Abstract
Regulation of brain-derived neurotrophic factor (BDNF) secretion plays a critical role in long-term potentiation (LTP). It is generally thought that the supply for this secretion is newly synthesized BDNF targeted to the synapse. Here we provide evidence that hippocampal neurons additionally recycle BDNF for activity-dependent secretion. Exogenously applied BDNF is internalized by cultured neurons and rapidly becomes available for activity-dependent secretion, which is controlled by the same mechanisms that regulate the secretion of newly synthesized BDNF. Moreover, BDNF recycling replaced the new synthesis pathway in mediating the maintenance of LTP in hippocampal slices: the late phase LTP, which is abolished by protein synthesis inhibition, was rescued in slices preincubated with BDNF. Thus, endocytosed BDNF is fed back to the activity-dependent releasable pool required for LTP maintenance.
Keywords: neuronal plasticity, neurotrophin, release, TIRF, vesicles
Introduction
The neurotrophin (NT) brain-derived neurotrophic factor (BDNF) is known to modulate neuronal plasticity by inducing changes in synaptic efficacy and morphology (McAllister et al, 1999; Thoenen, 2000; Poo, 2001; Lu, 2004). In particular, activity-dependent secretion of BDNF plays a critical role in long-term potentiation (LTP) (Bramham and Messoudi, 2005; Lu et al, 2005). The synaptic actions of BDNF depend on the specific sites (McAllister, 2002), the precise time window and extent of BDNF secretion (Lessmann et al, 2003), as well as the localization of its cognate receptors (Poo, 2001). Neuronal activity regulates BDNF availability at three different levels, namely by increasing transcription (Ernfors et al, 1991; Shieh et al, 1998; Tao et al, 1998; Chen et al, 2003), translation (Schratt et al, 2004) and secretion (Balkowiec and Katz, 2000; Hartmann et al, 2001; Gartner and Staiger, 2002; Aicardi et al, 2004). Recent studies have established that all these regulatory events are critical steps in the late, persistent phase of LTP, which is dependent on gene transcription and protein translation (Kandel, 2001). Overexpression of mRNA BDNF transcripts in transgenic mice carrying a constitutively activated form of CREB resulted in a late-phase LTP independent of new transcription in hippocampal CA1 neurons (Barco et al, 2005). Moreover, exogenous BDNF administered to mouse hippocampal slices rescued LTP impaired by protein synthesis inhibition (Pang et al, 2004). Thus, increased BDNF availability can overcome the physiological requirement for BDNF transcription and translation induced by high-frequency stimulation. In this respect, it is remarkable that critical levels of BDNF secreted for 5–12 min after theta-burst stimulation (TBS) strictly correlated with this form of LTP (Aicardi et al, 2004).
For the understanding of the activity-dependent regulation of BDNF availability, it is important to identify the pathways by which it is supplied to the site(s) of secretion. BDNF is synthesized in the soma, packaged into post-Golgi membrane-confined compartments and transported to either dendritic domains or axon terminals (Poo, 2001). BDNF synthesis has also been observed in dendrites of cultured hippocampal neurons (Tongiorgi et al, 1997). However, BDNF has been detected in neurons that lack BDNF mRNA transcripts (Conner et al, 1997), indicating that it has been taken up by these cells. Moreover, green fluorescent protein (GFP)-labeled BDNF can be synthesized in one neuron and transferred to another in an activity-dependent manner in cultured hippocampal neurons (Kohara et al, 2001). Thus, what is the fate of the endocytosed BDNF? Does it enter the pathway of newly synthesized BDNF leading to secretion? Does it contribute to the increased availability required for synaptic plasticity? Evidence for this process has been provided by studies on transneuronal transfer of NTs in the visual system of chick and rodents. Injection of BDNF or neurotrophin-3 (NT-3) into the eyes of chick embryos (von Bartheld et al, 1996; Butowt and von Bartheld, 2001; Wang et al, 2002) or adult rodents (Caleo et al, 2000, 2003; Butowt and von Bartheld, 2005) led to their uptake by retinal ganglion cells, transport to the axon terminals and secretion, resulting in survival of target neurons (von Bartheld et al, 1996; Caleo et al, 2000, 2003) and synaptic plasticity (Wang et al, 2002). However, it is still unclear whether NTs are only destined to long-range transport to distal synapses after internalization or can also gain rapid entry into a local recycling pathway (Poo, 2001; von Bartheld, 2003; Yano and Chao, 2003). The present study demonstrates that BDNF is internalized in large endocytic vesicles in cultured hippocampal neurons via a TrkB-dependent mechanism, and promptly becomes re-available for activity-dependent secretion. The recycled BDNF fulfills the function of newly synthesized BDNF in mediating the conversion of early- into late-phase LTP.
Results
BDNF endocytosis in hippocampal neurons is a TrkB-mediated process
The time course of BDNF endocytosis was analyzed by immunocytochemistry using anti-BDNF antibodies in cultured hippocampal neurons exposed to exogenous BDNF (100 ng/ml) for 10 and 60 min. Figure 1A shows only background levels of BDNF immunoreactivity in untreated neurons, whereas immunoreactive puncta appeared in the soma and neuronal processes that progressively increased in number and fluorescence intensity during BDNF exposure. In a second set of experiments, we investigated whether BDNF endocytosis depended on a receptor-mediated internalization process. Plasma membrane proteins from control or BDNF-incubated neurons were labeled with activated biotin and purified by immunoprecipitation using agarose-conjugated streptavidin. Two receptors, the full-length TrkB and its truncated form TrkB-t, which lacks the catalytic domains (Klein et al, 1990), were shown by Western blot analysis using an anti-TrkB antibody, which recognizes the extracellular domain of both receptors (Figure 1B). Membrane expression of TrkB estimated by densitometry was reduced by about 90% after BDNF treatment, and this effect was prevented by pretreating neurons with K252a, a relatively specific inhibitor of Trk phosphorylation. Conversely, TrkB-t surface expression did not change significantly following BDNF treatment. The total amount of both TrkB and TrkB-t precipitated by wheat germ agglutinin (WGA)-agarose in whole-cell lysates was similar in each sample, indicating that reduced plasma membrane levels of TrkB induced by BDNF reflect receptor internalization rather than specific degradation (Sommerfeld et al, 2000). Furthermore, we found that TrkB internalization correlated with cytoplasmic accumulation of BDNF quantified by conventional enzyme-linked immunosorbent assay (ELISA). The same lysate in which plasma membrane levels of TrkB were reduced by exogenous BDNF (Figure 1B) contained a high quantity of BDNF protein, which was drastically reduced by K252a pretreatment (Figure 1C). Only a small amount of BDNF bound to the cell surface was removed by acid treatment of intact neurons incubated with BDNF for 60 min (Figure 1C). Moreover, immunocytochemical analysis of K252a-pretreated neurons showed background BDNF immunoreactivity after 60 min exposure to exogenous BDNF (Figure 1A).
