Abstract
Background
Uncertainty remains about the extent to which findings from our previously published systematic review and meta-analysis of double-blind, randomised controlled trials of topical antibiotics compared with placebo in the management of patients with acute bacterial conjunctivitis treated in secondary care outpatient settings are generalisable to the management of the condition in primary care settings. We updated our review, undertaking searches, methodological assessment, data extraction and analysis according to a pre-defined protocol. In addition to the previous three included studies, we identified two additional double-blind primary care trials, one which compares fusidic acid gel with placebo gel and one which compares chloramphenicol eye drops with placebo eye drops in children. Meta-analyses of clinical and microbiological remission data reveal that topical antibiotics are of benefit in improving early (days 2–5) clinical (relative risk [RR] = 1.24, 95% confidence interval [CI] = 1.05 to 1.45) and microbiological (RR = 1.77, 95% CI = 1.23 to 2.54) remission rates; later (days 6–10) data reveal that these early advantages in clinical (RR = 1.11, 95% CI = 1.02 to 1.21) and microbiological cure rates are reduced (RR 1.56, 95% CI = 1.17 to 2.09), but persist. Most cases of acute bacterial conjunctivitis resolve spontaneously. While topical antibiotics are associated with significantly improved rates of early (days 2–5) clinical remission, this benefit is marginal for later remission (days 6–10).
Keywords: acute bacterial conjunctivitis, antibiotics, meta-analysis, randomised controlled trial, systematic review
INTRODUCTION
In 2001 we reported the findings of our systematic review and meta-analysis evaluating the effectiveness of topical antibiotics in the treatment of acute bacterial conjunctivitis and concluded that: ‘Acute bacterial conjunctivitis is frequently a self-limiting condition but the use of antibiotics is associated with significantly improved rates of early clinical remission, and early and late microbiological remission’.1 We also sounded a word of caution, arguing that ‘since trials to date have been conducted in selected specialist care patient populations, generalisations of these results to a primary care based population should be undertaken with a degree of caution’.1
We have since sought to update our review annually. The publication earlier this year of the trial by Rose et al2 and now by Rietveld et al, reported in this issue (page 924),3 represents the first new available evidence on the efficacy of topical antibiotic treatment in the management of acute bacterial conjunctivitis, and we welcome this opportunity to incorporate results from these new trials into our systematic review and meta-analysis.
METHOD
Searches were undertaken by the Cochrane Eyes and Vision Group using the search strategy detailed previously.1 The most recent searches of electronic databases were undertaken in November 2004.
Both authors were involved with the selection of studies, methodological assessment, and data extraction as previously detailed. Quantitative analyses of outcomes were performed on an intention-to-treat basis using random effects modelling with results expressed as relative risks with 95% confidence intervals [CIs].
RESULTS
Our previously reported review incorporated data from three eligible trials.1 The current round of searches identified two additional studies satisfying our inclusion criteria, bringing the total number of included studies to five.2,3 A total of 1034 patients were recruited into these five double-blind, placebo-controlled trials.
Rose et al studied 326 children aged 6 months to 12 years with a clinical diagnosis of infective conjunctivitis recruited from 12 UK general practices.2 Children were randomised to either 0.5% chloramphenicol eye drops or placebo eye drops with instructions to instil one drop into each child's affected eye 2 hourly for the first 24 hours when awake, and then four times daily until 48 hours after the infection had resolved. This study was judged methodologically of high quality (grade A); using an intention-to-treat analysis it found that at the primary end point of 7 days, parent assessed cure rates were 140/163 (85.9%) in the treatment group compared with 128/163 (78.5%) in the placebo group. The mean time to clinical cure was 5 days (standard deviation [SD] = 1.9) in those treated with antibiotics compared with 5.4 days (SD = 1.9) in those on placebo. This study also found that at day 7, antibiotic treatment resulted in improved rates of microbiological remission: 81/125 (64.8%) versus 69/125 (55.2%). A clinical audit conducted at 6 weeks on the 307 (94%) patients on whom data were available revealed that relapses or new episodes of infection were rare, involving 4% of those allocated to antibiotic treatment and 3% of those receiving placebo; similarly adverse events were rare in both groups, affecting 2% of those in each arm of the trial.
How this fits in
Trials of antibiotics conducted in specialist secondary care outpatient settings have shown that antibiotics are of limited value in improving clinical and microbiological outcomes in patients with acute bacterial conjunctivitis. Two new adequately powered trials conducted in primary care settings confirm that bacterial conjunctivitis is frequently self-limiting and that treatment with antibiotics offers only marginal benefits in improving clinical outcomes. The risk of adverse events in those treated with placebo eye drops appears to be low.
