Abstract
The broad field of gene therapy promises a number of innovative treatments that are likely to become important in preventing deaths from cancer. In this review, we discuss the history, highlights and future of three different gene therapy treatment approaches: immunotherapy, oncolytic virotherapy and gene transfer. Immunotherapy uses genetically modified cells and viral particles to stimulate the immune system to destroy cancer cells. Recent clinical trials of second and third generation vaccines have shown encouraging results with a wide range of cancers, including lung cancer, pancreatic cancer, prostate cancer and malignant melanoma. Oncolytic virotherapy, which uses viral particles that replicate within the cancer cell to cause cell death, is an emerging treatment modality that shows great promise, particularly with metastatic cancers. Initial phase I trials for several vectors have generated excitement over the potential power of this technique. Gene transfer is a new treatment modality that introduces new genes into a cancerous cell or the surrounding tissue to cause cell death or slow the growth of the cancer. This treatment technique is very flexible, and a wide range of genes and vectors are being used in clinical trials with successful outcomes. As these therapies mature, they may be used alone or in combination with current treatments to help make cancer a manageable disease.
Keywords: Cancer gene therapy, Gene transfer, Immunotherapy, Oncolytic virotherapy
New therapy options need to be developed if the National Cancer Institute’s bold plan1 of eliminating cancer death and suffering by 2015 is to be achieved. Five-year survival rates for pancreatic (4%), lung (15%), liver (7%) and glioblastoma (5%), a common form of brain cancer, remain abysmally low.2 Even prostate and breast cancers, which are highly amenable to treatment with 5-year survival rates better than 80%, still respond poorly to treatment at later stages and together result in more than 60,000 deaths a year.3 Current treatments often have far reaching negative side effects. The systemic toxicity of chemotherapy regimens, while not as severe as they once were, still often result in acute and delayed nausea, mouth ulcerations and mild cognitive impairments.4 In addition, long-term side effects from chemotherapy can include an increased risk of developing other types of cancers.5 Less serious, but potentially just as debilitating, side effects can also occur. Treatment for metastatic prostate cancer, while prolonging life, often causes hot flashes, impotence, incontinence and an increased risk of bone fractures.6 Therefore, entirely new treatment methods are called for in order to alleviate the death and suffering caused by cancers.
The emerging field of cancer gene therapy offers a number of exciting potential treatments. The term gene therapy encompasses a wide range of treatment types that all use genetic material to modify cells (either in vitro or in vivo) to help effect a cure.7 Numerous in vitro and preclinical animal models, testing a wide variety of gene therapy agents, have shown remarkable efficacy. In lung cancer models, for example, survival benefits have been demonstrated using gene therapy to create cancer vaccines, target viruses to cancer cells for lysis and death, decrease the blood supply to the tumor, and introduce genes into the cancer cells that cause death or restore normal cellular phenotype.8 Preclinical gene therapy tests have also been performed on gliomas,9 pancreatic cancer10 and liver cancer,11 as well as many other cancers.
As with any new type of therapy, there are serious safety concerns. Initial enthusiasm for gene therapy as a treatment modality was curtailed by the death of a patient participating in a dose escalation gene therapy trial in 1999.12 While this was a trial to use gene therapy to correct a metabolic disease (ornithine transcarbamylase deficiency) and not a cancer trial, all gene therapy trials were revaluated for safety.13 Since that time, newer and safer gene therapy delivery agents have been created and thousands of cancer patients globally have participated in gene therapy trials with remarkably few treatment side effects.14,15 The most frequent side effects are fever and symptoms that resemble a cold. If the agent is injected, there is often localized swelling and inflammation at the site of the injection.14,16–18 However, when compared with the side effects of conventional chemotherapeutic treatments, these side effects are minimal.
This review focuses on the gene therapy trials that have progressed beyond the preclinical stage and are now in clinical trials in the United States. In order to explain these treatments, we have broken the field of cancer gene therapy treatments into three broad categories: immunotherapy, oncolytic virotherapy and gene transfer. Each section includes a brief history of the gene therapy category, a brief discussion of the techniques being used, a discussion of the state of current clinical trials and the future directions for the therapy.
Immunotherapy
History
Immunotherapy, or the concept of boosting the immune system to target and destroy cancer cells, has been a goal of cancer treatment for over 100 years. However, limited success has been achieved with traditional immunotherapy, as cancer cells tend to evolve mechanisms that evade immune detection. A wide array of gene therapy techniques are being used to overcome this limitation.19
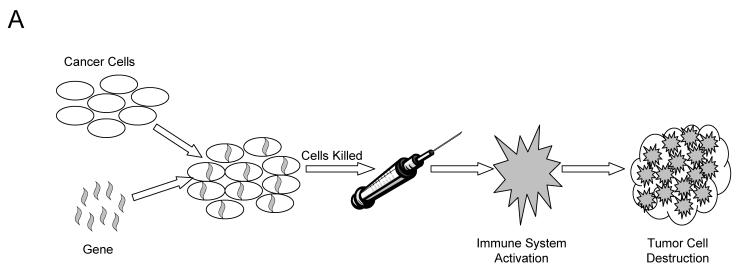
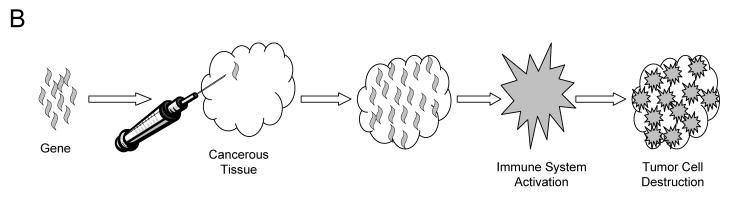
Currently gene therapy is being used to create recombinant cancer vaccines. Unlike vaccines for infectious agents, these vaccines are not meant to prevent disease, but to cure or contain it by training the patient’s immune system to recognize the cancer cells by presenting it with highly antigenic and immunostimulatory cellular debris. Initially cancer cells are harvested from the patient (autologous cells) or from established cancer cell lines (allogeneic) and then are grown in vitro. These cells are then engineered to be more recognizable to the immune system by the addition of one or more genes, which are often cytokine genes that produce pro-inflammatory immune stimulating molecules, or highly antigenic protein genes. These altered cells are grown in vitro and killed, and the cellular contents are incorporated into a vaccine (figure 1A ▶).20 Immunotherapy is also being attempted through the delivery of immunostimulatory genes, mainly cytokines, to the tumor in vivo. The method of introducing a gene to the tumor varies and is discussed in more detail in the gene transfer section of this review. Once in the cancer cell, these genes will produce proteins that unmask the cells from immune evasion and encourage the development of antitumor antibodies (figure 1B ▶).21
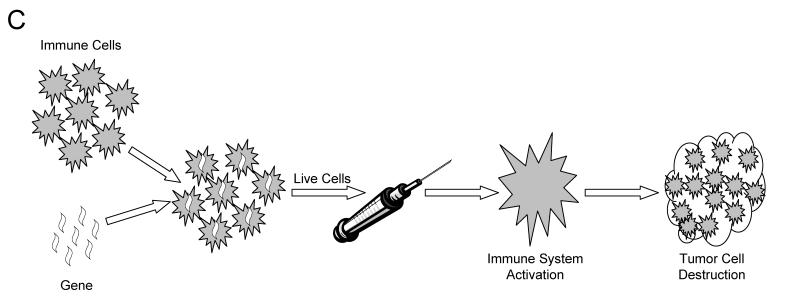
Figure 1.