Figure 1.
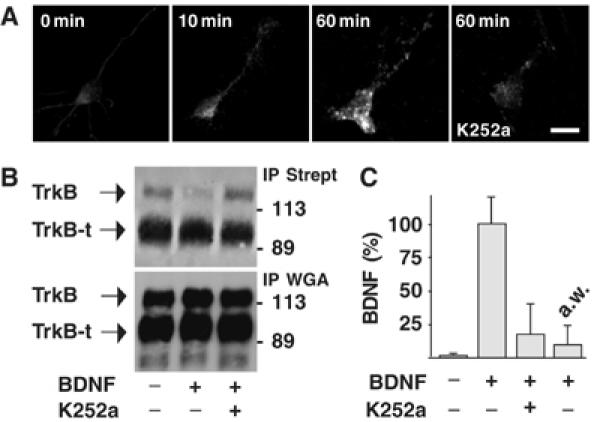
TrkB-mediated BDNF endocytosis in cultured hippocampal neurons. (A) Time course of BDNF endocytosis in untreated neurons (0 min) or neurons exposed to BDNF (10 and 60 min) in the presence or absence of K252a pretreatment, obtained by immunocytochemistry and analyzed by confocal microscopy. Images are representative of 50 neurons analyzed in four independent experiments. Bar, 20 μm. (B) Regulation of plasma membrane expression of TrkB by BDNF. Plasma membrane proteins labeled with activated biotin were isolated by precipitation using agarose-conjugated streptavidin (IP Strept) from lysates of control neurons or neurons treated with BDNF for 60 min. Western blot analysis showed that TrkB, but not TrkB-t, is reduced by BDNF administration; this effect was prevented by pretreating neurons with K252a. Glycoconjugate proteins precipitated by WGA-agarose (IP WGA) showed total amount of TrkB and TrkB-t in whole-cell lysates. (C) Intracellular accumulation of exogenous BDNF measured by ELISA in the same lysates as in panel B. a.w. indicates the amount of BDNF immunoreactivity stripped from the cell surface by acid treatment. Data are expressed as percentage of the means±s.e.m. (n=6).
We next immunopurified the vesicles containing BDNF/TrkB complexes using magnetic beads coated with pan-Trk antibodies, which recognize the carboxy-terminal region common to all Trks. Analysis of precipitated products by Western blotting using the specific anti-TrkB antibody confirmed the expression of TrkB (Figure 2A). The purified vesicles also contained BDNF, as shown by ELISA: BDNF was detectable in preparations obtained from untreated neurons, but its level was about two/three-fold higher in neurons incubated with BDNF for 60 min (Figure 2B). The specificity of the purification procedure was determined using magnetic beads coated with antibodies directed against the synaptic vesicle marker synaptophysin (anti-syp). Under these conditions, no BDNF immunoreactivity was detected in the purified vesicles (Figure 2B). The ultrastructural characterization of isolated vesicles obtained by electron microscopy revealed that pan-Trk-coated magnetic beads recruited a population of vesicles of 137±35 nm in diameter (Figure 2C). The number of BDNF-containing vesicles recovered by pan-Trk beads was more than doubled upon BDNF exposure, indicating that these vesicles indeed encompass endocytic vesicles. Conversely, anti-syp beads mostly recruit organelles with the typical dimensions of synaptic vesicles (63±24 nm), whose number did not change upon BDNF exposure (Figure 2D).
Figure 2.
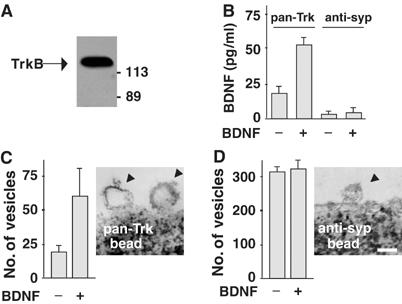
Internalization of BDNF–TrkB complexes in endocytic vesicles. (A) Magnetic beads coated with pan-Trk antibodies (pan-Trk bead) were used to trap Trk-containing vesicles from hippocampal neurons exposed to exogenous BDNF. Western blot analysis using anti-TrkB antibody revealed that purified vesicles express TrkB as a single immunoreactive protein of 135 kDa. (B) ELISA quantification of BDNF in pan-Trk bead and anti-syp bead purifications. Data are means±s.e.m. (n=4). (C) Quantification of vesicles isolated by pan-Trk beads. Vesicle number increased after BDNF exposure for 60 min. Representative image obtained by electron microscopy showing endocytic vesicles attached to a pan-Trk bead. (D) Quantification of vesicles isolated by anti-syp beads. Vesicle number did not change after BDNF exposure for 60 min. Representative image obtained by electron microscopy showing a vesicle with the typical size of a synaptic vesicle attached to an anti-syp bead. Bar, 100 nm. Data in panels C and D are means/100 beads±s.d.
Distinct intracellular distribution of endocytosed and newly synthesized BDNF
The intracellular distribution of endocytosed and newly synthesized BDNF was compared in colocalization studies based on BDNF labeling with two different tags. Neurons were transduced for 12 h with an adenoviral vector encoding BDNF tagged with the myc epitope (BDNF-myc) (Canossa et al, 1997) and then incubated for 10 or 60 min with BDNF tagged with yellow fluorescence protein (YFP), whose endocytosis did not differ from that of the native BDNF (Supplementary Figure 1). The extracellular BDNF scavenger TrkB-Fc was applied throughout the adenoviral infection and washed out before BDNF-YFP application, to avoid BDNF-myc secreted from infected neurons being internalized by infected or non-infected neurons. Direct YFP fluorescence and myc immunofluorescence were analyzed by confocal microscopy. Figure 3A shows that BDNF-YFP was distributed in the soma and neuronal processes with a punctate intracellular pattern similar to that of BDNF-myc, but individual puncta were positive for either BDNF-YFP or BDNF-myc without any overlap. This distinct intracellular distribution was confirmed in colocalization experiments using antibodies recognizing an early endosomal protein (EEA1), a resident protein (BiP) of the endoplasmic reticulum or a cis-Golgi matrix protein (GM130). BDNF-YFP colocalized with EEA1 but not with BiP or GM130 (Figure 3B); conversely, BDNF-myc colocalized with BiP and GM130 but not with EEA1 (Figure 3C).
