Abstract
Objective:
To better understand the factors associated with the well-established gender difference in survival for patients with melanoma.
Summary Background Data:
Gender is an important factor in patients with cutaneous melanoma. Male patients have a worse outcome when compared with females. The reasons for this difference are poorly understood.
Methods:
This prospective multi-institutional study included patients aged 18 to 70 years with melanomas ≥1.0 mm Breslow thickness. Wide excision and sentinel lymph node (SLN) biopsy was performed in all patients. Clinicopathologic factors, including gender, were assessed and correlated with disease-free survival (DFS), distant disease-free survival (DDFS), and overall survival (OS).
Results:
A total of 3324 patients were included in the covariate analyses; 1829 patients had follow-up data available and were included in the survival analyses. Median follow-up was 30 months. On univariate analysis, men (n = 1906) were more likely than women to be older than 60 years (P < 0.0001), have thicker melanomas (P < 0.0001), have primary tumor regression (P = 0.0054), ulceration (P < 0.0001), and axial primary tumor location (P < 0.0001). On multivariate analysis, age (P = 0.0002), thickness (P < 0.0001), ulceration (P = 0.015), and location (P < 0.0001) remained significant in the model. There was no difference in the rate of SLN metastasis between men and women (P = 0.37) on multivariate analysis. When factors affecting survival were considered, the prognosis was worse for men as validated by lower DFS (P = 0.0005), DDFS (P < 0.0001), and OS (P < 0.0001).
Conclusions:
Male gender is associated with a greater incidence of unfavorable primary tumor characteristics without an increased risk for nodal metastasis. Nonetheless, gender is an independent factor affecting survival.
Analysis of clinicopathologic factors of patients in the Sunbelt Melanoma Trial who underwent sentinel lymph node biopsy for melanoma demonstrated that men had a greater incidence of unfavorable primary tumor characteristics independent of nodal status. Gender was found to be an independent predictor of survival.
The most important predictor of outcome for patients with early melanoma is the status of the regional lymph nodes.1 Sentinel lymph node (SLN) biopsy, a widely accepted method for nodal staging, has supplanted elective lymph node dissection in the evaluation and management of early stage melanoma. SLN biopsy also has provided valuable prognostic information with minimal morbidity.2–6 Despite the attention that SLN biopsy has drawn to nodal status as an important prognostic factor in melanoma, other patient and tumor factors significantly affect prognosis.
Multiple studies have evaluated the impact of the following factors on prognosis: tumor thickness, ulceration, regression, mitotic rate, Clark's level of invasion, histopathologic subtype of melanoma, age, primary tumor site, and gender.2,7–14 Gender is well known to be an important prognostic factor for melanoma; it is well established that men have a worse outcome than women.1,5 Given this fact, one might suspect that men have a greater incidence of nodal metastasis (since nodal metastasis is the most powerful predictor of survival). However, several studies have shown that gender does not predict SLN status.4,12,14,15 The relationship of gender to other prognostic factors deserves further study. The aim of the current study was to determine the impact of primary tumor characteristics and SLN status on gender-specific survival for patients with cutaneous melanoma.
METHODS
The Sunbelt Melanoma Trial is a prospective, randomized trial involving 79 centers in North America. This study was approved by the Institution Review Boards of each participating institution. Patients aged 18 to 70 years with cutaneous melanoma ≥1 mm Breslow thickness and without clinical evidence of regional or distant metastasis were eligible.
Following informed consent, patients underwent wide local excision of the primary melanoma and SLN biopsy with peritumoral intradermal injection of 99mTc-labeled sulfur colloid; intradermal isosulfan blue dye was also used in most cases. Lymphoscintigraphy was performed to identify all draining nodal basins, including ectopic basins or in-transit SLN. Using a combination of a handheld gamma probe and visualization of blue lymphatic drainage, the SLN(s) were identified and excised. The protocol specified that all blue nodes and all nodes that were ≥10% of the most radioactive node were to be excised and designated as SLN.16
All SLNs were processed with serial sectioning (at least 5 sections per block) with hematoxylin and eosin staining as well as immunohistochemistry for S-100 protein. A histologically positive SLN was defined as evidence of metastatic tumor cells identified by either hematoxylin and eosin staining or immunohistochemistry. Patients with evidence of metastases to SLN underwent completion lymph node dissection (CLND). A central pathology review committee evaluated the first 10 cases from each participating institution, as well as all cases of SLN containing metastases. There was an independent data safety and monitoring committee for this study.
