Abstract
During clathrin-mediated endocytosis, the GTPase dynamin promotes formation of clathrin-coated vesicles, but its mode of action is unresolved. We provide evidence that a switch in three functional states of dynamin (dimers, tetramers, rings/spirals) coordinates its GTPase cycle. Dimers exhibit negative cooperativity whereas tetramers exhibit positive cooperativity with respect to GTP. Our study identifies tetramers as the kinetically most stable GTP-bound conformation of dynamin, which is required to promote further assembly into higher order structures such as rings or spirals. In addition, using fluorescence lifetime imaging microscopy, we show that interactions between dynamin and auxilin in cells are GTP-, endocytosis- and tetramer-dependent. Furthermore, we show that the cochaperone activity of auxilin is required for constriction of clathrin-coated pits, the same early step in endocytosis known to be regulated by the lifetime of dynamin:GTP. Together, our findings support the model that the GTP-bound conformation of dynamin tetramers stimulates formation of constricted coated pits at the plasma membrane by regulating the chaperone activity of hsc70/auxilin.
Keywords: assembly, auxilin, dynamin, endocytosis, GTP binding
Introduction
Clathrin-mediated endocytosis is a multistep process that involves formation of clathrin-coated vesicles (CCVs) from the plasma membrane (Kirchhausen, 2000). Based on current models, coat assembly starts with the recruitment and oligomerization of adaptor proteins and is followed by the recruitment of clathrin. Early stages of invagination are followed by a well-defined constriction step that requires the GTPase dynamin, whose exact role in this process is controversial. Based on the classical model, self-assembly of dynamin into higher order structures such as rings and spirals is thought to drive the constriction step, whereas assembly-stimulated GTPase activity is thought to be directly involved in severing the neck of a coated pit (for a review see Sever et al, 2000b). Alternatively, we have proposed that dynamin functions as a regulatory GTPase whose GTP-bound form regulates coat rearrangement and thereby drives constriction. In this view, assembly-stimulated GTP hydrolysis acts as a ‘switch off' mechanism for dynamin's active form (Sever et al, 1999; Narayanan et al, 2005).
After vesicle release, the clathrin coat is disassembled by hsc70 (Schlossman et al, 1984) and its cochaperones, aux1 (neuronal isoform) (Ungewickell et al, 1995) or auxilin-2 (aux2) (ubiquitous form) (Greener et al, 2000; Umeda et al, 2000). Although the N-terminus of aux2 is a functional protein kinase that is absent from auxillin-1, (aux1) the two proteins are otherwise highly homologous, sharing three key domains: the tensin domain, a clathrin-binding domain and a C-terminal DnaJ domain that interacts with hsc70. Both proteins can induce clathrin polymerization into baskets and they both support uncoating of CCVs by hsc70 in vitro (Ungewickell et al, 1995; Greener et al, 2000; Umeda et al, 2000).
The role of hsc70/auxilin in endocytosis does not appear to be restricted to the uncoating reaction. Dominant-negative hsc70 mutants have been shown to inhibit the formation of constricted coated pits (Newmyer and Schmid, 2001), the same step that requires dynamin. Furthermore, experiments in Drosophila identified genetic interactions between dynamin and hsc70 (Chang et al, 2002), and between dynamin and the DnaJ domain cochaperone of hsc70, Rme-8 (Chang et al, 2004), suggesting that dynamin and chaperones function in the same pathway in vivo. Further strengthening the connection between chaperones and dynamin, we have identified direct interactions between the GTP-bound form of dynamin and aux1, as well as hsc70 (Newmyer et al, 2003). Based on these results, we proposed that dynamin: GTP regulates hsc70/auxilin to drive CCV formation. This model was recently supported by two studies showing that downregulation of aux2 in HeLa cells inhibits clathrin-mediated endocytosis, but they differed with regard to the steps that required aux2. Downregulation of aux2 by shRNA resulted in the overall loss of clathrin-coated pits, suggesting a role for aux2 in de novo pit formation (Lee et al, 2005), whereas experiments performed using siRNA place the role of aux2, and specifically its cochaperone activity, somewhere between the initiation and the uncoating steps (Zhang et al, 2005). Live-cell imaging of clathrin (Merrifield et al, 2005) and downregulation of clathrin in cells (Iversen et al, 2003) together suggested that clathrin assembly may play a role in the final stages of invagination that lead to membrane scission. As there is no evidence that auxilin and hsc70 remove clathrin from clathrin-coated pits at the plasma membrane, but only from vesicles after budding (Ungewickell et al, 1995), our data suggest that interactions between dynamin and the chaperone machinery might suppress the uncoating activity.
Here we pinpoint constriction of clathrin-coated pits as the step in endocytosis that requires the cochaperone activity of auxilin. The identical step was previously shown to be regulated by the lifetime of dynamin:GTP (Sever et al, 2000a; Narayanan et al, 2005), consistent with auxilin being a bona fide dynamin downstream effector. Supporting this idea, fluorescence lifetime imaging microscopy (FLIM) shows that interactions between dynamin and auxilin are GTP- and endocytosis-dependent. In addition, we show that in order to interact with auxilin in vivo, dynamin must self-associate into tetramers. Together, the data support the model that dynamin tetramers regulate constriction of clathrin-coated pits by direct interactions with hsc70/auxilin.
Results
Endogenous dynamin-2 and aux2 occupy the same coated profiles
Our previous biochemical experiments showed that hsc70/auxilin interacts specifically with the GTP-bound form of dynamin, suggesting that this chaperone could be a dynamin effector. To further address this possibility, we asked whether auxilin and dynamin occupy the same coated profiles in vivo. We used the kidney cortex as a model system. The apical membranes of kidney proximal tubules support extensive clathrin-mediated endocytosis to retrieve proteins from the urine, and thus contain high levels of endocytic proteins (Sun et al, 2002). As shown in Figure 1A, left panel, anti-aux1 antibodies raised against auxilin405−814 specifically recognized auxilin-1 (aux1) in rat brain cytosol (RBC), and they also specifically recognized aux2 in inner medullary collecting duct (IMCD) cell extracts. Staining of kidney proximal tubules for aux2 showed that this protein is present in the apical membrane (Figure 1B, full arrow), as well as at the basal membrane, but at significantly lower levels (Figure 1B, open arrow). The same staining pattern was exhibited by dynamin (Figure 1B') and clathrin (data not shown). Because immunofluorescence cannot distinguish between auxilin on CCVs versus auxilin on coated pits, we performed immunogold electron microscopy. Kidney cortex slices were incubated with polyclonal anti-aux1 antibodies coupled to 15 nm gold particles (Figure 1C). Aux2 antigenic sites were concentrated on invaginated regions that could be readily identified as clathrin-coated pits on the basis of the thick, electron-dense coat of clathrin that characterizes these structures in electron micorgraphs (Figure 1C; Sun et al, 2002). Most coated profiles are connected with the membrane and thus do not represent budded vesicles. We next used monoclonal anti-dynamin antibodies in addition to polyclonal anti-aux1 antibodies (Figure 1D, E and F). In this case, aux2 (large gold particles) and dynamin-2 (dyn2) (small gold particles) antigenic sites were present on the same coated profiles (Figure 1D, E and F). In some instances, dynamin is found concentrated at the neck of invaginated coated pits (Figure 1E, arrow). It is important to note that dynamin is not present on CCVs after they have budded (Damke et al, 1994; and our data not shown). Therefore, the presence of aux2 on the same coated profiles as dyn2 indicates that it localizes to coated pits before budding.
Figure 1.
