Abstract
Male germ cell development includes mitotic and meiotic cell divisions that are followed by dramatic morphological changes resulting in the production of spermatozoa. Genetic evidence has indicated that the DAZ family genes are critical for successful male germ cell development in diverse animals as well as humans. In the present study, we investigated the cellular functions of Dazl in the mouse male germ cells. We identified a specific interaction of Dazl with the dynein light chain, a component of the dynein–dynactin motor complex. The subcellular distribution of Dazl was microtubule-dependent and a selected number of Dazl-bound mRNAs could accumulate in the perinuclear area. Based on these results, we propose that Dazl may play a role in transport of specific mRNAs via dynein motor complex. The Dazl-bound mRNAs may be stored at specific sites and would be available for future developmental processes. Our study revealed the presence of an active mRNA transport system in mouse male germ cells.
Keywords: Dazl, dynein, dynein light chain, male infertility, mRNA transport
Introduction
A significant proportion of the azoospermic male infertility patients have micro-deletions at specific loci called AZF in the Y chromosome, suggesting that some genes located at these loci are critical for spermatogenesis (Tiepolo and Zuffardi, 1976). Of all the genes located at the AZF loci, DAZ has been considered to be a male infertility gene (Reijo et al, 1995). Genetic evidence indicates that the DAZ family genes are critical for germ cell development in diverse animals as well as in humans. The disruption of the Dazl gene in mice leads to loss of germ cells and complete absence of gamete production (Ruggiu et al, 1997). A careful examination of the Dazl-deficient mice revealed that the leptotene–zygotene stage of meiotic prophase I was the final point reached in the male germ cell development (Saunders et al, 2003). A study on the Drosophila boule mutant revealed the primary defect in the meiotic cell division of male germ cells (Eberhart et al, 1996). In Caenorhabditis elegans, the loss of DAZ-1-function caused female sterility due to the blockage of oogenesis at the pachytene stage of meiotic prophase I (Karashima et al, 2000).
There are three DAZ family genes in the human genome. Four copies of the DAZ gene are located at the AZFc locus in the Y chromosome, and DAZL and BOULE are autosomal genes (Reijo et al, 1996; Saxena et al, 2000; Kuroda-Kawaguchi et al, 2001). The Y chromosomal DAZ is found only in humans and Old World primates, while the autosomal DAZ family genes are found to be present in all the tested organisms (Xu et al, 2003). Genetic rescue experiments were carried out to determine the functional redundancy among the DAZ family proteins. Male germ cell development in the Dazl-deficient mice was rescued partially by human DAZ or DAZL genes (Slee et al, 1999; Vogel et al, 2002). Similarly, the introduction of Xenopus Dazl or human BOULE into the boule mutant fly led to the completion of meiosis in male germ cells (Houston et al, 1998; Xu et al, 2003). These results suggest that the DAZ family proteins play related, yet distinct roles in male germ cell development.
The presence of an RNA-binding domain within the Dazl protein suggested its involvement in the RNA metabolism. Diverse strategies have been adopted to identify the mRNA species that are specifically bound to Dazl. A consensus sequence for the Dazl binding-element was defined and from among the candidate mRNA species, Cdc25C was confirmed to interact with Dazl (Venables et al, 2001). The mRNA species that interact with the GST-Dazl fusion protein was isolated and Cdc25A and Tpx1 from among candidate mRNAs were suggested to specifically interact with Dazl (Jiao et al, 2002). Fox et al (2005) identified the mRNAs to which both Dazl and a Dazl-interacting protein Pum2 were bound simultaneously, and Sdad1 was selected from the candidate mRNAs to be characterized further. Finally, the mRNA species that were coimmunoprecipitated with the endogenous Dazl protein were identified, and Mvh from among the candidate mRNAs was confirmed to interact with Dazl in vivo (Reynolds et al, 2005). These results indicate that Dazl interacts with a specific set of mRNA species and probably controls their expression.
The active transport of mRNAs along the cytoskeletal network has been described in selected model systems (reviewed in St Johnston, 2005). Perhaps, the best understood example of this process may be the transport of the ASH1 mRNA to the bud tip in Saccharomyces cerevisiae, thus repressing mating-type switching in daughter cells. This transport is actin-dependent and requires Myo4-a type V myosin-and additional coupling proteins such as She2 and She3 (reviewed in Cosma, 2004). Microtubule-dependent transport of mRNAs has been reported in Drosophila oocytes (Januschke et al, 2002). The oskar mRNA is transported from nurse cells to the posterior end of the oocytes by kinesin, a plus-end-directed microtubule motor protein (Brendza et al, 2000). On the other hand, the bicoid mRNA is transported and anchored to the anterior end of the oocytes. Dynein is responsible for bicoid transport, and a number of additional proteins such as Exuperantia, Swallow, γ-tubulin37C, Grip75 and Staufen are also involved in the transport and retention of bicoid at the anterior end of the oocytes throughout oogenesis (Ferrandon et al, 1994; Schnorrer et al, 2000, 2002; Cha et al, 2001). In particular, Swallow was known to interact with the dynein light chain (Dlc), suggesting its function as an adaptor for bicoid mRNA to dynein (Schnorrer et al, 2000). Other examples may be gurken mRNA that are moved within the oocytes in two dynein-dependent steps (MacDougall et al, 2003), and a group of pair-rule mRNAs that are transported in association with dynein and accumulated in the apical region of the syncytial blastoderm (Wilkie and Davis, 2001; Delanoue and Davis, 2005). The active transport of mRNAs through microtubules was also observed in Xenopus oocytes and mammalian neurons (reviewed in St Johnston, 2005; Carson and Barbarese, 2005). The primary goal of the active mRNA transport is the localization of mRNAs in a specific region of the cell. Translational regulation is always coupled with the active transport mechanisms.
The regulation of gene expression at translational levels has been emphasized in germ cell development (reviewed in Kuersten and Goodwin, 2003). In the present study, we report that Dazl interacts with Dlc, thereby suggesting that Dazl functions as an adaptor for a specific set of mRNAs on the dynein motor complex. Our study revealed the presence of an active mRNA transport system in mouse male germ cells.