Schematic diagram of immunotherapy. Pathway A represents immunotherapy with altered cancer cells. Pathway B represents immunotherapy with genes in vivo. Pathway C represents immunotherapy using altered immune cells.
Another unique immunotherapy strategy facilitated by gene therapy is to directly alter the patient’s immune system in order to sensitize it to the cancer cells. One approach uses mononuclear circulating blood cells or bone marrow gathered from the patient. A tumor antigen, or other stimulatory gene, is then added to the selected cell type. These altered cells are now primed to cause an immune reaction to the cancer cells leading to cancer eradication (figure 1C ▶).22 Alternatively, the gene can be added in vivo using a targeted delivery system, such as an altered viral particle.23
Initial trials using first generation vaccines have produced mixed results, highlighting both the potential for this therapy and the areas that still need to be perfected before these engineered cancer vaccines become part of standard cancer treatment. Early preclinical cancer vaccine models demonstrated positive results. Murine models using murine colon adenocarcinoma cells expressing human carcinoembryonic antigen (CEA) demonstrated tumor reduction and long-lasting immune response when immunized with a vaccinia virus engineered to express CEA.24 However, when this type of vaccine was used in patients with breast cancer, no clinical response was observed.25 This early experimental treatment highlights one of the limitations of using self-antigens for a vaccine. Although vaccines used for infectious agents generally lead to antigen-specific T-cell precursors in the range of 10%, with self-antigens of cancer this response is often <1%.26 Even when a successful immune response is mounted in clinical trials, it can be difficult to sustain. In a prostate cancer vaccine trial, a patient who achieved normal prostate specific antigen (PSA) levels for the year of the trial, developed rising PSA levels after vaccination was stopped. His PSA levels were stabilized again only with reinitiation of the vaccine therapy.27 These results, while not entirely positive, have given scientists a better understanding of the immune reaction to cancer and have led to the development of the next generation of cancer vaccines.
Current Clinical Trials
The next generation of vaccines is already in clinical trials for several cancer types. Table 1 ▶ provides a list of the more advanced clinical trials in this field, including phase, the type of cell used and the gene used to create a better immune response. These trials were picked to illustrate the fact that there are wide ranges of trials in different stages of efficacy testing using a variety of vectors for many cancer types.
Table 1.
Selected recent immunotherapy clinical trials
| Cancer | Stimulating genes | ClinicalTrials.gov47 identifier # | Description | Phase |
| Prostate | Murine α(1,3)- galactosyltranferase | NCT00105053 | Mouse protein-sugars are expressed on allogeneic prostate cells to induce a hyperacute rejection response | II |
| Pancreatic | CEA and MUC-1 | NCT00088660 | Replication incompetent vaccinia and fowlpox viruses engineered to produce CEA and MUC-1 given subcutaneously to produce an immune response to pancreatic cancer | III |
| Prostate | GM-CSF | NCT00122005 | Allogeneic prostate cells expressing the GM-CSF gene are used to induce immune response following chemotherapy and peripheral blood mononuclear cells infusion | I/II |
| Lymphoma | GM-CSF and CD40L | NCT00101101 | Autologous tumor cells are combined with allogeneic cells that express GM-CSF and CD40L and incorporated into a vaccine with low doses of IL-2 | II |
| Melanoma | IL-2 | NCT00059163 | Autologous tumor cells engineered to express IL-2 are incorporated into a vaccine. | II |
| Kidney | CD-80 | NCT00040170 | A modified replication incompetent adenovirus containing the tumor antigen CD-80 is injected subcutaneously along with the cytokine IL-2 to produce an immune response to the prostate cancer | II |
CD-80, cytokine co-stimulatory molecule; CEA, carcinoembryonic antigen; GM-CSF, granulocyte-macrophage colony stimulating factor; IL-2, interleukin-2; MUC-1, mucin-1
Vaccines using engineered cells are showing great promise for the treatment of many cancers that respond poorly to conventional therapy. Vaccine therapy for non-small cell lung cancer is an example of an autologous vaccine therapy that has had good results in clinical trials. Recent clinical trials with GVAX, a vaccine made from autologous tumor cells modified to express granulocyte-macrophage colony-stimulating factor (GM-CSF) have led to further clinical testing. The initial phase I/phase II trial resulted in 3 of 33 subjects experiencing complete remission and an additional 7 who achieved stable disease for an average of 7 months.28 A further phase II study comparing GVAX alone to GVAX combined with cyclophosphamide in advanced stage patients demonstrated a clinical effect with 14 of the 53 participants experiencing stable disease and 1 patient experiencing stable disease for over 2 years. Median overall survival was between 5.4 months and 9.5 months with longer survival times seen on the cyclophosphamide arm of the study.29 The vaccine is now being tested in at least two larger phase II trials and phase III testing is planned. Unlike past trials, these trials have shown a demonstrable, but somewhat modest, effect on patient survival and, if phase III studies continue to show this impact, have the potential to become part of a treatment regimen. In addition to lung cancer, GVAX is also being tested in other cancers. An allogeneic GVAX vaccine using a combination of prostate cell lines that are engineered to express GM-CSF is being tested as a treatment for prostate cancer. This vaccine has been shown to increase the PSA doubling time of patients with progressive prostate cancer and increase time to disease progression by several months. Currently, several large phase III trials are underway to determine if there is an increase in life expectancy as well.29
Other clinical trials are demonstrating the potential of unmasking the tumor from immune invasion using immunostimulatory genes inserted directly into the tumor tissue. For example, MDA-7 (IL-24), a cytokine that induces cancer cell death, is currently in clinical trials for its ability to cause a systemic immune reaction in malignant melanoma patients.30 Melanomas have long been observed to elicit an immune response from the patient and many attempts have been made over the years to bolster this reaction to effect a cure. After packaging in a replication incompetent adenovirus, the MDA-7 gene is injected intratumorally and induces apoptosis. A clinical trial demonstrated that this treatment lead to complete response and partial response in 2 of 28 patients.30 In 22 other patients, systemic immune activation was observed, as well as local apoptosis.31 The clinical response observed in these trials has led to a phase II study to determine if this response can induce apoptosis in distant metastasis via a systemic immune reaction when the vector is injected intratumorally.