Figure 3.
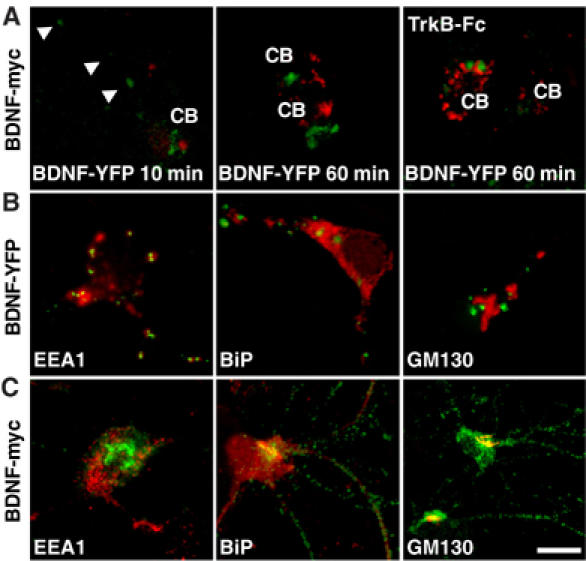
Intracellular localization of endocytosed and newly synthesized BDNF. (A) Hippocampal neurons infected for 12 h with adenoviral vector transducing BDNF-myc in the presence or absence of TrkB-Fc were incubated with BDNF-YFP for 10 or 60 min. YFP fluorescence (green) and myc immunofluorescence (red) showed distinct intracellular localization in cell soma (CB) and neuronal processes (arrows). Images are representative of 25 neurons analyzed in five independent experiments. Colocalization between BDNF-YFP (B) or BDNF-myc (C) immunoreactivity (green) with that of EEA1, BiP and GM130 (red). Images are representative of 20 neurons analyzed in three independent experiments. Bar, 20 μm.
Recycling of endocytosed BDNF by activity-dependent secretion
Time-lapse confocal imaging was used to investigate endocytosed BDNF-YFP secretion after exposing neurons to BDNF-YFP for 60 min. Figure 4A shows a representative experiment in which YFP fluorescence was analyzed in different areas located in the soma and neuronal processes, and plotted with time. Fluorescence intensity remained unchanged in the absence of stimuli, whereas KCl (50 mM) stimulation for 5 min led to a 20–40% reduction in some of these areas, reflecting BDNF-YFP secretion. Total internal reflection fluorescence (TIRF) microscopy was used to study BDNF-YFP secretion at the level of single vesicles with higher temporal and spatial resolution. YFP fluorescence is known to display marked pH dependence (Ashby et al, 2004): following endocytosis, BDNF-YFP accumulates in a self-quenched state inside acidic vesicles. Exocytosis is revealed by the occurrence of dequenching flashes caused by the rapid increase in pH upon diffusion of BDNF-YFP to the extracellular medium. Endocytic vesicles were also labeled with the pH-sensitive dye acridine-orange (Steyer et al, 1997; Avery et al, 2000; Tsuboi et al, 2000), a well-known marker for vesicle fusion events (Avery et al, 2000; Zenisek et al, 2000). A short (1–3 min) co-exposure of neurons to both BDNF-YFP and acridine-orange was sufficient to visualize double-labeled vesicles undergoing KCl-induced exocytosis. Time sequences document fusion of individual vesicles in different regions of a single neuron or neuronal network (Figure 4B; Supplementary movie). Changes in fluorescence intensity were analyzed to measure the exocytic pattern of an individual vesicle: when it fused with the plasma membrane, it produced a localized transient increase in brightness of both signals followed by a lateral spread in fluorescence (flash) leading to their disappearance. The number of light flashes observed during 3-min applications of KCl were increased roughly three-fold (26±4 mean flashes/7000 μm2 field of investigation±s.d.; n=36) with respect to buffer application only (6±2; n=12) or no application (8±3; n=42). Most (about 65%) fusion events took place during the first 20 s, indicating that the number of flashes increased more than 15-fold immediately after KCl application.
Figure 4.