Results
Dazl interacts with Dlc
In order to elucidate the biological functions of Dazl in male germ cells, we carried out yeast two-hybrid screenings with Dazl as the bait and isolated Dlc1 clones most predominantly. In addition, multiple clones of DAZAP2 (Tsui et al, 2000a) were also identified. The physical interaction of Dazl with Dlc1 was examined by the GST pull-down assay (Figure 1A). The results showed that the Flag-Dlc1 protein in the 293T cell lysates was coprecipitated with GST-Dazl, and the HA-Dazl protein was coprecipitated with GST-Dlc1. When the mouse testicular lysates were incubated with GST-Dazl, the endogenous Dlc was coprecipitated (Figure 1A). The physical association of endogenous Dazl and Dlc1 proteins was also confirmed by coimmunoprecipitation experiments (Figure 1B). When testicular lysates were incubated with antibodies against Dazl or Dlc, both the proteins were immunoprecipitated, thereby indicating a physical interaction between Dazl and Dlc in the mouse male germ cells.
Figure 1.
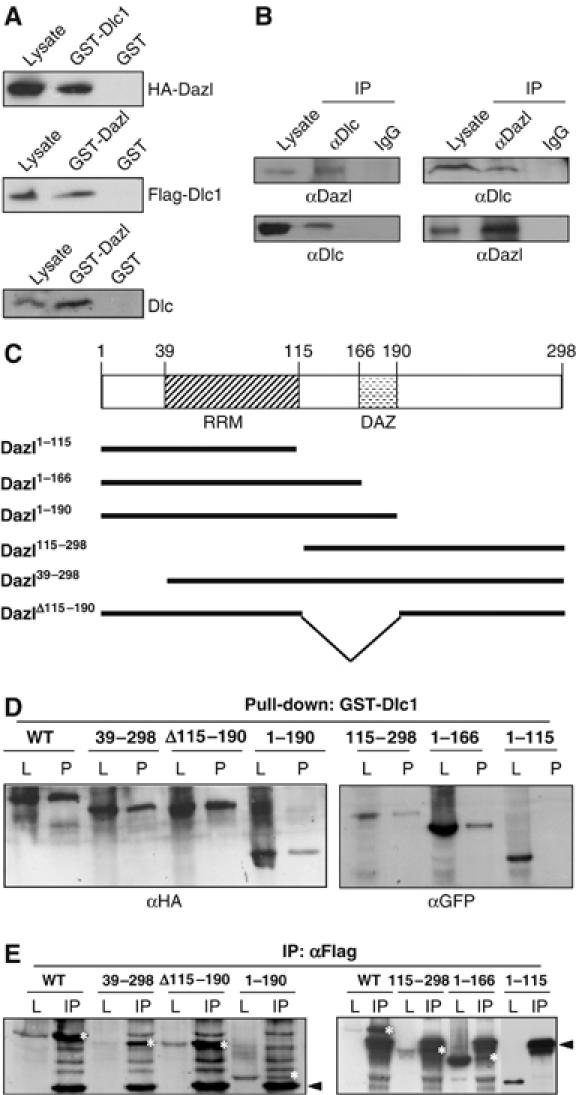
Interaction of Dazl with Dlc (A) GST pull-down assay. The cell lysates of the 293T cells in which the HA-Dazl or Flag-Dlc1 proteins were ectopically expressed were incubated with the GST-Dlc1 or GST-Dazl fusion protein and the coprecipitated proteins were detected using antibodies against the HA or Flag epitopes. Mouse testicular lysates were incubated with GST-Dazl, and the coprecipitated Dlc protein was detected using the Dlc antibody. (B) Coimmunoprecipitation assay. The endogenous Dazl or Dlc protein in the testicular lysates was immunoprecipitated, and the coimmunoprecipitated proteins were detected using the specific antibodies. IgG was used as a control for immunoprecipitation. (C) Truncated mutants of the mouse Dazl gene. The mouse Dazl protein contains an RNA-recognition motif (RRM) for RNA binding and a DAZ repeat (DAZ) that is characteristic to the DAZ family proteins. (D) The 293T cells were transfected with the expression vectors of truncated Dazl mutants tagged with HA (pHA-Dazl, pHA-Dazl39−298, pHA-DazlΔ115−190 and pHA-Dazl1−190) or with GFP (pGFP-Dazl115−298, pGFP-Dazl1−166 and pGFP-Dazl1−115) and were subjected to GST pulldown assays (P) with the GST-Dlc1 fusion protein. Antibodies against the HA or GFP epitopes were used for the detection of the Dazl mutant proteins. The cell lysates (L) were simultaneously loaded on the gels as the size markers of the Dazl mutant proteins. (E) The 293T cells cotransfected with the pFLAG-Dlc1 and Dazl mutant vectors were subjected to immunoprecipitation (IP) assays with the Flag antibody. The Dazl proteins were detected using antibodies against the HA or GFP epitopes. The cell lysates (L) were simultaneously loaded on the gels as the size markers of the Dazl mutant proteins. The Dazl protein bands are marked as white asterisks and the heavy and light chains of IgG are indicated with arrow heads.
In order to define the Dazl sequence that is responsible for the interaction of Dazl with Dlc1, we performed GST pull-down and coimmunoprecipitation assays with truncated Dazl mutant proteins. The mouse Dazl protein contains an RNA recognition motif and a so-called DAZ domain (Figure 1C). Truncated Dazl mutants tagged with HA or GFP were transfected into the 293T cells and subjected to GST pull-down assays using GST-Dlc1 fusion proteins. The results showed that all the tested Dazl mutants, except Dazl1−115, were coprecipitated with the GST-Dlc1 fusion protein (Figure 1D). Coimmunoprecipitation assays conducted with the same set of Dazl mutants also demonstrated positive physical interactions with Dlc1, except in the case of the Dazl1−115 mutant protein (Figure 1E). These results indicate that Dlc interacts with the C-terminal end of the Dazl protein. This binding activity is distinct from that of poly(A)-binding protein (PABP), which interacted with neither Dazl1−115 nor Dazl115−298 mutant proteins (Collier et al, 2005).
