Abstract
Objective:
Alterations of the endocrine system in patients following Roux-en-Y gastric bypass (GBP) are poorly described and have prompted us to perform a longitudinal study of the effects of GBP on serum calcium, 25-hydroxy-vitamin-D (vitamin D), and parathyroid hormone (PTH).
Methods:
Prospectively collected data were compiled to determine how GBP affects serum calcium, vitamin D, and PTH. Student t test, Fisher exact test, or linear regression was used to determine significance.
Results:
Calcium, vitamin D, and PTH levels were drawn on 243 patients following GBP. Forty-one patients had long-limb bypass (LL-GBP), Roux >100 cm, and 202 had short-limb bypass (SL-GBP), Roux ≤100 cm. The mean (±SD) postoperative follow-up time was significantly longer in the LL-GBP group (5.7 ± 2.5 years) than the SL-GBP group (3.1 ± 3.6 years, P < 0.0001). When corrected for albumin levels, mean calcium was 9.3 mg/dL (range, 8.5–10.8 mg/dL), and no difference existed between LL-GBP and SL-GBP patients. For patients with low vitamin D levels (<8.9 ng/mL), 88.9% had elevated PTH (>65 pg/mL) and 58.0% of patients with normal vitamin D levels (≥8.9 ng/mL) had elevated PTH (P < 0.0001). In individuals with vitamin D levels <30 ng/mL, 55.1% (n = 103) had elevated PTH, and of those with vitamin D levels ≥30 ng/mL 28.5% (n = 16) had elevated PTH (P = 0.0007). Mean vitamin D levels were lower in patients who had undergone LL-GBP as opposed to those with SL-GBP, 16.8 ± 10.8 ng/mL versus 22.7 ± 11.1 ng/mL (P = 0.0022), and PTH was significantly higher in patients who had a LL-GBP (113.5 ± 88.0 pg/mL versus 74.5 ± 52.7 pg/mL, P = 0.0002). There was a linear decrease in vitamin D (P = 0.005) coupled with a linear increase in PTH (P < 0.0001) the longer patients were followed after GBP. Alkaline phosphatase levels were elevated in 40.3% of patients and correlated with PTH levels.
Conclusion:
Vitamin D deficiency and elevated PTH are common following GBP and progress over time. There is a significant incidence of secondary hyperparathyroidism in short-limb GBP patients, even those with vitamin D levels ≥30 ng/mL, suggesting selective Ca2+ malabsorption. Thus, calcium malabsorption is inherent to gastric bypass. Careful calcium and vitamin D supplementation and long-term screening are necessary to prevent deficiencies and the sequelae of secondary hyperparathyroidism.
Routine monitoring of 25-OH-vitamin D and parathormone levels at annuals visits for gastric bypass patients disclosed a high incidence of vitamin D insufficiency and secondary hyperparathyroidism. This was progressive over time and was more severe in long-limb Roux-en Y gastric bypass patients.
The prevalence of obesity [body mass index (BMI ≥ 30 kg/m2)] doubled between 1976 and 1999 in the United States.1 A record number of morbidly obese patients are seeking surgery in an attempt to decrease their weight and ultimately prevent or decrease comorbid conditions associated with their obesity. A number of different weight reduction operations are performed throughout the world, but Roux-en-Y gastric bypass (GBP) is the leading weight reduction operation offered in the United States. It has been well documented that GBP provides long-term weight reduction with prevention or resolution of comorbid conditions,2–4 but the endocrine side effects of GBP remain incompletely studied.
Until recently, our bariatric surgery protocol included annual screening of calcium, phosphorus, magnesium, and albumin after GBP. With newer literature,5 suggesting that patients who undergo GBP are at increased risk for vitamin D deficiency, our screening was updated to include both 25-hydroxyvitamin D (vitamin D) and parathyroid hormone (PTH) levels. This is a longitudinal study of prospectively collected data that evaluates the endocrine effects of GBP on vitamin D, calcium and PTH levels.
METHODS
A total of 243 patients underwent routine laboratory testing after having GBP surgery. The testing included measurement of serum levels of vitamin D, PTH, calcium, and albumin levels. Statistical analysis using Student t test, Fisher exact test, or linear regression was used to determine the correlation of vitamin D levels to PTH levels and also determine whether vitamin D deficiency and hyperparathyroidism correlated to the length of bypass segment and time from initial surgery.
