Abstract
Objective:
In a prospective trial, to determine if eIF4E overexpression in breast cancer specimens is correlated with VEGF elevation, increased tumor microvessel density (MVD) counts, and a worse clinical outcome irrespective of nodal status.
Summary and Background Data:
In vitro, the overexpression of eukaryotic initiation factor 4E (eIF4E) up-regulates the translation of mRNAs with long 5′-untranslated regions (5′-UTRs). One such gene product is the vascular endothelial growth factor (VEGF).
Methods:
A total of 114 stage I to III breast cancer patients were prospectively accrued and followed with a standardized clinical surveillance protocol. Cancer specimens were quantified for eIF4E, VEGF, and MVD. Outcome endpoints were cancer recurrence and cancer-related death.
Results:
eIF4E overexpression was found in all cancer specimens (mean ± SD, 12.5 ± 7.6-fold). Increasing eIF4E overexpression correlated with increasing VEGF elevation (r = 0.24, P = 0.01, Spearman's coefficient), and increasing MVD counts (r = 0.35, P < 0.0002). Patients whose tumor had high eIF4E overexpression had shorter disease-free survival (P = 0.004, log-rank test) and higher cancer-related deaths (P = 0.002) than patients whose tumors had low eIF4E overexpression. Patients with high eIF4E had a hazard ratio for cancer recurrence and cancer-related death of 1.8 and 2.1 times that of patients with low eIF4E (respectively, P = 0.009 and P = 0.002, Cox proportional hazard model).
Conclusions:
In breast cancer patients, increasing eIF4E overexpression in the cancer specimens correlates with higher VEGF levels and MVD counts. Patients whose tumors had high eIF4E overexpression had a worse clinical outcome, independent of nodal status. Thus, eIF4E overexpression in breast cancer appears to predict increased tumor vascularity and perhaps cancer dissemination by hematogenous means.
eIF4E is a protein initiation factor that is overexpressed in breast cancer. This prospective trial of stage I to III breast cancer patients demonstrates that eIF4E overexpression is correlated with increased tumor vascularity and predicts a worse cancer outcome, irrespective of nodal disease.
It is estimated that a woman has close to a 12% risk of developing breast cancer by the time she reaches 80 years of age.1 This year, breast cancer will be responsible for more than 40,000 deaths, making it second only to lung cancer as the killer of women with malignancies.1 Breast cancer deaths are mostly from systemic disease, representing recurrences after definitive therapy. Recent research efforts have focused on identifying molecular markers to better stratify and more accurately predict risk for cancer outcome. However, nodal status continues to be the most relied on predictor of breast cancer recurrence. Unfortunately, even among node-negative patients, up to 20% will ultimately develop recurrence.2,3
One approach to better understand breast cancer outcome is to study the underlying biology of the tumor. Eukaryotic initiation factor 4E, or eIF4E, is a 25-kDa cap-binding protein. It recognizes and binds to the 7-methylguanosine cap in the 5′ untranslated regions (5′UTRs) of mRNAs. This binding facilitates the unwinding of long or complex 5′UTRs and subsequent attachment of the “RNA Helicase complex,” eIF4F.4,5 This recruitment of mRNA to the ribosomal apparatus constitutes a key event in the initiation of translation of mRNAs that are otherwise translationally repressed due to their long 5′UTRs.
A number of basic observations have been made of the role eIF4E plays in the progression of cancer. In vitro, overexpression of eIF4E up-regulates various cell cycle regulatory and cancer-related genes, such as cyclin D-1,6 Tousled-like kinase (TLK1B),7 and the angiogenic factors VEGF8 and FGF2.9 In addition, it has been demonstrated that transformed but nonmalignant cell lines acquire malignant phenotypic changes after transfection with a BK virus with eIF4E overexpression.10–12 A recent study reported the repression of eIF4E gene expression by the wild-type p53 at the transcriptional level, suggesting that the loss of normal p53 function may result in elevated levels of eIF4E.13
The overexpression of eIF4E has been reported in a number of human malignancies, including cancers of the breast,14 colon,15 head and neck,16 and sarcomas.17 Additionally, overexpression of eIF4E may have independent prognostic value.18,19 In a retrospective study of 59 stage I to III breast cancer patients, eIF4E was observed to be elevated 3- to 30-fold compared with benign breast tissue from noncancer patients. Furthermore, patients whose tumors had high eIF4E overexpression (defined as >7-fold elevated when compared with benign breast tissue) had a higher rate of cancer recurrence and cancer-related death.18
Subsequently, 191 patients with stage I to III breast cancer were accrued in a prospective trial designed to determine if high eIF4E predicts a higher risk for cancer recurrence. In this study, patients with tumors in the highest eIF4E tertile (defined as >14-fold elevation) were 7.2-fold (95% confidence interval [CI], 2.1–25, P = 0.011) more likely to have cancer recurrence than those patients with tumors in the lowest eIF4E tertile (defined as <7-fold elevation). On multivariate analysis, eIF4E overexpression and nodal status were independent predictors for cancer recurrence.19
To eliminate nodal status as a potential confounding factor, another prospective trial was designed to detect risk for recurrence in node-positive only breast cancer patients. A total of 174 node-positive patients were accrued; and after a median follow-up of 31 months, patients whose tumors were in the highest tertile of eIF4E overexpression had a statistically significant 2.4-fold (CI, 1.2–4.1, P = 0.011) increase in relative risk for cancer recurrence.20 Since this study specifically examined node-positive patients only, high eIF4E overexpression predicted cancer recurrence, independent of nodal status. Thus, this may represent a surrogate marker of a different mode of breast cancer metastasis, perhaps hematogenous dissemination.