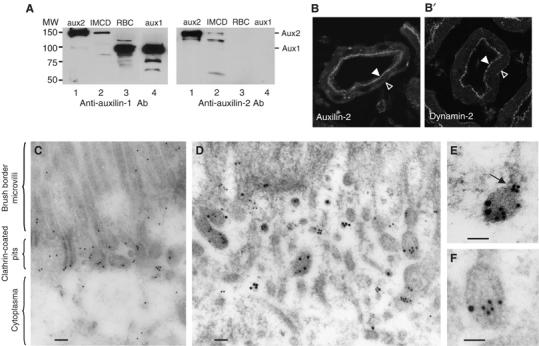
Endogenous aux2 and dyn2 colocalize at the same clathrin-coated profiles. (A) Aux1 antibodies recognize aux2. Western blot analysis of 100 μg cytosol prepared from IMCD cells transiently transfected with aux2 (lane 1), untransfected IMCD cells (lane 2), RBC (lane 3), and 0.1 μg purified recombinant aux1 (lane 4). The same samples were also blotted with anti-aux2 antibodies (Stressgene) (right panel). (B) Aux2 and dyn2 localize to the same apical region of the kidney proximal tubules. Rat kidneys were stained with aux1 antibodies (left panel) or hudy-1 (right panel). Closed arrow, apical membrane; open arrow, basal membrane of the polarized epithelial cells that form proximal kidney tubules. (C, D, E, F) Immunogold electron microscopy showing aux2 antigenic sites concentrated on clathrin-coated pits at the apical plasma membrane. Kidney cortex was labeled with only auxilin (15 nm gold) (C), or both auxilin (15 nm gold) and dynamin (10 nm gold) antibodies (D, E, F). The scale bars correspond to 0.1 μm.
Detection of GTP-dependent dynamin–auxilin interactions in cells using FLIM
To examine whether dynamin directly interacts with auxilin in vivo, we performed FLIM. The fluorescence lifetime of a high-energy donor fluorophore is influenced by its surrounding microenvironment, and it is shortened in the immediate vicinity of a lower energy acceptor fluorophore. Thus, detection of shortened lifetimes demonstrates fluorescence resonance energy transfer (FRET) and indicates spatial proximity of the two labeled molecules.
We first sought a cell type where adenoviral expression of wild-type aux1 (auxWT) has no negative effect on endocytosis. Overexpression of auxWT in IMCD cells did not cause accumulation of clathrin into cytosolic granules (Supplementary Figure 1A and C and compare with Supplementary Figure 3A) or inhibit endocytosis of rhodamine-transferrin (R-Tfn) (Figure 5C). Confocal microscopy of IMCD cells coinfected with adenoviruses expressing dyn1 and aux1 detected a similar distribution of both proteins in the cytoplasm, and enrichment at the edge of the cellular monolayer (Figure 2A, white arrows). A similar enrichment at the edge of the cellular monolayer was observed for clathrin but only after expression of auxWT (Supplementary Figure 1B), suggesting that this region supports active endocytosis. Association of the auxWT and dynWT with the membrane was further examined by subcellular fractionation. As shown in Figure 2B and C, endogenous dynamin was approximately equally distributed between the particulate (membrane bound) and soluble (cytoplasm) fractions, whereas endogenous auxilin was present predominantly in the cytoplasm (compare lanes 1 and 2). Overexpression of dynWT and auxWT led to an overall increase of these proteins in membrane-associated and soluble fractions (Figure 2), as previously observed for dynamin (van der Bliek et al, 1993). Although the exact mechanisms that target either dynamin or auxilin to the plasma membrane are not known (although binding of lipids (Lin et al, 1997) and clathrin-coated pits (Figure 1D; and Ungewickell et al, 1995) is likely involved), these data suggest that membrane targeting can be increased by overexpressing proteins.
Figure 5.
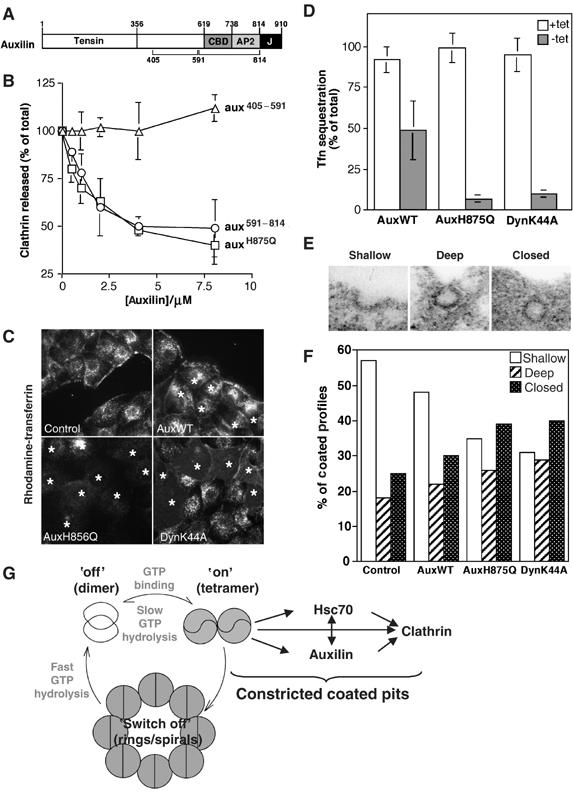
Mutation of auxilin's DnaJ domain inhibits endocytosis after the invagination step. (A) Domain structure of auxilin showing tensin, clathrin binding (CBD), adaptor binding (AP2) and DnaJ (J) domains. (B) Effects of auxH875Q on hsc70-mediated clathrin uncoating. CCVs were incubated in the presence of native hsc70 (0.4 μM) and increasing concentrations of auxH875Q (squares), aux591−814 (circles) or aux405−591 (triangle). Error bars reflect the ±s.d. from four independent experiments. (C) Effect of auxH875Q on R-Tfn internalization. IMCD cells infected with adenoviruses expressing auxWT, auxH875Q or dynK44A were incubated with R-Tfn for 10 min. Cells infected with virus are marked with*. (D) AuxH875Q inhibits a single round of endocytosis. tTA-HeLa cells overexpressing auxWT, auxH875Q or dynK44A were harvested 18 h after adenoviral infection. Single round kinetics of Tfn internalization was determined using avidin assay. Control cells (white columns) were infected with adenoviruses cultured in the presence of tetracycline to suppress expression. The error bars reflect the ±s.d. from four independent experiments. (E) Representative micrographs used to quantify coated profiles. (F) Distribution of coated endocytic structures in cells expressing WT and mutant proteins. (G) Schematic depiction of the GTPase cycle of dynamin. In its basal state dynamin is a dimer. Assembly into tetramers results in positive cooperativity for GTP binding. Tetramers are the kinetically most stable GTP-bound conformation, whose lifetime regulates endocytosis. Further assembly into rings or spirals potently increases the rate of GTP hydrolysis and leads to loss of positive cooperativity for GTP binding. Thus, rings/spirals have kinetic characteristics of a ‘switch off' mechanism for the active, GTP-bound conformation. The GTP-bound conformation of tetramers interacts with auxilin and hsc70. The network of interactions between dynamin, auxilin, hsc70 and clathrin could drive constriction of coated pits because of increased coat rigidity at the interface between lipids and the clathrin coat.
Figure 2.
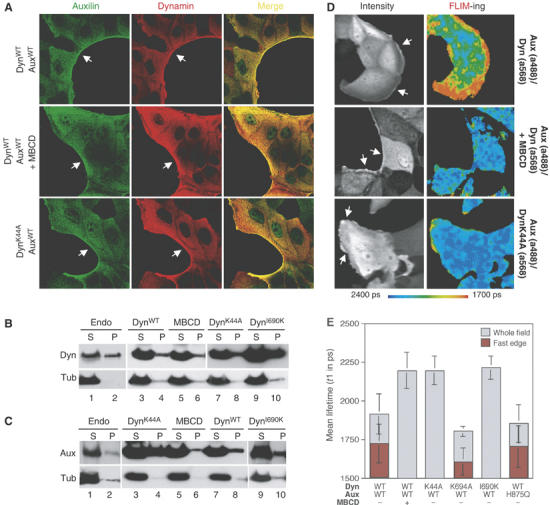
Identification of GTP- and endocytosis-dependent dynamin–auxilin interactions in IMCD cells. (A) Auxilin and dynamin colocalize at the edge of the cellular monolayer. IMCD cells were infected with adenoviruses encoding auxWT, dynWT or dynK44A, as indicated. Cells were optionally treated with MBCD before fixation. Cells were stained with polyclonal anti-auxilin antibodies followed by secondary IgG conjugated with the donor fluorophore a488, and monoclonal anti-dynamin antibodies hudy-1 followed by secondary IgG conjugated with acceptor fluorophore (a568. (B, C) Subcellular distribution of overexpressed dynamin and auxilin in IMCD cells. Cells were lysed and resolved into particulate and soluble fractions by centrifugation at 100 000 g. Total protein from both fractions was immunoblotted with hudy-1 (B) or anti-auxilin (C) antibodies. S, supernatant; P, pellet. (D) IMCD cells were infected and stained as described in (A). Intensity column shows auxilin distribution (a488). FLIM-ing column shows FRET between the donor and acceptor measured by the fluorescence lifetime, t1, of the donor fluorophore (a488) that is represented by a pseudo-colored image. (E) Lifetimes of the donor fluorophore (a488) under different experimental conditions. Each data point represents at least 10 images similar to those shown in (D).
