Abstract
Objective
This study was designed to investigate the effects of human bone marrow stromal cell (hMSC) administration in rats for three months after traumatic brain injury (TBI).
Methods
Adult male Wistar rats (n = 60) were injured with controlled cortical impact and divided into four groups. The three treatment groups (n = 10 each) were injected with 2 × 106, 4 × 106, and 8 × 106 hMSCs intravenously, whereas the control group (n = 30), received phosphate buffered saline (PBS). All injections were performed 1 day after injury into the tail veins of rats. Neurological functional evaluation of animals was performed before and after injury using Neurological Severity Scores (NSS). Animals were sacrificed 3 months after TBI and brain sections were stained by immunohistochemistry.
Results
Statistically significant improvement in functional outcome was observed in all three treatment groups when compared with control (p < 0.05). This benefit was visible 14 days after TBI and persisted until 3 months (end of trial). There was no difference in functional outcome among the three treatment groups. Histological analysis showed that hMSCs were present in the lesion boundary zone at 3 months with all three doses tested.
Conclusion
hMSCs injected in rats after TBI survive until 3 months and provide long-lasting functional benefit. Functional improvement may be attributed to stimulation of endogenous neurorestorative functions such as neurogenesis and synaptogenesis.
Keywords: functional improvement, human marrow stromal cells, traumatic brain injury
INTRODUCTION
The use of marrow stromal cells (MSCs) to promote tissue repair after cellular injury in different organ systems is an exciting new area of research, which has potential for great therapeutic dividends. The multipotency of MSCs to differentiate into different tissues has been recognized for nearly a decade (1, 4, 5, 7). In the last few years MSCs have been used to restore function after neural, cardiac and skeletal injury (2, 8–10, 12–15, 17, 26). We have used MSCs as a neurorestorative agent to improve functional outcome after traumatic brain injury (TBI). We initially used rat and then subsequently human MSCs (hMSCs) to treat TBI in rats and found significant functional improvement with both (10, 12, 14, 15, 16, 17).
However, this functional improvement was not due to MSCs replacing damaged nerve cells, since only a small percentage of transplanted MSCs differentiate into neurons or astrocytes (16,17), but rather by enhancement of growth factor production by MSCs. MSCs produce growth factors and also induce intrinsic brain cells to produce them (3,11,13), which promote the neurorestorative actions of neurogenesis and synaptogenesis.
All of our experimental studies to date have examined the MSC effect until 1 month after treatment (12, 14, 15, 16, 17). Long-term studies were needed to evaluate if functional improvement persists over time. Therefore, in the present study hMSCs were injected in rats one day after TBI and functional outcome was analyzed for three months after treatment.
MATERIALS AND METHODS
All procedures have been approved by the Henry Ford Hospital Animal Care Committee and Institutional Review Board.
Preparation of hMSCs
hMSCs were prepared, frozen in liquid nitrogen and transported to our laboratory by Cognate Therapeutics Inc. (Sunnyvale, CA). hMSCs were restored and a sample was selected for cell counting by using trypan blue stain at 0.4%. Nucleated marrow cells were counted using a cytometer to ensure adequate cell number for transplantation. The cell survival rate ranged from 87%–97%. Dead cells were exluded from the injected cell number. hMSCs suspended in phosphate-buffered saline (PBS) were injected into rats via the tail vein.
Animal model and injection of human MSCs
A controlled cortical impact model in rat was used (6). Male Wistar rats (n = 60) were anesthetized with chloral hydrate 350 mg/kg bodyweight intraperitoneally. Rectal temperature was controlled at 37° ± 0.5°C with a feedback-regulated water-heating pad. A controlled cortical impact device was used to induce the injury. Rats were placed in a stereotactic frame. Two 10-mm diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The contralateral craniotomy allowed lateral movement of cortical tissue (21). The dura was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm diameter tip at the rate of 4 m/s and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer (6).
Rats were anesthetized with chloral hydrate 350 mg/kg bodyweight intraperitoneally, and cells suspended in 1 ml of PBS were slowly injected into the tail vein of rats one day after TBI. Control animals received PBS only injected into their tail vein. Three different doses of MSCs were used, and animals were divided into four groups as follows:
Group 1 (10): TBI + 2 × 106 hMSCs
Group 2 (10): TBI + 4 × 106 hMSCs
Group 3 (10): TBI + 8 × 106 hMSCs
Group 4 (30): TBI + PBS
All rats were sacrificed three months after TBI.
