Abstract
Multiple studies indicate that mRNA processing defects cause mRNAs to accumulate in discrete nuclear foci or dots, in mammalian cells as well as yeast. To investigate this phenomenon, we have studied a series of GAL reporter constructs integrated into the yeast genome adjacent to an array of TetR-GFP-bound TetO sites. mRNA within dots is predominantly post-transcriptional, and dots are adjacent to but distinct from their transcription site. These reporter genes also localize to the nuclear periphery upon gene induction, like their endogenous GAL counterparts. Surprisingly, this peripheral localization persists long after transcriptional shutoff, and there is a comparable persistence of the RNA in the dots. Moreover, dot disappearance and gene delocalization from the nuclear periphery occur with similar kinetics after transcriptional shutoff. Both kinetics depend in turn on reporter gene 3′-end formation signals. Our experiments indicate that gene association with the nuclear periphery does not require ongoing transcription and suggest that the mRNPs within dots may make a major contribution to the gene–nuclear periphery tether.
Keywords: dot, mRNP, mRNA export, nuclear periphery, transcription
Introduction
mRNA export from the eukaryotic nucleus to the cytoplasm is preceded by multiple nuclear processing steps, many of which occur co-transcriptionally. Among these are three categories of covalent RNA modifications: addition of a 7′-methyl guanosine cap to the 5′-end, splicing, and addition of a 3′ stretch of adenosines (the poly-A tail). Nascent transcripts are also subject to numerous noncovalent processing events, that is, co-transcriptional recruitment of protein complexes that mediate these covalent alterations as well as proteins that contribute to messenger ribonucleoprotein particle (mRNP) export. Several mRNA export factors, including Npl3p, Sub2p and Yra1p, are recruited co-transcriptionally, whereas others such as Mex67p appear to associate with mRNP predominantly post-transcriptionally (Lei et al, 2001; Strasser et al, 2002; Zenklusen et al, 2002; Kim et al, 2004). Mex67p as well as other mRNP factors bind directly to nuclear pore components and facilitate mRNA egress to the cytoplasm (Segref et al, 1997; Strasser et al, 2000).
Much of our knowledge about mRNA export and its relationship to other processing steps comes from experiments utilizing yeast temperature-sensitive strains. Using this strategy, multiple factors have been identified that are required to promote efficient export and avoid substantial poly-A RNA accumulation within the nucleus. These include early factors such as those involved in 3′-end cleavage and polyadenylation (pA), and late factors such as nuclear pore proteins and mRNP export factors (Amberg et al, 1993; Gorsch et al, 1995; Murphy and Wente, 1996; Santos-Rosa et al, 1998; Snay-Hodge et al, 1998; Hurt et al, 2000; Sträßer and Hurt, 2000; Hammell et al, 2002). For example, an mRNA export factor mutant (yra1-1) results in retention of poly-A RNA in the nucleus that is visualized by fluorescent in situ hybridization (FISH) with an oligo-dT probe (Sträßer and Hurt, 2000). Surprisingly, similar experiments using gene-specific FISH probes revealed that individual mRNAs are retained in a discrete nuclear focus or ‘dot' under non-permissive conditions (Jensen et al, 2001). Furthermore, the number of dots correlates with the number of active genes, that is, one and two dots in haploid and diploid yeast strains, respectively (Jensen et al, 2001), and they appear similar to higher eukaryotic RNA foci that appear when RNA processing is impaired (Custodio et al, 1999). Some yeast dots appear related to a surveillance pathway that monitors mRNP quality, presumably to minimize nuclear export of faulty mRNPs (Hilleren et al, 2001). A number of studies indicate that surveillance and retention occur near the site of transcription (Thomsen et al, 2003; Dower et al, 2004), but the exact spatial and temporal relationship of actively transcribed genes and mRNP retention sites has never been addressed.
Another potentially export-relevant connection between subnuclear compartments is that some highly expressed genes localize to the nuclear periphery upon transcriptional induction. These include the galactose-inducible GAL genes, mating pheromone inducible genes, INOI, HXK1 and genes that are responsive to the transcription factor Rap1p (Brickner and Walter, 2004; Casolari et al, 2004, 2005; Menon et al, 2005; Cabal et al, 2006; Taddei et al, 2006). Recent studies indicate that the activated GAL locus moves to the nuclear periphery and dynamically interacts with nuclear pores, which effects a sliding along the nuclear membrane (Cabal et al, 2006). Components of the SAGA histone acetylation complex (Sus1p and Ada1p), an mRNA export factor (Sac3p) and a nuclear pore component (Nup1p) are required for GAL gene localization to the nuclear periphery (Cabal et al, 2006; Drubin et al, 2006). These observations may indicate that transcription and mRNA export from some genes are directly coupled: newly transcribed mRNA from a pore-adjacent gene is ideally positioned for nuclear export as initially proposed by Blobel (1985) in the gene-gating hypothesis.
A complication with the use of temperature-sensitive yeast strains for localization studies is that their interpretation can be complicated by indirect effects as well as the requisite use of heat-shock conditions, which may independently influence RNA export (Saavedra et al, 1997; Krebber et al, 1999). For these reasons, we previously carried out experiments to study export-related events in wild-type (WT) strain backgrounds. A set of high-copy reporter plasmids was used, which contain a GFP ORF under control of the strong TDH3 or GAL1 promoter, with 3′-end formation directed either by cleavage and pA signals from the corresponding gene (TDHpA or GALpA) or by a cis-acting hammerhead ribozyme element (RZ). The strategy was based in part on previous studies from our laboratory and others, which showed that a hammerhead ribozyme can cleave co-transcriptionally to generate an artificial 3′-end with no poly-A tail in vivo (Duvel et al, 2002; Dower et al, 2004; Lacadie et al, 2006). RZ-terminated RNAs (pTDH-GFP-RZ and pGAL-GFP-RZ) showed punctate nuclear staining by FISH, suggesting impaired RNA export and multiple nuclear accumulation sites from these high-copy plasmids. In contrast, pTDH-GFP-TDHpA and pGAL-GFP-GALpA-containing cells showed intense, uniform cell staining with only a hint of dots in the case of pGAL-GFP-GALpA (Dower et al, 2004).