Cells expressing auxWT and dynWT were first stained with rabbit anti-auxilin antibodies followed by goat-anti-rabbit (GAR) antibody conjugated to Alexa 488 (a488), the donor fluorophore. We measured changes in the lifetime of a488 under different experimental conditions. In the absence of an acceptor fluorophore, the lifetime of a488 was 2228±53 ps (Table I). If the cells were also stained with goat anti-mouse secondary antibodies conjugated with a568 (GAMa568), the lifetime remained almost unchanged at ∼2188±62 ps. In contrast, staining cells with dog anti-goat secondary antibodies conjugated with a568 (DAGa568) as a positive control, resulted in a very short lifetime of 972±153 ps. Cells stained with both anti-auxilin (a488) and anti-dynamin antibodies (a568) resulted in a statistically significant reduction of a488 fluorescence lifetime to 1922±131 ps. The fastest rates of a488 lifetime were situated at the edges of the cellular monolayer (τ1=1722±134 ps; Figure 2D, FLIM-ing panel). This spatially restricted lifetime shortening in cells suggests that dynamin–auxilin interactions occur at the plasma membrane at the edges of the cellular monolayer.
Table 1.
FLIM analysis for proximity between auxilin and dynamin in IMCD cells
| Donor | Acceptor | Mean lifetime (τ1 in ps) (mean ±s.d.) (whole field) | Lifetime (τ1 in ps) (mean ±s.d.) (fast edge-RDA) | Mean Lifetime (whole field) P compared to Aux (a488) |
|---|---|---|---|---|
| AuxWT (a488) | None | 2228±53 | ||
| AuxWT (a488) | GAM (a568) | 2188±62 | P>0.1 | |
| AuxWT (a488) | DAG (a568) | 972±153 | P<0.05 | |
| AuxWT (a488) | DynWT (a568) | 1922±131 | 1722±134 | P<0.05 |
| AuxWT (a488) | DynK44A (a568) | 2225±30 | None | P>0.1 |
| AuxWT (a488) | DynI690K (a568) | 2186±39 | None | P>0.1 |
| AuxWT (a488) | DynK694A (a568) | 1805±34 | 1605±187 | P<0.05 |
| AuxH875Q (a488) | DynWT (a568) | 1799±166 | 1708±136 | P<0.05 |
| AuxWT (a488) | DynWT (a568)+MBCD | 2281±63 | None | P>0.1 |
| Experiments were performed by staining cells with polyclonal anti-auxilin antibodies followed by goat anti-rabbit (GAR) secondary antibodies conjugated to Alexa-488 (a488, donor fluorophore), followed by monoclonal anti-dynamin antibody (hudy 1), followed by goat anti-mouse (GAM) secondary antibodies conjugated to Alexa-568 (a568, acceptor fluorophore). For positive control, cells were stained with donkey anti-goat (DAG) antibodies conjugated to Alexa-568 that recognize GAR-a488. τ2 was fixed to mean of Alexa-488 anti-auxilin antibody of 2207 picoseconds (ps). Range-dependent analysis (RDA) was performed by comparing only fast lifetimes at the edge of the cellular monolayer to the mean lifetime of 2207 ps, and it was determined as described in Materials and methods. For statistical analysis of residuals, one-way analysis of variance followed by a Tamhane post hoc test was performed with SPSS software. A P value of less than 0.05 constituted significance. | ||||
| Aux, auxilin; Dyn, dyanamin; IMCD, inner medullary collecting duct; MBCD, β-methyl-cyclodextrin. | ||||
To test whether dynamin–auxilin interactions were dependent on clathrin-mediated endocytosis, we treated cells with β-methyl-cyclodextrin (MBCD), a chemical that depletes cholesterol from the plasma membrane and inhibits endocytosis at the stage of open coated pits that fail to deeply invaginate (Subtil et al, 1999). MBCD did not diminish dynamin–auxilin colocalization on the edges of the cellular monolayer (Figure 2A), or their membrane association (in Figure 2B and 2C, lane 6), but it abolished the FLIM signal (Figure 2D and 2E). This result suggests that dynamin–auxilin interactions might occur among the subset of these proteins that are associated with clathrin-coated pits, and that these interactions require a certain degree of coat curvature. It is important to note that whereas short lifetime only occurs if fluorophores are within a distance well below optical resolution, the pixels that are pseudocolored to reflect the presence or absence of short lifetime species are large compared to the distances involved for FRET.
Next, we tested whether the observed dynamin–auxilin interactions are GTP-dependent. Cells were infected with adenoviruses expressing auxWT and dynK44A, a mutant of dynamin that cannot bind GTP. As shown in Figure 2D, there was no detectable FRET measured by FLIM. As dynamin targeting is not nucleotide dependent (Figure 2A and B; Song et al, 2004), loss of the signal cannot be attributed to an absence of dynK44A at the plasma membrane. The GTP dependence for dynamin–auxilin interactions in vivo is in agreement with in vitro binding experiments (Newmyer et al, 2003). Furthermore, these interactions were not dependent on a functional DnaJ domain, because an auxilin mutant with impaired J-domain function, auxH875Q, exhibited the same level of FRET as observed for auxWT (Figure 2E). This result is in agreement with our work that showed that auxilin fragments that lack the DnaJ domain, aux405−591 and aux591−814, both directly bind dynamin in vitro and potently inhibit endocytosis (Newmyer et al, 2003).
Next, we examined whether dynK694A, a mutant of dynamin that increases the rate of endocytosis owing to impaired assembly into higher order structures (Sever et al, 1999), can interact with auxilin. DynK694A exhibited FRET as measured by FLIM (Figure 2E). The decrease in the lifetime of a488 in cells expressing dynK694A (τ1=1605±187 ps) is greater than in cells expressing dynWT (τ1=1722±134 ps), suggesting that dynK694A interacts with auxilin more efficiently than dynWT. These data support our original interpretation (Sever et al, 1999, 2000a) that dynK694A increases the rate of endocytosis because of improved interactions with effector proteins.
A recent study showed that dynI690K is assembly incompetent and inactive for endocytosis (Song et al, 2004), and therefore concluded that dynamin self-assembly is essential for endocytosis. In our experiments, dynI690K exhibited no FRET as measured by FLIM (Figure 2E). The inability of this mutant to interact with auxilin could not be explained by defective targeting of dynI690K (Figure 2B, lane 10; Supplementary Figure 1D and Song et al, 2004). Interestingly, whereas dynI690K and dynK694A are both deficient in self-assembly, the former inhibits endocytosis whereas the latter is stimulatory. Our results therefore suggest that dynI690K inhibits endocytosis not due to a failure in self-assembly, but rather due to deficient interactions with auxilin.