Brain sample preparation
Brain tissue of all rats from each group was processed for preparation of paraffin-embedded sections, which were used for histological evaluation and immunostaining analysis procedures. Rat brains were removed and stored in 10% buffered formalin for 48–72 hours. Standard 2 mm-thick blocks of rat brain were cut on a rodent brain matrix (a total of 7 blocks from A to G) and embedded with paraffin. A series of adjacent 6 μm-thick sections were cut.
Immunohistochemistry
Brain sections were stained with single as well as double fluorescent immunostaining. Single staining was performed for identification of hMSCs, whereas double staining was used for detection of neuronal and astrocytic differentiation of hMSCs. For single staining, sections were immunostained with a primary mouse antihuman nuclei monoclonal antibody (MAB-1281) for detection of the human cell (16). Briefly, after being deparaffinized, the sections were placed in boiling citrate buffer (pH 6) in a microwave oven for 10 minutes. After cooling at room temperature, the sections were incubated overnight in cation solution at 4°C. After blocking in normal serum, sections were treated with the mouse monoclonal antibody MAB-1281 (dilution, 1:500; Chemicon International, Temecula, CA) at 4°C overnight. Cy5 conjugated anti-mouse antibody (1:100) was added and incubated at room temperature in absence of light for 2 hours and counterstained with 4′,6-diamino-2-phenylindole (DAPI). All slices were mounted with antifade Mounting medium (Dako Citomation California, Inc.; Carpinteria, CA) and observed under fluorescent microscopy. To identify neuronal or astrocytic differentiation, adjacent sections were subjected to double staining, as described previously (16). For this step, the brain sections were initially stained for the neuronal marker, NeuN or an astrocytic marker, glial fibrillary acidic protein (GFAP), and then subsequently double stained with MAB-1281. Briefly, for the identification of neurons, sections were incubated in 0.1% saponin PBS at 4°C overnight with the monoclonal antibody NeuN (dilution 1:400; Sigma Chemical Co.). Antimouse FITC-conjugated F(ab’)2 fragment (dilution 1:20; Calbiochem, San Diego, CA) was then added and incubated for 1 week. To identify astrocytes, the sections were treated with 0.1% pepsin at 37°C for 15 minutes and then polyclonal antibody (GFAP (dilution 1:400, Dakopatts AB, Stockholm, Sweden) was added on separated sections. The sections were incubated with antirabbit FITC-conjugated F(ab’)2 fragment (dilution 1:20, Calbiochem, CA) for 1 week. The above sections were subsequently processed for staining with MAB-1281. Because the primary antibodies used against MAB-1281 and NeuN were monoclonal antibodies, a nonspecific positive reaction could occur in double-labeled staining. Therefore, a series of negative controls were used to assess and evaluate the immunohistochemical staining results. Negative control sections from each animal underwent identical staining preparation, except that the primary or secondary antibodies were omitted.
hMSCs (MAB-1281 positive cells) were identified in brain sections using fluorescent microscopy (BX- 40; Olympus Optical Co., LTD, Tokyo, Japan). This cellular analysis was performed on blocks E and F which contain the lesion core. Three slides with a 50-μm interval from each rat were analyzed using the MCID (Micro Computer Imaging Device) system and the MAB-1281/DAPI positive cells were counted within the lesion boundary zone with a 100 × objective lens. Data were presented as cells/mm2. To detect if hMSCs differentiated into neurons or astrocytes, double-stained sections were examined to identify those MAB-1281 positive cells which colabeled with NeuN or GFAP.
Neurological Functional Evaluation
Neurological function in the rats was assessed using the neurological severity scores (NSS). The NSS is composed of motor (muscle status, abnormal movement), sensory (visual, tactile and proprioceptive), reflex, and beam walking tests. In the severity scores of injury, one point is awarded for the inability to correctly perform the tasks or for the lack of a tested reflex. The higher the NSS score is, the more severe the injury (22). The evaluation of all rats was started before TBI and performed after TBI at 1 and 2 weeks, and biweekly thereafter. All measurements were performed by observers blinded to individual treatment.
Statistical Analysis
Data were analyzed by ANOVA for multiple comparisons.
RESULTS
Presence of MAB-1281 positive cells
We did not find MAB-1281 positive cells in the brain sections of the PBS-treated group; however, MAB-1281 positive cells were found in the hMSC treated groups. These cells were mainly found in the boundary zone of injured cortex (Fig. 1), though a few scattered cells were also seen in other areas of ipsilateral cortex, corpus callosum, striatum and contralateral cortex. This finding confirmed the survival of injected hMSCs at 3 months. The number of cells was significantly more in animals receiving 4 × 106 and 8 × 106 hMSCs than those receiving 2 × 106 (Fig. 2); however, there was no significant difference between animals receiving 4 × 106 and 8 × 106 hMSCs.