Current clinical trials seeking to directly stimulate the immune system for cancer destruction also show promising results. One example of this type of immunotherapy is the current clinical trial using the TRICOM vaccines. These vaccines incorporate a cancer antigen into a modified virus, either vaccinia or fowlpox, that also contain three immunostimulatory genes: B-lymphocyte activation antigen B7-1 (B7-1), intercellular adhesion molecule 1 (ICAM-1) and lymphocyte function-associated antigen 3 (LFA-3).32 The PANVAC-VF vaccine is a vaccinia virus modified to deliver mucin-1 (Muc-1) and CEA, in addition to the immunostimulatory genes. The vaccine is injected subcutaneously and followed by boosting vaccines of a fowlpox virus modified in the same manner as the vaccinia virus.33 This vaccine strategy recently completed a phase III trial in pancreatic cancer. In addition Prostvac, a vaccine that uses the fowlpox virus engineered to express Muc-1 (a gene highly expressed in tumors) to induce an immune response, is exhibiting promising results. Phase I data revealed a 3- to 4-fold increase in PSA doubling time when patients were given the vaccine. Currently, large phase II studies are underway.18
Future Directions
While current clinical trials are progressing much better than earlier ones, there are still a few areas that could be improved. For example, many of the most promising vaccines rely on autologous cells for vaccine production. These vaccines do give the patient a truly personalized vaccine; however, they may also present a long-term problem because of the expense and effort needed to create it. Few hospitals contain a facility for vaccine production and substantial time and expertise are required to grow the cells and create a custom vaccine.34 One way around this obstacle is the creation of allogeneic alternative vaccines, though efforts to create an effective allogeneic alternative to GVAX have not been as successful in trials as the autologous GVAX vaccine.35 However, other allogeneic strategies, such as GM.CD40L, have been more successful. GM.CD40L is a vaccine composed of autologous tumor cells mixed with allogeneic tumor cells that have been engineered to produce both GM-CSF and CD40L. GM.CD40L is currently in a phase II trial for treatment of malignant melanoma.36 Combining these genes may lead to a stronger immune response than either gene used alone. In addition, in a pancreatic cancer trial, a vaccine of allogeneic pancreatic cancer cells engineered to produce GM-CSF combined with surgery has shown impressive phase II results with 76% survival at 2 years compared to the historic average of <50% at 2 years.37
As with any cancer monotherapy, combination therapy using vaccines may be more effective than vaccine therapy alone. Cancer vaccines that have presented only modest immune response may find usefulness as an adjuvant therapy for use after surgery or chemotherapy to eliminate any remaining cancer cells. With the current round of ongoing clinical trials, the potential of gene therapy cancer vaccines is close to being fulfilled. The initial phases of vaccine development are being completed and it is likely that there soon will be effective cancer treatments that incorporate vaccines into the therapy regimen.
Oncolytic Agents
History
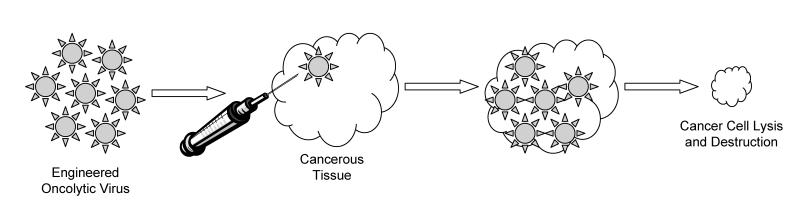
Another growing area of gene therapy treatment for cancer is the use of oncolytic vectors for cancer destruction. Like immunotherapy, this is a concept that has been around for almost a century and, like immunotherapy, it is undergoing a renaissance due to gene therapy.38 Oncolytic gene therapy vectors are generally viruses that have been genetically engineered to target and destroy cancer cells while remaining innocuous to the rest of the body. Oncolytic vectors are designed to infect cancer cells and induce cell death through the propagation of the virus, expression of cytotoxic proteins and cell lysis (figure 2 ▶).39 A number of different viruses have been used for this purpose, including vaccinia, adenovirus, herpes simplex virus type I, reovirus and Newcastle disease virus.38 These viruses have been chosen, in many cases, for their natural ability to target cancers, as well as the ease at which they can be manipulated genetically.
Figure 2.