Dlc mediates the association of Dazl with the dynein–dynactin complex
We examined the association of Dazl with the dynein–dynactin complex. When the testicular lysates were immunoprecipitated with the Dazl antibody, the dynein intermediate chain (Dic) and p150Glued were coimmunoprecipitated, thus indicating that Dazl is associated with the dynein–dynactin complex in the mouse male germ cells (Figure 2A). A GST pull-down assay was carried out with GST-Dazl and ectopically expressed Dic proteins. The results showed that HA-Dic1 was coprecipitated with GST-Dazl only in the presence of Flag-Dlc1 (Figure 2B). The indirect interaction of Dic1 with Dazl was also confirmed by the coimmunoprecipitation assay using the 293T cells co-transfected with a combination of pMyc-Dazl, pHA-Dic1 and pFlag-Dlc1. The HA-Dic1 proteins were coimmunoprecipitated with Myc-Dazl only in the presence of Flag-Dlc1 (Figure 2C). Identical results were obtained on the same set of experiments conducted with Dic2 (data not shown). These results strongly suggest that Dlc mediates the association of Dazl with the dynein–dynactin complex.
Figure 2.
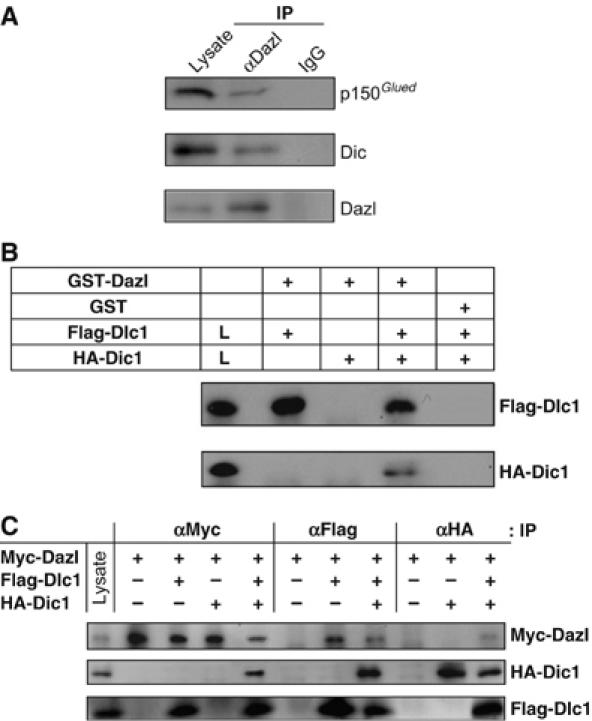
Association of Dazl with the dynein–dynactin complex. (A) In vivo interaction of Dazl with Dic and p150Glued in the testis. The endogenous Dazl protein in the testicular lysates was immunoprecipitated (IP), and the coimmunoprecipitated proteins were detected using the specific antibodies against p150Glued and Dic. IgG was used as a control for immunoprecipitation. Testicular lysates were used for detection of the endogenous p150Glued, Dic and Dazl proteins in the immunoblots. (B) Indirect interaction of Dazl with Dic: GST pull-down assay. The 293T cell lysates transfected with pFlag-Dlc1 and/or pHA-Dic1 were incubated with the GST-Dazl fusion protein or with the GST protein as a control. The precipitated Dlc1 and Dic1 proteins were detected using the Flag and HA antibodies, respectively. The HA-Dic1 protein was precipitated with GST-Dazl only in the presence of Flag-Dlc1. (C) Indirect interaction of Dazl with Dic: immunoprecipitation assay. The 293T cell lysates cotransfected with pFlag-Dlc1 and/or pHA-Dic1 along with the Dazl expression vector (pMyc-Dazl) were immunoprecipitated, followed by immunoblotting using the indicated antibodies. The cell lysates were loaded on the gels to confirm the expression of Myc-Dazl, HA-Dic1 and Flag-Dlc1 proteins in the cells. The HA-Dic1 protein was coimmunoprecipitated with Myc-Dazl only in the presence of Flag-Dlc1.
Dazl travels via the microtubule network
In vivo association of Dazl with dynein–dynactin complex allowed us to propose that Dazl travels via the microtubule network. In the COS7 cells, the ectopic Dazl proteins were located in the perinuclear area in either a diffused or an aggregated form and endogenous Dlc colocalized with the Dazl proteins (Figure 3A). When nocodazole was added to the culture medium to disrupt the microtubule network, it caused the dispersion of both ectopic Dazl and endogenous Dlc proteins throughout cytoplasm; this suggests that Dazl is linked to the microtubule network in cells (Figure 3A). Little change was observed in the subcellular distribution of Dazl in the cytochalisin D-treated cells; this suggests that the Dazl distribution is not actin-dependent (Figure 3A). The overexpression of dynamintin, a component of the dynactin complex, has been known to induce the disruption of the dynein–dynactin complex from the microtubule network (Echeverri et al, 1996). As expected, HA-Dazl had a scattered distribution throughout the cytoplasm in the dynamitin-overexpressed COS7 cells (Figure 3B). Finally, endogenous Dazl coprecipitated with taxol-stabilized microtubules along with Dic, while Rbm, another male infertility protein, did not (Figure 3C). Taken together, these results indicate that Dazl is associated with the microtubule network via the dynein–dynactin complex.
Figure 3.