Fig. 1.
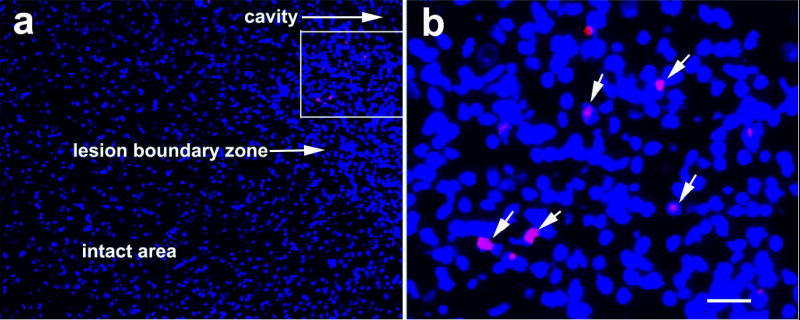
Photomicrographs showing the immunohistochemical staining for identification of hMSCs using MAB 1281 (red) counterstained with DAPI (blue) for nuclear staining. MAB 1281-positive cells are seen in the lesion boundary zone (a) with inset magnified in b to show the cells (arrow) more clearly. Scale bar (shown in b) equals 100 μm in a and 25 μm in b.
Fig. 2.
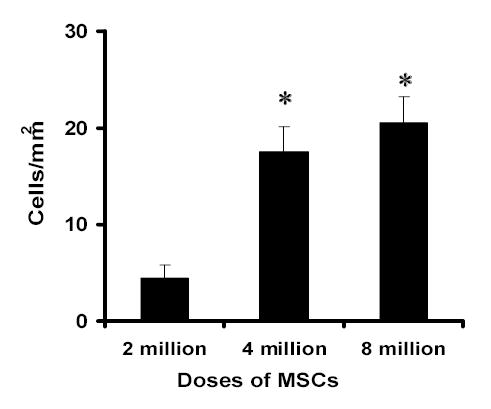
Bar graph showing the number of MAB1281-positive cells in injured brain 3 months after TBI. Significantly more MAB1281-positive cells were measured in 4 × 106 and 8 × 106 hMSC-treated groups than in the 2 × 106 treated group (*< 0.05). No significant difference was found between 4 × 106 and 8 × 106 groups.
Double fluorescent immunostaining showed that none of the MAB-1281 positive cells showed positive staining for GFAP or NeuN in any of the treatment groups. This demonstrates that hMSCs do not express neuronal or astrocytic markers three months after transplantation.
Neurological and motor function evaluation
Injury in the left hemispheric cortex of rats caused neurological functional deficits, as measured by the NSS, which is a composite of motor, sensory, reflex and beam walking tests. These rats presented with high scores on motor, sensory, reflex, and beam balance tests in the early phase after injury (first week postinjury). Recovery began in the second week and persisted at all subsequent evaluation times in both PBS-treated and MSC-treated groups. Motor function tested by the NSS recovered faster than sensory and beam balance functions. From 8 w to 12 w postinjury, residual deficit scores were present mainly on the beam balance and sensory tests of the NSS. NSS scores for the all hMSC-treated groups were significantly lower than the NSS scores of the PBS-treated groups, indicating an improvement in the functional outcome (Fig. 3). The treated animals uniformly showed this improvement with little variation among themselves and this improvement was seen as early as 14 days after TBI and was still evident at day 84. However, no significant difference in NSS scores was observed between different doses.
Fig. 3.