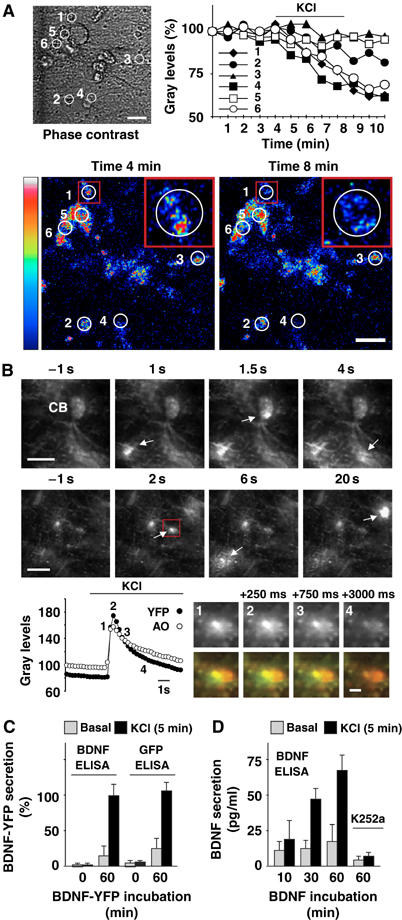
Activity-dependent secretion of endocytosed BDNF. (A) BDNF-YFP secretion was analyzed by time-lapse confocal imaging in circular areas 1–6 (phase contrast). Fluorescence intensity was expressed as percentage of gray levels and plotted with time. In resting conditions, fluorescence was unchanged, whereas KCl treatment induced a reduction in areas 1, 4 and 6. Confocal images document fluorescence intensity displayed in pseudo-color (scale palette on the left) before (min 4) and after (min 8) KCl treatment. Three-fold magnifications of area 1 (insets) showed BDNF-YFP fluorescence in a neuronal process. Note that fluorescence decreased within the white circle, and did not increase in the surrounding area delimited by the red square. This indicates that decreases in fluorescence intensity were due to BDNF-YFP secretion and not to its possible movements in the x–y axis. Data are representative of six independent experiments. Bars, 20 μm. (B) Exocytic fusion of vesicles analyzed by TIRF imaging in hippocampal neurons incubated with BDNF-YFP for 3 min. Sequential images document dequenching flashes of YFP fluorescence (arrows) in cell body (CB) and neuronal processes of a single neuron (upper sequence), or neuronal network (lower sequence). Times represent seconds before and after perfusion with KCl. Bars, 10 μm. Changes in fluorescence intensity (gray levels) of a single vesicle (red square) fusing to the plasma membrane. YFP and acridine-orange (AO) fluorescence was measured in a circular mask with an area of 120 pixels centered over the spots. Numbers (1–4) on the graph correspond to sequential images of YFP signal shown in gray on the right. The same sequential images showed overlap (yellow) of YFP (green) and acridine-orange (red) fluorescence. Times represent milliseconds from appearance (1), increase in brightness and diffusion (2), decrease (3) and disappearance (4) of the fluorescent signals. Bar, 2 μm. (C) ELISA quantification of BDNF-YFP in perfusates of control neurons or neurons previously treated with BDNF-YFP for 60 min. Cultured neurons did not show immunoreactivity for BDNF or GFP in sufficient amounts to be detected by two-site BDNF or GFP ELISAs, respectively. KCl treatment (5 min) increased BDNF-YFP immunoreactivity over the basal levels in BDMF-YFP-treated neurons. (D) ELISA quantification of BDNF in perfusates of neurons previously exposed to BDNF for 10, 30 or 60 min. KCl treatment (5 min) increased BDNF immunoreactivity over the basal levels and was prevented by K252a pretreatment. Data in panels C (n=5) and D (n=8) are means±s.e.m.
Lastly, two different ELISAs were used to measure BDNF-YFP in the perfusion medium of control neurons or neurons treated with BDNF-YFP for 60 min. These are two-site BDNF and GFP ELISAs made using a specific primary anti-BDNF and anti-GFP (recognizing all GFP isoforms including YFP) antibody, respectively. Both ELISAs shared the same secondary anti-BDNF-POD antibody. Untreated neurons did not show immunoreactivity for BDNF or GFP, whereas comparable levels of immunoreactivity were detected by both ELISAs after 5 min KCl stimulation of BDNF-YFP-treated neurons (Figure 4C). This indicates that endocytosed BDNF-YFP secretion is activity-dependent, and that endogenous BDNF was not secreted in sufficient amounts to be detected. Thus, the subsequent experiments were carried out by exposing neurons to native BDNF (Figure 4D). Challenge with KCl for 5 min resulted in increased BDNF secretion in neurons exposed to BDNF for 10–60 min, and this effect was abolished when BDNF internalization was prevented by K252a pretreatment. On the contrary, basal (non-stimulated) BDNF secretion did not change after the different incubation times.
Secretion of endocytosed and synthesized BDNF is regulated by common mechanisms
We investigated whether the mechanisms regulating the secretion of newly synthesized BDNF also apply to endocytosed BDNF. We initially analyzed the role of glutamate, known to regulate the secretion of synthesized NTs through a mechanism involving calcium release from intracellular stores (Blochl and Thoenen, 1995; Canossa et al, 1997, 2001; Griesbeck et al, 1999; Balkowiec and Katz, 2002; Wang et al, 2002). Endocytosed BDNF secretion was evaluated by ELISA in the perfusion medium of neurons previously exposed to BDNF for 60 min. Glutamate applied for 5 min in both calcium-containing and calcium-free extracellular medium supplemented with ethylene glycol-bis-(2-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA) elicited an increase in endocytosed BDNF, which was partially prevented by chelating cytosolic calcium with the membrane-permeable calcium chelator bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) in calcium-free conditions (Figure 5A). Moreover, a 5 min treatment with caffeine, known to mobilize calcium from intracellular stores, induced massive secretion of endocytosed BDNF. The effect of glutamate on endocytosed BDNF secretion was mimicked by treating neurons for 5 min with α-amino-3-hydroxyl-5-methyl-4-isoxazolpropionic acid (AMPA) or 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD), which activate AMPA or metabotropic group I/II glutamate receptors, respectively. These effects were prevented by pretreating with specific antagonists: 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX) for the AMPA receptor and 1-aminoindan-1,5-dicarboxylacid (AIDA) for metabotropic group I receptor. N-methyl-D-aspartate (NMDA) was not effective.
Figure 5.
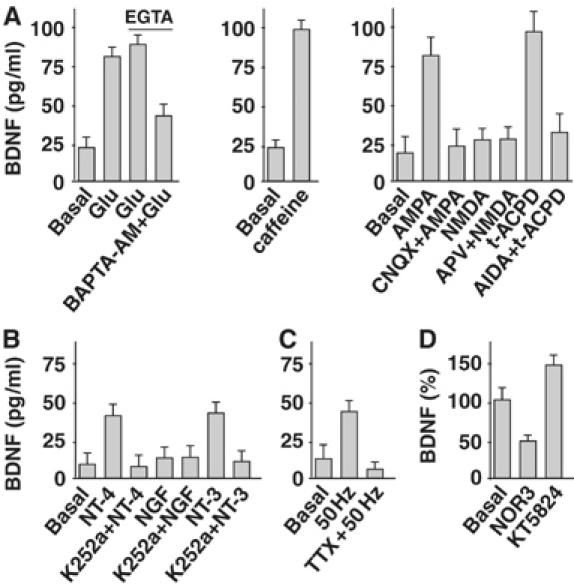
Pharmacology of endocytosed BDNF secretion. (A) BDNF secretion was evaluated by ELISA in perfusates of hippocampal neurons previously exposed to BDNF for 60 min. Glutamate (Glu) application elicited the secretion of endocytosed BDNF in both calcium-containing and calcium-free medium (EGTA). The effect was partially prevented by BAPTA-AM. BDNF secretion was also triggered by caffeine. AMPA- and t-ACPD-induced BDNF secretion was prevented by CNQX and AIDA, respectively. NMDA was not effective either in the presence or absence of APV. (B) BDNF secretion was induced by NT-4 and NT-3 but not NGF. This effect was prevented by K252a. (C) High-frequency stimulation (50 Hz) triggers BDNF secretion. This effect was prevented by TTX. (D) The NO donor NOR3 administered to BDNF-treated neurons elicited a decrease in BDNF secretion. Conversely, KT5823 triggered BDNF secretion. Data in panels A (n=12), B (n=9), C (n=12) and D (n=6) are means±s.e.m.