Dynamin exhibits positive cooperativity for GTP binding
Although the K694A and I690K mutations are both situated within the GAP domain, which also functions as an assembly domain, dynI690K and dynK694A exhibit different biochemical properties; dynI690K is completely unable to assemble into higher order structures, even on lipids and microtubules (MT) (Song et al, 2004), whereas dynK694A exhibits a six-fold lower propensity to self-assemble, which is overcome by lipids or MT (Sever et al, 1999). Regardless of this difference, the FLIM results were puzzling as it was not obvious why differences in self-assembly should influence the interaction with auxilin. We therefore speculated that dynI690K was impaired not only for self-assembly, but also for GTP binding. This idea was inspired by the dynamin family member Dnm1, whose GTP binding is assembly dependent (Ingerman et al, 2005); to test it, we examined the interaction of dynI690K and dynK694A with GTP.
GTP binding of dynamin was studied using a ‘coupled GTPase assay' (Ingerman et al, 2005), in which GTP is continuously regenerated from guanosine 5′-diphosphate. Measurement of the GTPase activity of dynWT and dynK694A at high ionic strength (150 mM NaCl) revealed that the maximal rate of GTP hydrolysis for both proteins was ∼2.5 min−1 (Figure 3A and Table II). These data are similar to values determined using fixed time-point assays (Sever et al, 1999; Narayanan et al, 2005). Addition of the isolated GAP domain of dynamin, which mimics self-assembly into higher order structures (Sever et al, 1999), potently increased the rate of GTP hydrolysis by dynWT (Vmax=77 min−1), whereas it only partially increased the rate of GTP hydrolysis by dynK694A (Vmax=29 min−1, Figure 3B), in agreement with previous studies (Sever et al, 1999). In contrast to dynWT or dynK694A, dynI690K exhibited a three-fold lower basal rate of GTP hydrolysis, which could not be further stimulated by the addition of the GAP domain (Vmax=1.1 min−1, Figure 3A and B). All three proteins exhibited similar affinities for GTP under different conditions, in agreement with previously published data (Table II and Warnock et al, 1996). Importantly, the coupled assay revealed that dynWT and dynK694A both exhibit positive cooperativity with respect to GTP binding (Hill coefficient of ∼2, Supplementary Figure 2). This positive cooperativity was lost when dynamin self-assembly was promoted in low ionic strength, or through addition of the GAP domain (Hill coefficient=1, Table II). In contrast, dynI690K exhibited negative cooperativity with respect to GTP binding under all three experimental conditions (Hill coefficient=0.6–0.8, Supplementary Figure 2). Thus, the I690K mutation impaired the enzyme's ability to efficiently bind GTP, whereas K694A did not have this effect.
Figure 3.
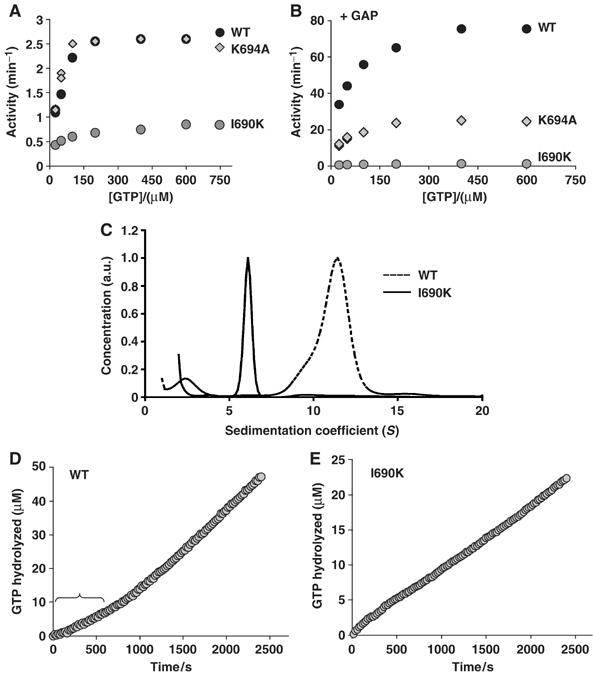
Assembly-dependent GTP binding by dynamin. Steady-state kinetics of dynWT, dynK694A and dynI690K at 150 mM NaCl (A), or with 3 μM GAP domain at 50 mM NaCl (B). 0.06 mg/ml (0.6 μM) dynamin was assayed for GTPase activity. The difference between rates in three different experiments was less than 5%. (C) Analytical ultracentrifugation of 5 μM dynWT or dynI690K. (D, E) GTPase assays were initiated by the dilution of dynWT (D) or dynI690K (E) from 200 into 40 mM NaCl in the presence of 100 μM of GTP, and GTPase activity was measured over time.
Table 2.
Kinetic parameters of dynamin wild-type and assembly mutants
| Enzymes | Vmax (min−1) | Km (μM) | Hill coefficient |
|---|---|---|---|
| DynWT | |||
| −High salt | 2.3±0.2 | 40±2 | 1.9 |
| −Low salt | 4.8±0.5 | 35±4 | 1 |
| +GAP | 77±8 | 31±2 | 1 |
| DynK694A | |||
| −High salt | 2.1±0.1 | 44±4 | 2.1 |
| −Low salt | 3.1±0.2 | 25±5 | 1 |
| +GAP | 29±5 | 33±2 | 1 |
| DynI690K | |||
| −High salt | 0.7±0.1 | 25±5 | 0.6 |
| −Low salt | 0.8±0.1 | 25±4 | 0.7 |
| +GAP | 1.1±0.1 | 28±3 | 0.8 |
| Dyn, dynamin. | |||
In its basal state at high ionic strength, dynamin is best described by a monomer–tetramer or dimer–tetramer equilibrium (Muhlberg et al, 1997; Binns et al, 1999). Differences in cooperativity of GTP binding between dynWT and dynI690K suggested that the I690K mutation might have changed the oligomeric state of dynI690K. As shown in Figure 3C, analytical ultracentrifugation revealed that in contrast to dynWT, which formed homotetramers, dynI690K was a homodimer. Thus, the I690K mutation abolished the ability of dynI690K to form tetramers, the first level of higher order structure. Together, the biochemical analysis suggests that to from a kinetically stable GTP-bound conformation, dynamin dimers need to oligomerize into a tetrameric state. In light of dynamin's oligomerization-dependent GTP-binding properties, we interpret the inability of dynI690K to bind auxilin as determined by FLIM as a consequence of its inability to form a kinetically stable GTP-bound conformation.
Monitoring the rates of GTP hydrolysis continuously revealed that tetramer formation might be a prerequisite to assemble into higher order structures such as rings in solution. Dilution of dynamin from a high ionic strength buffer (tetramers) into a low ionic strength condition induces formation of higher order structures such as rings in solution (Hinshaw and Schmid, 1995), and leads to 2–3-fold increase in the rate of GTP hydrolysis by dynamin (Table II and Warnock et al, 1996). As shown in Figure 3D, when dynWT was diluted into low salt, there was a 300–600 s lag before the enzyme reached the increased, steady-state GTPase velocity. The kinetic lag likely reflects the time required for assembly into higher order structures. In contrast, the GTPase activity of dynI690K revealed no lag in reaching steady-state GTPase velocity (Figure 3E). Together, these data suggest that dynamin nucleates its own self-assembly into rings, and that nucleation requires formation of tetramers.
Auxilin is required for constriction, but not fission of clathrin-coated pits
The GTP-specific interaction of dynamin with auxilin in vitro (Newmyer et al, 2003) and in vivo (Figures 1 and 2) strongly suggests that auxilin is a dynamin effector. If this model is correct, auxilin should be required for vesicle constriction, as this step is known to be regulated by the lifetime of dynamin:GTP (Sever et al, 2000a; Narayanan et al, 2005). To test this prediction, we performed stage-specific semi-in-vitro assays for endocytosis (Schmid and Smythe, 1991) that use perforated cells and are dependent on RBC and dynamin (Hill et al, 2001).