To investigate the relationship between genes, transcripts, 3′-end formation and the nuclear periphery in more detail and with better resolution, we constructed strains containing these reporter genes, all integrated at the same chromosomal location. Although the FISH results were similar to those observed with high-copy plasmid reporters including the lack of an Rrp6p effect (Dower et al, 2004; see below), we were now able to clearly detect a single nuclear dot with the GAL-GFP-GALpA gene. Further characterization also indicated that GAL-GFP-GALpA as well as the GAL-GFP-RZ dot consisted of non-nascent RNA, which is near but not coincident with the gene and persists after transcriptional shutoff. As GAL genes become more rim-associated upon transcriptional induction, we verified that our GAL1 promoter-driven reporter genes localize to the nuclear periphery in a manner indistinguishable from what has been described for natural GAL genes. Surprisingly, both the GAL-GFP-GALpA and GAL-GFP-RZ genes dissociate from the periphery well after transcriptional repression effected by glucose addition, indicating that ongoing transcription is not necessary to maintain a link between GAL genes and the nuclear periphery. As the kinetics of RNA dot disappearance parallels gene departure from the periphery, our experiments suggest that the mRNPs within the dot contributes to gene tethering at the nuclear periphery.
Results
To enable a comparison with our previous work utilizing high-copy (2μ) plasmids (Dower et al, 2004), we generated integrating constructs that express GFP under the control of two strong promoters: the regulated GAL1 promoter and the constitutive TDH3 promoter (Figure 1A). Transcript 3′-ends were directed by WT pA signals (e.g., GAL-GFP-GALpA), or by a self-cleaving hammerhead RZ (e.g., GAL-GFP-RZ). In this initial set of experiments, all constructs were integrated into the TRP1 locus of haploid Saccharomyces cerevisiae, and all strains contained single integrants as determined by both Southern blot analysis and PCR (data not shown). As previously shown, GFP mRNAs from reporters containing a pA signal (TDHpA or GALpA) have poly-A tails but the ribozyme terminated constructs do not (Dower et al, 2004 and data not shown).
Figure 1.

FISH of cells expressing GFP reporter constructs. (A) Diagram of four GFP reporter constructs used in this study. All constructs contain the GFP open reading frame under the control of either the constitutive TDH3 promoter or the inducible GAL1 promoter. The 3′-end of the constructs has either the TDH3 or GAL1 3′UTR or a self-cleaving hammerhead ribozyme (RZ). The position of the GFP FISH probes is shown. (B) FISH with a GFP-specific probe shows that GFP mRNA with a ribozyme generated 3′-end are retained as dots within the nucleus (TDH-GFP-RZ and GAL-GFP-RZ). In contrast, TDH3-promoted GFP RNAs that contain a TDH3 pA signal are diffusely localized throughout the cell (TDH-GFP-TDHpA). Surprisingly, GAL1-promoted GFP mRNAs that are polyadenylated via a GAL1 pA signal are retained dots within the nucleus (GAL-GFP-GALpA).
RNA localization was assayed by FISH with antisense probes to GFP, and results were largely consistent with those previously reported for the 2μ plasmid reporters (Dower et al, 2004). The TDH3 promoter-driven gene with a WT TDH3 pA signal (TDH-GFP-TDHpA) showed cytoplasmic signal indicative of normal export and cytoplasmic stability, whereas the presence of an RZ 3′-end signal (TDH-GFP-RZ) gave rise to a single strong nuclear dot (Figure 1B). The RZ 3′-end also causes a slightly weaker cytoplasmic signal due to decreased RNA stability as well as nuclear processing difficulties (Dower et al, 2004). Similar results were obtained with the GAL promoter-driven genes, except that a nuclear dot was visible even with a WT GAL1 pA sequence (Figure 1B).
The observation that GAL-GFP-GALpA mRNAs are retained in a dot, but TDH-GFP-TDHpA mRNAs are not, did not distinguish between a promoter effect or a 3′-end formation effect or both. To address this issue, we analyzed two additional integrated chimeric constructs with swapped promoters (Figure 2A): one contained the GAL1 promoter and the TDH3 pA signal (GAL-GFP-TDHpA), and the other contained the TDH3 promoter and the GAL1 pA signal (TDH-GFP-GALpA). Because the TDH-GFP-GALpA strain had a dot in both glucose and galactose, whereas the GAL-GFP-TDHpA strain had no expression in glucose and only diffuse cytoplasmic staining in galactose (Figure 2), this assigns the difference to the 3′-end signals (see Discussion). We conclude that both RZ and GALpA 3′-end signals generate a nuclear dot in an otherwise WT strain and therefore focused most subsequent experiments on a comparison of these two genes, GAL-GFP-RZ and GAL-GFP-GALpA.
Figure 2.

Dot formation is dependent on the nature of the 3′UTR. (A) Diagram of the two chimeric GFP reporter constructs. These constructs contain the GFP open reading frame and have either a TDH3 promoter and a GAL1 3′UTR (TDH-GFP-GALpA) or a GAL1 promoter and a TDH3 3′UTR (GAL-GFP-TDHpA). The position of the GFP FISH probes is shown. (B) FISH with a GFP-specific probe (red) and DAPI staining (blue) of the DNA was performed on TDH-GFP-GALpA and GAL-GFP-TDHpA cells grown either in glucose (GLU) or galactose (GAL). TDH-GFP-GALpA mRNAs are expressed and retained in dots in the nucleus when cells are grown in either glucose or galactose. GAL-GFP-TDHpA mRNAs are not expressed in glucose and in galactose they are diffusely localized throughout the cell.
GAL-GFP-GALpA and GAL-GFP-RZ dots are independent of Rrp6p
Previous studies showed that specific mRNAs are retained in nuclear dots when mRNA processing or export is impaired by shifting a number of conditional mutants to the non-permissive temperature (37°C; Amberg et al, 1993; Gorsch et al, 1995; Murphy and Wente, 1996; Santos-Rosa et al, 1998; Snay-Hodge et al, 1998; Hurt et al, 2000; Sträßer and Hurt, 2000; Hammell et al, 2002). This nuclear retention is dependent on the nuclear exosome component Rrp6p (Hilleren et al, 2001; Thomsen et al, 2003; Lund and Guthrie, 2005). In contrast, both GAL-GFP-GALpA and GAL-GFP-RZ dots are unaffected by a deletion of RRP6 (Figure 3). This result cannot be attributed to temperature; the GAL-GFP-GALpA, Δrrp6 and GAL-GFP-RZ, Δrrp6 strains still form dots even under heat-shock conditions (data not shown). This distinction suggests that not all dots are equivalent.
Figure 3.