RESULTS
Of the 243 patients who underwent GBP, 41 had long limb bypasses (LL-GBP), Roux >100 cm, and 202 patients had a short limb bypass (SL-GBP), Roux <100 cm. BMI was higher in those who underwent LL-GBP (60.6 ± 8.3 kg/m2) as opposed to those who had a SL-GBP (49.1 ± 8.8 kg/m2) (P < 0.0001). The average postoperative follow-up was longer in the LL-GBP group, and the average age was higher in the SL-GBP group (Table 1). Mean corrected calcium levels were 9.3 mg/dL (8.5–10.8 mg/dL), and no difference existed between the LL-GBP and SL-GBP groups. The average vitamin D level for the entire group was 21.7 ± 11.3 ng/mL (laboratory normal >8.9 ng/mL). Individuals who underwent LL-GBP had lower vitamin D levels and higher PTH levels (laboratory normal range, 12.0–65 pg/mL) than those who had a SL-GBP (Table 1). Of the individuals with low vitamin D levels (n = 36), 88.9% had an elevated PTH (P < 0.0001), and 42.1% of those with laboratory normal vitamin D levels (n = 207) had an elevation in PTH (P < 0.0001).
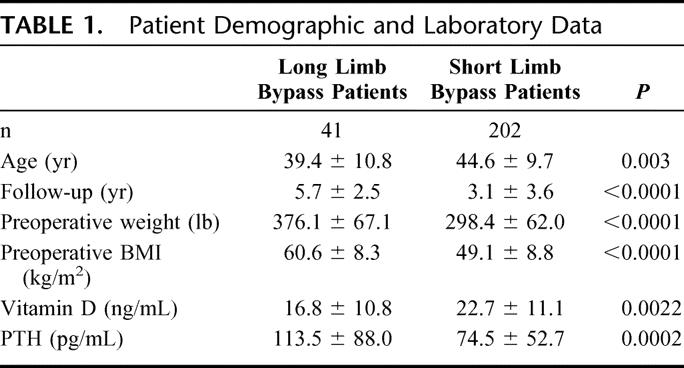
TABLE 1. Patient Demographic and Laboratory Data
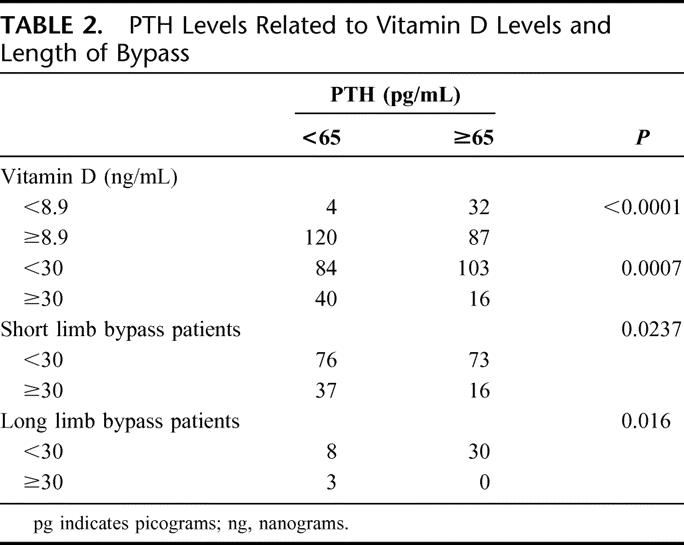
Recently, it has been recognized that vitamin D concentration of 30 ng/mL or higher are optimal for good bone health, even in those who have not had GBP and that quoted “normal” reference range include individuals with significant vitamin D insufficiency.6,7 When making 30 ng/mL the cutoff for normal vitamin D levels, the majority (78.9%) of patients who underwent a LL-GBP and whose vitamin D levels were <30 ng/mL also had elevated PTH levels. Only 3 of 41 LL-GBP patients had vitamin D levels ≥30 ng/mL. Almost half (49.0%) of SL-GBP patients with a vitamin D <30 ng/mL had an elevated PTH level (Table 2). Fully, 28.5% of SL-GBP patients with vitamin D levels ≥30 ng/mL had elevated PTH levels.