In the perforated-cell assay, roughly 50% of cell surface-bound Tfn was rendered avidin inaccessible, and most of this inaccessibility was dependent on the presence of RBC, ATP and physiological reaction temperature (Figure 4C, columns 1–4). We next compared mock-depleted and aux1-depleted RBC. Greater than 99% of aux1 was immunodepleted from RBC after 4 h using an anti-auxilin antibody (Figure 4A; Newmyer et al, 2003). Aux1-depleted cytosol was ∼50% less efficient than mock-depleted cytosol in stimulating Tfn uptake (Figure 4C, compare columns 5 and 6). A similar extent of inhibition was previously observed with dynamin-depleted cytosol (Hill et al, 2001). Quantitative Western blotting showed that the concentration of auxilin in RBC is approximately 0.1 μM (Figure 4B). Readdition of cytosolic levels of purified aux1 to auxilin-depleted RBC fully restored and reproducibly stimulated endocytosis above mock levels (Figure 4C, compare columns 5–7). Higher concentrations of aux1 inhibited endocytosis (Figure 4C, column 8), presumably because of sequestration of clathrin into nonfunctional cytosolic granules, as seen in vivo (Zhao et al, 2001). Finally, recombinant aux1 by itself was not able to replace RBC in the assay (Figure 4C, column 9).
Figure 4.
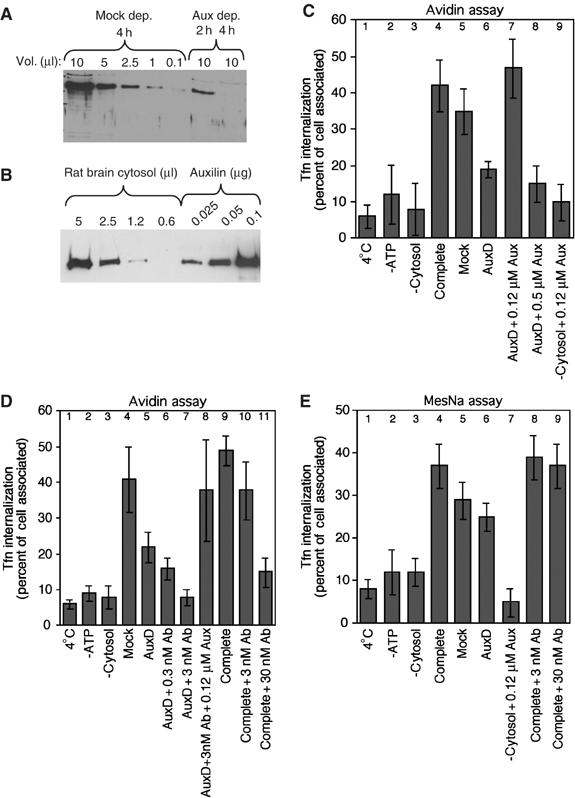
Auxilin is essential for formation of constricted-coated pits but not fission. (A) RBC depleted of auxilin for 2 or 4 h was blotted with anti-aux1 antibody alongside a dilution series of mock-depleted RBC. (B) A dilution series of RBC was blotted with anti-aux antibody alongside known amounts of purified aux1. (C, D) Endocytosis of Tfn in perforated A431 cells is inhibited by immunodepletion of auxilin (C) or addition of anti-aux antibodies (D). The amount of internalized Tfn after 7 min at 37°C was determined by avidin inaccessibility. For rescue experiments, antibodies were preincubated with 0.5 or 2 μg of purified auxilin (0.12 or 0.5 μM final concentration in the assay, respectively) for 15 min on ice, before addition of membrane. Results are the average of 10 independent experiments±s.d. (E) Budding of coated pits in perforated A431 cells was not inhibited by immunodepletion of auxilin (columns 5, 6) or addition of anti-auxilin antibodies (compare column 4 with 8, 9). The amount of Tfn internalized after 7 min was determined. Results are the average of six independent experiments ±s.d.
The remaining endocytosis observed in aux1- (Figure 4C, column 6) and dynamin-depleted extracts (Hill et al, 2001) likely involves membrane-bound aux2 and dyn2. To test this possibility, we preincubated the membrane fraction with increasing concentrations of auxilin antibodies before addition of auxilin-depleted cytosol. Auxilin antibodies recognize the ubiquitous aux2 (Figure 1A), and thus might be expected to interfere with its function. Indeed, addition of these antibodies reduced Tfn sequestration to background levels (Figure 4D, compare columns 1, 2, 3 and 7). Preincubation of the antibodies with purified aux1 rescued the inhibitory effects of antibody addition and auxilin depletion, restoring endocytosis to the mock-depleted level (Figure 4D, compare columns 4 and 8). Significant inhibition of endocytosis was also observed when auxilin antibodies were added to the complete RBC (Figure 4D, columns 10 and 11). Together, these data demonstrate that auxilin is required but not sufficient to form constricted coated pits.
We next examined whether auxilin is required for the fission step. Although vesicle constriction is measured by avidin inaccessibility, the fission reaction is assayed by the acquired inaccessibility of labeled Tfn to MesNa. Importantly, constricted coated pits formed in vivo or de novo in vitro do not proceed to bud off in the permeable cell assay (Supplementary Figure 3C, cartoon 1). Conversely, only coated pits primed for budding in vivo undergo fission (Supplementary Figure 3C, cartoon 2 and Schmid and Smythe, 1991). Thus, constriction and fission occur independently of one another in the perforated-cell assay, and the role of auxilin in fission can be measured independently of its function in upstream events. As shown in Figure 4E, depletion of aux1 from RBC did not significantly decrease the amount of Tfn that became inaccessible to MesNa when compared to mock-depleted RBC (compare columns 5 and 6). Therefore, in contrast to vesicle constriction, fission does not require cytosolic auxilin. As shown in Figure 4E, preaddition of auxilin antibodies to the membrane fraction also did not impair budding of coated pits (compare columns 4 with 8 and 9), suggesting that neither membrane associated nor cytosolic auxilin is required for the fission step. Thus, stage-specific permeable cell assays for CCV formation place the role of auxilin before formation of constricted coated pits.
The cochaperone activity of auxilin is required after coated pit invagination
Identification of interactions between dynamin:GTP and auxilin and hsc70 in vitro suggested that auxilin exerts its function in early endocytosis by regulating hsc70 (Newmyer et al, 2003). To directly examine this hypothesis, we generated a point mutation in the J-domain of auxilin, H875Q, which is predicted to disrupt hsc70-DnaJ interactions (Tsai and Douglas, 1996). We first examined whether auxH875Q could inhibit auxWT function in an uncoating assay. Recombinant auxH875Q was titrated into an uncoating reaction in which coat removal is mediated by endogenous aux1 that copurifies with coated vesicles isolated from bovine brain (Newmyer et al, 2003). As expected, auxH875Q potently inhibited uncoating (Figure 5B, squares). As a positive control for inhibition, we used aux405−814 (Newmyer et al, 2003), a fragment that contains an intact clathrin-binding domain but lacks the DnaJ domain (Figure 5B, circles). In contrast, aux405−591, which lacks both the DnaJ and clathrin-binding domains, did not inhibit (Figure 5B, triangles). We next examined whether auxH875Q can act as a dominant negative for endocytosis when overexpressed in IMCD cells. As shown in Figure 5C, expression of auxH875Q potently inhibited endocytosis of R-Tfn (see auxH875Q-expressing cells marked with asterisks). The inhibition was equivalent to that seen in cells expressing dynK44A (Figure 5C). Note that relatively low levels of virus were used in Figure 5C so that differences between infected and noninfected cells could be shown in the same panel.