Activation of Trk receptors stimulates secretion of newly synthesized BDNF (Canossa et al, 1997; Kruttgen et al, 1998). We found that this mechanism also applied to endocytosed BDNF: as shown in Figure 5B, its secretion was induced by a 5 min treatment with neurotrophin-4 (NT-4) and NT-3. This induction was prevented by pretreating neurons with K252a. Nerve growth factor (NGF) was not effective. These data are consistent with TrkB and TrkC, but not TrkA, expression in these neurons (Supplementary Figure 2).
Secretion of BDNF could also be induced by high-frequency (50 Hz) electrical stimulation (Balkowiec and Katz, 2000). This effect was abolished by the sodium channel blocker tetrodotoxin (TTX) (Figure 4D).
Lastly, we found that the secretion of endocytosed BDNF was reduced by nitric oxide (NO) via activation of protein kinase G (PKG) signaling, as previously shown for newly synthesized BDNF (Canossa et al, 2002). Figure 5C shows that a 10 min treatment with the NO donor (±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide (NOR3) diminished the basal secretion of endocytosed BDNF by about 50%. Conversely, acute inhibition of PKG by KT5823 applied for 10 min elicited an increase in endocytosed BDNF secretion.
Thus, all the investigated mechanisms regulating the secretion of synthesized BDNF also apply to endocytosed BDNF.
Secretion of endocytosed BDNF rescues LTP impaired by protein synthesis inhibition
We initially confirmed in adult (4–6 weeks old) rat hippocampal slices that BDNF is a necessary protein synthesis product for LTP maintenance, as already observed in mice by Pang et al (2004). Field excitatory post-synaptic potentials (EPSPs) evoked by stimulation of Schaffer collaterals were recorded in CA1 area (Figure 6A). TBS induced LTP that persisted for more than 180 min. When the slices were treated for 30 min before stimulation and throughout the recording with the protein synthesis inhibitor anisomycin, the duration of LTP decreased to about 70–100 min. We found that this effect was fully reversed by exogenous BDNF (100 ng/ml) application from 5 min before to 15 min after TBS. The same BDNF treatment was ineffective in the absence of TBS (Supplementary Figure 4C).
Figure 6.
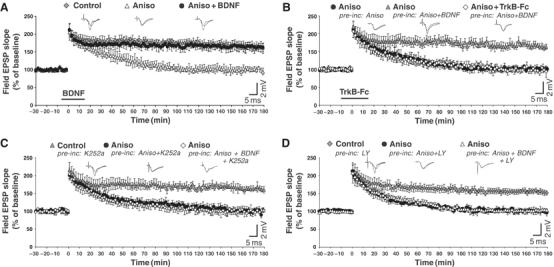
Endocytosed BDNF rescues LTP impaired by protein synthesis inhibition. (A) Field EPSPs evoked in CA1 area by Schaffer collaterals stimulation. TBS induced LTP that is maintained for 180 min (Control) (five slices, five rats). In slices perfused with anisomycin (Aniso) from 30 min before TBS to the end of the recording, LTP persisted for only 70–100 min (five slices, five rats). The effect of anisomycin was fully reversed by applying BDNF from 5 min before to 15 min after TBS (Aniso+BDNF) (six slices, five rats). (B) In slices incubated (pre-inc: Aniso) and perfused (Aniso) with anisomycin throughout the recording, LTP persisted for only 70–100 min (nine slices, seven rats). LTP was rescued in slices preincubated with BDNF (pre-inc: Aniso+BDNF) (12 slices, nine rats), an effect that was prevented by TrkB-Fc (Aniso+TrkB-Fc) (eight slices, six rats). (C) Pretreatment with K252a did not affect LTP maintenance (Control; pre-inc: K252a) (10 slices, seven rats). The presence of anisomycin in both preincubation and perfusion fluid reduced LTP duration to 70–100 min (Aniso; pre-inc: Aniso+K252a) (nine slices, six rats). The blocking action of anisomycin on LTP persisted in slices preincubated with BDNF and K252a (Aniso; pre-inc: Aniso+BDNF+K252a) (11 slices, eight rats). (D) Pretreatment with LY294002 (LY) (five slices, five rats) led to the same effects observed in the presence of K252a (panel C). Data in panels A–D are means±s.e.m. of field EPSP slopes plotted as percentages of the baseline. Representative field EPSP traces recorded before and 180 min after TBS are shown on the top of the panels.
To verify whether secretion of endocytosed BDNF could rescue LTP impaired by protein synthesis inhibition, we incubated the slices with both anisomycin (90 min) and BDNF (60 min) before transferring them to the recording chamber in which BDNF was no longer present. Immunohistochemical analysis revealed that this preincubation augmented intracellular BDNF immunoreactivity in neurons in comparison with untreated slices or slices incubated only with anisomycin (Supplementary Figure 4A). High-magnification confocal analysis of single optical images (z resolution <1 μm) revealed that immunostaining for BDNF was localized in CA1 pyramidal neuron cell bodies and processes as a punctate intracellular pattern (Supplementary Figure 4B).
Notably, the blocking action of anisomycin on LTP was fully prevented in BDNF-incubated slices (Figure 6B), suggesting that LTP maintenance could be due to TBS-induced secretion of previously endocytosed BDNF. This interpretation is confirmed by the observation that the effect of BDNF incubation on LTP was abolished (Figure 6C and D) by preventing BDNF internalization in slices with K252a or with the PI3K inhibitor LY294002 (Supplementary Figure 4A), which prevents BDNF and TrkB internalization in cultured neurons (Supplementary Figure 3). A similar effect was obtained by scavenging extracellular BDNF with TrkB-Fc applied from 10 min before to 15 min after TBS (Figure 6C).