TABLE 2. PTH Levels Related to Vitamin D Levels and Length of Bypass
There was a decrease in vitamin D levels and a progressive increase in PTH level (Fig. 1) the longer patients were followed after their surgery. Beyond 5 years of follow-up, few patients had vitamin D levels ≥30 ng/mL. Additionally, there was an inversed linear relationship between PTH and vitamin D such that the lower the vitamin D levels the higher the PTH levels (Fig. 2).
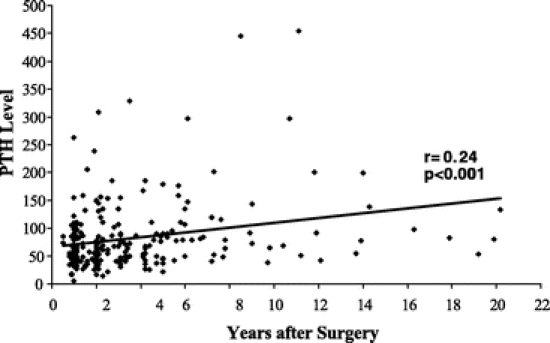
FIGURE 1. Parathormone levels at annual intervals following Roux-en-Y gastric bypass. PTH levels increased in a linear fashion with time.
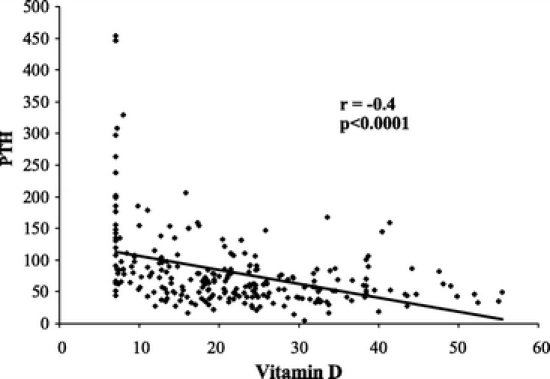
FIGURE 2. Parathormone levels as a function of 25-hydroxy-vitamin D levels. A significant negative linear correlation is evident.
Alkaline phosphatase levels were elevated in 40.3% of patients, overall. Patients with elevated PTH levels had a higher incidence of elevated alkaline phophatase levels than those with normal PTH levels (48.7 versus 32.3%, P < 0.01 by χ2 test). There was a significant correlation of alkaline phosphatase with PTH levels (Fig. 3).
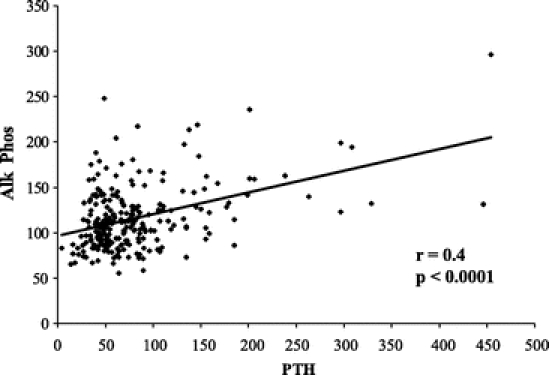
FIGURE 3. Linear relationship between PTH levels and serum alkaline phosphatase in gastric bypass patients. The relationship suggests active bone loss with secondary hyperparathyroidism.
DISCUSSION
As the rate of obesity continues to rise in the United States, the demand for weight-reduction surgery will continue to increase. It has been estimated that the number of obesity procedures performed in the United States will top 130,000 in 2005, and may even exceed 200,000 by 2010.8 It has been repeatedly demonstrated that GBP provides sustained weight loss with either prevention or resolution of comorbid conditions.3,4 However, the effect of GBP on vitamin D metabolism remains incompletely studied.