GAL-GFP-GALpA and GAL-GFP-RZ dots are independent of the nuclear exosome component, Rrp6p. Cells expressing integrated GAL-GFP-GALpA or GAL-GFP-RZ in either a WT or Δrrp6 background were grown in galactose and message specific FISH was performed using oligos specific to GFP (see Figures 1 and 2). The ability of GAL-GFP-GALpA and GAL-GFP-RZ dots to form is not dependent on Rrp6p.
mRNA in GAL-GFP-GALpA and GAL-GFP-RZ dots is post-transcriptional
Previous results suggested that nuclear dots were at or near their genes of origin but did not discriminate between these two possibilities (Thomsen et al, 2003; Dower et al, 2004). The difference is important, as ‘near' but not ‘at' indicates that dots contain a substantial fraction of non-nascent RNA. This is especially relevant for GAL-GFP-GALpA dots; the normal 3′-end signal of this construct might indicate that an in situ signal reflects predominantly nascent RNA. To address this issue and to localize the gene simultaneously, we integrated GAL-GFP-RZ and a slightly modified GAL-GFP-GALpA (GFPkD200pA; the GFP fluorophore was mutated to generate a non-fluorescent GFP) at a different location, 5 kb from a pre-existing tandem TetO array at the BMH1 gene (Ciosk et al, 1998; see Materials and methods for details). By co-expressing a TetR-GFP fusion, we could simultaneously monitor RNA dots (by FISH; Figure 4A, shown in red) and the GAL-GFP gene (marked by TetR-GFP tethered to the TetO array; Figure 4A, shown in green).
Figure 4.

GFP dots are adjacent to but not overlapping the site of transcription and persist in the absence of transcription. (A) The GFP reporter genes were integrated adjacent to a TetO operator array to which TetR-GFP binds. When transcription is activated by steady-state growth in galactose, the GFP dots (FISH; shown in red) are adjacent to but not overlapping the site of transcription (TetR-GFP; shown in green). At 60 min after transcription is repressed by the addition of glucose, the dots are still visible and maintain a similar spatial relationship with the DNA locus. (B) Cells expressing GAL-GFP-GALpA or GAL-GFP-RZ were grown in galactose and at time zero, glucose was added to repress transcription. Cells were harvested for ChIP prior to glucose addition, 30 and 60 min after glucose addition and from cells maintained in glucose. RNA PolII occupancy on GAL-GFP-GALpA (blue) and GAL-GFP-RZ (red) was monitored at each timepoint. After 30 min, PolII levels are similar to those observed when transcription is repressed in glucose. (C) FISH using a GFP-specific probe was performed on the same cells used in (B). The number of cells containing GFP dots was counted (see Materials and methods). Both GAL-GFP-GALpA (blue) and GAL-GFP-RZ (red) expressing cells contain dots after transcription has been repressed by the addition of glucose. The GAL-GFP-RZ dots are more stable than the GAL-GFP-GALpA dots.
Dots from both integrated genes were always distinct from—albeit always adjacent to—the marked chromosomal locus (Figure 4A). Because TetraSpeck beads (Invitrogen) examined under the identical conditions showed completely overlapping red/green images (data not shown; see Materials and methods), the dot–gene separation cannot be attributed to chromatic aberration. Given the 5 kB distance from the integrated constructs to the TetO array and the compaction ratio in yeast interphase chromatin (Bystricky et al, 2004), the non-overlapping signals therefore indicate that even the GAL-GFP-GALpA dots contain predominantly non-nascent RNA.
The non-nascent nature of both dot RNAs was substantiated by the results of glucose chase experiments. Both reporter genes have a GAL1 promoter, which is rapidly inhibited by glucose addition (Mason and Struhl, 2005; see below), yet many dots are still easily visible by FISH after 60 min of growth in glucose (Figure 4A). Similar stability of nuclear mRNAs has been reported in mammalian systems (Weil et al, 2000). Moreover, both dots always remain adjacent to their genes, implying the existence of a persistent tether between mRNP within the dot and the locus of origin.
To perform a more careful analysis of dot disappearance from GAL-GFP-GALpA and GAL-GFP-RZ strains, a quantitative glucose time course shutoff experiment was performed. The kinetics of dot disappearance was compared to the kinetics of RNA PolII clearance from the two GAL genes as assayed by chromatin immunoprecipitation (ChIP; Figure 4B). As expected (Mason and Struhl, 2005), transcription is inhibited by 30 min after glucose addition, and PolII occupancy at that time is comparable to steady-state glucose levels (no transcription). Indeed, 10 min is sufficient for a complete shutoff (data not shown). Although dot intensity does get weaker as a function of time after glucose addition (especially in the case of the GALpA-containing strain), dots persist much longer than expected for nascent RNA. Moreover, dot decay from the RZ gene is slower than from the GALpA gene. Forty-eight percent of GAL-GFP-RZ cells contain dots after 60 min in glucose, whereas only 27% of GAL-GFP-GALpA cells contain dots (Figure 4C). The differences in pA and RZ dot stability must reflect relative rates of GALpA and RZ RNA departure from dots after transcriptional repression, due to either turnover or export to the cytoplasm.
The nature of the 3′UTR can alter co-transcriptional recruitment of an export factor
To explore what might be responsible for the more persistent dots of the GAL-GFP-RZ gene, we compared the two genes by ChIP and assayed the association of PolII and two RNP proteins to different regions of nascent transcription complexes (5′, middle, 3′) under transcription-permissive (galactose) conditions. Both PolII and the RNP protein Sub2p were essentially indistinguishable across the genes, indicating that basic transcription and recruitment of at least some RNP proteins are unaffected by the RZ 3′-end (Figure 5B and C). However, GAL-GFP-RZ has higher levels of Yra1p at the 3′-end, showing that some feature of RNP protein recruitment or transfer to the nascent RNA is altered (Figure 5D). This suggests that the GAL-GFP-RZ mRNPs contain excess Yra1p in addition to lacking a pA tail (Duvel et al, 2002; Dower et al, 2004). mRNP differences more generally may be responsible for the differences in the dot stability from these two reporters.
Figure 5.