The new trial, reported in this issue of the Journal,3 is a primary care study that recruited 181 adult patients from 25 Dutch primary care centres presenting with a red eye (whether mucopurulent discharge or glued eyelid(s)) who were randomly allocated to either one drop of fusidic acid gel 1% or placebo gel, four times daily for a week. This study is methodologically of high quality (grade A); using an intention-to-treat analysis it found that at 7 days patient assessed cure rates were 45/73 (61.6%) in the treatment group and 53/90 (58.9%) in the placebo group.
Meta-analysis of early (days 2–5) and late (days 7–10) clinical and microbiological outcomes reveals that topical antibiotics are of benefit in improving early clinical (relative risk [RR] = 0.24, 95% CI = 1.05 to 1.45) and microbiological (RR = 1.77, 95% CI = 1.23 to 2.54) remission, with these benefits being reduced, but nonetheless persisting for late clinical (RR = 1.11, 95% CI = 1.02 to 1.21) and microbiological (RR = 1.56, 95% CI = 1.17 to 2.09) remission (Tables 1–4).
Table 1.
Random effects meta-analysis of efficacy of topical antibiotics versus placebo in improving early (days 2–5) clinical remission.
| Study or sub-category | Treatment n/N | Control n/N | RR (random) 95% CI | Weight % | RR (random) 95% CI |
|---|---|---|---|---|---|
| Gigliotti 1984 | 21/1984 | 9/32 | 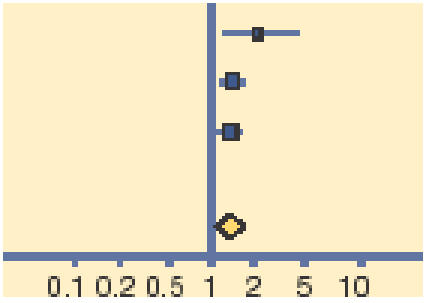 |
6.18 | 2.20 (1.19 to 4.06) |
| Miller 1992 | 126/143 | 101/141 | 49.31 | 1.23 (1.09 to 1.39) | |
| Rose 2005 | 123/163 | 107/163 | 44.52 | 1.15 (1.00 to 1.32) | |
| Total (95% CI) | 340 | 336 | 100.00 | 1.24 (1.05 to 1.45) | |
| Total events: 270 (treatment), 217 (control) | |||||
| Test for heterogeneity:χ2 = 4.33, degrees of freedom = 2 (P = 0.12), l2 = 53.8% | |||||
| Test for overall effect: Z = 2.61 (P = 0.009) | |||||
n = number of subjects in remission. N = number of subjects tested. RR = relative risk.
Table 4.
Random effects meta-analysis of efficacy of topical antibiotics versus placebo in improving late (6–10 days) microbiological remission.
| Study or sub-category | Treatment n/N | Control n/N | RR (random) 95% CI | Weight % | RR (random) 95% CI |
|---|---|---|---|---|---|
| Gigliotti 1984 | 27/34 | 10/32 | 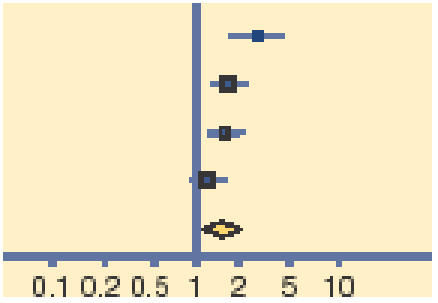 |
16.88 | 2.54 (1.48 to 4.37) |
| Miller 1992 | 59/76 | 35/67 | 30.67 | 1.49 (1.15 to 1.93) | |
| Rietveld 2005 | 16/21 | 12/29 | 18.68 | 1.84 (1.12 to 3.02) | |
| Rose 2005 | 81/125 | 69/125 | 33.76 | 1.17 (0.96 to 1.44) | |
| Total (95% CI) | 256 | 253 | 100.0 | 1.56 (1.17 to 2.09) | |
| Total events: 183 (treatment), 126 (control) | |||||
| Test for heterogeneity:χ2 = 9.01, degrees of freedom = 3 (P = 0.03), l2 = 66.7% | |||||
| Test for overall effect: Z = 3.01 (P = 0.003) | |||||
n = number of subjects in remission. N = number of subjects tested. RR = relative risk.
DISCUSSION
These two adequately powered new trials represent a welcome addition to the evidence base for the treatment of acute bacterial conjunctivitis.4 They confirm findings from previous outpatient-based secondary care studies that indicate that topical antibiotics are of limited efficacy in improving clinical outcomes for acute bacterial conjunctivitis. While topical antibiotics clearly have an impact on microbiological remission and (less so) on early clinical remission rates, by 6–10 days their clinical advantage is marginal. Although unanswered questions about risk of serious adverse events in those who do not receive antibiotic treatment remain to be fully addressed, on balance our earlier conclusion is strengthened by this updated systematic review and meta-analysis: acute bacterial conjunctivitis is frequently a self-limiting condition and topical antibiotic use offers only marginal benefit in improving clinical outcomes.4,5
Table 2.