When patients undergo GBP, the preferential sites for the absorption of calcium, the duodenum and proximal jejunum are bypassed, placing them at an increased risk for hypocalcemia.9,10 Under circumstances of low dietary calcium intake, the duodenum can absorb as much as 80% to 100% by a vitamin D-dependent active transport, transcellular mechanism. In the absence of the duodenum and proximal jejunum, the remaining small intestine can absorb calcium through a less efficient paracellular mechanism, which takes in as little as 20% of oral calcium. A necessary component of calcium absorption within the intestines is vitamin D. Crowley et al11 have shown that gastric bypass patients who don't take regular vitamin D supplementation consume only about 50% of the recommended daily requirements of vitamin D. The decreased intake of vitamin D then leads to a decrease in the absorption of calcium from the intestinal lumen. Furthermore, it has been suggested that the creation of a Roux anastomosis in itself causes malabsorption of fat-soluble vitamins due to poor mixing with bile salts, thus creating a further decrease in the amount of vitamin D available and still less absorption of calcium.12,13
One of the defense mechanisms of the body for the maintenance of normocalcemia, in the setting of decreased absorption of calcium, is the up-regulation of PTH, which directly causes an increased production of 1,25-dihydroxy vitamin D (calcitriol) and, more importantly, an increase in calcium reabsorption from the bone.10 Calcitriol enhances both the absorption of calcium in the intestine and the bone-resorptive effects of PTH on bone. A significant number of our patients experienced an elevated PTH after their GBP (Table 1). This is of great concern because, if left neglected over long periods of time, vitamin D deficiency and secondary hyperparathyroidism can result in osteopenia, osteoporosis, and ultimately osteomalacia.12
Recent studies suggest that patients, whose 25-OH-vitamin D levels are in the lower portion of most labs’ reference range for vitamin D, are already profoundly deficient.6,7 By the time the vitamin D level of patients in our study fell below the reported reference range at Virginia Commonwealth University (<8.9 ng/mL), 89% of them demonstrated hyperparathyroidism (PTH >65 pg/mL), while only 28% of the patients who had serum vitamin D ≥30 ng/mL had an elevated PTH level (Table 2). As one might anticipate, there was an inverse, linear relationship between PTH levels and serum vitamin D levels such that the lower the vitamin D, the higher the PTH levels (Fig. 3). The longer patients were followed from their original surgery, the more prone they were to deficiencies of vitamin D (Fig. 1). Additionally, those who had a LL-GBP (Roux >100 cm) had a significantly lower serum vitamin D level when compared with those who had an SL-GBP (Table 1).
Our data underscore the importance of aggressively supplementing, screening, and treating post-GBP patients for vitamin D deficiencies. Currently, most authorities recommend that 25-hydroxyvitamin D levels should be maintained greater than 25 to 30 ng/mL in an attempt to prevent problems associated with vitamin D deficiencies.7 We have instituted an aggressive screening and vitamin D supplementation program follow GBP. Preoperatively and at each annual visit, patients are screened for deficiencies of vitamin D along with hyperparathyroidism and aggressive supplementation is given in an attempt to keep the serum 25-hydroxyvitamin D levels ≥30 ng/mL. The fact that a significant number of SL-GBP patients with normal vitamin D levels were also hyperparathyroid suggests that some patients selectively malabsorb calcium after GBP. Another possibility is that due to a low acid environment after GBP, calcium carbonate may be poorly absorbed; calcium citrate may be a better option in these patients. Since morbidly obese patients have a higher incidence of elevated PTH levels than normal weight individuals, even before surgery, it is apparent that patients should be screened quite early in the therapeutic process and that patients should be educated as to the importance of compliance with vitamin and mineral supplementation.
We currently recommend that all patients be supplemented with at least 1200 mg of calcium per day and an additional 800 IU of vitamin D per day following GBP. On the other hand, Goode et al14 reported that replacement of calcium and vitamin D in GBP patients with elevated PTH levels failed to suppress PTH or bone resorption over a 6-month period. Since the paracellular absorptive process in the distal jejunum and ileum is less vitamin D-dependent than the transcellular process in the duodenum and proximal jejunum, there is reason to fear that supplementation may be ineffective. It remains to be seen whether more aggressive therapy with high doses of vitamin D can correct secondary hyperparathyroidism. Further follow-up studies are necessary to determine whether routine supplementation with calcium and vitamin D for GBP patients will prevent secondary hyperparathyroidism and whether screening for and aggressively correcting low vitamin D levels (<30 ng/mL) will suppress established disease.
Discussions
Dr. Kenneth G. MacDonald (Greenville, North Carolina): Many of the findings in this paper have been confirmed by other reports, although there is not complete unanimity of the results, and there remain some unanswered questions that I would like to ask of the authors, although I am not sure there are definite answers.
Number 1, you mention in the manuscript that the morbidly obese have a higher incidence of elevation of PTH and documented decrease in vitamin D levels even before surgery compared with normal weight individuals, and some further report that these levels don't change appreciably after bariatric surgery, which is in opposition to your data and a lot of others. A possible explanation is the increased uptake and clearance of calcidiol by adipose tissue, which we all know is a pretty active metabolic organ. If this is the case, though, should not the bioavailability of vitamin D increase with the decrease in adiposity after surgery? That is question 1.