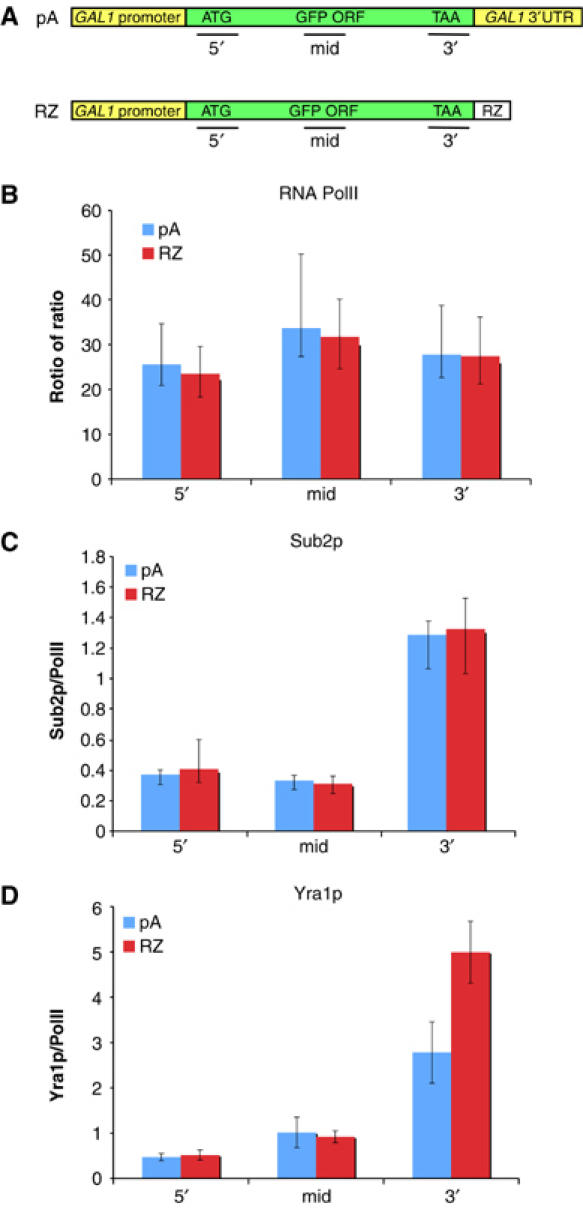
Co-transcriptional recruitment of mRNP proteins to GAL-GFP-GALpA and GAL-GFP-RZ. ChIPs were performed with RNA PolII and two different mRNP proteins (Sub2p and Yra1p) and the levels of each factor on GAL-GFP-GALpA and GAL-GFP-RZ was determined. (A) Diagram of the GFP reporters and the location of the PCR primers used in ChIP experiments. (B) RNA PolII recruitment is similar on GAL-GFP-GALpA and GAL-GFP-RZ. (C) The mRNP protein, Sub2p, is recruited similarly to both reporter genes. (D) Yra1p is enriched on the 3′-end of the GAL-GFP-RZ gene in comparison to the GAL-GFP-GALpA gene.
GAL-GFP-GALpA and GAL-GFP-RZ associate with the nuclear periphery
Because the GAL locus increases its association with the nuclear periphery in a transcription-dependent fashion, we addressed whether there might be a relationship between dots and intranuclear gene localization. We compared the nuclear localization of three different reporter constructs: GAL-GFP-GALpA (dot forming), GAL-GFP-RZ (dot forming) and GAL-GFP-TDHpA (non-dot forming). All three of these reporters were integrated into the genome adjacent to the Tet0-GFP array (see Materials and methods for details). To visualize the nuclear rim, Nup49p fused to CFP (NUP49-CFP; Shor et al, 2005) was expressed in the cells. Cells were fixed and visualized using a DeltaVisionTM (Applied Precision) optical sectioning microscope (see Materials and methods). The TetR-GFP mark was scored as internal (I), subperipheral (S; touching the nuclear rim from interior of nucleus) or peripheral (P; coincident with the rim), and a distribution among the three positions was calculated (Figure 6A).
Figure 6.

Intranuclear localization of the GAL-GFP-GALpA, GAL-GFP-RZ and GAL-GFP-TDHpA genes. The GAL reporter genes were integrated into the genome adjacent to a TetO array. TetR-GFP was co-expressed to visualize the gene and NUP49-CFP was expressed to mark the nuclear periphery. (A) The intranuclear localization of the gene (TetR-GFP) relative to the nuclear periphery was categorized as being internal (I), subperipheral (S; proximal to but not overlapping the nuclear membrane), or peripheral (P; adjacent to the nuclear membrane). Representative images of each category are shown. (B) The intranuclear localization of the GAL-GFP-GALpA, GAL-GFP-RZ and GAL-GFP-TDHpA genes was determined during transcriptionally active (GAL; galactose) or repressed (GLU; glucose) states. Both GAL-GFP-GALpA and GAL-GFP-RZ become more associated with the nuclear periphery when grown in galactose. In contrast, there was no substantial difference in the localization of the GAL-GFP-TDHpA gene in glucose compared to galactose. (C) The intranuclear localization of the GAL-GFP-RZ gene was examined 0, 15, 30 and 60 min after transcriptional shutoff by the addition of glucose as well as in steady-state glucose. The GAL-GFP-RZ gene is enriched in the nuclear periphery for up to 60 min after transcriptional shutoff suggesting that the GAL-GFP-RZ gene does not require active transcription to maintain its peripheral localization. (D) The intranuclear localization of the GAL-GFP-GALpA gene was examined 0, 15, 30 and 60 min after transcriptional shutoff by the addition of glucose as well as in steady-state glucose. The GAL-GFP-GALpA remains at the nuclear periphery for 30 min after transcriptional shutoff illustrating that the gene does not require active transcription to maintain its peripheral localization. However, the GAL-GFP-GALpA is released from the nuclear periphery more quickly than the GAL-GFP-RZ gene.
Both the GAL-GFP-GALpA gene and the GAL-GFP-RZ gene show an enhanced peripheral localization when transcription of these genes is induced by growth in galactose (compare galactose to glucose; Figure 6B). The quantitative results are essentially identical to what has been reported previously for an endogenous GAL locus (Casolari et al, 2004; Cabal et al, 2006). In contrast to endogenous loci where multiple neighboring genes might undergo activation, these data indicate that activation of this single, added gene is sufficient for enhanced peripheral localization of the region. In contrast to GAL-GFP-GALpA, the GAL-GFP-TDHpA gene shows little change in nuclear localization. It has an intermediate phenotype on both carbon sources, that is, not as peripheral as the other two genes on galactose and not as central as on glucose (Figure 6B). This difference is not due to an alteration of transcriptional induction in galactose: RNA polymerase II (PolII) ChIPs indicate that this chimeric gene is induced similarly to GAL-GFP-GALpA (data not shown). This result suggests that the GAL1 promoter is insufficient to localize a gene to the nuclear periphery and indicate that the nature of the 3′UTR can impact gene localization.
