopharmacology. . 1997;36:295–303. doi: 10.1016/s0162-3109(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 12.Lalmanach G., et al. Congopain from Trypanosoma congolense: drug target and vaccine candidate. Biol. Chem. 2002;383:739–749. doi: 10.1515/BC.2002.077. [DOI] [PubMed] [Google Scholar]
- 13.Scory S., Caffrey C.R., Stierhof Y.D., Ruppel A., Steverding D. Trypanosoma brucei: killing of bloodstream forms in vitro and in vivo by the cysteine protease inhibitor Z-Phe-Ala-CHN2. . Exp. Parasitol. 1999;91:327–333. doi: 10.1006/expr.1998.4381. [DOI] [PubMed] [Google Scholar]
- 14.Troeberg L., et al. Cysteine protease inhibitors kill cultured bloodstream forms of Trypanosoma brucei brucei. . Exp. Parasitol. 1999;91:349–355. doi: 10.1006/expr.1998.4386. [DOI] [PubMed] [Google Scholar]
- 15.Scharfstein J., et al. Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B2 receptors. . J. Exp. Med. 2000;192:1289–1330. doi: 10.1084/jem.192.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todorov A.G., et al. Trypanosoma cruzi induces edematogenic responses in mice and invades cardiomyocytes and endothelial cells in vitro by activating distinct kinin receptor (B1/B2) subtypes. . FASEB J. 2003;17:73–75. doi: 10.1096/fj.02-0477fje. [DOI] [PubMed] [Google Scholar]
- 17. Scharfstein, J. 2003. Activation of bradykinin-receptors by Trypanosoma cruzi: a role for cruzipain in microvascular pathology. In Molecular mechanisms of pathogenesis in Chagas disease. J.M. Kelly, editor. Springer. New York, New York, USA. 111–137. [Google Scholar]
- 18.Aparicio I.M., Scharfstein J., Lima A.P.C.A. A new cruzipain-dependent pathway of human cell invasion by Trypanosoma cruzi requires trypomastigote membranes. . Infect. Immun. 2004;72:5892–5902. doi: 10.1128/IAI.72.10.5892-5902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caffrey C.C., et al. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. . Mol. Biochem. Parasitol. 2001;118:61–73. doi: 10.1016/s0166-6851(01)00368-1. [DOI] [PubMed] [Google Scholar]
- 20.Palmer J.T., Rasnick D., Klaus J.L., Bromme D. Vinyl sulfones as mechanism-based cysteine protease inhibitors. J. Med. Chem. 1995;38:3193–3196. doi: 10.1021/jm00017a002. [DOI] [PubMed] [Google Scholar]
- 21.Paemeleire K., de Hemptinne A., Leybaert L. Chemically, mechanically, and hyperosmolarity-induced calcium responses of rat cortical capillary endothelial cells in culture. Exp. Brain Res. 1999;126:473–481. doi: 10.1007/s002210050755. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y.V., DiCello F., Hillaire C.S., Kim K.S. Protease-activated receptors of human brain microvascular endothelial cells: expression and differential Ca2+ signaling induced by thrombin and protease-activated receptor-1 activating peptide. . Am. J. Physiol. Cell Physiol. . 2004;286:C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- 23.Mbawa Z.R., Gumm I.D., Fish W.R., Lonsdale-Eccles J.D. Endopeptidase variations among different life-cycle stages of African trypanosomes. Eur. J. Biochem. 1991;195:183–190. doi: 10.1111/j.1432-1033.1991.tb15693.x. [DOI] [PubMed] [Google Scholar]
- 24.Mackey Z.B., O’Brien T.C., Greenbaum D.C., Blank R.B., McKerrow J.H. A cathepsin B like protease is required for host protein degradation in Trypanosoma brucei. . J. Biol. Chem. 2004;279:48426–48433. doi: 10.1074/jbc.M402470200. [DOI] [PubMed] [Google Scholar]
- 25.Girard M., Giraud S., Courtioux B., Jauberteau-Marchan M.O., Bouteille B. Endothelial cell activation in the presence of African trypanosomes. Mol. Biochem. Parasitol. 2005;139:41–49. doi: 10.1016/j.molbiopara.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Miffert M.K., Baltimore D. Physiological functions for brain NF-κB. Trends Neurosci. 2005;28:37–42. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Karin M., Greten F.R. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. . 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 28.Nikolskaia O.V., Kim Y.V., Kovbasnjuk O., Kim K.J., Grab D.J. Entry of Trypanosoma brucei gambiense into microvascular endothelial cells of the human blood-brain barrier. . Int. J. Parasitol. 2006;36:513–519. doi: 10.1016/j.ijpara.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Burleigh B.A., Andrews N.W. Signaling and host cell invasion by Trypanosoma cruzi. . Curr. Opin. Microbiol. 1998;1:461–465. doi: 10.1016/s1369-5274(98)80066-0. [DOI] [PubMed] [Google Scholar]
- 30.Persidsky Y., et al. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 31.Grab D.J., et al. Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier. . Infect. Immun. 2005;73:1014–1022. doi: 10.1128/IAI.73.2.1014-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Deursen F.J., et al. Characterisation of the growth and differentiation in vivo and in vitro of bloodstream-form Trypanosoma brucei strain TREU 927. . Mol. Biochem. Parasitol. 2001;112:163–171. doi: 10.1016/s0166-6851(00)00359-5. [DOI] [PubMed] [Google Scholar]
- 33.Dempsey W.L., Mansfield J.M. Lymphocyte function in experimental African trypanosomiasis. VI. Parasite-specific immunosuppression. J. Immunol. 1983;130:2896–2898. [PubMed] [Google Scholar]
- 34.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. . J. Biol. Chem. 1985;260:3440–3450.. [PubMed] [Google Scholar]