Schematic diagram of oncolytic virotherapy.
Initial trials of oncolytic therapies have highlighted both its incredible power, as well as unique obstacles to treatment implementation. Mammalian models of oncolytic gene therapy have worked remarkably well. In murine models, both colon and bladder cancer have shown survival benefits and reduced metastasis using oncolytic viral agents.40,41 In a canine model, using an oncolytic virus designed to destroy osteosarcoma, survival was prolonged even in immunocompetent dogs with syngenic osteosarcoma.42 However, there are several unique stumbling blocks for oncolytic virotherapy in humans. Most people have antibodies to the common viruses used for therapy development which often leads to an immune response that clears the viral agent before it has had time to infect cells. In addition, the use of replication competent viral particles often calls for increased safety precautions, making clinical trials more expensive and cumbersome.43 In a trial using a modified vaccinia virus to treat breast and prostate cancer, patients were required to be isolated in a specialized hospital facility for a week to ensure that the virus had completely cleared before being allowed back into the general population.18 Because of these limitations, there have been relatively few trials with oncolytic therapy. However, new vectors are being created and past experience is being incorporated into current trials to enhance results so that they mimic those in animal studies.
Current Clinical Trials
Even in this early stage, oncolytic viral therapy has demonstrated some success. Both adenovirus and herpes virus agents have ongoing clinical trials for intractable cancers. The most notable adenoviral therapy is the ONYX-015 viral therapy. ONYX-015 is an adenovirus that has been engineered to lack the viral E1B protein.44 Without this protein, the virus is unable to replicate in cells with a normal p53 pathway. In addition, the E1B protein is essential for RNA export during viral replication.45 Cancer cells often have deficiencies in the p53 pathway due to mutations and thus, allow ONYX-015 to replicate and lyse the cells.44 Cancer cells also exhibit altered RNA export mechanisms that allow for the export of viral RNA even in the absence of the E1B protein.45 ONYX-015 has been tested in phase I and II trials on squamous cell carcinoma of the head and neck that resulted in tumor regression which correlated to the p53 status of the tumor. Tumors with an inactive pathway demonstrated a better response.46 Phase II trials of ONYX-015, in combination with chemotherapy, demonstrated even better tumor response and have led to a phase III study.47 In addition to squamous cell carcinoma, ONYX-015 is currently being tested as a preventative treatment for precancerous oral tissue, the theory being that even in the precancerous state, there are p53 pathway inactivating mutations that will allow the oncolytic adenovirus to replicate and eliminate the cells before they become cancerous.48
The second type of oncolytic virotherapy undergoing clinical trials uses herpes simplex virus type 1 (HSV-1). Two vectors, G207 and NV1020, are currently in phase I and phase II trials for treatment of intractable cancers. Mutations in several genes of these herpes viruses ensure that they replicate efficiently only in cancerous cells. G207 is mutated so that it has attenuated neurovirulence and cannot replicate in nondividing cells.41 NV1020, a derivative originally used for vaccine studies, has multiple mutations, including a deletion in the thymidine kinase region and a deletion across the long and short components of the genome, and an insertion of the thymidine kinase gene under the control of the α4 promoter.41 These viral vectors have two distinct cell killing mechanisms. The lytic portion of the life cycle directly kills cells and the thymidine kinase that is expressed from the viral genes sensitizes cells to ganciclovir. These viral therapy vectors have been used with great success in vitro and in model animals against a wide number of solid cancers.49–51 Clinical trials using these vectors include a phase I trial of G207 for treatment of malignant glioma52 and a phase I/II trial of NV1020 for treatment of colorectal cancer metastases to the liver.53 In addition, NV1020 has also been tested for treatment of glioblastoma.53
Future Directions
Because oncolytic virotherapy is not yet a mature technology, there is plenty of room for improved treatment vectors. In order for virotherapy to be successful, viral particle production rates in the infected cancer cells must outstrip the growth rate of the uninfected cancer cells. This may be difficult to achieve with large established tumors54 and may mean that virotherapy must be combined with an existing therapy, such as surgery, to decrease the number of cancer cells in the initial treatment. In addition, the most effective treatment delivery method is yet to be determined. In preliminary studies, systemic injection required 1000x the viral load necessary to achieve results than injection intratumorally.55
However, once these factors are overcome, there are many benefits to oncolytic therapy. The selective nature of the virotherapy ensures that healthy tissue will be minimally impacted. In addition, when combined with cytotoxic gene expression, this therapy can affect not only rapidly dividing cells, but those in the surrounding tissue making the microenvironment less favorable for cancer growth. The combination of the powerful killing nature of these vectors combined with the selectivity makes them an exciting avenue for lowering the number of cancer deaths.
Gene Transfer
History
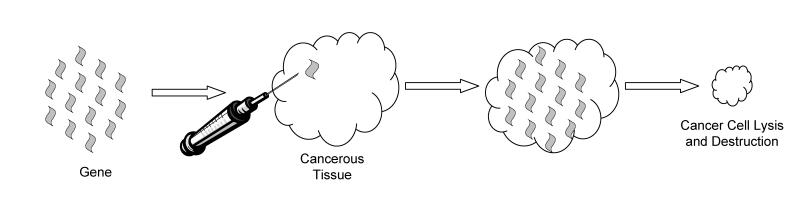
One of the most exciting treatments to emerge from the concept of gene therapy is that of gene transfer or insertion. This is a radically new treatment paradigm involving the introduction of a foreign gene into the cancer cell or surrounding tissue. Genes with a number of different functions have been proposed for this type of therapy, including suicide genes (genes that cause cellular death when expressed), antiangiogenesis genes and cellular stasis genes (figure 3 ▶). A number of different viral vectors have been used in clinical trials to deliver these genes, but most commonly have used a replication incompetent adenovirus. Nonviral methods, including naked DNA transfer and oligodendromer DNA coatings, as well as electroporation are also viable modes of gene delivery.56 The type of delivery vehicle chosen depends on the desired specificity of the gene transfer therapy, as well as the length of time the gene must be expressed in order to be effective. For instance, a replication incompetent adenoviral vector containing the herpes simplex virus thymidine kinase (HSVtk) gene needs only transient expression to accomplish cell death and is generally delivered via an adenoviral vector.57 However, antiangiogenesis genes, such as sFLT-1 and statin-AE, need continuous expression for therapeutic effect and have been delivered using plasmids that contain a transposon to insert the gene into the cellular DNA.58
Figure 3.