Univariate analyses to determine factors correlating with gender were performed using χ2 tests. A multivariate analysis to determine which clinicopathologic factors were independently associated with gender was performed using a binary logistic regression model. Log rank tests were used to assess the impact of gender and other clinicopathologic variables on disease-free survival (DFS), distant disease-free survival (DDFS), and overall survival (OS). Survival curves were generated using the method of Kaplan and Meier. A Cox proportional hazard model was then performed to determine which clinicopathologic variables, including gender, were independent predictors of DFS, DDFS, and OS. All analyses were performed with JMP software (SAS Institute, Cary, NC). Survival curves were generated using GraphPad Prism Software (GraphPad Software, San Diego, CA).
RESULTS
Study Population
The Sunbelt Melanoma Trial was open for accrual between June 1997 through October 2003. A total of 3324 patients (1906 males, 1418 females) were included in the analyses of which clinicopathologic factors were associated with gender. Survival analyses were performed on a subset of 1829 patients on whom follow-up data were available; these are the patients who underwent randomization or assignment to treatment arms in the study. The median follow-up in this subset was 30 months.
Primary Tumor Characteristics and Gender
The clinicopathologic characteristics of the study population comparing men and women are shown in Table 1. On univariate analysis, men were more likely to have thicker tumors (P < 0.0001), evidence of tumor ulceration (P < 0.0001), and regression (P = 0.005). Men were more likely to be older than 60 years of age (P < 0.0001), have axial melanomas (P < 0.0001), and have a positive SLN (P = 0.02) than women (Table 1). There were no differences in the Clark level of invasion (P = 0.55), the histologic subtype of melanoma (P = 0.51), lymphovascular invasion (P = 0.18), or vertical growth phase (P = 0.38) on univariate analysis.