In recent years, substantial evidence on the importance of angiogenesis in the progression of cancer has accumulated.21–24 In experimental models, tumor cells are unable to grow beyond a few millimeters without neovascularization.21 Vascular endothelial growth factor (VEGF) is widely considered to be the most significant of all the angiogenic peptides.21–24 In addition, elevation of VEGF and microvessel density (MVD) has been reported to impact on breast cancer outcome.25,26
In vitro, cell lines that overexpress eIF4E up-regulate VEGF expression.8 In human breast cancer specimens, there are varying degrees of eIF4E, VEGF, and MVD elevation. Additionally, patients with cancers that are in the highest tertile of eIF4E overexpression do worst. Thus, this study was designed as a prospective trial to accrue patients with stage I to III breast cancer and test the following hypotheses: 1) eIF4E overexpression in breast cancer correlates with elevation of VEGF expression and increase in MVD counts, and 2) eIF4E overexpression results in a higher risk for cancer recurrence and cancer-related death, independent of nodal status.
METHODS
Patients were counseled and consented to participate in this Institutional Review Board (IRB) approved study prior to enrollment. Treatment and surveillance protocols were standardized to ensure study homogeneity and compliance. Surgical treatment consisted of either a modified radical mastectomy or breast conservation therapy (BCT, lumpectomy with tumor-free margin, axillary lymph node dissection, and breast irradiation; a subset of patients with T1 lesion underwent sentinel node biopsy, followed by a complete axillary lymph node dissection for those with positive sentinel nodes). Adjuvant axillary irradiation, systemic chemotherapy, and antiestrogen therapy were offered and administered as indicated per current standard of care.
Surveillance protocol consisted of a history and physical examination every 3 months for 3 years, every 6 months in years 4 and 5, and annually thereafter. Annual chest x-ray, mammogram, complete blood count, and liver function test were obtained. Any additional radiologic and/or histologic evaluation was performed based on the patient's examination and history. Primary endpoints for this study were cancer recurrence and cancer-related death. Clinical data were accrued and recorded prospectively and included age at diagnosis, comorbid conditions, stage of disease, treatment protocol, surveillance protocol compliance, and study endpoints.
Tissue Procurement
A cancer specimen of at least 100 mg was obtained from the tumor core at the time of surgery from each patient. The specimen was verified by the study pathologist (F.A.) to be an invasive mammary carcinoma. It was then immediately frozen in liquid nitrogen and stored at −70°C. The specimen was given a randomly generated code that links the specimen to the clinical data, available to investigators only at the end of the study period.
Assay for eIF4E
The Western blot assay for eIF4E protein level has been previously described in detail.14,18 In brief, a protein lysate was prepared using a 10-mg portion of tumor tissue cut into tiny pieces, suspended in 0.5 mL RIPA buffer (150 mmol/L NaCl; 1% NP-40; 0.5% DOC; 0.1% SDS; 50 mmol/L Tris [pH 8.0]; 0.1 mmol/L PMSF), and mechanically homogenized using a Savant Bio 101 Fastprep FP120 system (Savant Instruments, Inc., Holbrook, NY). The lysate was then centrifuged at 10,000g for 10 minutes (at 4°C) and total protein content was determined using a standard BCA (bicinchoninic acid) copper reduction assay kit (Pierce, Rockford, IL) (Figure 1).
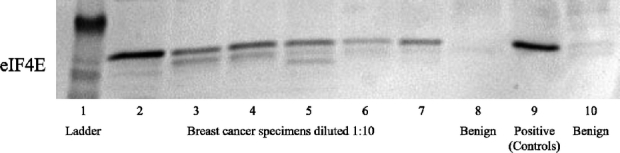
FIGURE 1. Representative Western blot for eIF4E, demonstrating varying degrees of overexpression in tumor specimens (lanes 2–7), a positive control (lane 9 [SK-N-MC]), and 2 negative controls (lanes 8 and 10 [benign breast tissue from noncancer patients]). eIF4E quantification in cancer specimens is calculated as x-fold over the benign breast tissue.
An equal amount of protein lysate from each specimen as well as benign control breast tissue (20 μg diluted in 1:10 RIPA) were loaded onto and separated by using 4% to 20% denaturing gel Tris HCl polyacrylamide gel electrophoresis. Electroblotting onto a nylon membrane (Immobilon PVDF, Millipore, Bedford, MA) was then performed, and the membranes blocked with 5% nonfat milk for 1 hour. Primary incubation of the membrane was carried out using monoclonal mouse antieIF4E antibody (Transduction Laboratories, San Diego, CA). Secondary incubation of the membrane was carried out using a goat anti-mouse horseradish peroxidase conjugate. Blot development was then accomplished using Opti 4CN (4-chloro-1-naphthol, Bio-Rad Laboratories, Hercules, CA). Using the Biophotonics system (Biophotonics Corp., Ann Arbor, MI), the blots were scanned and the band intensity was evaluated using Intelligent Quantifier software (Bio Image, Ann Arbor, MI). Quantification of eIF4E level in each cancer specimen was expressed as x-fold elevated over a control from a breast tissue specimen of a noncancer patient. This process was repeated twice for each specimen and the results were averaged.
Immunostaining for VEGF
Immunohistochemical (IHC) staining was used to grade VEGF expression.27 Cancer specimen slides were cut from paraffin-embedded tissue blocks, deparaffinized in xylene, and rehydrated in graded ethanol. The slides were incubated overnight in monoclonal anti-VEGF165 (Labvision Corporation, Neomarkers, Fremont, CA) antibody at 4°C. The sections were then incubated in Biogenex Link and Label QP900-9L streptavidin peroxidase (Biogenex, San Ramon, CA) as secondary antibody for 30 minutes. The slides were then counterstained with hematoxylin using the Biogenex I-6000 Auto stainer (Biogenex).