Studies to date show that transcriptional induction is required for the association between GAL genes and the nuclear periphery, but it is not clear whether continued transcription induction is necessary for its maintenance. For these reasons, we compared the intranuclear localization of GAL-GFP-GALpA and GAL-GFP-RZ after shifting from galactose to glucose (Figure 6C and D). Remarkably, the data indicate that the two genes maintain their peripheral nuclear localization for different amounts of time after glucose addition: the RZ gene maintains a peripheral localization much longer than the GALpA gene. Indeed, the intranuclear localization of the RZ gene may not change at all after 60 min in glucose (Figure 6C). In contrast, the GAL-GFP-GALpA gene becomes progressively more interior and less peripheral; by 60 min, localization is almost indistinguishable from steady-state glucose grown cells (Figure 6D). Nonetheless, even this gene has a more peripheral localization 30 min after transcription shutoff than under steady-state repressed conditions. As 30 min is sufficient to completely inhibit transcription from the GAL promoter (Figure 4B; data not shown; Mason and Struhl, 2005), this indicates that continued transcription is not necessary to maintain peripheral gene localization. Moreover, the relative locus dissociation kinetics (Figure 6C and D) resembles the relative dot decay time courses of the two genes in glucose (Figure 4C), further suggesting a relationship between mRNP in retained foci and peripheral gene localization.
Endogenous GAL1 mRNA accumulates in the nucleus in dots that are independent of RRP6
The movement of the GAL locus to the nuclear periphery has been well documented in a number of studies (Casolari et al, 2004; Cabal et al, 2006; Schmid et al, 2006). If there is a general role of post-transcriptional mRNP in tethering active loci to the nuclear periphery, GAL mRNAs should accumulate in nuclear dots. Indeed, FISH with GAL1-specific probes show that GAL1 mRNAs accumulate in dots in both a WT and a Δrrp6 background (Figure 7). Furthermore, similar to the GAL reporter genes, GAL1 dots are still evident 60′ after transcription has been repressed (data not shown). This suggests that like the GAL-GFP-GALpA and GAL-GFP-RZ reporter genes, the GAL1 gene could be tethered to the nuclear periphery at least in part through post-transcriptional mRNP.
Figure 7.

GAL1 mRNAs accumulate in dots that are independent of the nuclear exosome component, Rrp6p. WT or Δrrp6 cells were grown in galactose and message specific FISH was performed using oligos specific to GAL1. Dapi staining (blue), GAL1-specific FISH (red) and the overlapping signals are shown.
Our key results are summarized in a model, which suggests a role of post-transcriptional mRNPs in tethering GAL genes to the nuclear periphery (Figure 8). Activation of GAL genes causes mRNPs to accumulate in a dot near the gene, and the gene as well as the dot relocalizes to the nuclear periphery (Thomsen et al, 2003; Casolari et al, 2004; Dower et al, 2004; Schmid et al, 2006). As shown recently by Cabal et al (2006), the GAL gene then maintains a dynamic association with the nuclear periphery. This relationship is likely mediated by transient interactions between nuclear pore proteins and chromatin (Schmid et al, 2006), nascent mRNP (Casolari et al, 2005) and/or post-transcriptional mRNP as proposed here (see above). After transcriptional shutoff (Figure 8B), the dot persists and remains in close proximity to the gene suggesting the existence of a dot–gene tether independent of transcription (Figure 8B). The GAL reporter genes also remain tethered to the nuclear periphery long after active transcription has ceased. This indicates that features of the gene–nuclear periphery tether must be independent of active transcription. The 3′-ends of the two reporters, GALpA versus RZ, modulate these effects. Although these differences could be mediated by chromatin effects that persist much longer than active transcription, we suggest that they are due to alterations in the quantity or quality of post-transcriptional mRNPs within the dots.
Figure 8.

Model. (A) When GAL genes are actively transcribed, mRNPs accumulate in a dot that is adjacent to but not overlapping with the gene. Consistent with the observations of others, we show that GAL genes localize to the nuclear periphery and that a gene/nuclear periphery tether exists that is likely to include actively transcribing chromatin and its associated proteins. (B) When transcription of the GAL genes is repressed, dots persist and maintain the same spatial relationship with the gene. In addition, the gene remains associated with the nuclear periphery. These results suggest the existence of both a dot/gene and a gene/nuclear periphery tether that are independent of transcription. Both dot persistence and maintenance of nuclear periphery association are dependent on the 3′end (pA versus RZ). Although these differences could be affected by chromatin modifications that are maintained in the absence of transcription, we suggest that they are due to changes in the quantity or quality of the post-transcriptional mRNPs. It is also possible that post-transcriptional mRNPs also play a role in the gene/nuclear periphery tether during active transcription (see Discussion).
Discussion
Our experiments indicate that GAL-GFP-GALpA and GAL-GFP-RZ mRNAs accumulate in the nucleus in discrete foci or dots, which contain predominantly post-transcriptional RNA. The dots are positioned adjacent to but not coincident with the gene locus. This relationship persists after transcription repression suggesting the existence of a transcription independent gene–dot tether. Promoter swapping experiments illustrate that different 3′UTRs (GAL1 versus TDH3 versus RZ) differentially affect dot formation and/or stability. Additional experiments illustrate that like endogenous GAL genes, the GAL-GFP-GALpA and GAL-GFP-RZ genes are localized to the nuclear periphery when transcription is activated by growth in galactose. Surprisingly, the peripheral localization of GAL-GFP-GALpA and GAL-GFP-RZ genes also persists after transcriptional shutoff. In addition, the loss of dots and peripheral localization of GAL reporters shows similar kinetics after glucose addition: the GAL-GFP-RZ dots are more stable, and this gene also maintains its peripheral localization for a longer time after transcriptional shutoff. As the RZ 3′-end affects these two features in parallel as well as mRNP composition assayed by ChIP, the data suggest that the mRNAs within dots (and associated proteins) may act as a tether between the gene and nuclear periphery after transcription is shutoff.
In mammalian cells as well as in yeast, gene-proximal dots have only been observed with mutant reporter genes or in mutant strains under non-permissive conditions (Custodio et al, 1999; Jensen et al, 2001). In this study, the GAL-GFP-GALpA reporter gene as well as the endogenous GAL1 gene contains a WT 3′UTR and generate properly cleaved and adenylated RNAs, which accumulate in nuclear dots (Figures 1B and 7; data not shown). This localization of dot mRNAs indicates reduced diffusion compared to most nuclear RNAs (Shav-Tal et al, 2004), presumably due to a tether that restrains the RNA from moving far from its transcription site. The observation of WT mRNA in dots makes it unlikely that these tethers include proteins involved in nuclear mRNA surveillance or degradation.