Random effects meta-analysis of efficacy of topical antibiotics versus placebo in improving late (6–10 days) clinical remission.
| Study or sub-category | Treatment n/N | Control n/N | RR (random) 95% CI | Weight % | RR (random) 95% CI |
|---|---|---|---|---|---|
| Gigliotti 1984 | 31/34 | 23/32 | 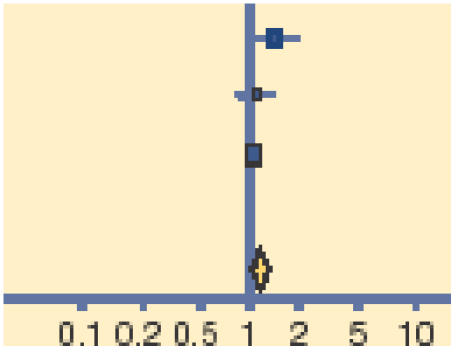 |
13.26 | 1.27 (1.00 to 1.61) |
| Rietveld 2005 | 45/73 | 53/90 | 12.28 | 1.05 (0.82 to 1.21) | |
| Rose 2005 | 140/163 | 128/163 | 74.46 | 1.09 (0.99 to 1.21) | |
| Total (95% CI) | 270 | 285 | 100.00 | 1.11 (1.01 to 1.21) | |
| Total events: 216 (treatment), 204 (control) | |||||
| Test for heterogeneity:χ2 = 1.49, degrees of freedom = 2 (P = 0.47), l2 = 0% | |||||
| Test for overall effect: Z = 2.31 (P = 0.02) | |||||
n = number of subjects in remission. N = number of subjects tested. RR = relative risk.
Table 3.
Random effects meta-analysis of efficacy of topical antibiotics versus placebo in improving early (days 2–5) microbiological remission.
| Study or sub-category | Treatment n/N | Control n/N | RR (random) 95% CI | Weight % | RR (random) 95% CI |
|---|---|---|---|---|---|
| Gigliotti 1984 | 24/34 | 6/32 | 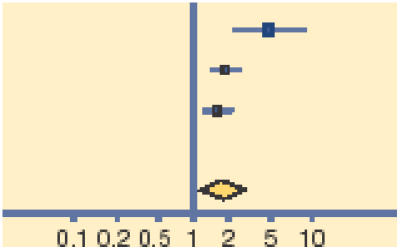 |
16.46 | 3.76 (1.77 to 8.00) |
| Leibowitz 2005 | 132/140 | 22/37 | 42.57 | 1.59 (1.21 to 2.08) | |
| Miller 1992 | 53/76 | 32/67 | 40.97 | 1.46 (1.09 to 1.95) | |
| Total (95% CI) | 250 | 136 | 100.00 | 1.77 (1.23 to 2.54) | |
| Total events: 209 (treatment), 60 (control) | |||||
| Test for heterogeneity:χ2 = 5.64, degrees of freedom = 2 (P = 0.06), l2 = 64.5% | |||||
| Test for overall effect: Z = 3.06 (P = 0.002) | |||||
n = number of subjects in remission. N = number of subjects tested. RR = relative risk.
Acknowledgments
We thank Karen Blackhall and Anupa Shah of the Cochrane Eyes and Vision Group for their help with conducting searches, retrieving and organising translation of manuscripts.
Competing interests
Aziz Sheikh was a co-author on one of the new trials incorporated into this review update
REFERENCES
- 1.Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review. Br J Gen Pract. 2001;51:473–477. [PMC free article] [PubMed] [Google Scholar]
- 2.Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial. Lancet. 2005;366:37–43. doi: 10.1016/S0140-6736(05)66709-8. [DOI] [PubMed] [Google Scholar]
- 3.Rietveld RP, ter Riet G, Bindels PJE, et al. The treatment of acute infectious conjunctivitis with fusidic acid: a randomised trial. Br J Gen Pract. 2005;55:924–930. [PMC free article] [PubMed] [Google Scholar]
- 4.Sheikh A, Wormald R, Smeeth L, Henshaw K, editors. Evidence based ophthalmology. London: BMJ Books; 2004. Acute bacterial conjunctivitis; pp. 33–35. [Google Scholar]
- 5.Sheikh A, Hurwitz B. Bacterial conjunctivitis. In: Hampton F, Fraunfelder FT, Fraunfelder FW, editors. Current ocular therapy. 7th edn. London: Elsevier Ltd; (in press) [Google Scholar]