Each slide was reviewed by 2 individuals, 1 of whom is the study pathologist. Each slide was viewed at 200× magnification. The stain intensity was graded from 0 to III, grade 0 being nonstaining and grade III being most intense staining (Fig. 2). Two slides from each specimen were scored, and their average was used.
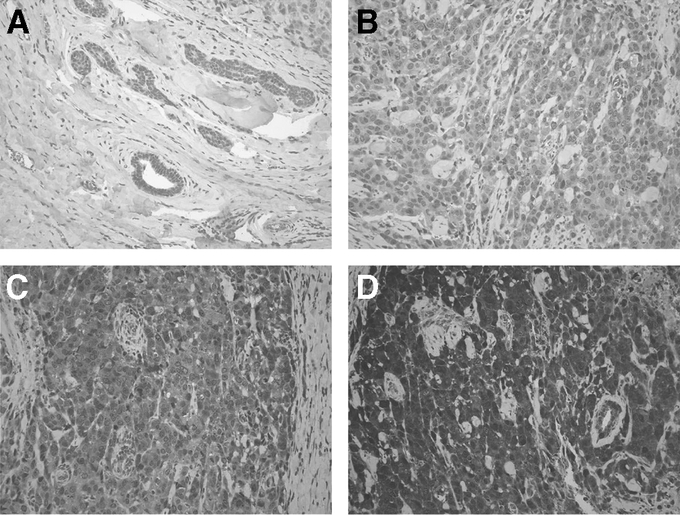
FIGURE 2. Representative slides immunostained for VEGF expression. A, Benign breast tissue, representing grade 0 staining. B–D, Representative panels for 3 breast cancer specimens, demonstrating, respectively, grade I, grade II, and grade III VEGF staining.
Immunostaining for MVD Count
MVD count was determined using the Chalkley method.28 In brief, tissue sections were immunostained with a monoclonal anti-CD34 antibody (Labvision Corporation, Neomarkers, Fremont, CA). MVD count was determined by identifying the areas of highest vascular density for each cancer slide at 100× magnification. These areas were then examined at 200× magnification with the 25 point Chalkley eyepiece graticule oriented to permit maximum number of points to hit on blood vessels. The number of vessels to hit upon a Chalkley point represented the Chalkley score (Fig. 3). Two slides per cancer specimen were scored, and their average score was used.
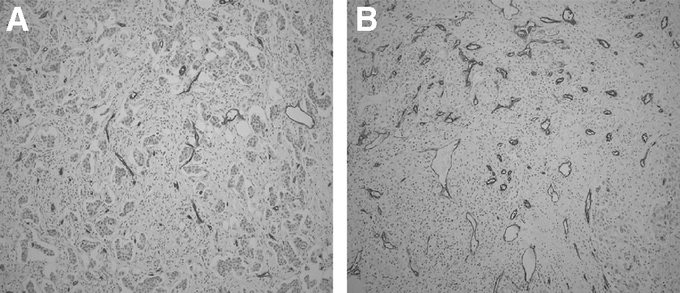
FIGURE 3. Representative slides of 2 different cancer specimens immunostained for CD34 to assess microvessel density (MVD). A, Low MVD (Chalkley score of 3). B, High MVD (Chalkley score of 10).
Estrogen and Progesterone Receptor Status
Estrogen receptor (ER) and progesterone receptor (PR) status was determined using immunohistochemical methods. Slides were stained and evaluated using the Dako Autostainer and the Automated Cellular Imaging System. Activity ≥ 10% was considered positive.
Statistical Analysis
Statistical analyses were performed using MedCalc software (Microsoft, Inc.). The level of eIF4E overexpression, VEGF grade, and MVD counts were correlated using the Spearman rank correlation method. χ2 test was used to assess the association between T stage and N stage of tumor with the degree of eIF4E overexpression as grouped by tertile distribution. Survival analysis was performed using the Kaplan-Meier method and the log-rank test. Hazard ratio calculations for cancer recurrence and cancer-related death were performed using the Cox proportional hazard model.
RESULTS
A total of 114 patients were prospectively accrued for this study. The mean age at diagnosis was 52 years. Table 1 demonstrates the T and N stage distribution of the study patients. The T stage distribution was as follows: T1 lesions (n = 39 patients), T2 (n = 56 patients), T3 (n = 11 patients), and T4 (n = 8 patients). There were 53 node-positive patients (N1 = 30 patients, N2 = 16 patients, and N3 = 7 patients) and 61 node-negative patients. Eighty-four patients underwent modified radical mastectomy, while 30 patients underwent BCT. Compliance with treatment and surveillance protocol was 95% and 99%, respectively.
TABLE 1. T Stage, N Stage, and eIF4E

Figure 1 is a representative blot for eIF4E quantification. Lane 1 represents the standard kDa ladder. Lanes 2 thru 7 represent breast cancer specimens of varying degrees of eIF4E overexpression diluted at 1:10 concentration. Lanes 8 and 10 represent negative benign controls (benign noncancer breast tissue). Lane 9 represents the positive control, a neuroepithelioma cell line (SK-N-MC, American Type Culture Collection) known to overexpress eIF4E. The mean level of eIF4E overexpression was 12.5 ± 7.6-fold (mean ± standard deviation).