Indeed, neither the accumulation of the GAL-GFP-GALpA, the GAL-GFP-RZ mRNP, nor the GAL1 mRNP is dependent on the nuclear exosome component Rrp6p (Figures 3 and 7), which contrasts with several previous studies showing that Rrp6p is required for dot formation of yeast heat-shock transcripts (Hilleren et al, 2001; Thomsen et al, 2003; Lund and Guthrie, 2005), This difference suggests that the nuclear exosome is not an essential dot–gene tether component in WT cells. This suggestion may even extend to heat-shock dots, for example, the exosome may be involved in the generation of heat-shock dot RNA in mutant strains rather than its maintenance or tethering (T Heick-Jensen, personal communication).
Our experiments show that these GAL reporter genes move to the nuclear periphery upon activation, indistinguishably from what has been reported for endogenous GAL genes (Casolari et al, 2004; Schmid et al, 2006). Recent studies have shown that gene activation is crucial for this relocalization and have implicated promoter regions (Schmid et al, 2006) as well as nascent mRNP (Casolari et al, 2005) in transcription-dependent tethering.
However, gene localization has not been previously reported during a transcription repression time course. The fact that GAL1-driven reporter genes remain at the nuclear periphery well after transcriptional shutoff indicates that there are aspects of the gene–periphery tether(s) that are transcription-independent, and we predict that ongoing transcription is not necessary for the maintenance of peripheral localization of all GAL genes. Although a somewhat similar conclusion was recently based on a biochemical assay (DNA cleavage by a nuclear pore-tethered nuclease; Schmid et al, 2006), peripheral localization in this case was strongly dependent on preinitiation complex formation. Our observations, however, are based entirely on the visual assay and indicate the opposite, namely, a reversal much slower than glucose repression of preinitiation complex formation as well as transcription. Although transcriptionally active chromatin may be required to establish the gene–periphery tether, it is possible that distinct or additional tethers act to maintain this association after transcriptional shutoff (Figure 8).
Our data also point to an important role of 3′-end formation signals in dictating gene localization to the nuclear periphery. First, the nature of the 3′UTR affects the ability of genes to establish a nuclear periphery association. The GAL-GFP-GALpA and GAL-GFP-TDHpA constructs are identical except for their 3′UTRs, and both constructs show similar transcriptional activation in galactose (data not shown). However, the GAL-GFP-GALpA gene associates with the nuclear periphery, whereas the GAL-GFP-TDHpA gene does not (Figure 6B). Furthermore, replacement of the 3′UTR with a hammerhead ribozyme still localizes to the nuclear periphery. In contrast to this result, a recent study found that deleting the HXK1 3′UTR abolished its ability to localize to the nuclear periphery (Taddei et al, 2006). These data suggest that certain 3′UTRs or cryptic pA signals (in the case of the HXK1 gene) could restrict genes from moving to the nuclear periphery. Additionally, the nature of the 3′UTR affects gene maintenance at the periphery after transcriptional shutoff. This is because the GAL-GFP-RZ shows slower kinetics of peripheral delocalization than GAL-GFP-GALpA (Figure 6C and D).
Moreover, our data indicate that the effects of 3′-end signals on dots and peripheral localization upon transcriptional shutoff are correlated: loss of dots and peripheral delocalization show similar kinetics, for example, 1 h after the addition of glucose, 27.3% of the GAL-GFP-GALpA dots persist and 21% of the GAL-GFP-GALpA genes are still associated with the nuclear periphery. The GAL-GFP-RZ construct shows slower kinetics of dot and localization loss: 1 h after the addition of glucose, 47.5% of the dots persist and 48.4% of the GAL-GFP-RZ genes are associated with the nuclear periphery. Since PolII occupancy levels (in galactose as well as in glucose repression) are virtually identical between these two constructs (Figures 4B and 5B), we favor a model in which the differences between the GAL reporters are due to differences in the quantity or quality of the post-transcriptional mRNPs within dots. We propose that the dot or more precisely the dot mRNPs helps tether chromatin to the nuclear periphery in the absence of transcription. Although previous studies from the Silver lab suggested a role for mRNPs in the gene–pore tether, these studies focused on nascent mRNPs (Casolari et al, 2005).
An extrapolation of this view suggests that dot mRNPs are the physical link to the nuclear periphery, that is, the chromosome is tethered indirectly through this post-transcriptional mRNP. Genes would be released from the periphery when the quantity of mRNA decreases, either through degradation and/or by exiting the nucleus. If a dot contains fewer mRNPs, the mRNP–pore interaction would be less stable and lead to gene release from the periphery. The lack of a poly-A tail on RZ RNA slows RZ RNA export to the cytoplasm (Dower et al, 2004). Slower export of dot RNA might help explain the increased stability of GAL-GFP-RZ dots and the increased peripheral localization of this gene relative to GAL-GFP-GALpA. In addition, we have shown that the levels of at least one mRNA export factor, Yra1p, are different between the GAL-GFP-GALpA and GAL-GFP-RZ genes. It is therefore possible that mRNP compositional differences directly affect tethering strength and therefore the shutoff kinetics.
Recent studies have identified several candidate proteins that might tether the gene to the nuclear pore as well as facilitate an interaction between the post-transcriptional mRNP and the nuclear periphery. Tethering of the endogenous GAL genes is abolished by the deletion of either SUS1 or SAC3 (Cabal et al, 2006; Drubin et al, 2006; Chekanova et al, in preparation). Both of these proteins are members of the Sac3-Thp1-Sus1-Cdc31 protein complex that localizes to the nuclear pore and is believed to help facilitate mRNA export by binding to the mRNA export factor Mex67p. These proteins could bind to the mRNPs in the dot either directly or through Mex67p and function to dock the gene to the pore via the post-transcriptional mRNPs.
Although our data primarily address gene localization after transcriptional shutoff, mRNPs within dots might also complement the tethers that mediate the gene–pore connection during active transcription (Figure 8A, question mark). Indeed, the 3′UTR (GAL1 versus TDH3) modulates gene–periphery association, suggesting this broader role of mRNA processing or export. Understanding the roles of mRNP in tethering genes to the nuclear periphery as well as the function and composition of dots and dot–gene tethers are major goals for the near future and should be amenable to genetic screens that assay gene–dot and/or gene–periphery colocalization.