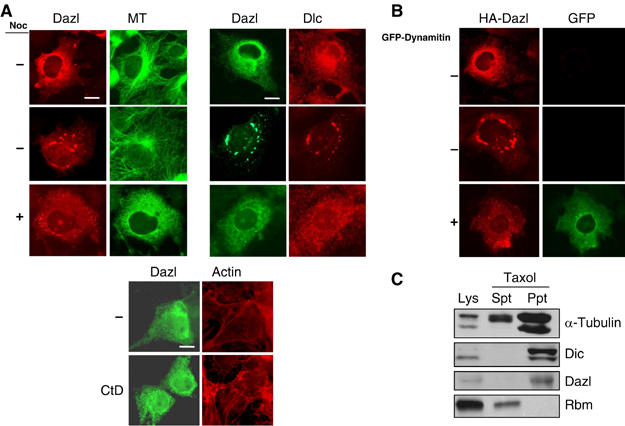
Dazl travels via the microtubule network. (A) The COS7 cells transfected with pHA-Dazl were treated with either 10 μM nocodazole (Noc) or 1 μM cytochalasin D (CtD) for 1 h and immunostained with antibodies against Dazl, α-tubulin and Dlc. Actin was stained with phaloidin. Scale bars, 10 μm. (B) Subcellular distribution of HA-Dazl in the COS7 cells was observed in the absence or presence of GFP-Dynamitin. Scale bar, 10 μm. (C) Association of Dazl with microtubules. The soluble fraction of the mouse testicular lysates (Lys) was treated with 20 μM taxol and 0.5 mM GTP and centrifuged to separate the precipitate (Ppt) from the supernatant (Spt). Immunoblotting analyses were performed using antibodies against α-tubulin, Dic, Dazl and the Rbm proteins.
The real-time movement of the ectopic Dazl protein was measured in the cultured cells. The CFP-Dazl protein moved along straight or curvilinear tracks intermittently, whereas the GFP-Dazl1−115 mutant protein remained stationary (Figure 4A and Supplementary videos). The polarity and speed of movement of Dazl and Dazl1−115 were quantitated. Dazl moved in a retrograde direction more frequently, while the Dazl1−115 mutant protein remained stationary and did not have any directional preferences (Figure 4B). The median speed of the CFP-Dazl particles was approximately 0.3 μm/s (Figure 4C); this is comparable to that of the dynein motor complex (Gross et al, 2000). These results are consistent with the hypothesis that Dazl travels via the microtubule network in association with the cytoplasmic dynein motor complex.
Figure 4.
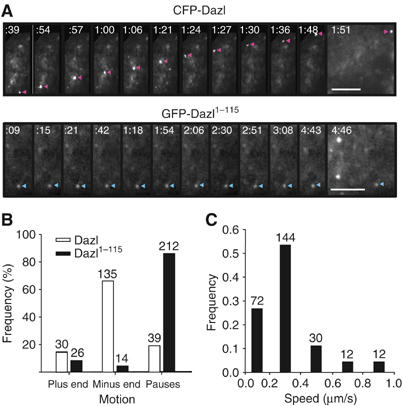
Live cell images of the Dazl proteins in cultured cells. (A) The COS7 cells were transfected with pCFP-Dazl or pGFP-Dazl1−115. The movement of the CFP-Dazl and GFP-Dazl1−115 proteins was recorded 24 h after the transfection. The Dazl particles are indicated by arrowheads. The elapsed times of sequences was recorded (ms). Scale bars, 5 μm. (B) Directional movement of the Dazl proteins in cultured cells. The directions of the Dazl protein movements were analyzed using the COS7 cells transfected with pCFP-Dazl (blank bar) or pGFP-Dazl1−115 (solid bar). Signals slower than 0.05 μm/s were considered to be in pause. The number of signals that moved toward the periphery (plus end) or nucleus (minus end) was counted and indicated at the top of the bars. (C) Velocity distribution of the retrograde Dazl movements. The velocity distribution of the CFP-Dazl protein was analyzed in the COS7 cells. The number of particles counted was indicated on the top of the bar.
Dazl controls the subcellular distribution of a specific set of mRNAs
Dazl was reported to bind to a specific set of mRNAs (Venables et al, 2001; Jiao et al, 2002; Fox et al, 2005; Reynolds et al, 2005). In order to examine whether Dazl functions as an mRNA transporter in cells, we determined the subcellular distribution of the target mRNAs by using a system involving the MS2-GFP fusion protein (Fusco et al, 2003). This protein was located in the nucleus due to the presence of a nuclear localization signal within the protein (Figure 5A (a–d)). In the presence of an mRNA with the MS2-binding sites, a fraction of the MS2-GFP fusion proteins was transported to the cytoplasm, following the MS2-fusion mRNA to which it bound (panels e, i, and m). In the presence of Dazl, the fluorescent signals aggregated in the perinuclear area of cells with the MS2-Tpx1 and MS2-Cdc25C fusion mRNAs (panels f and n) but not in cells with the MS2-Cdc25A fusion mRNA (panel j). The Dazl protein was also colocalized in the MS2-Tpx1 and MS2-Cdc25C aggregates (data not shown). Such perinuclear aggregates were absent in cells with Dazl1−115 that does not bind to Dlc1 (panels g and o) as well as in cells with Dazl115−298 that lacks the RNA-binding motif (panels h and p). These results suggest that the Dazl-bound mRNAs such as Tpx1 and Cdc25C are linked to the cytoplasmic dynein motor complex and accumulate at specific sites within a cell.
Figure 5.