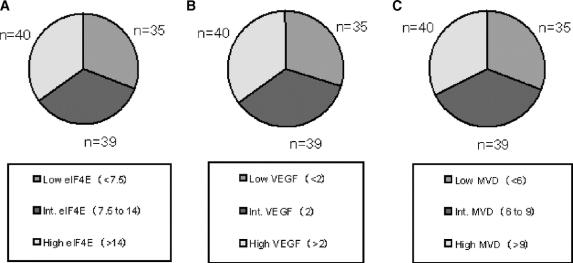
Using previously established eIF4E tertile distribution,20 patients were distributed into 3 groups based on eIF4E overexpression (Fig. 4A): 1) low eIF4E group (<7.5-fold elevation, n = 35 patients), 2) intermediate eIF4E group (7.5- to 14-fold elevation, n = 39 patients), and 3) high eIF4E group (>14-fold elevation, n = 40 patients). As shown in Table 1, there was no association between T or N stage and the degree of eIF4E overexpression (χ2 test).
FIGURE 4. Tertile distribution of patients based on eIF4E overexpression (A), VEGF expression (B), and MVD counts (C). In the pie chart, low expression is blue, intermediate expression is red, and high expression is yellow.
Representative panels for VEGF immunostaining are shown in Figure 2. The intensity for VEGF stain is graded as 0 (Fig. 2A, benign breast tissue), grade I for low intensity (Fig. 2B), grade II for intermediate intensity (Fig. 2C), and grade III for high intensity (Fig. 2D) in breast cancer slides. Patients were divided into tertiles: 1) low VEGF group (grade <II, n = 34 patients), 2) intermediate VEGF group (grade II, n = 40 patients), and 3) high VEGF group (grade >II, n = 40 patients) (Fig. 4B).
Assessing MVD count is shown in Figure 3. A representative slide demonstrating low MVD staining in a tumor specimen (Chalkley count of 3, Fig. 3A) is contrasted with a slide with high MVD staining (Chalkley count of 10, Fig. 3B). MVD counts ranged from 3 to 11, with a mean of 7.8 ± 2 (mean ± SD). Based on the MVD counts, patients were grouped into tertiles: 1) low MVD group (<6, n = 35 patients), 2) intermediate MVD group (6 to 9, n = 42 patients), and 3) high MVD group (>9, n = 37 patients) (Fig. 4C).
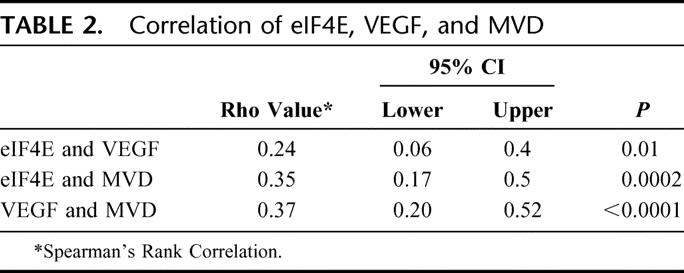
Table 2 displays the correlation of eIF4E overexpression with VEGF elevation and MVD count. In human breast cancer specimens, the degree of VEGF elevation was correlated with the degree of eIF4E overexpression (r = 0.24, P = 0.01 Spearman's correlation). Additionally, increasing tumor MVD counts were also highly correlated with the degree of eIF4E overexpression (r = 0.35, P = 0.0002). Finally, the elevation of tumor VEGF was highly correlated with tumor MVD count (r = 0.37, P = <0.0001).
TABLE 2. Correlation of eIF4E, VEGF, and MVD
Of the 114 patients in this study, there were 33 recurrences (Table 3). Six of these recurrences were locoregional, 25 were systemic, while 2 were both locoregional and systemic. Five recurrences were in the low eIF4E group, while 20 were in the high eIF4E group (15% versus 61%). Twenty-nine cancer-related deaths were observed; 4 were in the low eIF4E group, while 18 were in the high eIF4E group (14% versus 62%).
TABLE 3. Cancer Outcomes
Disease-free survival (DFS) and cancer survival (CS) analysis by the Kaplan-Meier method for eIF4E overexpression (Fig. 5), VEGF elevation (Fig. 6), and MVD count (Fig. 7) are shown. Patients whose tumors were in the highest tertile of eIF4E overexpression demonstrated a statistically significant higher rate of cancer recurrence (P = 0.004, log-rank test) and cancer-related death (P = 0.002, log-rank test) when compared with patients whose tumors were in the lowest eIF4E tertile (Fig. 5A, B). Comparing DFS and CS among the 3 groups of VEGF expression (Fig. 6A, B, respectively) and MVD count (Fig. 7A, B, respectively) demonstrated that statistical significance has not been reached (VEGF DFS, P = 0.07; VEGF CS, P = 0.24; MVD DFS, P = 0.11; and MVD CS, P = 0.15).
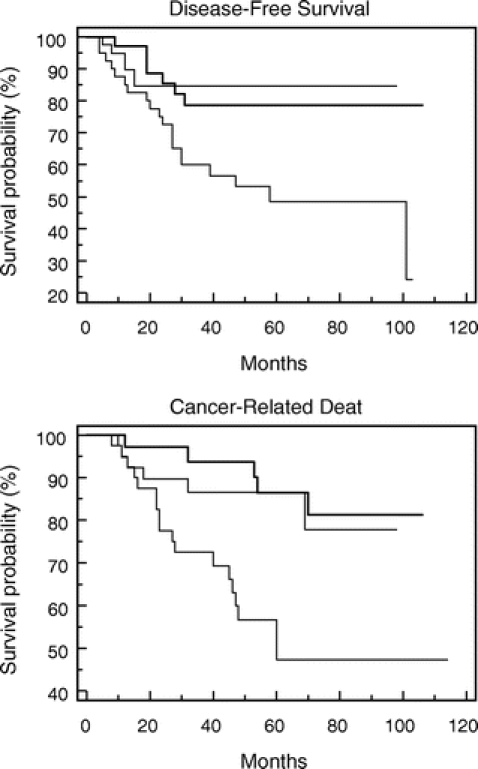
FIGURE 5. Kaplan-Meier graphs comparing disease-free survival (DFS) and cancer survival (CS) for patients based on the tertile distribution of tumor eIF4E overexpression.