Materials and methods
Yeast strains and media
All yeast strains used in this study are from the W303 background and are derivatives of Y368 except where noted (Supplementary Table 1). All plasmids used in this study are listed in Supplementary Table 1 and are described in detail in Supplementary Materials. To integrate pKA8, pKA11, pKA23, pKA24, pKA51 and pKA52 into the TRP1 locus, the plasmids were digested with Bsu36I (cuts in the middle of TRP1) and the resulting DNA was transformed into Y368 to generate FY775, FY776, FY811, FY812, KAY968 and KAY971 (see Supplementary Table 1). TRP+ isolates were obtained and verified to contain a single copy of the reporter gene integrated at the TRP1 locus using either PCR or Southern blotting.
To integrate the reporter genes adjacent to the BMH1 locus, pDB716, pDB719 and pKA58 were digested with NotI and transformed into a variant of K7022 called AE3 (generated by backcrossing K7022 into a WT W303 strain to remove the his4 locus to generate a his3, HIS4 strain). TRP+ transformants were isolated and the integration was verified to be between the ECM32 and BMH1 loci. The resulting strains were then transformed with pWJ1323 (NUP49-CFP; Shor et al, 2005) to generate KAY977, KAY978 and KAY993 for localization experiments (see Supplementary Table 1).
For glucose shutoff time course experiments, cells were grown overnight in either in 2% galactose or 2% galactose, 1% sucrose and at time zero, glucose was added to a final concentration of 2%.
FISH
GFP and GAL1 FISH was performed using Cy3-conjugated oligonucleotides as described previously with some modifications (see Supplementary Table 2; Vainberg et al, 2000; Jensen et al, 2001; Dower et al, 2004). Cells were fixed for 15 min in 4% paraformaldehyde (electron microscopy sciences) and 10% acetic acid and washed twice in Sorb/KPi (1.2 M sorbitol, 0.1 M potassium phosphate, pH 6.5). Fixed cells were allowed to adhere onto multiwell slides and were spheroplasted using a combination of Lyticase (Sigma) and Zymolyase 20T (Seikagaku). Slides were visualized using an Olympus AX70 with a × 100 objective using a rhodamine or DAPI filter set. Images were captured using Openlab software (Improvision) and the exposure times used to capture FISH or DAPI images were kept consistent throughout each experiment.
For experiments involving the colocalization of the GFP RNA by FISH and the TetR-GFP fusion marking the gene locus, acetic acid was omitted from the fixation step. To control chromatic aberration, a mixture of 100- and 500 nm-diameter TetraSpeck beads (Invitrogen) were mounted in the same media and imaged alongside with the fixed cells under identical conditions. We found that the extent of spectral (red/green) signal separation was negligible and could not account for the separation of RNA and locus signals observed in our strains. For dot quantitation experiments (Figure 4C), 100–150 cells were counted for the presence or absence of dots in three independent experiments.
Intranuclear localization experiments
To score the fraction of cells with the reporter locus positioned internally, subperipherally or peripherally in the nucleus, KAY977, KAY978 and KAY993 were grown in the appropriate selective media containing either 2% glucose or 2% galactose, 1% sucrose. Adenine was added at twice the usual concentration in order to minimize autofluorescence caused by an adenine metabolic precursor in vacuoles. Cells were grown to OD ∼0.5, fixed 15 min in 4% paraformaldehyde without acetic acid, harvested, washed in sorbitol/KPi and mounted in the solution of 50% glycerol, 0.1% (1 mg/ml) r-phenylenediamine antifade in PBS. Between 100 and 150 cells with the reporter locus positioned in the z-section that cuts through the middle of the nucleus were scored per sample/timepoint, as described (Brickner and Walter, 2004). Cells were scored into three categories (with the reporter locus positioned in the nucleus internally, peripherally and subperipherally), by two independent operators.
Images were acquired with a CoolSnapHQ (Photometrics) camera mounted onto a DeltaVisionTM (Applied Precision) optical sectioning microscope on a TE200 base (PlanApo 100X, 1.4 NA objective lens; Nikon; Muhlemann et al, 2001). Data sets were obtained as 16–20 optical sections per wavelength spaced 0.2 m apart along the Z-axis using CFP, FITC, rhodamine/Cy3 and GFP filters. Out of focus information was removed using a constrained iterative deconvolution algorithm (Agard et al, 1989; softWoRxTM; Applied Precision).
ChIPs
ChIPs were performed as previously described (Abruzzi et al, 2004). For glucose shutoff experiments, PolII ChIPs and FISH were performed from identical cultures (Figure 4). Monoclonal antibodies against total RNA PolII (8WG16) were purchased from Covance. The anti-HA monoclonal (12CA5) was purchased from Roche. The primers used in this study were chosen using Primer3 from the Whitehead Institute (Rozen and Skaletsky, 2000) and are described in Figure 5 and Supplementary Table 2.
The calculations and normalizations for each ChIP were carried out as previously described (Komarnitsky et al, 2000; Abruzzi et al, 2004).
Supplementary Material
Supplementary Table 1
Supplementary Table 2
Supplemental Materials and Methods
Acknowledgments
We thank the Nasmyth, Rothstein and Stutz Labs for yeast strains and plasmids. We appreciate the technical assistance of S Jennings, B Shkolnik and P Mallik and are grateful to Kristine O'Brien and other members of the M. Moore Laboratory for help and guidance with the microscopy. We thank Francoise Stutz, Torben Heick Jensen and members of the Rosbash Lab for valuable discussions. KCA was supported in part by a post-doctoral fellowship from the NIH. This work was supported in part by a grant to MR from the NIH (Grant GM23549), a grant to DAB and JC from the NIH (Grant GM073872) and a grant to DAB from the NSF (Grant MCB0424651).