Schematic diagram of gene transfer therapy.
Initial attempts to implement gene transfer therapy have highlighted its promise, as well as some delivery difficulties. Delivery of the therapeutic gene to the target cells has to be effective enough to elicit a response and has been difficult to achieve with many of the current technologies. In addition, extra precautions must be taken to ensure the therapeutic gene does not integrate into unwanted cell types, such as reproductive tissues. Earlier gene transfer trials suffered from gene silencing so that even if the gene was effectively introduced into the cell, it was not expressed or was expressed only for a limited length of time.59 Despite these hurdles, solid tumors such as prostate, lung and pancreatic tumors have been treated successfully in animal models using a variety of genes and transfer methods.60–62 Special precautions must be taken if DNA is inserted into the cell chromosome. The insertional site must be in an area of the genome that does not promote cancer. Retrotransposons, such as sleeping beauty (an artificially constructed retrotransposon that is used to insert genes into vertebrate chromosomes) often insert into actively transcribed genes causing potential problems for cellular function.63 Preclinical models using gene insertion techniques, such as murine models for glioma, showed significantly greater survival when they administered antiangiogenic genes via a retrotransposon system injected intracranially.58
Current Clinical Trials
Because gene transfer technology encompasses such a diverse set of therapeutic options, it is impossible to describe examples for every treatment. However, a partial list of treatments in significant current clinical trials with a brief description of each is presented in table 2 ▶. Below, we highlight several of the late stage clinical trials, as well as some exciting innovative approaches that further highlight the promise of gene transfer therapy.
Table 2.
Selected recent gene transfer clinical trials.
| Cancer | Transferred genes | ClinicalTrials.gov47 identifier # | Description | Phase |
| Pancreatic | Rexin-G | NCT00121745 | A cytocidal cyclin G1 construct accumulates preferentially in the tumor cells to block the action of cyclin G1 and initiate cell death | I |
| Glioblastoma | HSVtk | NCT00001328 | The HSVtk gene is introduced into glioblastoma cells via a mouse retrovirus. Glioblastoma cells with the HSVtk gene are then sensitive to the drug glanciclovir which is administered | I |
| Head and neck | p53 | NCT00041613 | Transfer of the p53 gene via a replication incompetent adenovirus to tumor cells to inhibit cell growth and induce apoptosis | III |
| Melanoma | MDA-7 | NCT00116363 | MDA-7 a novel tumor suppressor molecule is introduced into the melanoma cells and overexpression inhibits cellular proliferation and induces apoptosis | II |
| Pancreatic | TNF-α | NCT00051467 | The TNF-α gene under the control of a radiation inducible promoter is introduced into tumor cells and in combination with the radiation therapy induces cell death | II |
HSVtk, herpes simplex virus thymidine kinase; TNF-α, tumor necrosis factor alpha
TNFerade is one such treatment option that is currently in late stage II trials. This agent is a replication incompetent adenoviral vector that delivers the tumor necrosis factor-α (TNF-α) gene under the transcriptional control of a radiation inducible promoter. TNF-α is a cytokine with potent anticancer properties and high systemic toxicity, and TNF-α gene therapy provides a way to target this molecule to only the cancer cells through the use of intratumoral injections and a promoter that is activated by radiation therapy.64 Once TNFerade is injected, the patient then receives radiation therapy to the tumor to activate the gene. The gene then produces the TNF-α molecule which in combination with the radiation therapy promotes cell death in the affected cancer cells and surrounding cells.64 A phase I study of patients with soft tissue sarcoma using TNFerade demonstrated an 85% response rate including 2 complete responses.65 In another large phase I study of patients with histologically confirmed advanced cancer, 43% of the patients demonstrated an objective response with 5 of 30 exhibiting complete response to the treatment.66 Larger studies are being conducted using TNFerade for treatment in pancreatic, esophageal, rectal cancer and melanoma.66,67
Another exciting gene therapy treatment agent is Rexin-G, the first injectable gene therapy agent to achieve orphan drug status from the Food and Drug Administration for treatment of pancreatic cancer.68 This gene therapy agent contains a gene designed to interfere with the cyclin G1 gene and is delivered via a retroviral vector. The gene integrates into the cancer cell’s DNA to disrupt the cyclin G1 gene and causes cell death or growth arrest. In a phase I trial, 3 of 3 patients experienced tumor growth arrest with 2 patients experiencing stable disease. These results have led to larger phase I and II trials.69 Rexin-G is also being evaluated for colon cancer that has metastasized to the liver.
A gene transfer technology that shows great promise is the replication incompetent adenovirus delivering the HSVtk gene to a tumor followed by ganciclovir treatment. Ganciclovir is not toxic unless metabolized by the HSVtk gene,70 and therefore only the cancer cells that are treated with the gene and the surrounding cells will be affected by treatment. In a large phase I study involving glioblastoma patients, the HSVtk-engineered viral treatment increased median survival from 39 weeks to 70.6 weeks and was the first glioblastoma gene therapy trial to show any measurable improvement in survival.71
Several agents that use a replication incompetent adenoviral vector to deliver the p53 gene to cancer cells are also currently in phase II and III trials. The p53 gene is an important cell cycle regulator that has been extensively studied and is mutated in 50% to 70% of human tumors.72 Mutations in this gene are often linked to aggressiveness. It has been shown that restoration of a functional p53 gene in cancer cells results in tumor cell stasis and often apoptosis.72 Using this information, INGN 201, an adenoviral vector containing p53 for gene transfer, is in current phase III testing for squamous cell carcinoma of the head and neck, and has completed phase I studies on prostate, ovarian, glioma and bladder cancer.73–75
Future Directions
Gene transfer, while a radical new type of treatment, is also the only gene therapy product to obtain regulatory approval in any global market, as demonstrated by China’s 2003 approval of Gendicine for clinical use.76 Gendicine is a modified adenovirus that delivers the p53 gene to cancer cells and is approved for the treatment of head and neck squamous cell carcinoma. Since approval, thousands of patients have been treated in China; some with repeated injections. As yet, large-scale efficacy trial results have not been published; the results of which are eagerly awaited.