Do you have any data on preoperative vitamin D and PTH levels in your patients and do you have any thoughts about this issue of vitamin D bioavailability versus changes in the gut absorption after gastric bypass?
Dr. Kellum mentioned why you chose 30 ng/mL as the cutoff for the vitamin D levels. My second question is: do you think that you have enough data to make this the standard that we ought to shoot for when we are supplementing our patients after surgery?
Question 3, just to further confuse the picture, I have read articles about the purely restrictive laparoscopic banding operation that report no increase in PTH levels, which might make some sense, although bone marker studies still show increased bone resorption. While you didn't study any patients, I assume, with restrictive operations, do you have an opinion from your work and your interest in this topic whether there is any incidence of secondary hyperparathyroidism with purely restrictive operations?
You reported a progressive, essentially linear increase in PTH levels with time after surgery, but the data on the graph out to 10 years show few data points that are widely scattered. Do you think there is enough long-term data to show the PTH levels continue to increase with time? Or, perhaps, because of increased food intake which occurs with all of these people and the decreased adiposity increasing bioavailability, is there a chance that perhaps there is increased calcidiol available and perhaps PTH levels will be moderated in the future?
Finally, my last question, do you have any evidence from your studies that oral supplementation of vitamin D and calcium citrate at the doses you recommended effectively prevent bone disease? You discussed this very briefly in the conclusion of your manuscript, but I want to know if you can elaborate any further.
Dr. James W. Maher (Richmond, Virginia): This study proceeded from studies of my patients at Iowa where we had looked at serial bone densities in patients undergoing laparoscopic gastric bypass and showed a significant decrease in bone density at 1 and 2 years. Now, that varied a little bit depending on where the site was. The lumbosacral spine and the hip seemed to be most affected, the radius and forearm less affected. And we saw a significant but small augmentation of parathormone in those patients.
The purpose of this study was really to get a snapshot of what we were dealing with. We really didn't think it was going to be much of a problem, but we thought with the volume of gastric bypasses we see in follow-up on a yearly basis that it was a question that we could pretty easily answer. And we were really astonished at the magnitude of what we saw here.
As far as the question about decrease in adiposity leading to an increased bioavailability of vitamin D, I really don't know what the answer is to that question. I think that is something that is going to require clinical studies in the future to determine the nature of this problem. Our purpose was really to bring this to the attention of the sort of bariatric surgical community as a potential problem. What we don't want to see is people 30 years out developing osteopenia, osteomalacia, and those sorts of problems in the future from an operation designed to correct the comorbidities they started with. I think, as with most studies, this opens up new questions that are going to have to be answered in future studies.
And I think that goes to the essence of your last question: is oral supplementation effective in these people? We know that this paracellular mechanism that takes over when the duodenum and proximal jejunum are excluded is rather inefficient, absorbing only about 20% of ingested calcium, and even that is a vitamin D-dependent mechanism. We are planning right now in conjunction with our endocrinologists to do a clinical research center study where we take some of these patients and see whether this regimen of vitamin D supplementation is effective, and those will be coming in the near future.
As far as your question about whether or not 30 is a proper goal for vitamin D levels in these patients, this is derived from the endocrinology literature. I think it is a place to start. If you look at our patients with a vitamin D level greater than 30, still about 25% of them had chemical increases in their parathormone levels, so it may be that the proper vitamin D level is even higher than that.
As far as your question about laparoscopic gastric banding as well as the secondary hyperparathyroidism that is seen in obese patients before surgery, in fact, that is the case. I believe about 15% to 20% of patients have very mild elevations in parathormone to begin with, but very few of those patients are either hypo or hypercalcemic. So that is another unknown sort of confounding variable in here that we have to dissect in the future. These are really areas as surgical gastroenterologists that are beyond our usual level of knowledge, and we are having to acquire this expertise as we go along.
The lap band, I don't think this has been studied in any sort of reasonable way. There have been studies in the past on a small level, almost anecdotal level in some cases, of all these factors before. But this was sort of a snapshot of patients over a year and a half, and I think it gives us a good baseline of what we are dealing with in that it increases over time. So it is a base for new studies in the future.