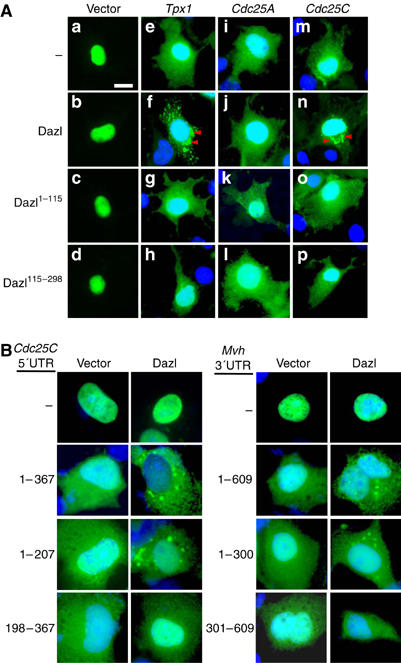
Specific association of Dazl with the Cdc25C, Tpx1 and Mvh mRNAs. (A) The COS7 cells were transfected with pSL-MS2 linked to Tpx1 3′UTR (pMS-Tpx1), Cdc25A 3′UTR (pMS2-Cdc25A) or Cdc25C 5′UTR (pMS2-Cdc25C), along with pHA-Dazl, pHA-Dazl1−115, or pHA-Dazl115−298. The subcellular localization of the fusion mRNAs was traced using the fluorescent signals of the MS2-GFP protein. The MS2–Tpx1 and MS2-Cdc25C fusion mRNAs formed aggregates in the perinuclear area of the cells (arrowheads) scale bar, 10 μm. (B) The COS7 cells were transfected with pSL-MS2 linked to Cdc25C 5′UTR of full-length (1–367) or truncated (1–207 and 198–367) sequences or to Mvh 3′UTR of full-length (1–609) or truncated (1–300 and 301–609) sequences along with pHA or pHA-Dazl. The subcellular localization of the fusion mRNAs was traced as described.
Dazl-binding elements have been defined in selected mRNAs. The (GUn)n stretch located between nucleotides 123–160 of the 5′UTR of Cdc25C was designated as a possible binding site of Dazl (Venables et al, 2001). Dazl-binding elements were also defined in the first 300 nucleotide residues of the 3′UTR of the Mvh mRNA (Reynolds et al, 2005). In order to confirm that the perinuclear aggregation of mRNAs depends on their specific recognition by Dazl, we prepared MS2 mRNAs linked to the 5′UTR of Cdc25C or the 3′UTR of the Mvh sequences and determined their distribution. The results showed that only the mRNA species that possessed the Dazl-binding elements could form perinuclear aggregates (Figure 5B). These results confirmed that Dazl binding is required for the specific accumulation of mRNAs in perinuclear areas.
Ectopic Dazl accumulates at the stress granule
In response to an assortment of environmental stresses, cells limit translation activity by accumulating mRNAs at discrete cytoplasmic foci called stress granules (Anderson and Kedersha, 2002; Kimball et al, 2003). The mRNAs in the stress granules are not degraded; they are stored and are available for re-initiation as soon as the cell recovers from the stress. Since we observed that only specific mRNAs formed granules in the perinuclear area along with ectopic Dazl (Figure 5A (f and n)), we investigated whether the Dazl-mRNA aggregates in cultured cells were indeed stress granules. In HeLa cells with the dispersed Dazl proteins, TIA1, a stress-granule marker, was detected mostly at the nucleus (Figure 6A). However, in cells with Dazl aggregates, TIA1 was found to be colocalized in the perinuclear area along with Dazl (Figure 6A). When the cells were treated with arsenite for the induction of the stress granule, most of the intracellular Dazl formed aggregates along with TIA1 (Figure 6A). In fact, the ectopic expression of Dazl, but not of Dazl1−115 or Dazl115−298, induced significant stress granule formation (Figure 6B). These results suggest that Dazl transports specific mRNAs to a stress granule-like body in mouse male germ cells.
Figure 6.
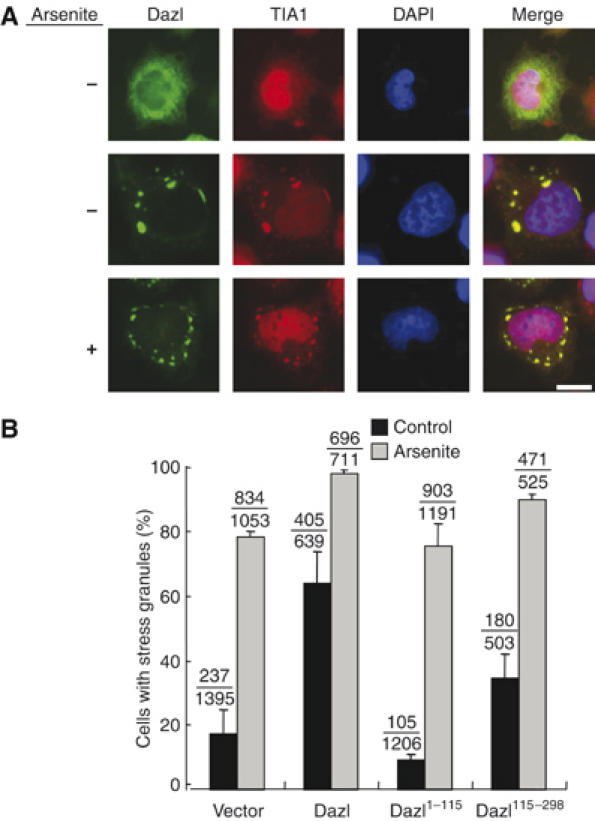
Subcellular localization of Dazl. (A) The HeLa cells with HA-Dazl were coimmunostained with antibodies against HA and TIA1, a stress-granule marker. The cells were treated with arsenite (0.5 mM) to induce the formation of the stress granules. The DNA was stained with DAPI. Scale bar, 10 μm. (B) The HeLa cells transfected with HA-Dazl, HA-Dazl1−115 and HA-Dazl115−298 were cultured in the presence or absence of 0.5 mM arsenite for 45 min and immunostained with the TIA1 and HA antibodies. These experiments were repeated three times. Total number of the cells with stress granules is indicated on the top of the bars.
Discussion
In the present study, we observed a specific interaction between Dazl and Dlc in mouse male germ cells. Based on the results, we propose that Dazl functions as an adaptor for mRNAs located on the cytoplasmic dynein motor complex. In other words, Dazl recognizes a specific set of mRNAs, links them to the dynein–dynactin complex, and transports them to an RNA granule that is analogous to the stress granules in male germ cells (Figure 7). The Dazl-bound mRNAs in the RNA granules may be stable but are translationally dormant. When the Dazl-bound mRNAs are released from the RNA granules, they may become translationally active. We do not rule out the possibility that some of these Dazl-bound mRNAs may be directly transported to the ribosomes.