Gene transfer technology allows an incredible diversity of treatment possibilities. This diversity can be used to complement traditional therapies, as well as provide radically new frontiers for treatment. Gene transfer therapy can rely on the current information known about the genetics of cancer formation, bringing a more sophisticated and personalized approach to therapy. Current gene transfer trials have demonstrated statistically significant survival improvements for cancers such as glioblastoma and pancreatic cancer, as discussed previously. These studies have provided very encouraging signs that current research is on the right path. New delivery methods and more sophisticated gene expression cassettes will create better therapeutic alternatives to make the goal of cancer treatment and eradication achievable.
Conclusions
The field of cancer gene therapy is rapidly maturing and will no doubt be part of the future of cancer therapeutics. Several very exciting cancer vaccine treatments are in late stage trials, thanks to the advent of genetic engineering. In addition, gene transfer technology for cancer treatment holds great promise for increasing the effectiveness of current chemotherapeutic treatment regimens. Significant advances have been made in the field of oncolytic virotherapy, and trials are in progress that incorporate this technique for precancerous, as well as cancerous treatment. Many of the past obstacles to treatment are being actively overcome and current second and third generation therapeutics are being tested. While not all the current trials will lead to a viable therapeutic agent, there is great hope that these advances will help relegate cancer to a manageable chronic disease without severe suffering and death.
References
- 1.National Cancer Institute. The nation’s investment in cancer research: A plan and budget proposal for fiscal year 2007. Washington, DC: US National Institutes of Health, October 2005. NIH Publication No. 06-5856.
- 2.Cancer Facts and Figures 2006. American Cancer Society Web site. Available at: http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf. Accessed July 31, 2006.
- 3.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst 2005;97:1407–1427. [DOI] [PubMed] [Google Scholar]
- 4.Chemotherapy and you: A guide to self-help during cancer treatment. National Institutes of Health Web site. Available at: http://www.cancer.gov/PDF/b21d0a74-b477-41ec-bdc0-a60bbe527786/chemoandyou.pdf. Accessed July 31, 2006.
- 5.Bassal M, Mertens AC, Taylor L, Neglia JP, Greffe BS, Hammond S, Ronckers CM, Friedman DL, Stovall M, Yasui YY, Robison LL, Meadows AT, Kadan-Lottick NS. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2006;24:476–483. [DOI] [PubMed] [Google Scholar]
- 6.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005;294:238–244. [DOI] [PubMed] [Google Scholar]
- 7.Mulligan RC. The basic science of gene therapy. Science 1993;260:926–932. [DOI] [PubMed] [Google Scholar]
- 8.Vattemi E, Claudio PP. Gene therapy for lung cancer: practice and promise. Ann Ital Chir 2004;75:279–289. [PubMed] [Google Scholar]
- 9.Prasad G, Wang H, Hill DL, Zhang R. Recent advances in experimental molecular therapeutics for malignant gliomas. Curr Med Chem Anticancer Agents 2004;4:347–361. [DOI] [PubMed] [Google Scholar]
- 10.Tseng JF, Mulligan RC. Gene therapy for pancreatic cancer. Surg Oncol Clin N Am 2002;11:537–569. [DOI] [PubMed] [Google Scholar]
- 11.Prieto J, Qian C, Hernandez-Alcoceba R, Gonzalez- Aseguinolaza G, Mazzolini G, Sangro B, Kramer MG. Gene therapy of liver diseases. Expert Opin Biol Ther 2004;4:1073–1091. [DOI] [PubMed] [Google Scholar]
- 12.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003;80:148–158. [DOI] [PubMed] [Google Scholar]
- 13.Guidance for FDA review staff and sponsors: Content and review of chemistry manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs). Food and Drug Web site. Available at: http://www.fda.gov/CbER/gdlns/gtindcmc.pdf. Accessed July 31, 2006.