Discussion
A major finding of the present study is that BDNF endocytosed in hippocampal neurons promptly undergoes activity-dependent secretion. Optical and biochemical techniques were used to investigate this process in real time. Time-lapse confocal imaging showed KCl-induced secretion of endocytosed BDNF-YFP in the soma and processes of cultured neurons. Single vesicle dynamics, studied by TIRF imaging, revealed the rapid (milliseconds) fusion of BDNF-YFP-containing vesicles to the plasma membrane already 1 min after exogenous BDNF-YFP administration. Thus, the whole recycling process can occur on a rapid timescale. Finally, ELISA quantification of BDNF in the perfusate of neurons previously incubated with exogenous BDNF disclosed increased BDNF levels upon KCl application or high-frequency electrical stimulation. We also observed a small basal (non-stimulated) secretion of endocytosed BDNF, recalling the spontaneous re-exocytosis of internalized NGF previously reported in sympathetic neurons (Weible et al, 2001).
The recycling process described here is distinctly different from the long-lasting transfer of endocytosed NTs to distal synapses previously described in the visual system (von Bartheld et al, 1996; Butowt and von Bartheld, 2001; Wang et al, 2002; Butowt and von Bartheld, 2005). The latter requires many hours of NTs transport following internalization, and passage through the same sorting pathway (Golgi system) of the newly synthesized NTs before secretion (reviewed by Lessmann et al, 2003; von Bartheld, 2003). Instead, here we document a process in which BDNF internalized in large TrkB-containing vesicles avoids ER and Golgi and never colocalizes with newly synthesized BDNF before secretion. Endocytic vesicles may thus represent the main storage compartment for endocytosed BDNF, before routing to the secretory pathway. Since recycling can occur rapidly after exogenous BDNF administration, it is likely that endocytic vesicles containing BDNF can enter the exocytic process directly, in contrast with the general assumption that they are not designated for regulated exocytosis. This might take place either by recycling of BDNF–TrkB complexes to the surface, or by BDNF recycling upon its dissociation from TrkB. The rapid recycling of endocytic vesicles also implies that BDNF is re-secreted locally, near the site of endocytosis. Given that NTs cannot diffuse far from the site of secretion (Blochl and Thoenen, 1995; Wang et al, 1998), recycling may provide a mechanism that contributes to the tight control of BDNF availability in close proximity to TrkB localization. TrkB receptors are known to be expressed in both dendrites and axons (McAllister et al, 1999; Poo, 2001); therefore, BDNF is expected to be recycled by both synaptic partners. This would imply that the amount of recycled BDNF can be controlled by the number of TrkB transferred to the cell surface. Membrane insertion (Meyer-Franke et al, 1998; Du et al, 2000) and internalization (Du et al, 2003) of these receptors at active synapses change dynamically depending on neuronal activity. Thus, active synapses would benefit more from BDNF recycling. This is of particular importance as both NT availability and expression of Trk receptors must be tightly regulated and synchronized to transduce NT signaling in synaptic plasticity (McAllister et al, 1999; Poo, 2001).
It is generally accepted that high-frequency neuronal activity stimulates the secretion of BDNF (Balkowiec and Katz, 2000; Hartmann et al, 2001; Gartner and Staiger, 2002; Aicardi et al, 2004), strengthening and maintaining active synapses (reviewed by Snider and Lichtman, 1996; Poo, 2001; McAllister, 2002). In this context, recycling may help to maintain the size of the activity-dependent releasable pool of BDNF. Although obviously dependent on newly synthesized BDNF secretion for an initial supply, activity-dependent recycling may endow neurons with the capacity to re-use BDNF on a rapid timescale depending on demand. This might be particularly important for LTP maintenance, which requires threshold BDNF levels (Korte et al, 1995, 1996; Patterson et al, 1996) secreted during a limited time window after TBS (Aicardi et al, 2004). The present findings support the competence of BDNF recycling in this process. We demonstrated that secretion of endocytosed BDNF is regulated by the same mechanisms as the secretion of synthesized BDNF. In hippocampal cultures, we found that endocytosed BDNF secretion depended on high-frequency electrical stimulations, glutamate-mediated intracellular calcium mobilization from internal stores, TrkB and TrkC receptor activation, and was downregulated by NO through a cGMP-mediated signaling pathway. The common mechanism underlying the secretion from these two sources is important from a functional point of view: it can ensure that newly synthesized and endocytosed BDNF are recruited by the same patterns of neuronal activity, and thus both BDNF supplies may synergistically regulate synaptic modifications. More direct evidence supporting the role of recycling in LTP maintenance comes from the experiments carried out in hippocampal slices. After preincubation with exogenous BDNF, TBS induced the secretion of endocytosed BDNF that rescued LTP impaired by protein synthesis inhibition. Endocytosed BDNF was secreted in tight temporal conjunction with the LTP-inducing electrical stimulation: TrkB-Fc applied from 5 min before up to 15 min after TBS to BDNF-treated slices abolished the rescue of LTP maintenance. This finding is in accordance with previous investigations showing that this time window is critical for BDNF action on LTP (Chen et al, 1999; Aicardi et al, 2004; Pang et al, 2004; for review see Pang and Lu, 2004; Bramham and Messoudi, 2005). Thus, recycling can replace newly synthesized BDNF secretion in maintaining LTP when adequately supplied.
Another interesting aspect concerns the possible functional difference between endocytosed and newly synthesized BDNF, due to the fact that a substantial portion of the latter is secreted as pro-form (Mowla et al, 1999, 2001; Egan et al, 2003; Chen et al, 2004). In contrast to mature processed NTs, the pro-form shows preferential binding affinity for the pan-NT p75NTR with respect to Trk receptors (Lee et al, 2001). There is evidence that secreted pro-BDNF participates in synaptic modifications only upon its conversion into the mature protein by extracellular proteolysis, a process dependent on the concomitant release of tissue plasminogen activator (Pang et al, 2004). The finding that endocytosis of BDNF occurs in hippocampal neurons selectively via full-length TrkB indicates that BDNF is predominantly internalized in its mature form. Thus, BDNF recycling may represent a mechanism for selectively supplying BDNF without further processing to induce synaptic modifications.
Materials and methods
Cell cultures
COS-7 cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS) (GIBCO) and incubated at 37°C in 5% CO2. Primary cultures of hippocampal neurons were prepared from embryonic day 17 Wistar rats (Zafra et al, 1991).
Slice preparation
Hippocampal slices were prepared from male Sprague–Dawley rats (4–6 weeks old) as previously described (Aicardi et al, 2004). A detailed description of this section is available online as Supplementary data.