References
- Abruzzi KC, Lacadie S, Rosbash M (2004) Biochemical analysis of TREX complex recruitment to intronless and intron-containing yeast genes. EMBO J 23: 2620–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agard DA, Hiraoka Y, Shaw P, Sedat JW (1989) Fluorescence microscopy in three dimensions. Methods Cell Biol 30: 353–377 [DOI] [PubMed] [Google Scholar]
- Amberg DC, Fleischmann M, Stagljar I, Cole CN, Aebi M (1993) Nuclear PRP20 protein is required for mRNA export. EMBO J 12: 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G (1985) Gene gating: a hypothesis. Proc Natl Acad Sci USA 82: 8527–8529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner JH, Walter P (2004) Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol 2: e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystricky K, Heun P, Gehlen L, Langowski J, Gasser SM (2004) Long-range compaction and flexibility of interphase chromatin in budding yeast analyzed by high-resolution imaging techniques. Proc Natl Acad Sci USA 101: 16495–16500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, Nehrbass U (2006) SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441: 770–773 [DOI] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Drubin DA, Rando OJ, Silver PA (2005) Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev 19: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA (2004) Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117: 427–439 [DOI] [PubMed] [Google Scholar]
- Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K (1998) An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 93: 1067–1076 [DOI] [PubMed] [Google Scholar]
- Custodio N, Carmo-Fonseca M, Geraghty F, Pereira HS, Grosveld F, Antoniou M (1999) Inefficient processing impairs release of RNA from the site of transcription. EMBO J 18: 2855–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower K, Kuperwasser N, Merrikh H, Rosbash M (2004) A synthetic A tail rescues yeast nuclear accumulation of a ribozyme-terminated transcript. RNA 10: 1888–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DA, Garakani AM, Silver PA (2006) Motion as a phenotype: the use of live-cell imaging and machine visual screening to characterize transcription-dependent chromosome dynamics. BMC Cell Biol 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvel K, Valerius O, Mangus DA, Jacobson A, Braus GH (2002) Replacement of the yeast TRP4 3′ untranslated region by a hammerhead ribozyme results in a stable and efficiently exported mRNA that lacks a poly(A) tail. RNA 8: 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsch LC, Dockendorff TC, Cole CN (1995) A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J Cell Biol 129: 939–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell CM, Gross S, Zenklusen D, Heath CV, Stutz F, Moore C, Cole CN (2002) Coupling of termination, 3′ processing, and mRNA export. Mol Cell Biol 22: 6441–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleren P, McCarthy T, Rosbash M, Parker R, Jensen TH (2001) Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 413: 538–542 [DOI] [PubMed] [Google Scholar]
- Hurt E, Sträber K, Segref A, Bailer S, Schlaich N, Presutti C, Tollervey D, Jansen R (2000) Mex67p mediates nuclear export of a variety of RNA polymerase II transcripts. J Biol Chem 12: 8361–8368 [DOI] [PubMed] [Google Scholar]
- Jensen TH, Patricio K, McCarthy T, Rosbash M (2001) A block to mRNA nuclear export in S. cerevisiae leads to hyperadenylation of transcripts that accumulate at the site of transcription. Mol Cell 7: 887–898 [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn SH, Krogan NJ, Greenblatt JF, Buratowski S (2004) Transitions in RNA polymerase II elongation complexes at the 3′ ends of genes. EMBO J 23: 354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnitsky P, Cho EJ, Buratowski S (2000) Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev 14: 2452–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebber H, Taura T, Lee MS, Silver PA (1999) Uncoupling of the hnRNP Np13p from mRNA during the stress-induced block in mRNA export. Genes and Development 13: 1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie SA, Tardiff D, Kadener S, Rosbash M (2006) Cotranscriptional Intron Definition and Yeast Transcription Elongation Mutants. Genes Dev 20: 2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei EP, Krebber H, Silver PA (2001) Messenger RNAs are recruited for nuclear export during transcription. Genes Dev 15: 1771–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MK, Guthrie C (2005) The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol Cell 20: 645–651 [DOI] [PubMed] [Google Scholar]
- Mason PB, Struhl K (2005) Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol Cell 17: 831–840 [DOI] [PubMed] [Google Scholar]
- Menon BB, Sarma NJ, Pasula S, Deminoff SJ, Willis KA, Barbara KE, Andrews B, Santangelo GM (2005) Reverse recruitment: the Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc Natl Acad Sci USA 102: 5749–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlemann O, Mock-Casagrande CS, Wang J, Li S, Custodio N, Carmo-Fonseca M, Wilkinson MF, Moore MJ (2001) Precursor RNAs harboring nonsense codons accumulate near the site of transcription. Mol Cell 8: 33–43 [DOI] [PubMed] [Google Scholar]
- Murphy R, Wente SR (1996) An RNA-export mediator with an essential nuclear export signal. Nature 383: 357–360 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386 [DOI] [PubMed] [Google Scholar]
- Saavedra C, Felber B, Izaurralde E (1997) The simian retrovirus-1 constitutive transport element, unlike the HIV-1 RRE, uses factors required for cellular mRNA export. Curr Biol 7: 619–628 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E (1998) Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol 18: 6826–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, Laemmli UK (2006) Nup-PI: the nucleopore–promoter interaction of genes in yeast. Mol Cell 21: 379–391 [DOI] [PubMed] [Google Scholar]
- Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E (1997) Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J 16: 3256–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, Singer RH (2004) Dynamics of single mRNPs in nuclei of living cells. Science 304: 1797–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E, Weinstein J, Rothstein R (2005) A genetic screen for top3 suppressors in Saccharomyces cerevisiae identifies SHU1, SHU2, PSY3 and CSM2: four genes involved in error-free DNA repair. Genetics 169: 1275–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snay-Hodge CA, Colot HV, Goldstein AL, Cole CN (1998) Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. EMBO J 17: 2663–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Bassler J, Hurt E (2000) Binding of the Mex67p/Mtr2p heterodimer to FXFG, GLFG, and FG repeat nucleoporins is essential for nuclear mRNA export. Journal of Cell Biology 150: 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sträßer K, Hurt E (2000) Yra1p, a Conserved Nuclear RNA Binding Protein, Interacts Directly with Mex67 and is Required for mRNA Export. EMBO J 19: 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E (2002) TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417: 304–308 [DOI] [PubMed] [Google Scholar]
- Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM (2006) Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441: 774–778 [DOI] [PubMed] [Google Scholar]
- Thomsen R, Libri D, Boulay J, Rosbash M, Jensen TH (2003) Localization of nuclear retained mRNAs in Saccharomyces cerevisiae. RNA 9: 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainberg IE, Dower K, Rosbash M (2000) Nuclear export of heat shock and non-heat-shock mRNA occurs via similar pathways. Mol Cell Biol 20: 3996–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, Boutain S, Audibert A, Dautry F (2000) Mature mRNAs accumulated in the nucleus are neither the molecules in transit to the cytoplasm nor constitute a stockpile for gene expression. RNA 6: 962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen D, Vinciguerra P, Wyss JC, Stutz F (2002) Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol 22: 8241–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Supplementary Table 2
Supplemental Materials and Methods