To examine whether the observed inhibition in endocytosis was due to a defect in the formation of constricted coated pits, as suggested by semi-in-vitro experiments (Figure 4), a single round of endocytosis was measured in the avidin assay (Carter et al, 1993). In this assay, cells are allowed to prebind R-Tfn at 4°C (amount of ligand bound at the 0 time point represents 100%). Excess Tfn is removed, and endocytosis is initiated by placing the cells at 37°C. As only prebound ligand is internalized, the assay is independent of receptor recycling and thus uncoating of free vesicles. HeLa cells stably expressing a tetracycline-repressible transcription activator (tTA-HeLa) were infected with adenoviruses encoding different aux1 cDNAs under the control of tTA. To rule out effects of infection, cells infected with each construct were grown in the presence of tetracycline (construct not expressed) or in its absence (construct expressed). In agreement with the complete inhibition of R-Tfn internalization in IMCD cells (Figure 5C), expression of auxH875Q almost completely eliminated a single round of B-Tfn internalization in HeLa cells (Figure 5D, compare white with gray column). The degree of inhibition was the same as for dynK44A. Expression of auxWT resulted in a significant but much less severe impairment of Tfn internalization, likely because of auxilin's ability to sequester clathrin into nonfunctional cytosolic granules in HeLa cells (Supplementary Figure 3A and Zhao et al, 2001). Importantly, expression of auxH875Q did not induce formation of clathrin aggregates inside the cytosol as reported previously (Supplementary Figure 3A and Zhao et al, 2001), suggesting that none of the inhibition caused by this mutant is the result of clathrin sequestration. These data demonstrate that a functional J-domain of auxilin is required for the formation of constricted coated pits.
The observed block in R-Tfn uptake could be due to a defect in the early stages of invagination or constriction. To distinguish between these possibilities, we performed a morphological analysis using thin section electron microscopy. Figure 5E shows representative electron micrographs of coated profiles, which were divided into three categories: shallow or wide-mouthed pits, deeply invaginated or omega-shaped coated pits and closed coated profiles with no connection to the surface. The strongest effect of overexpressing auxH875Q was a shift in the distribution of endocytic intermediates from early stages, seen as shallow pits, to later stages, seen as deeply invaginated pits and closed vesicles (Figure 5F and Table III). The data show that auxH875Q arrests endocytosis after the invagination step, either before vesicle constriction or before vesicle budding. Importantly, all closed vesicles were in close proximity to the plasma membrane, and limited serial section analysis revealed that all of them had connections to the surface in subsequent sections (data not shown). In addition, a shift of similar magnitude towards late stages was observed in cells overexpressing dynK44A (Figure 5F and Damke et al, 2001a). Taken together, these data suggest that a majority of closed coated profiles actually represent deeply invaginated coated pits, arguing against a major increase in the number of free CCVs. In agreement with our data, the lack of a major defect in CCV uncoating was also observed in aux2-deficient cells (Zhang et al, 2005).
Table 3.
Quantitative analysis of coated pits in IMCD cells
| Cell type | Diameter of coated profiles (nm) | Coated profiles/mm of surface | Shallow coated profiles | Deep coated profiles | Closed coated profiles |
|---|---|---|---|---|---|
| Control | 163±38 (n=20) | 109 | 43 | 14 | 18 |
| AuxWT | 161±31 (n=24) | 104 | 36 | 17 | 23 |
| AuxH875Q | 160±27 (n=26) | 94 | 27 | 20 | 30 |
| DynK44A | 165±33 (n=20) | 102 | 20 | 19 | 26 |
| The membrane surface sampled was 689, 734, 823, 638 μm for control, auxWT, auxH875Q and dynK44A cells, respectively. | |||||
| Aux, auxilin; Dyn, dyanamin; IMCD, inner medullary collecting duct. | |||||
Despite the change in distribution of pit profiles, the morphology and size of the coated structures and their total abundance per cell did not differ significantly between control, auxWT, dynK44A and auxH875Q (Table III). Thus, the inhibition of endocytosis observed in cells overexpressing auxH875Q was not due to a decrease in the size or the number of coated pits formed. In summary, the avidin assays (Figures 4C and D and 5D) argue that auxilin is required upstream of vesicle fission (invagination or constriction), whereas the morphological analysis demonstrates that chaperone activity is required downstream of invagination. Together, the data strongly argue that the J-domain of auxilin is required for vesicle constriction, the same step regulated by the lifetime of dynamin:GTP.
Discussion
Here we show that dynamin dimers need to oligomerize into tetramers for efficient GTP binding. A mutation within the GAP domain that impairs assembly into higher order structures also impairs dynamin oligomerization into tetramers, identifying tetramer formation as a nucleation event required for assembly into higher order structures as well as efficient GTP binding. In turn, GTP binding by tetramers is a prerequisite for nucleotide- and endocytosis-dependent interactions between dynamin and auxilin in vivo. Biochemical as well as morphological analysis pinpoint vesicle constriction as the primary step in endocytosis that requires the cochaperone activity of auxilin. Precisely, the same step is known to be regulated by the lifetime of dynamin:GTP. Together, our data suggest a specific model by which the oligomerization state of dynamin regulates its interaction with effector molecules.
Auxilin, a dynamin downstream effector
The function of dynamin in CCV formation is well established (for a review see Sever et al, 2000b). Morphological analyses using electron microscopy show that when dynamin activity is compromised, clathrin-coated signatures are arrested after pit invagination, and biochemical analysis using stage-specific endocytosis assays shows that dynamin is required upstream of constriction. Furthermore, stage-specific assays in vivo identified constriction as the rate-limiting step in CCV formation, which is regulated by the lifetime of dynamin:GTP (Sever et al, 2000a; Narayanan et al, 2005). Together, the above studies pinpoint vesicle constriction as the primary step in endocytosis that is regulated by dynamin.
Here, we demonstrate that auxilin is also required for vesicle constriction. AuxH875Q, a mutant that is impaired in cochaperone activity, inhibits endocytosis after the invagination step (EM analysis) and upstream of the constriction step (single round endocytosis assay). Immunodepletion of auxilin from a semipermeable endocytosis assay corroborates the requirement for auxilin upstream of constriction, as does the localization of auxilin to invaginated endocytic vesicles (EM analysis). Together, the data clearly show that the cochaperone activity of auxilin is required for vesicle constriction. Thus, on the basis of strikingly similar outcomes in analogous experiments, dynamin and auxilin exert their functions at the same well-defined and highly specific step in endocytosis.
Further supporting our model, FLIM has identified a GTP- and endocytosis-dependent interaction between auxilin and dynamin specifically at the edge of the cellular monolayer. Mutants of dynamin with impaired GTP binding (dynK44A and dynI690K) did not interact with auxilin. In contrast, dynK694A, a mutant that increases the rate of endocytosis because of longer lifetime of its GTP-bound conformation, exhibited increased interactions with auxilin. In addition, dynamin–auxilin interactions were lost by addition of MBCD (Figure 2D). Because targeting of the proteins to the plasma membrane at the edge of the cellular monolayer was not affected (Figure 2A, B and C), the data suggest that interactions might require a certain degree of coat curvature in order to be promoted, thus making them temporally and spatially regulated. Thus, whereas dynamin–auxilin interactions are most likely transient, the presence of both proteins on the coated profiles is most likely not. Indeed, it has been shown by dual-color total-internal-reflection fluorescence microscopy (TIR-FM) that the amount of dynamin associated with clathrin-coated pits is linearly proportional to the amount of clathrin before and during endocytosis (Rappoport and Simon, 2003). Given the significant presence of auxilin on the coated profiles observed by EM (Figure 1D), its colocalization at the edge of the cellular monolayer with clathrin and dynamin (Figure 2 and Supplementary Figure 1B), as well as the inhibitory effect of anti-auxilin antibodies on the membranes in semi-permeable cell assays (Figure 4D), our data suggest that association of auxilin with the clathrin-coated pits is also not transient. Taken together, our data provide additional strong evidence that auxilin has characteristics of a bona fide downstream dynamin effector, and they suggest a very specific model for how dynamin interacts with this effector (Figure 5G).
Importantly, our data demonstrate that dynamin–auxilin interactions are not sufficient for endocytosis; auxH875Q can interact with dynamin in vivo, but its expression inhibits endocytosis. The inhibitory phenotype of auxH875Q further suggests that dynamin's, as well as auxilin's role in endocytosis, is dependent on functional hsc70. The model of dynamin as a GTPase that regulates the chaperone machinery need not be restricted to formation of CCVs at the plasma membrane. In support of this view, the dynamin homolog in yeast, Vps1p, promotes fusion of yeast vacuoles in conjunction with the chaperone NSF (Peters et al, 2004). Thus, a growing body of evidence suggests that a common theme among dynamin and its family members is to act as timers for the assembly of multiprotein complexes that drive membrane fission or fusion.