Production of BDNF-YFP
The cDNA coding for BDNF-YFP was transfected into COS-7 cells cultured in reduced (5%) FBS using a conventional diethylaminoethyl-dextran procedure. The supernatant containing the secreted proteins was collected and concentrated to obtain standard solutions of BDNF-YFP ranging from 10 to 100 pg/ml.
Recombinant adenovirus
Adenoviral vectors containing the cDNA sequence coding for mouse pre-proBDNF tagged with the myc epitope at the C-terminus were prepared as previously described (Canossa et al, 1997).
Biotinylation assay
Hippocampal neurons were incubated with recombinant BDNF (100 ng/ml) or BDNF-YFP (10–100 pg/ml) for 60 min in the presence or absence of K252a (200 nM). After acid wash, cell-surface biotinylation of intact neurons was performed on ice using an immunolabeling kit (Pierce). Neurons were then lysed and half of each sample was subjected to overnight precipitation using agarose-conjugated streptavidin (Sigma), which recognizes biotin-labeled proteins. The second half was subjected to overnight precipitation using WGA-agarose (Sigma), which recognizes glycoconjugate proteins. The precipitates were analyzed by Western blot and aliquots of the cell lysates by ELISA.
Purification of vesicles
Vesicle immunopurification was performed as described previously (Schenk et al, 2003). In brief, hippocampal neurons were incubated with recombinant BDNF (100 ng/ml) on Petri dishes and harvested by scraping and re-suspended in homogenization buffer (10 mM HEPES–KOH pH 7.4, 250 mM sucrose, 1 mM Mg-actetate). The cells were homogenized using a cell cracker (European Molecular Biology Laboratory) and centrifuged at 1000 g for 10 min to prepare the post-nuclear supernatant. Vesicles were immunoisolated from the post-nuclear supernatant with Dynabeads (M-280) sheep anti-rabbit coated with pan-Trk antibody. Bound vesicles were further analyzed by Western blot and ELISA and processed for electron microscopy.
Western blot
Immunoprecipitation and immunopurification products were separated in 8% SDS–PAGE and transferred to nitrocellulose membranes (0.45 μm). using standard procedures. After blocking unspecific sites, the membranes were incubated overnight at 4°C with primary mouse anti-TrkB (BD Transduction Laboratories). Detection was performed after 60 min incubation with anti-mouse secondary antibody conjugated to horseradish peroxidase (Pierce) and subsequent conversion with a chemiluminescent substrate (Pierce).
Electron microscopy
For ultrastructural characterization, the bead pellets were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 30 min at 4°C, and post-fixed in 1% OsO4 in the same buffer for 60 min at room temperature. After dehydration, the samples were embedded in Epon 812 and counterstained with uranyl acetate–lead citrate. Sections were examined with a Zeiss EM 109 electron microscope and pictures were taken with an EM digital camera system, DXM1200F (Nikon). A detailed description of statistical analysis is available online as Supplementary data.
Immunocytochemistry and immunohistochemistry
Immunolabeling of BDNF or BDNF-myc was performed as described previously (Riccio et al, 2005). Colocalization studies were performed by using antibodies recognizing EEA1, BiP, GM130 and NeuN proteins. Immunoreactivity was evaluated by confocal analysis using a Radiance 2000 confocal laser scanning microscope (Bio-Rad), equipped with a Nikon × 60, 1.4 NA objective and with a krypton–argon and a red diode laser. A detailed description of this section is available online as Supplementary data.
Optical imaging of BDNF-YFP
Time-lapse confocal imaging: Hippocampal neurons were incubated with BDNF-YFP for 60 min and transferred into a perfusion chamber. Samples were perfused at a rate of 0.1 ml/min in Hank's modified medium at 37°C. Time-lapse imaging was conducted using a confocal microscope equipped with a × 60 objective 1.2 NA water immersion (Nikon) and a temperature-controlled stage (Bioptechs). Time-lapse TIRF imaging: Hippocampal neurons (300 000 neurons/glass coverslip) were incubated with BDNF-YFP and/or acridine-orange for 1–3 min and perfused as described above. Time-lapse imaging was conducted using an Olympus inverted microscope IX71 equipped with a × 60 objective Plapon/TIRFM-SP1 oil immersion and diode solid state lasers (488 and 532 nm). Images were analyzed by CellR software. A detailed description of this section is available online as Supplementary data.
Characteristics of the perfusion setup and release experiments
BDNF release experiments were performed as described previously (Canossa et al, 1997, 2001). A detailed description of this section is available online as Supplementary data.
Enzyme immunoassay (ELISA)
BDNF ELISA was performed as described previously (Canossa et al, 1997). GFP ELISA differs from BDNF ELISA in the use of anti-GFP (Invitrogen) primary antibody. The BDNF ELISA showed a sensitivity of 1–3 pg/ml of BDNF.
Electrophysiology
At least 60 min after the slicing procedure, a single slice was transferred into a submersion recording chamber perfused (3 ml/min) with oxygenated ACSF at 32±0.2°C. Field EPSPs evoked by Schaffer collaterals stimulation were recorded in CA1 stratum radiatum with ACSF-filled glass pipettes connected to a DC current amplifier. Test stimuli (square pulses, 200 μs, intensity inducing about 50% of the maximal synaptic response, 0.03 Hz) were delivered with a concentric bipolar electrode. TBS stimulation was applied 30 min after baseline stabilization. A detailed description of this section is available online as Supplementary data.
Supplementary Material
Supplementary movie
Supplementary data
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Acknowledgments
We thank Benedikt Berninger for comments on the manuscript. We also thank Oliver Griesbeck for providing the cDNA coding for BDNF-YFP, Regeneron Pharmaceuticals for providing TrkB-Fc and Olympus Italia SRL for technical assistance. This work was supported by MIUR-PRIN (2005057070), MIUR-FIRB (RBAU01EJM3; RBNE01ARR4) and RFO (ex 60%) to MC; by MIUR-FIRB (RBAU01Z2P5) and RFO (ex 60%) to GA; by EC (QLGR3-CT-2000-01343), MIUR-PRIN 2003, MIUR-FIRB (RBNE01RHZM), MIUR-CNR Functional Genomics, FISR-CNR Neurobiotecnologia 2003 and HFSPO (RGY0027/2001) to MM. SC was supported by MIUR-Cofin and MIUR-FIRB fellowships.