Figure 7.
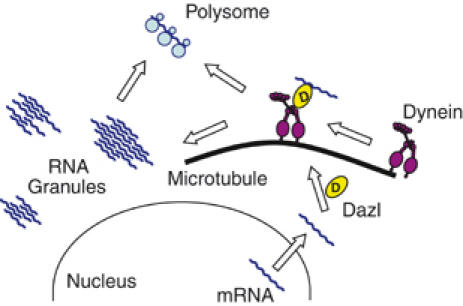
Model. Dazl recognizes a specific set of mRNAs, links them to the cytoplasmic dynein motor complex and transports them to an RNA granule within the cytoplasm of male germ cells. Some of the Dazl-bound mRNAs may be directly transported to ribosomes.
The presence of an active mRNA transport system was rather unexpected in mouse male germ cells wherein the polarized distribution of mRNAs has not yet been reported. Rather, its significance may reside in the translational control of the transported mRNAs. The Dazl-bound mRNAs may be stored to prevent them from being degraded until a proper developmental cue necessitates for their translation. Thus, the spermatocytes can undergo complex and highly elaborate developmental processes such as meiosis with precision.
It remains to be investigated where the Dazl-bound mRNAs are stored within the mouse male germ cells. One candidate site may be the nuage structure, which was initially reported in the oocytes of selected organisms and also in the cytoplasm of the mouse spermatocytes (Fawcett et al, 1970; Russell and Frank 1978; reviewed in Ikenishi, 1998). It was known that nuage is essentially an RNA granule with mRNAs for the development and specification of early embryonic cells and proteins for the regulation of mRNA translation and decay (reviewed in Anderson and Kedersha, 2006). The chromatoid body precursor may be the best characterized nuage structure in the mouse male germ cells (Noce et al, 2001; Kotaja et al, 2006). However, the Dazl-bound mRNAs may be accumulated not only in a single type of RNA granules but also in other types of nuage structures present in spermatocytes, such as Rnf17 granules (Pan et al, 2005). Further, we do not rule out the possibility that the Dazl-bound mRNAs accumulate in structures other than RNA granules in male germ cells. Therefore, a thorough investigation of the subcellular distribution of the Dazl-bound mRNAs appears to be critical.
Previous reports in which the Dazl protein was cofractionated with a polysome pool indicated that the Dazl-bound mRNA may be translationally active (Tsui et al, 2000b; Maegawa et al, 2002). Consistent with this viewpoint, Collier et al (2005) reported that Dazl interacted with the PABP and augmented the translation of target mRNAs in vivo. On the other hand, the interaction of Dazl with Pum2, a potential translation repressor, was also observed (Moore et al, 2003). These results suggest that Dazl functions as a platform for the translational regulation of the bound mRNA. We think that Dazl can perform both functions for the bound mRNAs, that is, active transport and translational regulation. Both these functions may not be required in a competitive situation, as suggested by the fact that the Dazl-binding sites for Dlc are distinct from those for PABP (Collier et al, 2005; Figure 1D and E). Therefore, we propose that the translation activity of the Dazl-bound mRNAs may be affected by subcellular location as well as by the translational regulators. For example, the Dazl-bound mRNAs may be translationally active only when a translational activator is in association with Dazl outside the RNA storage granule. Further studies are required to define the physiological role of Dazl in the active transport and translational regulation of mRNA during male germ cell development.
Materials and methods
Yeast two-hybrid screening
The Matchmaker Two-Hybrid System (Clontech, Palo Alto, CA) was used to isolate the genes that encode the Dazl-interacting proteins. Full-length or truncated mutants of the Dazl proteins were used as baits for screening the mouse testis and HeLa cDNA libraries. Approximately 5 × 106 colonies were screened with a cotransformation efficiency of approximately 5 × 103 CFU/μg. The transformants were grown at 30°C for 3–6 days on a medium with the following composition: SD/−His/−Leu/−Trp+5 mM 3-amino-1,2,4-triazole. Further, the specificity of interaction was tested by a β-galactosidase assay.
Plasmid construction
Mouse Dazl, Dic1 and Dic2 genes were subcloned into the pcDNA3.1-HA vector (Invitrogen) and the mouse Dlc1 gene was subcloned into the p3XFLAG-CMV-10 vector (Sigma). Truncated Dazl mutants were subcloned into pGEM-T, pCMV-Tag3B and pcDNA3.1-HA, or pEGFP-C1 (Clontech). The mouse Dynamitin gene was subcloned into pEGFP-C1 (Clontech). The Cdc25A 3′UTR, Cdc25C 5′UTR, Tpx1 3′UTR and Mvh 3′UTR sequences were subcloned into the pSL-MS2-6X and pSL-MS2-12X plasmids (Fusco et al, 2003). The pMS2-GFP and pSL-MS2 plasmids were generously provided by Dr R Singer (Albert Einstein College of Medicine, USA).
Antibodies
The Dazl polyclonal rabbit antibody was raised against the GST-Dazl fusion protein and was affinity-purified. Monoclonal Dlc antibodies were purchased from Alexis (Lausanne, Switzerland) and BD Transduction Laboratories (NJ, USA). Monoclonal antibodies against the Flag and HA epitopes were purchased from Sigma (St Louis, MO, USA) and the Berkeley Antibody Company (Richmond, CA, USA), respectively. Antibodies against GFP, Dic, α-tubulin, Myc, TIA1 and p150Glued were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and transfection
HeLa, 293T and COS7 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and maintained in 5% CO2 at 37°C. The plasmids were transfected into the 293T cells by the calcium phosphate method and into the COS7 and HeLa cells by using the LipofectAMINE Plus™ reagent in accordance with the manufacturer's instruction (Invitrogen).