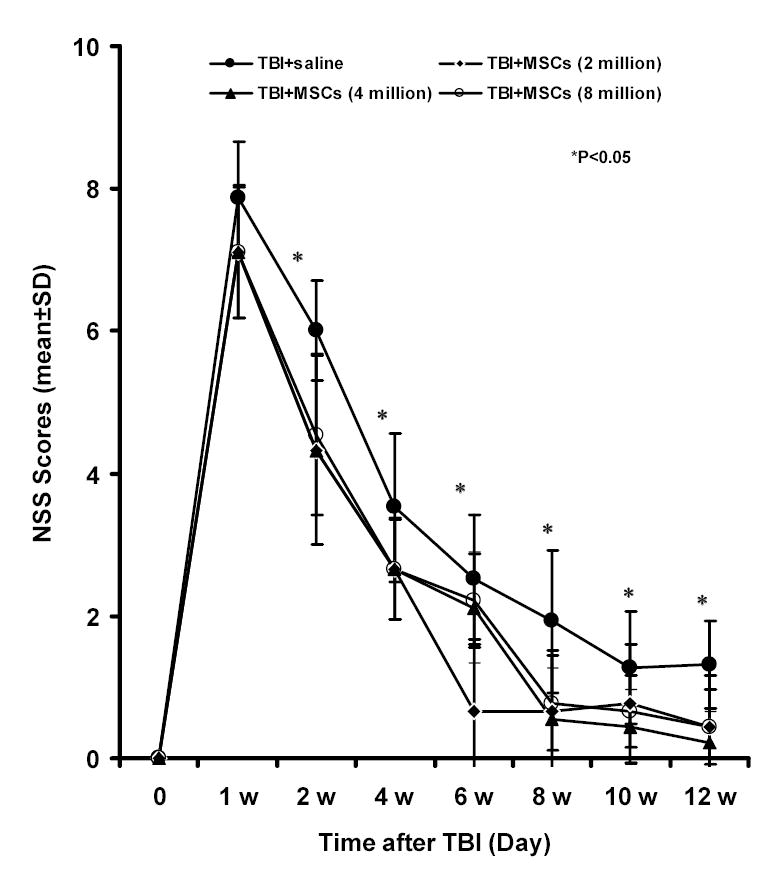
Results of Neurological Severity Scores (NSS) before and after TBI. Significant functional recovery was detected in rats treated with 3 different doses of hMSCs (2 x106, 4 × 106, and 8 × 106) compared with controls. This recovery was evident at 2 weeks after TBI and persisted at all subsequent time points. There was no difference among the three treatment groups.
DISCUSSION
In the current study we found that hMSCs injected intravenously in rats survive until 3 months after treatment, and that hMSC treatment promotes functional recovery until 3 months after treatment.
In a previous study we demonstrated functional improvement after treatment with 2 × 106 hMSCs at 1 month after TBI (16), whereas no functional benefit was detected with 1 × 106 hMSCs. To date, this study (16) is the only report investigating the effects of hMSCs on TBI. Further studies were needed to substantiate the initial findings and to investigate if the benefit seen acutely after treatment persists over time, since unless hMSCs can provide long-lasting benefit, their clinical application is limited. It was also necessary to evaluate other doses of MSCs to determine the optimum dose. Our data show that functional benefit induced by MSC treatment was obvious at 2 weeks after treatment and persisted until the end of the trial (3 months). This long-lasting benefit of hMSC treatment demonstrates the efficacy of hMSCs in promoting functional recovery.
Histologic analysis showed that 3 months after administration, hMSCs were still visible in the recipient brain, primarily around the lesion site; however, their number was less compared to a postmortem analysis performed at 1 month (16). With 2 × 106 hMSCs injected, the average number of cells found in the recipient brain at 1 month was 25 cells/mm2 (16) compared to 4 cells/mm2 at 3 months. Also at three months none of the hMSCs found in the brain showed astrocytic or neuronal differentiation. At one month post-transplantation the number of hMSCs showing astrocytic or neuronal differentiation was also very low (6–13%) (16). A preponderance of data indicate that MSC-induced functional recovery from stroke and brain injury is not due to MSCs replacing the damaged neurons but rather by MSCs inducing growth factor production and promoting intrinsic neurorestorative functions of the brain (9, 11, 13). The vital role of neurotrophic growth factors in neural repair and regeneration has been well established (9, 11, 13, 18–20, 25). We have previously shown that MSCs express as well as induce intrinsic parenchymal cells to express multiple growth factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), fibroblast growth factor (bFGF), and vascular endothelial growth factors (VEGF) both in vitro as well as in vivo (3, 13). Also, this induction of growth factor production persists over time and is evident at least until 3 months after treatment (report submitted for publication). In addition to growth factors, MSCs have recently been shown to produce brain natriuretic peptide (BNP), an important vasoactive factor that exerts powerful natriuretic, diuretic and vasodilatory effects (23, 24). BNP may facilitate recovery from neural injury by improving cerebral perfusion, decreasing intracranial pressure and reducing cerebral edema (23). BNP production by MSCs is enhanced by adding growth factors to their culture media (23) and since MSCs intrinsically produce growth factors, this may serve as an autoinductive stimulus to increase the expression of BNP.
Our data also showed that all three doses of hMSCs (i.e., 2 × 106, 4 × 106 and 8 × 106) were equally effective in improving functional outcome with no significant difference between them, even though the number of cells found in recipient brain was significantly more with 4 × 106 and 8 × 106 hMSCs than with 2 × 106 hMSCs. This may be due to a ceiling effect with 2 × 106 hMSCs providing an optimal number of cells to induce neurorestorative function with no added benefit observed by increasing the cell number. However, we also acknowledge that this lack of observed enhanced effect may be due to the limitation of functional tests. The NSS, though comprehensive, still do not encompass all aspects of neurological function, and dose increment may improve certain facets of neurological performance that remain undetected by NSS.