FIGURE 6. Kaplan-Meier graphs comparing disease-free survival (DFS) and cancer survival (CS) for patients based on the tertile distribution of tumor VEGF expression.
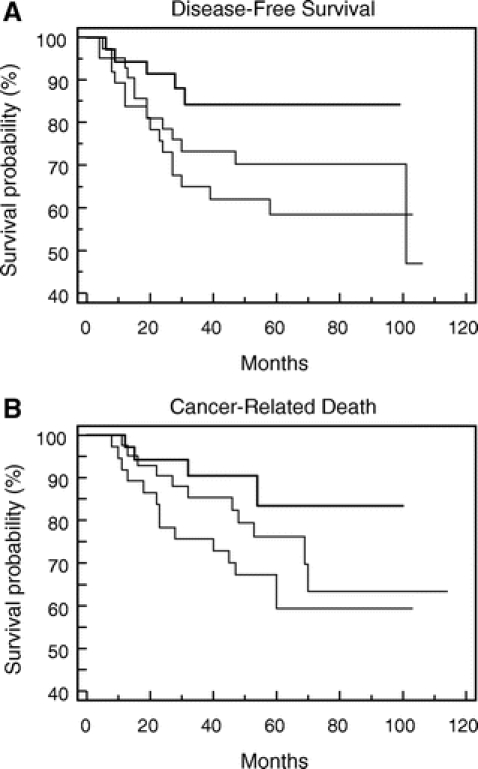
FIGURE 7. Kaplan-Meier graphs comparing disease-free survival (DFS) and caner survival (CS) for patients based on the tertile distribution of tumor MVD counts.
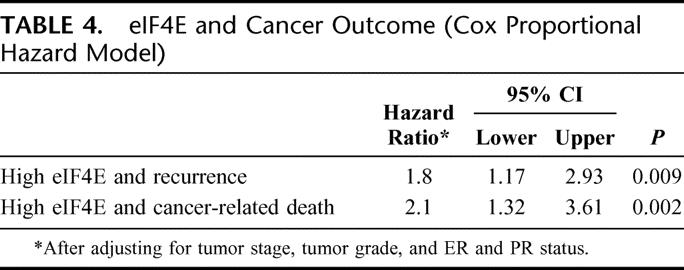
The risk for cancer recurrence and cancer-related death was determined using Cox proportional hazard model. After adjusting for tumor stage and grade, ER and PR status, only tumor stage and eIF4E overexpression were independent predictors for cancer recurrence and cancer-related death. When compared with patients whose tumors were in the lowest tertile of eIF4E overexpression, patients whose tumors had the highest tertile of eIF4E overexpression were more likely to recur (hazard ratio [HR] = 1.8; 95% confidence interval [CI], 1.2–2.9, P = 0.009) and die of their cancer (HR = 2.1; CI, 1.3–3.6, P = 0.002) (Table 4).
TABLE 4. eIF4E and Cancer Outcome (Cox Proportional Hazard Model)
DISCUSSION
As our understanding of tumor biology evolves, our ability to predict cancer outcome will likely improve. Presently, the American Joint Commission on Cancer staging by tumor size and nodal status continues be the accepted method to predict prognosis.29 The primary determinant of whether to offer adjuvant therapy continues to be the T and N stage of the primary tumor. Unfortunately, approximately 35% of node-positive patients will remain disease free, while 20% of node-negative patients will recur systemically.2,3 Thus, an understanding of tumor biology to more accurately stratify risk for recurrence is needed to target high-risk patients for treatment while sparing those who are unlikely to benefit from it.
In this study, 114 patients with stage I to III breast cancer were prospectively accrued to determine: 1) whether the overexpression of eIF4E in tumor specimens correlates with VEGF and MVD elevation, and 2) whether high eIF4E overexpression predicts cancer recurrence and death, independent of nodal status. Our line of inquiry originates from the observation that in vitro, cell lines induced to overexpress eIF4E, up-regulates VEGF.8 Thus, the first objective in this study was to determine whether VEGF elevation and MVD count correlated with the degree of eIF4E overexpression in human breast cancer specimens. Using Western blots to quantify eIF4E elevation and IHC to grade tumor VEGF expression and MVD count, we observed that both VEGF elevation and MVD count were correlated with the degree of eIF4E overexpression (VEGF and eIF4E, r = 0.24, P = 0.01; MVD and eIF4E, r = 0.35, P = 0.0002, Spearman's rank correlation).