Dr. J. Patrick O'Leary (New Orleans, Louisiana): The study is a snapshot of patients at one point in time and not a longitudinal study. We don't even have the preoperative data to act as a point for comparison. So my first question would be: were there any patients in this group who were studied longitudinally preop and then yearly in the postoperative period?
I was also struck that after 6 years (I counted up the little dots on the scanograms) and it seemed to me that there were many more patients who had normal PTH levels than had abnormal PTH levels. That sort of fits with Scopinaro's work published many years ago. When he did a biliopancreatic procedure in his Italian patients, he studied their calcium metabolism in over 100 patients. He did find that the patients showed transient hypocalcemia followed by normalization of calcium levels. Then he did bone densitometry on these patients. He did not find that they had substantial losses of bone density. They were in the normal range.
Now, heavy patients, in my opinion, probably have different bone densitometer readings than patients who are not heavy. That may explain why your lumbosacral patients lost density where the radial arm did not. It may be a matter of which bones were exposed to less stress. Did you look at bone densitometer in your patients? Did you document any parathyroid disease? In other words, did you find any adenomas or masses in the neck?
Dr. James W. Maher (Richmond, Virginia): As far as your question about the longitudinal nature of this study, it is not of a longitudinal nature. The patients that I studied at the University of Iowa started in 2000, and that was a longitudinal study; we looked at all of these measurements in those patients. But frankly, at the time I left, about a year and a half ago, we really only had 3-year data on about 12 patients at that point in time. We did see the same sort of things.
The augmentations in parathormone were of a lesser magnitude than we have seen in these patients followed longer periods of time out. And actually the studies on the patients at Iowa are continuing in a longitudinal fashion. They are attempting to get bone density studies every year. But, of course, we run into the problems there of insurance companies that don't want to pay for it. You would think an insurance company would be interested in that sort of information even if it does cost them a bit of money. But that, of course, is not the case.
We don't know whether or not this is a clinical problem. It very well may not be. Scopinaro has really studied his patients extraordinarily well. On the other hand, there is a series of about 100 biliopancreatic diversions by George Fielding that showed a pretty high incidence of secondary hyperparathyroidism in their patients. And, of course, he has sort of found religion now and given up biliopancreatic bypass in favor of the lap band.
So I think it is a worrisome thing, but we don't know whether it is a problem or not. We would like to find out whether it is a problem, and we are designing studies to try to follow up on that.
Footnotes
Reprints: John M. Kellum, MD, P.O. Box 980519, Department of Surgery, Virginia Commonwealth University, Richmond VA, 23298-0519. E-mail: jmkellum@vcu.edu.
REFERENCES
- 1.Anonymous. 2000 National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States, 1999–2000. Health E-Stats(http://www.cdc.gov/nchs/products/pubs/pubd/hestats/obese/obse99.htm).
- 2.NIH Technology Assessment Conference Panel. NIH Conference: methods for voluntary weight loss and control. Ann Intern Med. 1992;116:942–949. [DOI] [PubMed] [Google Scholar]
- 3.Biertho L, Steffen R, Ricklin T, et al. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding: a comparative study of 1200 cases. J Am Coll Surg. 2003;197:536–544. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman HJ, Kellum JM, Engle KM, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr. 1992;55(suppl):560–566. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JM, Maher JW, Heitshusen D, et al. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg. 2005;9:1106–11. [DOI] [PubMed] [Google Scholar]
- 6.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(suppl):1706–1709. [DOI] [PubMed] [Google Scholar]
- 7.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporosis Int. 2005 Mar 18; [Epub ahead of print]. [DOI] [PubMed]
- 8.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. [DOI] [PubMed] [Google Scholar]
- 9.de Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. [DOI] [PubMed] [Google Scholar]
- 10.Hamoui N, Kim K, Anthone G, et al. The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg. 2003;138:891–897. [DOI] [PubMed] [Google Scholar]
- 11.Crowley LV, Seay J, Mullin G. Late effects of gastric bypass for obesity. Am J Gastroenterol. 1984;79:850–860. [PubMed] [Google Scholar]
- 12.Goldner WS, O'Dorisio TM, Dillon JS, et al. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg. 2002;12:685–692. [DOI] [PubMed] [Google Scholar]
- 13.Slater GH, Ren CF, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55. [DOI] [PubMed] [Google Scholar]
- 14.Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–47. [DOI] [PubMed] [Google Scholar]