There was no histologic or clinical evidence of immunorejection observed with hMSC transplantation in rats. hMSCs have been used before in rat models of stroke, TBI and cardiac ischemia (8, 9, 16) without any problems of graft rejection. However, the duration of all these studies was short (2–4 weeks) and the probability of late graft rejection could not be ruled out. The present study addressed this important question and demonstrated that hMSC transplantation does not cause early or late xenograft rejection. This may be because of weak immunogenicity of hMSCs (16). Li et al (9) studied mixed lymphocyte reaction and the development of cytotoxic T lymphocytes after injecting MSCs in a stroke model in rats. They did not find any evidence of immune rejection or development of cytotoxic T cell response to hMSC administration.
Biological transplantation using MSCs as a means of inducing neuroplasticity to treat TBI and neural injury is a promising new therapy. However, before these laboratory insights can be translated into clinical therapeutic interventions, questions such as optimum dose and long-term effects both toxic and beneficial have to be investigated. Our present data addresses some of these questions and brings us closer to the clinical applications of these agents. However, other questions such as the molecular and genetic basis of this neural restoration induced by hMSCs require further investigation.
References
- 1.Borlongan CV, Stahl CE, Cameron DF, Saporta TB, Freeman DW, Cahill PR. CNS modulation of graft survival and rejection. Neurol Res. 1996;18:297–304. doi: 10.1080/01616412.1996.11740425. [DOI] [PubMed] [Google Scholar]
- 2.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Katakowski M, Li Y. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. J Neurosci Res. 2002;69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 4.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: Induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 5.Clark BR, Keating A. Biology of bone marrow stroma. Ann N Y Acad Sci. 1995;770:70–78. doi: 10.1111/j.1749-6632.1995.tb31044.x. [DOI] [PubMed] [Google Scholar]
- 6.Dixon E, Clifton G, Lighthall JW. A controlled cortical impact model of traumatic brain injury in rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 8.Kocher A, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chen J, Chen XJ. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 10.Lu D, Li Y, Mahmood A, Wang L, Rafiq T, Chopp M. Neural and marrow-derived stromal cell sphere transplantation in a rat model of traumatic brain injury. J Neurosurg. 2002;97:935–940. doi: 10.3171/jns.2002.97.4.0935. [DOI] [PubMed] [Google Scholar]
- 11.Lu D, Mahmood A, Chopp M. Biological transplantation and neurotrophic induced neuroplasticity after traumatic brain injury. J Head Trauma Rehabil. 2003;18:357–376. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lu D, Mahmood A, Li Y, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood A, Lu D, Li Y, Chen JL, Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improves functional outcome in adult rats. J Neurosurg. 2001;94:589–595. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- 15.Mahmood A, Lu D, Li Y, Chopp M. Intracerebral transplantation of marrow stromal cells (MSCs) improves outcome after traumatic brain injury. J Neurotrauma. 2002;19:1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- 16.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rat with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–703. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood A, Lu D, Wang L, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1204. [PubMed] [Google Scholar]
- 18.Memberg SP, Hall AK. Proliferation, differentiation, and survival of rat sensory neuron precursors in vitro require specific trophic factors. Mol Cell Neurosci. 1995;6:323–335. doi: 10.1006/mcne.1995.1025. [DOI] [PubMed] [Google Scholar]
- 19.Newman MP, Feron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience. 2000;99:343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 20.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 21.Postmantur R, Kampel A, Simon R. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:765–787. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- 22.Shohami E, Novikov M, Bass R. Long term effect of HU-211, a novel non-competitive NMD antagonist, on motor and memory functions after closed head injury in rat. Brain Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- 23.Song S, Kamath S, Mosquera D, Zigova T, Sanberg P, Vesely DL, Sanchez-Ramos J. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp Neurol. 2004;185:191–197. doi: 10.1016/j.expneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Vesely DL, Sallman AL, Bayliss JM. Specific binding site for proatrial natriuretic factors 1–30, 31–67, and 99–126 on distal nephrons, proximal tubules, renal cortical and medullary membranes. Renal Physiol Biochem. 1992;15:23–32. doi: 10.1159/000173438. [DOI] [PubMed] [Google Scholar]
- 25.Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao LR, Duan WM, Reyes M, Keene CD, Verfai Uie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]