Elevated levels of VEGF have been observed in a number of tumors, and the degree of elevation has been found to have prognostic significance.25,26 Linderholm et al demonstrated the significance of VEGF, specifically in the setting of p53 mutation, in predicting recurrence and death in 833 breast cancer patients.25 Gasparini reported the significance of elevated VEGF expression and a worse breast cancer outcome.26
In this study, the degree of eIF4E overexpression was not associated with tumor T or N stage. Survival analysis demonstrated a statistically significant decrease in disease-free survival (P = 0.004, log-rank test) and cancer survival (P = 0.002 log-rank test) for patients whose tumors were in the highest tertile of eIF4E overexpression compared with those whose tumors were in the low eIF4E tertile. Additionally, patients whose tumors were in the highest tertile of eIF4E overexpression were more likely to recur (HR = 1.8; CI, 1.2–2.9, P = 0.009, Cox proportional hazard model) and die of their cancer (HR = 2.1; CI, 1.3–3.6, P = 0.002) than patients whose tumors were in the lowest tertile of eIF4E overexpression. This was the case even after adjusting for tumor grade and stage, ER, and PR status. Therefore, it appears that high eIF4E overexpression in breast cancer is a predictor of cancer recurrence independent of nodal status. Thus, our current study confirms the findings of our 2 previous studies: 1) the prospective trial of 191 patients with stage I to III breast cancer patients, and 2) a prospective trial of 174 node-positive only breast cancer patients.
Presently, we find that the degree of eIF4E overexpression is correlated with elevation of VEGF expression and MVD count in cancer specimens. Additionally, patients with tumors in the highest eIF4E tertile had higher risk for recurrence and cancer-related death, independent of nodal status. Since VEGF and MVD count are markers for angiogenesis, and high eIF4E predicts cancer outcome independent of nodal status, eIF4E overexpression in breast cancer appears to predict increased tumor vascularity, and perhaps may shed light on the process of cancer dissemination by hematogenous means.
At present, our study fails to demonstrate increased risk for cancer recurrence and/or cancer-related deaths from VEGF elevation and increased MVD count. Possible explanations for this observation include the low number of cancer events overall, as well as the relatively short median follow-up for a breast cancer study. A longer follow-up may allow us to address the present limitations.
Others have reported the association of angiogenesis and a worse cancer outcome.25,26 In a study by Braun et al, patients with bone marrow aspirate-positive, but node-negative breast cancer have similar clinical outcome as node-positive patients.30 The growth and dissemination of cancer are thought to be closely linked to its angiogenic potential.21,23,24 Supporting this is the observation that experimental tumors do not achieve a size greater than a couple of millimeters without neovascularization.21 This neovascularization is thought to be a potential means for hematogenous dissemination of tumor cells.31 Our findings that high eIF4E overexpression was correlated with increased tumor VEGF and MVD count, but not nodal disease, perhaps lends support to this conjecture. Our next series of studies will examine whether high eIF4E overexpresion is correlated with bone marrow-positive disease and hematogenous metastasis.
CONCLUSION
Increasing eIF4E overexpression in cancer specimens from stage I to III breast cancer is highly correlated with increasing VEGF levels and MVD counts, both markers of tumor angiogenesis. Using multivariate analysis, patients with tumors in the highest tertile of eIF4E overexpression had a hazard ratio of 1.8× (confidence interval, 1.2–2.9, P = 0.009) that of the lowest eIF4E tertile group for cancer recurrence and hazard ratio of 2.1× (confidence interval, 1.3–3.6, P = 0.002) for cancer-related death. Thus, eIF4E overexpression in breast cancer is correlated with increased tumor vascularity and predicts a non-nodal mode of metastasis, perhaps by hematogenous means.
Discussions
Dr. B. Mark Evers (Galveston, Texas): This work represents a nice extension of previous work by Dr. Li and his investigative group showing the potential clinical utility of Initiation Factor 4E as a prognostic factor for breast cancer recurrence and an indicator of an overall worse prognosis. Furthermore, the correlation with 4E expression and increasing tumor vascularity is intriguing. I have four questions for Dr. Li.
First, why do you use Western blot to evaluate expression as opposed to staining techniques that you have utilized for VEGF and microvessel density? This could give you additional information regarding patterns and location of the expression that Western blot does not provide or at least provide additional qualitative information.
Along those lines, do you know where the increased expression of 4E is located? That is, do you see expression limited to the cancer cells or do you also see expression in the surrounding stromal tissue you?
The third question relates to findings in patients on the other end of the spectrum. For example, do you see overexpression in patients with ductal carcinoma in situ? And how about the patients with stage IV disease?
Finally, how are you incorporating this information into your current practice for patients with breast cancer at your institution? Is this a technique that you are now doing on all patients with breast cancer and, if so, do you use this information when you counsel your patients regarding prognosis and further treatments?
Dr. Benjamin D.L. Li (Shreveport, Louisiana): Why do we not use immunohistochemical staining? We have tried using IHC to quantify 4E, but it has never been very quantitative. Using Western blots, we can quantify 4E overexpression relative to benign breast tissue. We have tried the automated cellular imaging system (or ACIS). Unfortunately, that is also not sufficiently quantitative.
Where does the staining of eIF4E overexpression localize in cancer? There is no overexpression of 4E in the stromal element. It is mostly in the cytoplasm of cancer cell, as you might expect, of a factor that works on mRNA translation. If you look at a slide of normal breast tissue, there is very seldom overexpression of 4E by immunohistochemical staining. If you look at the margin of a tumor, or in the transitional zone between cancer and normal epithelium, you will see varying degree of 4E overexpression in the ductal cells. In fact, if one looks at ductal carcinoma in situ (DCIS) 4E overexpression is intermediate in intensity compared to invasive carcinoma. Additionally, comedonecrosis in the center of DCIS, though the cells may look necrotic, in fact, those very cells will have high 4E overexpression. If one stains those cells for VEGF, interestingly, VEGF overexpression is also noted. Additionally, eIF4E overexpression is seen in stage IV breast cancer, ie, in the metastatic tumors.
Finally, on the issue of current practice, and how do we apply the result from this study? Well, the studies reported to date are all relatively small single institutional studies even though they are prospective trials. As such, multicenter validation trials are needed. We look forward to having this study be introduced into one of the oncology trial groups to validate our observations.