Immunoprecipitation, GST pulldown and microtubule precipitation analyses
For immunoprecipitation, the cells were lysed in the RIPA buffer and centrifuged at 5000 g for 30 min at 4°C. Next, 1 mg protein from the supernatant was incubated with the indicated antibodies and precipitated with protein A-Sepharose (Amersham Pharmacia, Buckinghamshire, UK), as recommended by the manufacturer.
For GST pull-down analysis, 5 μg of the GST fusion proteins were incubated with the cell lysates for up to 3 h at 4°C and precipitated with glutathione-Sepharose beads. The beads were washed three times with the lysis buffer (50 mM HEPES (pH 7.4), 150 mM NaCl, 1% NP-40, 25 mM NaF, 2 mM EDTA, 1 mM Na3VO4, 10% glycerol, 100 mM PMSF and protease inhibitor cocktails). The precipitated proteins were resolved by 10–15% SDS–PAGE and immunoblotted using the indicated antibodies.
The microtubule precipitation assay was carried out by a previously described method (Tavares et al, 1996).
Immunocytochemistry and stress granule formation
The cells were fixed with methanol at −20°C and washed with phosphate-buffered saline. The samples were then incubated with the primary antibodies in 3% BSA for 1 h, washed three times, incubated with fluorescein- or rhodamine-conjugated secondary antibodies, mounted with a mounting solution with DAPI, and observed under a fluorescence microscope (Olympus IX51). The images were photographed using a CoolSnap-Hq CCD camera (Roper Scientific).
In order to induce stress granule formation, HeLa cells were treated with 0.5 mM sodium arsenite for 45 min. The cells were fixed with 3% paraformaldehyde followed by −20°C methanol for 10 min each and immunostained as described.
Live cell imaging of the minus-end-directed proteins
The COS7 cells containing CFP-Dazl or GFP-Dazl1−115 were immersed in a phenol red-free medium and imaged on an Olympus IX71 inverted microscope equipped with differential interference contrast optics, a × 60 fluorite objective (NA 1.2) and a 100 W mercury lamp. Images were acquired at every 2- or 3-s interval using the CoolSnap-Hq CCD camera driven by the MetaMorph imaging software (Universal Imaging, West Chester, PA, USA). The imaging chambers were prepared on glass slides with a border of silicone grease; the slides were sealed with nail polish. The samples were maintained at 37°C by a heater that directed the heat flow across the microscope stage. Image analysis and generation of video clips were performed using the MetaMorph software.
Supplementary Material
Supplementary videos
Supplementary videos
Acknowledgments
We thank Dr EJ Choi (Korea University, Korea) for the HeLa yeast two-hybrid library and Dr R Singer (Albert Einstein College of Medicine, USA) for the pMS2-GFP and pSL-MS2 plasmids. This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ10-PG6-01GN13-0002). S Lee was supported by BK21 Research Fellowship from the Ministry of Education and Human Resources Development.
References
- Anderson P, Kedersha N (2002) Stressful initiations. J Cell Sci 115: 3227–3234 [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N (2006) RNA granules. J Cell Biol 172: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Serbus LR, Duffy JB, Saxton WM (2000) A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science 289: 2120–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JH, Barbarese E (2005) Systems analysis of RNA trafficking in neural cells. Biol Cell 97: 51–62 [DOI] [PubMed] [Google Scholar]
- Cha B, Koppetsch BS, Theukauf WE (2001) In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell 106: 35–46 [DOI] [PubMed] [Google Scholar]
- Collier B, Gorgoni B, Loveridge C, Cooke HJ, Gray NK (2005) The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J 24: 2656–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma MP (2004) Daughter-specific repression of Saccharomyces cerevisiae HO: Ash1 is the commander. EMBO Rep 10: 953–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoue R, Davis I (2005) Dynein anchors its mRNA cargo after apical transport in the Drosophila blastoderm embryo. Cell 122: 97–106 [DOI] [PubMed] [Google Scholar]
- Eberhart CG, Maines JZ, Wasserman SA (1996) Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia. Nature 381: 783–785 [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB (1996) Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 132: 617–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Eddy EM, Phillips DM (1970) Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod 2: 129–153 [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Elphick L, Nusslein-Volhard C, St Johnston D (1994) Staufen protein associates with the 3′UTR of bicoid mRNA to form particles which move in a microtubule-dependent manner. Cell 79: 1221–1232 [DOI] [PubMed] [Google Scholar]
- Fox M, Urano J, Reijo RA (2005) Identification and characterization of RNA sequences to which human PUMILIO-2 (PUM2) and deleted in Azoospermia-like (DAZL) bind. Genomics 85: 92–105 [DOI] [PubMed] [Google Scholar]
- Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard JM, Singer RH, Bertrand E (2003) Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol 13: 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP, Welte MA, Block SM, Wieschaus EF (2000) Dynein-mediated cargo transport in vivo: a switch controls travel distance. J Cell Biol 148: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DW, Zhang J, Maines JZ, Wasserman SA, King ML (1998) A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development 125: 171–180 [DOI] [PubMed] [Google Scholar]
- Ikenishi K (1998) Germ plasm in Caenorhabditis elegans, Drosophila and Xenopus. Dev Growth Differ 40: 1–10 [DOI] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Dass S, Kaltschmidt JA, Lopez-Schier H, St Johnston D, Brand AH, Roth S, Guichet (2002) Polar transport in the Drosophila oocyte requires dynein and kinesin I cooperation. Curr Biol 12: 1971–1981 [DOI] [PubMed] [Google Scholar]