References
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M (2004) Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci USA 101: 15788–15792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Ibaraki K, Hemley JM (2004) It's green outside: tracking cell surface proteins with pH-sensitive GFP. Trends Neurosci 27: 257–261 [DOI] [PubMed] [Google Scholar]
- Avery J, Ellis DJ, Lang T, Holroyd P, Riedel D, Henderson RM, Edwardson JM, Jahn R (2000) A cell-free system for regulated exocytosis in PC12 cells. J Cell Biol 148: 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM (2000) Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci 20: 7417–7423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM (2002) Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci 22: 10399–10407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER (2005) Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 48: 123–137 [DOI] [PubMed] [Google Scholar]
- Blochl A, Thoenen H (1995) Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci 7: 1220–1228 [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76: 99–125 [DOI] [PubMed] [Google Scholar]
- Butowt R, von Bartheld CS (2001) Sorting of internalized neurotrophins into an endocytic transcytosis pathway via the Golgi system: ultrastructural analysis in retinal ganglion cells. J Neurosci 21: 8915–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, von Bartheld CS (2005) Anterograde axonal transport of BDNF and NT-3 by retinal ganglion cells: roles of neurotrophin receptors. Mol Cell Neurosci 29: 11–25 [DOI] [PubMed] [Google Scholar]
- Caleo M, Medini P, von Bartheld CS, Maffei L (2003) Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J Neurosci 23: 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caleo M, Menna E, Chierzi E, Cenni MC, Maffei L (2000) Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr Biol 5: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Canossa M, Gartner A, Campana G, Inagaki N, Thoenen H (2001) Regulated secretion of neurotrophins by metabotropic glutamate group I (mGluRI) and Trk receptor activation is mediated via phospholipase C signalling pathways. EMBO J 20: 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S (2002) Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Natl Acad Sci USA 99: 3282–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H (1997) Neurotrophin release by neurotrophins: implications for activity-dependent neuronal plasticity. Proc Natl Acad Sci USA 94: 13279–13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A (1999) Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci 19: 7983–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 31: 885–889 [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS (2004) Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24: 4401–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17: 2295–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Yang F, Lu B (2000) Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol 150: 1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B (2003) Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J Cell Biol 163: 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269 [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O (1991) Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron 7: 165–176 [DOI] [PubMed] [Google Scholar]
- Gartner A, Staiger V (2002) Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA 99: 6386–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck O, Canossa M, Campana G, Gartner A, Hoener MC, Nawa H, Kolbeck R, Thoenen H (1999) Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc Res Tech 45: 262–275 [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V (2001) Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J 20: 5887–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038 [DOI] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M (1990) The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell 61: 647–656 [DOI] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T (2001) Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291: 2419–2423 [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92: 8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T (1996) Virus-mediated gene transfer into hippocampal CA1 region restores longterm potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA 29: 12547–12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruttgen A, Moller JC, Heymach JV, Shooter EM (1998) Neurotrophins induce release of neurotrophins by the regulated secretory pathway. Proc Natl Acad Sci USA 95: 9614–9619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945–1948 [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M (2003) Neurotrophin secretion: current facts and future prospects. Prog Neurobiol 69: 341–374 [DOI] [PubMed] [Google Scholar]
- Lu B (2004) Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res 146: 137–150 [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6: 603–614 [DOI] [PubMed] [Google Scholar]
- McAllister AK (2002) Spatial restricted actions of BDNF. Neuron 36: 549–550 [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC (1999) Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22: 295–318 [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Reichardt LF, Barres BA (1998) Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 21: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276: 12660–12666 [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA (1999) Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci 19: 2069–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Lu B (2004) Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev 3: 407–430 [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306: 487–489 [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16: 1137–1145 [DOI] [PubMed] [Google Scholar]
- Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32 [DOI] [PubMed] [Google Scholar]
- Riccio M, Santi S, Dembic M, Di Giaimo R, Cipollini E, Costantino-Ceccarini E, Ambrosetti D, Maraldi NM, Melli M (2005) Cell-specific expression of the epm1 (cystatin B) gene in developing rat cerebellum. Neurobiol Dis 20: 104–114 [DOI] [PubMed] [Google Scholar]
- Schenk U, Verderio C, Benfenati F, Matteoli M (2003) Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO J 22: 558–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg M (2004) BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24: 9366–9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A (1998) Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20: 727–740 [DOI] [PubMed] [Google Scholar]
- Snider WD, Lichtman JW (1996) Are neurotrophins synaptotrophins? Mol Cell Neurosci 7: 433–442 [DOI] [PubMed] [Google Scholar]
- Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E (2000) Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem 275: 8982–8990 [DOI] [PubMed] [Google Scholar]
- Steyer JA, Horstmann H, Almers W (1997) Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature 388: 474–478 [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709–726 [DOI] [PubMed] [Google Scholar]
- Thoenen H (2000) Neurotrophins and activity-dependent plasticity. Prog Brain Res 128: 183–191 [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Righi M, Cattaneo A (1997) Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J Neurosci 17: 9492–9505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Zhao C, Terakawa S, Rutter GA (2000) Simultaneous evanescent wave imaging of insulin vesicle membrane and cargo during a single exocytotic event. Curr Biol 10: 1307–1310 [DOI] [PubMed] [Google Scholar]
- von Bartheld CS (2003) Axonal transport and neuronal transcytosis of trophic factors, tracers, and pathogens. J Neurobiol 58: 295–314 [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Byers MR, Williams R, Bothwell M (1996) Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature 379: 830–833 [DOI] [PubMed] [Google Scholar]
- Wang X, Berninger B, Poo MM (1998) Localized synaptic actions of neurotrophin-4. J Neurosci 18: 4985–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Butowt R, Vasko MR, von Bartheld CS (2002) Mechanisms of the release of anterogradely transported neurotrophin-3 from axon terminals. J Neurosci 22: 931–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible MW, Bartlett SE, Reynolds AJ, Hendry IA (2001) Prolonged recycling of internalized neurotrophins in the nerve terminal. Cytometry 43: 182–188 [DOI] [PubMed] [Google Scholar]
- Yano H, Chao MV (2003) Mechanisms of neurotrophin receptor vesicular transport. J Neurobiol 58: 244–257 [DOI] [PubMed] [Google Scholar]
- Zafra F, Castren E, Thoenen H, Lindholm D (1991) Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA 88: 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Almers W (2000) Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature 406: 849–854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary movie
Supplementary data
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4