Dr. Frederick L. Greene (Charlotte, North Carolina): In his presentation today, Dr. Byrnes has prospectively assessed eIF4E in a group of patients with stage I to III breast cancer and has demonstrated that this overexpression is correlated with both VEGF and microvessel density measured in breast cancer. Importantly, this group has shown that eIF4E is a marker for tumor vascularity and may be useful as a factor in the staging and prognostication of breast cancer.
In 1999, as we began our work on the sixth edition of the AJCC Cancer Staging Manual, our group dedicated to breast cancer staging reviewed the traditional TNM categories as well as data on over 80 prognostic factors relating to breast cancer that had been reported to that time in the peer-reviewed literature. It was apparent that none of these reported factors had achieved a significant independent status which would recommend addition to the tumor-node-metastasis strategy. As we begin work on the seventh edition due for publication in 2009, perhaps several of these prognostic factors are gaining increasing importance. The ability of gene markers and protein products to stratify patients into subsets that predict recurrence and survival is compelling. Dr. Li and his colleagues have today given us more compelling data.
While not mentioning HER-2/neu oncogene overexpression in the presentation today, you have commented on the nonconcordance of immunohistochemical staining of HER-2/neu and the levels of eIF4E in previous work. Since most analyses of HER-2/neu are now performed using fluorescent hybridization, have you re-looked at the association of this oncogene using that technique and the eIF4E subsets?
Since hypoxia is a potent angiogenic factor, is eIF4E induced by hypoxia and is this factor merely a marker for hypoxic cells?
Your data show that about 15% of the lowest eIF4E group both recurred and died of their disease. Were there other specific adverse prognostic factors in these patients?
Finally, is eIF4E really a predictive factor and not a prognostic factor? If this protein is overexpressed, perhaps these patients will respond to newer anti-angiogenic drugs and we can use this marker to predict a good treatment response by targeting therapy.
Dr. Benjamin D.L. Li (Shreveport, Louisiana): We have studied HER-2/neu overexpression by immunohistochemical staining as well as by Western blots. There is no correlation between HER-2 overexpression, which is seen in about 20% to 30% of breast cancer, and eIF4E overexpression, which is seen in just about 100% of breast cancer.
You are absolutely right; hypoxia seems to induce eIF4E, as seen in comedonecrosis in DCIS. Now, whether it is truly a marker for hypoxia, I don't know. That is an intriguing question.
I do not know at this time if eIF4E is purely a prognostic marker or a therapeutic marker. We are currently engaged in a study that examines eIF4E overexpression and TLK1B elevation. TLK1B is downstream of eIF4E and, in vitro, TLK1B seems to confer radiation resistance in cancer cell lines. A paper that we have submitted and has been accepted by the Society of University Surgeons will report on whether patients with high eIF4E overexpression also have high elevation of TLK1B. It then examines whether this predicts cancer recurrence, ie, resistance to breast irradiation. Therefore, whether high 4E is strictly a prognostic marker or a therapeutic marker remains to be resolved with subsequent studies.
Dr. Kirby L. Bland (Birmingham, Alabama): This marker you call IGF4E, we changed the name, “we” meaning several molecular scientists, now call this GKLF4. And this is one of the projects in the UAB Breast Spore grant, as you probably know.
Combining our database at UAB with Yale and Baylor, we have looked at over 200 patients upregulating this messenger protein and have shown virtually almost an identical score that you showed with cytoplastic localization. You did not correlate these data with the TNM system but did complete correlative analysis with MVD, as you showed, and VEGF expression. My group has published extensively on this finding.
So I want to congratulate you. And you refer to yourself as a small institution, but we would like to add you to our database and maybe we could look prospectively at this important marker. The NCI last year considered GKLF-4 as one of the better oncologic biomarkers of the year. So it may be more important than we previously considered as there are no valid biomarkers in breast cancer. We talk about ER/PR receptors, HER-2neu expression, proliferation indices, etc., but there are really no valid markers. I congratulate you and your group and encourage you to continue with this important work.
Dr. Courtney M. Townsend, Jr. (Galveston, Texas): There are too many initials for me to remember what to call this stuff, but what I really want to know is what is the mechanism of action by which this agent regulates VEGF expression? As Dr. Greene mentioned, novel therapeutic targets are I think where we are all wanting to go with translational research. And this is upstream of many factors. Would it be the logical target itself? Or is it downstream products?
Dr. Benjamin D.L. Li (Shreveport, Louisiana): The exact mechanism how 4E overexpression upregulates VEGF is not entirely worked out. What is known is that VEGF mRNA has a long and complex 5′UTR. And since eIF4E binds to the cap of the untranslated region and allows for the RNA helicase to unwind and reduces the steric hindrance, that's the reason VEGF is upregulated by 4E overexpression. We have not specifically looked at VEGF upregulation mechanistically in human breast cancer specimens.
Footnotes
Supported in part by a Department of Defense Grant (DAMD17-99-1-9256) and the Powers Foundation.
Reprints: Benjamin D. Li, MD, FACS, Department of Surgical Oncology, Louisiana State University Health Sciences Center, and the Feist-Weiller Cancer Center, 1501 Kings Highway, Shreveport, LA 71130. E-mail: bli@lsuhsc.edu.