- 14.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002;9:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther 2005;16:1016–1027. [DOI] [PubMed] [Google Scholar]
- 16.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther 2005;12:585–598. [DOI] [PubMed] [Google Scholar]
- 17.Malaeb BS, Gardner TA, Margulis V, Yang L, Gillenwater JY, Chung LW, Macik G, Koeneman KS. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology 2005;66:830–834. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Acres B, Balloul JM, Bizouarne N, Paul S, Slos P, Squiban P. Gene-based vaccines and immunotherapeutics. Proc Natl Acad Sci U S A 2004;101:14567–14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong AC, Eaton D, Ewing JC. Science, medicine, and the future: Cellular immunotherapy for cancer. BMJ 2001;323:1289–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalczyk DW, Wysocki PJ, Mackiewicz A. Cancer immunotherapy using cells modified with cytokine genes. Acta Biochim Pol 2003;50:613–624. [PubMed] [Google Scholar]
- 21.Nawrocki S, Wysocki PJ, Mackiewicz A. Genetically modified tumour vaccines: an obstacle race to break host tolerance to cancer. Expert Opin Biol Ther 2001;1:193–204. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T. Genetically modified dendritic cells for therapeutic immunity. Tohoku J Exp Med 2006;208:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Hodge JW, Greiner JW, Tsang KY, Sabzevari H, Kudo-Saito C, Grosenbach DW, Gulley JL, Arlen PM, Marshall JL, Panicali D, Schlom J. Costimulatory molecules as adjuvants for immunotherapy. Front Biosci 2006;11:788–803. [DOI] [PubMed] [Google Scholar]
- 24.Greiner JW, Zeytin H, Anver MR, Schlom J. Vaccine-based therapy directed against carcinoembryonic antigen demonstrates antitumor activity on spontaneous intestinal tumors in the absence of autoimmunity. Cancer Res 2002;62:6944–6951. [PubMed] [Google Scholar]
- 25.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, Tsang KY, Schlom J. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol 2000;18:3964–3973. [DOI] [PubMed] [Google Scholar]
- 26.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res 2005;65:8059–8064. [DOI] [PubMed] [Google Scholar]
- 27.Pantuck AJ, van Ophoven A, Gitlitz BJ, Tso CL, Acres B, Squiban P, Ross ME, Belldegrun AS, Figlin RA. Phase I trial of antigen-specific gene therapy using a recombinant vaccinia virus encoding MUC-1 and IL-2 in MUC-1-positive patients with advanced prostate cancer. J Immunother 2004;27:240–253. [DOI] [PubMed] [Google Scholar]
- 28.Nemunaitis J. GVAX (GMCSF gene modified tumor vaccine) in advanced stage non small cell lung cancer. J Control Release 2003;91:225–231. [DOI] [PubMed] [Google Scholar]
- 29.Nemunaitis J, Schiller J, Ross H, Jablons D, Harper H, Sterman D, Kelly K, Carbone D, Lin A, Maslyar D, Hege K. A phase 2 randomized study of GM-CSF gene-modified autologous tumor cell immunotherapy (CG8123) with and without low-dose cyclophosphamide in advanced stage non-small cell lung cancer (NSCLC) [abstract]. Mol Ther 2006;13:S243. Abstract 630. [Google Scholar]
- 30.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther 2005;11:149–159. [DOI] [PubMed] [Google Scholar]
- 31.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther 2005;11:160–172. [DOI] [PubMed] [Google Scholar]
- 32.Garnett CT, Greiner JW, Tsang KY, Kudo-Saito C, Grosenbach DW, Chakraborty M, Gulley JL, Arlen PM, Schlom J, Hodge JW. TRICOM vector based cancer vaccines. Curr Pharm Des 2006;12:351–361. [DOI] [PubMed] [Google Scholar]
- 33.Petrulio CA, Kaufman HL. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert Rev Vaccines 2006;5:9–19. [DOI] [PubMed] [Google Scholar]
- 34.Gordon NF, Clark BL. The challenges of bringing autologous HSP-based vaccines to commercial reality. Methods 2004;32:63–69. [DOI] [PubMed] [Google Scholar]
- 35.Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, Jablons D, Aimi J, Lin A, Hege K. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006;13:555–562. [DOI] [PubMed] [Google Scholar]
- 36.Vaccine therapy in treating patients with stage IIIC or stage IV malignant melanoma. ClinicalTrials.gov Web site. Available at: http://www.clinicaltrials.gov/ct/show/NCT00101166?order=1. Accessed July 31, 2006.
- 37.Laheru D, Yeo C, Biedrzycki B, Onners B, Tartakovsky I, Solt S, Hruban R, Lillemoe K, Cameron J, Abrams R, Garrett-Mayer E, Jaffee E. A Safety and efficacy trial of lethally irradiated allogeneic pancreatic tumor cells transfected with the GM-CSF gene in combination with adjuvant chemoradiotherapy for the treatment of adenocarcinoma of the pancreas [abstract]. In: Proceedings of AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; November 14–18, 2005; Philadelphia, Pennsylvania, USA. Abstract C28.
- 38.Mullen JT, Tanabe KK. Viral oncolysis. Oncologist 2002;7:106–119. [DOI] [PubMed] [Google Scholar]
- 39.Mullen JT, Tanabe KK. Viral oncolysis for malignant liver tumors. Ann Surg Oncol 2003;10:596–605. [DOI] [PubMed] [Google Scholar]
- 40.Yoon SS, Nakamura H, Carroll NM, Bode BP, Chiocca EA, Tanabe KK. An oncolytic herpes simplex virus type 1 selectively destroys diffuse liver metastases from colon carcinoma. FASEB J 2000;14:301–311. [PubMed] [Google Scholar]
- 41.Cozzi PJ, Malhotra S, McAuliffe P, Kooby DA, Federoff HJ, Huryk B, Johnson P, Scardino PT, Heston WD, Fong Y. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and Nv1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. FASEB J 2001;15:1306–1308. [DOI] [PubMed] [Google Scholar]
- 42.Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, Wang M, Desmond RA, Keriel A, Barnett B, Baker HJ, Siegal GP, Curiel DT. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther 2003;7:163–173. [DOI] [PubMed] [Google Scholar]
- 43.Dobbelstein M. Viruses in therapy—royal road or dead end? Virus Res 2003;92:219–221. [DOI] [PubMed] [Google Scholar]
- 44.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996;274:373–376. [DOI] [PubMed] [Google Scholar]
- 45.O’Shea CC, Soria C, Bagus B, McCormick F. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell 2005;8:61–74. [DOI] [PubMed] [Google Scholar]
- 46.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, Reid T, Kaye S, Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res 2000;60:6359–6366. [PubMed] [Google Scholar]
- 47.Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience). Ann Surg Oncol 2000;7:588–592. [DOI] [PubMed] [Google Scholar]
- 48.Rudin CM, Cohen EE, Papadimitrakopoulou VA, Silverman S Jr, Recant W, El-Naggar AK, Stenson K, Lippman SM, Hong WK, Vokes EE. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol 2003;21:4546–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R, Varghese S, Rabkin SD. Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res 2005;65:1532–1540. [DOI] [PubMed] [Google Scholar]
- 50.Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J, Petrowsky H, Mastorides S, Federoff H, Fong Y. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther 2002;9:935–945. [DOI] [PubMed] [Google Scholar]
- 51.McAuliffe PF, Jarnagin WR, Johnson P, Delman KA, Federoff H, Fong Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J Gastrointest Surg 2000;4:580–588. [DOI] [PubMed] [Google Scholar]
- 52.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000;7:867–874. [DOI] [PubMed] [Google Scholar]
- 53.Information on clinical trials and human research studies. ClinicalTrials.gov Web site. Available at: http://www.clinicaltrials.gov/. Accessed July 31, 2006.