Dynamin, an unusual but still canonical regulatory GTPase
Further supporting the role of dynamin as a canonical regulatory GTPase is our mechanistic insight into the molecular switch that allows dynamin to change its functional states. Our data suggest that dynamin cycles between several oligomeric forms that in turn determine its functional state (Figure 5G). Given their negative cooperativity for GTP binding (Table II), dynamin dimers probably oscillate between the empty and GTP-bound states. Assembly of dimers into tetramers induces a conformational change within the GTPase domain, which results in positive cooperativity for GTP binding, and thus drives the equilibrium to the GTP-bound state (illustrated by changes in the symbols for dynamin in Figure 5G). Positive cooperativity and a relatively low rate of GTP hydrolysis make tetramers the most kinetically stable GTP-bound form of dynamin. Finally, assembly into higher order structures such as rings and spirals results in a further conformational change within the GTPase domain of dynamin that leads to GAP-stimulated GTP hydrolysis (Figure 5G). Because GTP hydrolysis is the rate-limiting step in dynamin's GTPase cycle (Eccleston et al, 2002), and because the GTP-bound form is active for endocytosis (see above), assembly into rings and/or spirals has biochemical characteristics of a ‘switch-off' mechanism. We propose that analogous to other GTPases, the switch in three functional states of dynamin (dimers, tetramers, rings/spirals) coordinates its GTPase cycle.
Steady-state kinetic parameters (Vmax and Km for GTP) would predict that dynI690K could bind GTP in vivo, yet it potently inhibits endocytosis. We interpret the inhibition of endocytosis as an inability of dynI690K to form a kinetically stable complex with GTP in vivo, owing to negative cooperativity for GTP binding (Figure 3C). This interpretation is supported by dynI690K's inability to interact with auxilin (Figure 2), which requires GTP, and its inhibition of endocytosis at the same step that is blocked by expression of dynK44A (Song et al, 2004). Based on these results, the original suggestion that this mutant demonstrates a requirement for higher order dynamin assembly in endocytosis must be re-evaluated. A similar mechanism by which assembly states coordinate the GTPase cycle has been reported for the dynamin family member, Dnm1p (Ingerman et al, 2005). Thus, it seems that the classical view for coordinating the GTPase cycle should be extended to include complex formation/oligomerization in addition to GAPs and GEFs.
Materials and methods
Cells, antibodies, reagents and standard assays
Stable IMCD cell lines expressing the human FLAG-tagged vasopressin receptor were established as described for LLC-PK1 cell line (Bouley et al, 2003). The following antibodies were used: anti-clathrin TD1 (a generous gift from SL Schmid, TSRI, La Jolla, CA, USA with permission from FM Brodsky, UCSF, San Francisco, CA, USA); hudy-1 from StressGen Biotechnologies; monoclonal anti-HA tag antibody HA.11 from Covance. Polyclonal anti-auxilin antibodies were prepared against bovine auxilin405−814 (Newmyer et al, 2003). Recombinant His-tagged full-length auxilin was expressed using the baculovirus expression system in Tn5 cells based on the standard protocol (Damke et al, 2001b). After purification on NTA agarose, 1 l of insect cell culture yielded ∼5 mg of auxilin with minimal proteolytic contamination.
The perforated-cell assay for formation of endocytic coated vesicles using A431 cells was performed as described (Schmid and Smythe, 1991). The continuous, regenerative coupled GTPase assay was preformed using 0.6 μM dynamin as described (Ingerman and Nunnari, 2005). Assays performed in the presence of GAP domain (3 μM) were as described (Damke et al, 2001b). Immunofluorescence in tTA HeLa cells, clathrin release assays and single round of endocytosis were performed as described (Newmyer et al, 2003). When IMDC cells were used, cells were coinfected with adenoviruses expressing the chimeric tet-regulatable transcription activator in addition to viruses expressing auxilin and/or dynamin. Internalization of R-Tfn was performed using 20 μg/ml R-Tfn in phosphate-buffered saline (PBS) containing 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose and 0.2% bovine serum albumin (BSA) for 10 min at 37°C.
Antibody inhibition experiments
Experiments were performed essentially as described in Schmid and Smythe (1991) except that increasing concentrations of affinity-purified anti-auxilin antibodies were incubated with membranes for 20 min on ice, before addition of the ATP-regenerating mixture and cytosol. For rescue experiments, antibodies were preincubated with 0.5 or 2 μg of purified auxilin (0.12 or 0.5 μM final concentration in the assay, subsequently) for 15 min on ice, before addition of membrane. After addition of cytosol and nucleotides, cell membranes were incubated at 37°C for 10 min to allow for endocytosis. For auxilin depletion and rescue experiments, Protein-A sepharose beads (80 μl) were preincubated with affinity-purified anti-auxilin polyclonal antibodies (150 μl). Affinity matrix was washed with PBS and ∼40 μl of affinity matrix was incubated with cytosolic fractions derived from RBC (0.2 ml containing ∼3 mg total proteins). Two consecutive 2 h incubations at 4°C with rotation were performed. Preimmune serum served as negative control.
Fluorescence lifetime imaging microscopy
FLIM was performed as described (Sever et al, 2005), except that no vasopressin was added to the cells. Use of 1 μM of vasopressin as reported in Sever et al (2005) can induce non-clathrin-dependent endocytosis in IMCD cells, and thus lead to Tfn internalization in dynK44A or dynI690K background. In addition, cells were permeablized using three different procedures: 0.3% Triton X-100 in PBS for 20 min; 1% sodium dodecyl sulfate in PBS for 3 min; 0.1% Triton X-100 in PBS with 1% BSA and 5% normal goat serum for 1 h (simultaneous permeablization and blocking). Signal for the wild-type proteins was identical regardless of the permeablization procedure. As the spatial lifetime map showed that FRET was localized to subcellular components, the t1 mean for the whole image could be convolved by spatial artifacts (i.e. the relative amount of non-FRETing pixels). By performing a range-dependent analysis, which excluded all pixels where t1>2 ns (technical note: actually 1 ns>t1>2 ns, t1<1 ns is considered abnormally fast), the t1 matrix mean represented exclusively the average lifetime of the population of pixels exhibiting detectable FRET.
Analytical ultracentrifugation
Analytical ultracentrifugation was performed on a Beckman XL-I analytical ultracentrifuge using both interference and absorbance optics. AUC cells were constructed with sapphire windows, 1.2 mm charcoal-filled Epon centerpieces and used with either an An60 Ti 4-hole or An50 Ti 8-hole rotor. Samples (5 μM WT or I690K dynamin in 20 mM N-2-hydroxyl piperazine-N′-2-ehane sulfonic acid, pH 7.5, 150 mM KCl, 1 mM ethylene diaminetetra acetic acid, 1 mM ethylene glycol-bis (b-aminoethyl ether), 1 mM dithiothreitol) for velocity experiments were equilibrated to 20°C before being spun at 50 000 r.p.m. for 4 h, recording absorbance and interference spectra as quickly as instrumentation allowed (∼every 2–10 min). Scans were analyzed using the c(s) method in SedFit (Schuck et al, 2002).
Transmission electron microscopy
Electron microscopy of kidney. Ultrathin sections of normal dog kidney cortex were embedded (London Resin white, medium hard grade, Electron Microscopy Sciences, Fort Washington, PA) and collected onto formvar-coated, gilded nickel grids. The sections were blocked for 10 min on drops of 5% normal goat serum plus 1% BSA in phosphate-buffered saline and immunostained using polyclonal anti-auxilin antibodies and monoclonal hudy-1. The primary antibodies were detected with either 15 nm GAR or 10 nm goat-anti-mouse gold (Ted Pella, Inc., Redding, CA). Sections were observed in a Philips CM10 transmission electron microscope at 80 kV. Pictures were taken at 21 000 and enlarged × 2.75.