- Jiao X, Trifillis P, Kiledjian M (2002) Identification of target messenger RNA substrates for the murine deleted in azoospermia-like RNA-binding protein. Biol Reprod 66: 475–485 [DOI] [PubMed] [Google Scholar]
- Karashima T, Sugimoto A, Yamamoto M (2000) Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis. Development 127: 1069–1079 [DOI] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP (2003) Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol 284: C273–C284 [DOI] [PubMed] [Google Scholar]
- Kotaja N, Bhattacharyya SN, Jaskiewicz L, Kimmins S, Parvinen M, Filipowicz W, Sassone-Corsi P (2006) The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc Natl Acad Sci USA 103: 2647–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuersten S, Goodwin EB (2003) The power of the 3′UTR: translational control and development. Nat Rev Genet 4: 626–637 [DOI] [PubMed] [Google Scholar]
- Kuroda-Kawaguchi T, Skaletsky H, Brown LG, Minx PJ, Cordum HS, Waterston RH, Wilson RK, Silber S, Oates R, Rozen S, Page DC (2001) The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet 29: 279–286 [DOI] [PubMed] [Google Scholar]
- MacDougall N, Clack A, MacDougall E, Davis I (2003) Drosophila gurken (TGFα) mRNA localizes as particles that move within the oocyte in two dynein-dependent steps. Dev Cell 4: 307–319 [DOI] [PubMed] [Google Scholar]
- Maegawa S, Yamishita M, Yasuda K, Inoue K (2002) Zebrafish DAZ-like protein controls translation via the sequence ‘GUUC'. Genes Cells 7: 971–984 [DOI] [PubMed] [Google Scholar]
- Moore FL, Jaruzelska J, Fox MS, Urano J, Firpo MT, Turek PJ, Dorfman DM, Reijo Pera RA (2003) Human Pumilio-2 is expressed in embryonic stem cells and germ cells and interacts with DAZ (Deleted in AZoospermia) and DAZ-Like protein. Proc Natl Acad Sci USA 100: 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noce T, Okamoto-Ito S, Tsunekawa N (2001) Vasa homolog genes in mammalian germ cell development. Cell Struct Funct 26: 131–136 [DOI] [PubMed] [Google Scholar]
- Pan J, Goodheart M, Chuma S, Nakatsuji N, Page DC, Wang PJ (2005) RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis. Development 132: 4029–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijo R, Lee T-Y, Salo P, Allagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O, de la Chapelle A, Silber S, Page DC (1995) Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 10: 383–393 [DOI] [PubMed] [Google Scholar]
- Reijo R, Seligman J, Dinulos MB, Jaffe T, Brown LG, Disteche CM, Page DC (1996) Mouse autosomal homolog of DAZ, a candidate male sterility gene in humans, is expressed in male germ cells before and after puberty. Genomics 35: 346–352 [DOI] [PubMed] [Google Scholar]
- Reynolds N, Collier B, Maratou K, Bingham V, Speed RM, Taggart M, Semple CA, Gray NK, Cooke HJ (2005) Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum Mol Genet 14: 3899–3909 [DOI] [PubMed] [Google Scholar]
- Ruggiu M, Speed R, Taggart M, KcKay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ (1997) The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389: 73–77 [DOI] [PubMed] [Google Scholar]
- Russell L, Frank B (1978) Ultrastructural characterization of nuage in spermatocytes of the rat testis. Anat Rec 190: 79–97 [DOI] [PubMed] [Google Scholar]
- Saunders PT, Turner JM, Ruggiu M, Taggart M, Burgoyne PS, Elliott D, Cooke HJ (2003) Absence of mDazl produces a final block in germ cell development at meiosis. Reproduction 126: 589–597 [DOI] [PubMed] [Google Scholar]
- Saxena R, de Vries JWA, Repping S, Alagappan RK, Skaletsky H, Brown LG, Ma P, Chen E, Hoovers JMN, Page DC (2000) Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics 67: 256–267 [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Bohmann K, Nusslein-Volhard C (2000) The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat Cell Biol 2: 185–190 [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Luschnig S, Koch I, Nusslein-Volhard C (2002) γ-tubulin37C and γ-tubulin ring complex protein 75 are essential for bicoid RNA localization during Drosophila oogenesis. Dev Cell 3: 685–696 [DOI] [PubMed] [Google Scholar]
- Slee R, Grimes B, Speed RM, Taggart M, Maguire SM, Ross A, McGill NI, Saunders PT, Cooke HJ (1999) A human DAZ transgene confers partial rescue of the mouse Dazl null phenotype. Proc Natl Acad Sci USA 96: 8040–8045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D (2005) Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol 6: 363–375 [DOI] [PubMed] [Google Scholar]
- Tavares AA, Glover DM, Sunkel CE (1996) The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J 15: 4873–4883 [PMC free article] [PubMed] [Google Scholar]
- Tiepolo L, Zuffardi O (1976) Localisation of factors controlling spermatogenesis in the non-fluorecent portion of the human Y chromosome long arm. Hum Genet 34: 119–124 [DOI] [PubMed] [Google Scholar]
- Tsui S, Dai T, Roettger S, Schempp W, Salido EC, Yen PH (2000a) Identification of two novel proteins that interact with germ-cell-specific RNA-bindind proteins DAZ and DAZL1. Genomics 65: 266–273 [DOI] [PubMed] [Google Scholar]
- Tsui S, Dai T, Warren ST, Salido EC, Yen PH (2000b) Association of the mouse infertility factor DAZL1 with actively translating polyribosomes. Biol Reprod 62: 1655–1660 [DOI] [PubMed] [Google Scholar]
- Venables JP, Ruggiu M, Cooke HJ (2001) The RNA-binding specificity of the mouse Dazl protein. Nucleic Acids Res 29: 2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T, Speed RM, Ross A, Cooke HJ (2002) Partial rescue of the Dazl knockout mouse by the human DAZL gene. Mol Hum Reprod 8: 797–804 [DOI] [PubMed] [Google Scholar]
- Wilkie GS, Davis I (2001) Drosophila wingless and pair-rule transcripts localize apically by dynein-mediated transport of RNA particles. Cell 105: 209–219 [DOI] [PubMed] [Google Scholar]
- Xu EY, Lee DF, Klebes A, Turek PJ, Kornberg TB, Reijo Pera RA (2003) Human BOULE gene rescues meiotic defects in infertile flies. Hum Mol Genet 12: 169–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary videos
Supplementary videos