- 54.Wein LM, Wu JT, Kirn DH. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res 2003;63:1317–1324. [PubMed] [Google Scholar]
- 55.Demers GW, Johnson DE, Tsai V, Wen SF, Quijano E, Machemer T, Philopena J, Ramachandra M, Howe JA, Shabram P, Ralston R, Engler H. Pharmacologic indicators of antitumor efficacy for oncolytic virotherapy. Cancer Res 2003;63:4003–4008. [PubMed] [Google Scholar]
- 56.Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J 2005;7:E61–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadeghi H, Hitt MM. Transcriptionally targeted adenovirus vectors. Curr Gene Ther 2005;5:411–427. [DOI] [PubMed] [Google Scholar]
- 58.Ohlfest JR, Demorest ZL, Motooka Y, Vengco I, Oh S, Chen E, Scappaticci FA, Saplis RJ, Ekker SC, Low WC, Freese AB, Largaespada DA. Combinatorial antiangiogenic gene therapy by nonviral gene transfer using the sleeping beauty transposon causes tumor regression and improves survival in mice bearing intracranial human glioblastoma. Mol Ther 2005;12:778–788. [DOI] [PubMed] [Google Scholar]
- 59.Frank O, Rudolph C, Heberlein C, von Neuhoff N, Schrock E, Schambach A, Schlegelberger B, Fehse B, Ostertag W, Stocking C, Baum C. Tumor cells escape suicide gene therapy by genetic and epigenetic instability. Blood 2004;104:3543–3549. [DOI] [PubMed] [Google Scholar]
- 60.Satoh T, Irie A, Egawa S, Baba S. In situ gene therapy for prostate cancer. Curr Gene Ther 2005;5:111–119. [DOI] [PubMed] [Google Scholar]
- 61.Vattemi E, Claudio PP. Gene therapy for lung cancer: practice and promise. Ann Ital Chir 2004;75:279–289. [PubMed] [Google Scholar]
- 62.Tseng JF, Mulligan RC. Gene therapy for pancreatic cancer. Surg Oncol Clin N Am 2002;11:537–569. [DOI] [PubMed] [Google Scholar]
- 63.Hackett PB, Ekker SC, Largaespada DA, McIvor RS. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv Genet 2005;54:189–232. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen H, Rasmussen C, Lempicki M, Durham R, Brough D, King CR, Weichselbaum R. TNFerade Biologic: preclinical toxicology of a novel adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene. Cancer Gene Ther 2002;9:951–957. [DOI] [PubMed] [Google Scholar]
- 65.Mundt AJ, Vijayakumar S, Nemunaitis J, Sandler A, Schwartz H, Hanna N, Peabody T, Senzer N, Chu K, Rasmussen CS, Kessler PD, Rasmussen HS, Warso M, Kufe DW, Gupta TD, Weichselbaum RR. A Phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res 2004;10:5747–5753. [DOI] [PubMed] [Google Scholar]
- 66.Senzer N, Mani S, Rosemurgy A, Nemunaitis J, Cunningham C, Guha C, Bayol N, Gillen M, Chu K, Rasmussen C, Rasmussen H, Kufe D, Weichselbaum R, Hanna N. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol 2004;22:592–601. [DOI] [PubMed] [Google Scholar]
- 67.McLoughlin JM, McCarty TM, Cunningham C, Clark V, Senzer N, Nemunaitis J, Kuhn JA. TNFerade, an adenovector carrying the transgene for human tumor necrosis factor alpha, for patients with advanced solid tumors: surgical experience and long-term follow-up. Ann Surg Oncol 2005;12:825–830. [DOI] [PubMed] [Google Scholar]
- 68.Gordon EM, Hall FL. Nanotechnology blooms, at last (Review). Oncol Rep 2005;13:1003–1007. [PubMed] [Google Scholar]
- 69.Gordon EM, Cornelio GH, Lorenzo CC 3rd, Levy JP, Reed RA, Liu L, Hall FL. First clinical experience using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int J Oncol 2004;24:177–185. [PubMed] [Google Scholar]
- 70.Fillat C, Carrio M, Cascante A, Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther 2003;3:13–26. [DOI] [PubMed] [Google Scholar]
- 71.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttuala S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 2004;10:967–972. [DOI] [PubMed] [Google Scholar]
- 72.Cheah PL, Looi LM. p53: an overview of over two decades of study. Malays J Pathol 2001;23:9–16. [PubMed] [Google Scholar]
- 73.Merritt JA, Roth JA, Logothetis CJ. Clinical evaluation of adenoviral-mediated p53 gene transfer: review of INGN 201 studies. Semin Oncol 2001;28:105–114. [DOI] [PubMed] [Google Scholar]
- 74.Swisher SG, Roth JA, Komaki R, Gu J, Lee JJ, Hicks M, Ro JY, Hong WK, Merritt JA, Ahrar K, Atkinson NE, Correa AM, Dolormente M, Dreiling L, El-Naggar AK, Fossella F, Francisco R, Glisson B, Grammer S, Herbst R, Huaringa A, Kemp B, Khuri FR, Kurie JM, Liao Z, McDonnell TJ, Morice R, Morello F, Munden R, Papadimitrakopoulou V, Pisters KM, Putnam JB Jr, Sarabia AJ, Shelton T, Stevens C, Shin DM, Smythe WR, Vaporciyan AA, Walsh GL, Yin M. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res 2003;9:93–101. [PubMed] [Google Scholar]
- 75.INGN 201: Ad-p53, Ad5CMV-p53, Adenoviral p53, INGN 101, p53 gene therapy—Introgen, RPR/INGN 201. BioDrugs 2003;17:216–222. [DOI] [PubMed] [Google Scholar]
- 76.China OK’s gene therapy drug. Genetic Engineering News 2003;6.