Electron microscopy of IMCD cells. IMCD cells were grown in 10 cm dishes. At 18 h postinfection, the cells were fixed using 4% paraformaldehyde in PBS with 4% sucrose. The cells were permeabilized with 0.05% CHAPS in PBS for 1 h. Following a final rinse in PBS, the cells were fixed in 1% glutaraldehyde in PBS for 30 min at room temperature. The cells were then scraped, pelleted and osmicated in 1% OsO4 in 0.1 M sodium cacodylate buffer, pH 7.4 for 1 h, room temperature. The pellets were embedded in 2% agarose and dehydrated through a graded series of ethanol, infiltrated in an ethanol:epon mixture and finally embedded in pure epon (EMS, Fort Washington, PA) overnight in a 60°C oven. Ultrathin sections were collected onto formvar-coated slot grids and poststained with lead citrate and uranyl acetate. Quantification of the coated pit was performed by photographing individual cell profiles at a low magnification (× 1200) to measure the surface length on negatives and then counting the number of coated pits and classifying their morphology at a higher magnification (× 10 500). At least 10 cell profiles were counted for each condition. The dimensions of randomly photographed deeply curved or sealed pits having clearly defined diameters were measured on EM negatives at × 21 000 using a × 15 magnifying glass and a ruler with 0.1 mm subdivisions.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Legends to Supplementary Figures
Acknowledgments
We are indebted to Elena Ingerman and Jodi Nunnari (UC Davis) for help in setting up the continuous GTPase assay, and to Sandy Schmid (TSRI) for adenovirus expressing dynI690K and helpdul discussion. The fluorescence and EM work was performed in the Microscopy Core facility of the MGH Program in Membrane Biology, supported by NIH Grants DK38452, DK57521, DK43341. RR was supported by Grant GM42455. SS was supported by the CSIBD Pilot and Feasibility Grant (5P30DK43351-14), M James Scherbenske Grant from ASN and RO1 (DK064787).
References
- Binns DD, Barylko B, Grichine N, Atkinson MA, Helms MK, Jameson DM, Eccleston JF, Albanesi JP (1999) Correlation between self-association modes and GTPase activation of dynamin. J Protein Chem 18: 277–290 [DOI] [PubMed] [Google Scholar]
- Bouley R, Sun TX, Chenard M, McLaughlin M, McKee M, Lin HY, Brown D, Ausiello DA (2003) Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol 285: C750–C762 [DOI] [PubMed] [Google Scholar]
- Carter LL, Redelmeier TE, Woollenweber LA, Schmid SL (1993) Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J Cell Biol 120: 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Hull M, Mellman I (2004) The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J Cell Biol 164: 1055–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Newmyer SL, Hull MJ, Ebersold M, Schmid SL, Mellman I (2002) Hsc70 is required for endocytosis and clathrin function in Drosophila. J Cell Biol 159: 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Binns DD, Ueda H, Schmid SL, Baba T (2001a) Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell 12: 2578–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Muhlberg AB, Sever S, Sholly S, Warnock DE, Schmid SL (2001b) Expression, purification, and functional assays for self-association of dynamin-1. Methods Enzymol 329: 447–457 [DOI] [PubMed] [Google Scholar]
- Eccleston JF, Binns DD, Davis CT, Albanesi JP, Jameson DM (2002) Oligomerization and kinetic mechanism of the dynamin GTPase. Eur Biophys J 31: 275–282 [DOI] [PubMed] [Google Scholar]
- Greener T, Zhao X, Nojima H, Eisenberg E, Greene LE (2000) Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles form non-neuronal cells. J Biol Chem 275: 1365–1370 [DOI] [PubMed] [Google Scholar]
- Hill E, van der Kaay J, Downes CP, Smythe E (2001) The role for dynamin and its binding partners in coated pit invagination and scission. J Cell Biol 152: 309–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE, Schmid SL (1995) Dynamin self assembles into rings suggesting a mechanism for coated vesicle budding. Nature 374: 190–192 [DOI] [PubMed] [Google Scholar]
- Ingerman E, Nunnari J (2005) A continuous, regenerative coupled GTPase assay for dynamin-related proteins. Methods Enzymol 404: 611–619 [DOI] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J (2005) Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol 170: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen TG, Skretting G, van Deurs B, Sandvig K (2003) Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA. Proc Natl Acad Sci USA 100: 5175–5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T (2000) Clathrin. Annu Rev Biochem 69: 699–727 [DOI] [PubMed] [Google Scholar]
- Lee DW, Zhao X, Zhang F, Eisenberg E, Greene LE (2005) Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors. J Cell Sci 118: 4311–4321 [DOI] [PubMed] [Google Scholar]
- Lin HC, Barylko B, Achiriloaie M, Albanesi JP (1997) Phosphatidylinositol (4,5)-bisphosphate-dependent activation of dynamins I and II lacking the proline/arginine-rich domains. J Biol Chem 272: 25999–26004 [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D (2005) Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121: 593–606 [DOI] [PubMed] [Google Scholar]
- Muhlberg AB, Warnock DE, Schmid SL (1997) Domain structure and intramolecular regulation of dynamin GTPase. EMBO J 16: 6676–6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Leonard M, Song BD, Schmid SL, Ramaswami M (2005) An internal GAP domain negatively regulates presynaptic dynamin in vivo: a two-step model for dynamin function. J Cell Biol 169: 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmyer SL, Christensen A, Sever S (2003) Auxilin–ynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev Cell 4: 929–940 [DOI] [PubMed] [Google Scholar]
- Newmyer SL, Schmid SL (2001) Dominant-interfering hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J Cell Biol 152: 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Baars TL, Buhler S, Mayer A (2004) Mutual control of membrane fission and fusion proteins. Cell 119: 667–678 [DOI] [PubMed] [Google Scholar]
- Rappoport JZ, Simon SM (2003) Real-time analysis of clathrin-mediated endocytosis during cell migration. J Cell Sci 116: 847–855 [DOI] [PubMed] [Google Scholar]
- Schlossman DM, Schmid SL, Braell WA, Rothman JE (1984) An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol 99: 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, Smythe E (1991) Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol 114: 869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck P, Perugini MA, Gonzales NR, Howlett GJ, Schubert D (2002) Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys J 82: 1096–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S, Damke H, Schmid SL (2000a) Dynamin:GTP controls formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J Cell Biol 150: 1137–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S, Damke H, Schmid SL (2000b) Garrotes, springs, ratchets, and whips: putting dynamin models to the test. Traffic 1: 385–392 [DOI] [PubMed] [Google Scholar]
- Sever S, Muhlberg AB, Schmid SL (1999) Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature 398: 481–486 [DOI] [PubMed] [Google Scholar]
- Sever S, Skoch J, Bacskai BJ, Newmyer S (2005) Assays and functional properties of auxilin–dynamin interactions. Methods Enzymol 404: 570–585 [DOI] [PubMed] [Google Scholar]
- Song BD, Yarar D, Schmid SL (2004) An assembly-incompetent mutant establishes a requirement for dynamin self-assembly in clathrin-mediated endocytosis in vivo. Mol Biol Cell 15: 2243–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA 96: 6775–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D (2002) Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011 [DOI] [PubMed] [Google Scholar]
- Tsai J, Douglas MG (1996) A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem 271: 9347–9354 [DOI] [PubMed] [Google Scholar]
- Umeda A, Meyerholz A, Ungewickell E (2000) Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur J Cell Biol 79: 336–342 [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature 378: 632–635 [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL (1993) Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol 122: 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock DE, Hinshaw JE, Schmid SL (1996) Dynamin self assembly stimulates its GTPase activity. J Biol Chem 271: 22310–22314 [DOI] [PubMed] [Google Scholar]
- Zhang CX, Engqvist-Goldstein AE, Carreno S, Owen DJ, Smythe E, Drubin DG (2005) Multiple roles for cyclin G-associated kinase in clathrin-mediated sorting events. Traffic 6: 1103–1113 [DOI] [PubMed] [Google Scholar]
- Zhao X, Greener T, Al-Hasani H, Cushman SW, Eisenberg E, Greene LE (2001) Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J Cell Sci 114: 353–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Legends to Supplementary Figures