REFERENCES
- 1.Jemal A, Murray T, Ward E, et al. Cancer Statistics 2005. CA Cancer J Clin. 2005;55:10–30. [DOI] [PubMed] [Google Scholar]
- 2.Weidner N, Cady B, Goodson WH III. Pathologic prognostic factors for patients with breast carcinoma: which factors are important. Surg Oncol Clin North Am. 1997;6:415–453. [PubMed] [Google Scholar]
- 3.Jatoi I, Hilsenbeck SG, Clark GM, et al. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–2340. [DOI] [PubMed] [Google Scholar]
- 4.DeBenedetti A, Harris AL. EIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. [DOI] [PubMed] [Google Scholar]
- 5.Rhoads RE, Joshi-Barve S, Rinker-Schaeffer C. Mechanism of action and regulation of protein synthesis initiation factor 4E: effects of mRNA discrimination, cellular growth rate, and oncogenesis. Prog Nucleic Acid Res Mol Biol. 1993;46:183–219. [DOI] [PubMed] [Google Scholar]
- 6.Rosenwald IB, Lazaris-Karatzas A, Sonenberg N, et al. Elevated levels of Cyclin D1 in response to increased expression of Eukaryotic Initiation Factor 4E. Mol Cell Biol. 1993;13:7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, DeFatta R, Anthony C, et al. A translationally regulated Tousled Kinase phosphorylates histone H3 and confers radioresistance when overexpressed. Oncogene. 2001;20:726. [DOI] [PubMed] [Google Scholar]
- 8.Kevil C, DeBenedetti A, Payne DK, et al. Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. Int J Cancer. 1996;65:786. [DOI] [PubMed] [Google Scholar]
- 9.Kevil C, Carter P, Hu B, et al. Translational enhancement of FGF-2 by eIF-4 factors, and alternate utilization of CUG and AUG codons for translation initiation. Oncogene. 1995;11:2339–2348. [PubMed] [Google Scholar]
- 10.DeBenedetti A, Joshi-Barve S, Rinker-Schaeffer C, et al. Expression of antisense RNA against initiation factor eIF4E mRNA in HeLa cells results in lengthened cell division times, diminished translation rates, and reduced levels of both eIF4E and p220 component of eIF4F. Mol Cell Biol. 1991;11:5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBenedetti A, Rhoads RE. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa Cells results in aberrant growth and morphology. Proc Natl Acad Sci USA. 1990;87:8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBennedetti A, Joshi B, Graff JR, et al. CHO cells transformed by the translation factor eIF-4E display increased c-myc expression, but requires overexpression of max for tumorigenicity. Mol Cell Diff. 1994;2:347. [Google Scholar]
- 13.Zhu N, Gu L, Findley H, et al. Transcriptional repression of the Eukaryotic Initiation Factor 4E gene by wild type p53. Biochem Biophys Res Commun. 2005;335:1272–1279. [DOI] [PubMed] [Google Scholar]
- 14.Kerekatte V, Smiley K, Hu B, et al. The proto-oncogene/translation factor eIF4E: a survey of its expression in breast carcinomas. Int J Cancer. 1995;64:27. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez SH, Byrnes K, Chu Q, et al. Eukaryotic Factor 4E (eIF4E) expression in malignant versus inflammatory colon tissue. Ann Surg Oncol. 2005;12(suppl):90–91. [Google Scholar]
- 16.Nathan CO, Liu L, Li BDL, et al. Detection of the proto-oncogene eIF4E in surgical margins may predict recurrence in head and neck cancer. Oncogene. 1997;15:579. [DOI] [PubMed] [Google Scholar]
- 17.Chu QD, Turnage R, McClusky D, et al. Overexpression of Eukaryotic Initiation Factor 4E (eIF4E) in soft tissue neoplasms. Proceedings of the AACR. 2004;45:4285. [Google Scholar]
- 18.Li BDL, McDonald JC, Nassar R, et al. Clinical outcome in stage I to III breast carcinoma and eIF4E overexpression. Ann Surg. 1998;227:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li BDL, Gruner JS, Abreo F, et al. Prospective study of Eukaryotic Initiation Factor 4E protein elevation and breast cancer outcome. Ann Surg. 2002;235:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClusky D, Chu Q, Yu H, et al. A prospective trial on Initiation Factor 4E (eIF4E) overexpression and cancer recurrence in node-positive breast cancer. Ann Surg. 2005;242:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1989;82:4–6. [DOI] [PubMed] [Google Scholar]
- 22.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. [DOI] [PubMed] [Google Scholar]
- 23.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- 24.Gimbrone M, Leapman S, Cotran R, et al. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linderholm B, Lindh B, Tavelin B, et al. p53 and Vascular-Endothelial-Growth-Factor (VEGF) expression predicts outcome in 833 patients with primary breast carcinoma. Int J Cancer. 2000;89:51–62. [PubMed] [Google Scholar]
- 26.Gasparini G. Prognostic value of Vascular Endothelial Growth Factor in breast cancer. Oncologist. 2000;10:37–44. [DOI] [PubMed] [Google Scholar]
- 27.Jennbacken K, Valbo C, Wang W, et al. Expression of Vascular Endothelial Growth Factor C (VEGF-C) and VEGF Receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005;65:110–116. [DOI] [PubMed] [Google Scholar]
- 28.Benoy I, Salgado R, Elst H, et al. Relative microvessel area of the primary tumour, and not lymph node status, predicts the presence of bone marrow micrometastases detected by reverse transcriptase polymerase chain reaction in patients with clinically non-metastatic breast cancer. Breast Cancer Res. 2005;7:R210–R219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AJCC Staging Manual, 6th ed. Springer-Verlag, 2002.
- 30.Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, of III breast cancer. N Engl J Med. 2000;342:525–533. [DOI] [PubMed] [Google Scholar]
- 31.Weidner N, Semple J, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. [DOI] [PubMed] [Google Scholar]