TABLE 1. Univariate Analysis of Clinicopathologic Factors Associated With Gender Status
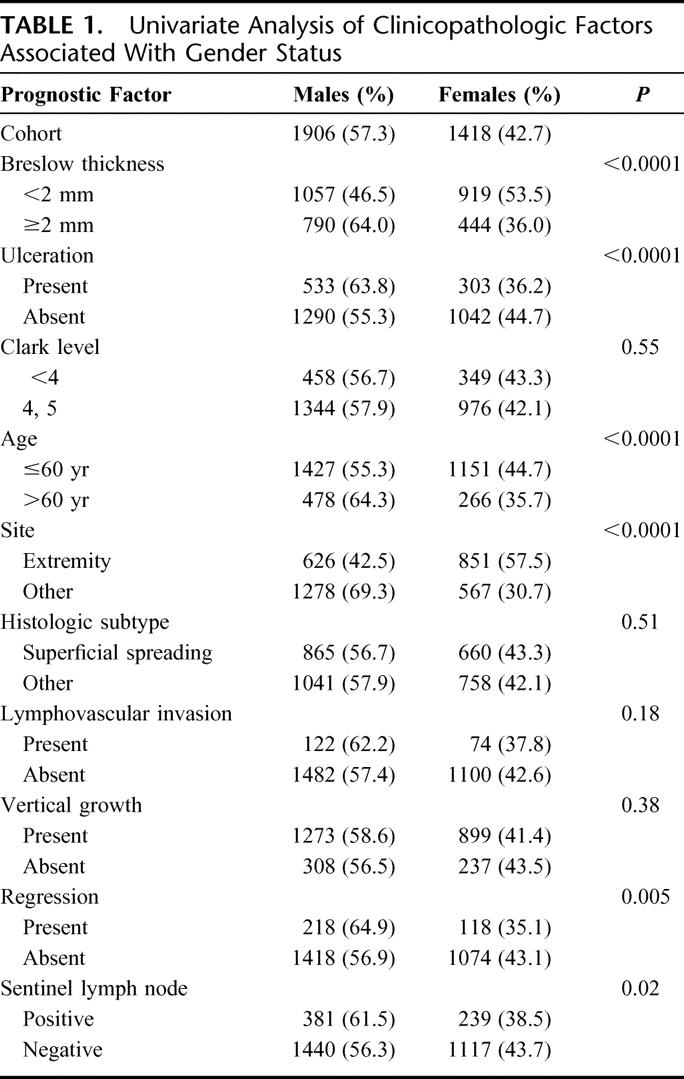
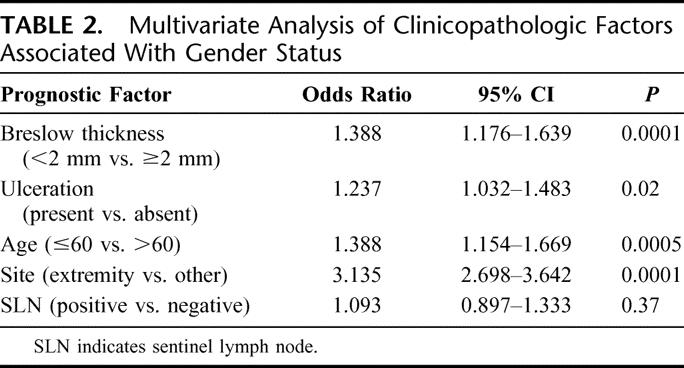
On multivariate analysis, only tumor thickness (P < 0.0001), ulceration (P = 0.02), age (P = 0.0005), and primary tumor site (P < 0.0001) remained significantly different between men and women (Table 2). Notably, there was no significant difference in the incidence of SLN metastasis between men and women on multivariate analysis (P = 0.37).
TABLE 2. Multivariate Analysis of Clinicopathologic Factors Associated With Gender Status
Survival Analysis
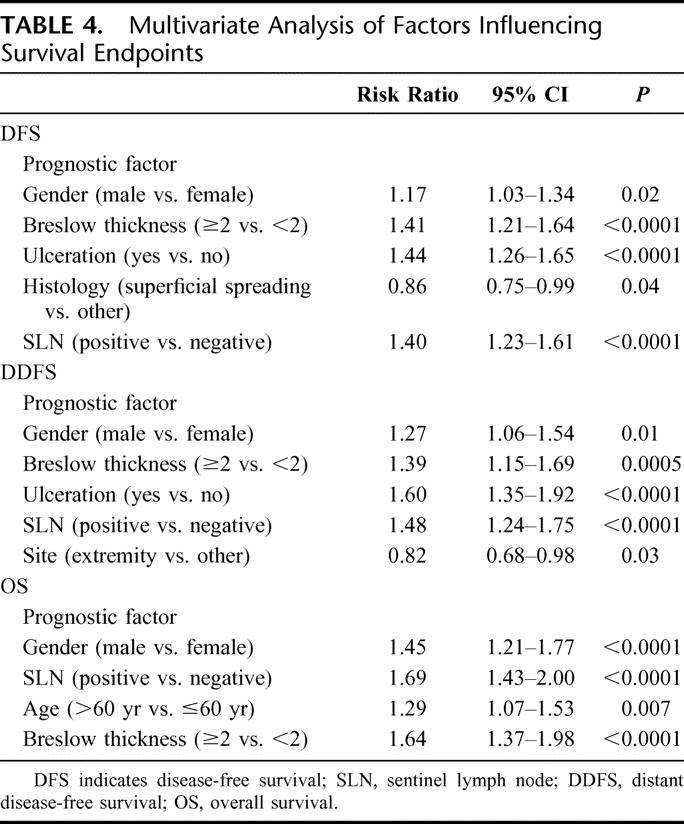
Overall, men had a worse DFS (P = 0.0005), DDFS (P < 0.0001), and OS (P < 0.0001) compared with women (Fig. 1). Gender-based differences were also evaluated among node-negative and node-positive patients. When considering patients with a negative SLN, men had a significantly worse DFS (P = 0.003), DDFS (P = 0.005), and OS (P < 0.0001) compared with women (Fig. 2). When considering patients with a positive SLN, men also had a worse DDFS (P = 0.004) and OS (P = 0.01); DFS, however, did not reach statistical significance (P = 0.088; Fig. 3). Table 3 considers the effect of clinicopathologic variables on DFS, DDFS, and OS; gender, tumor thickness, ulceration, and nodal status were the most significant factors by univariate analysis. In Table 4, Cox proportional hazards modeling (when initially considering all variables from Table 3) demonstrates that gender is a predictor of DFS (P = 0.02), DDFS (P = 0.01), and OS (P < 0.0001), independent of other clinicopathologic variables.
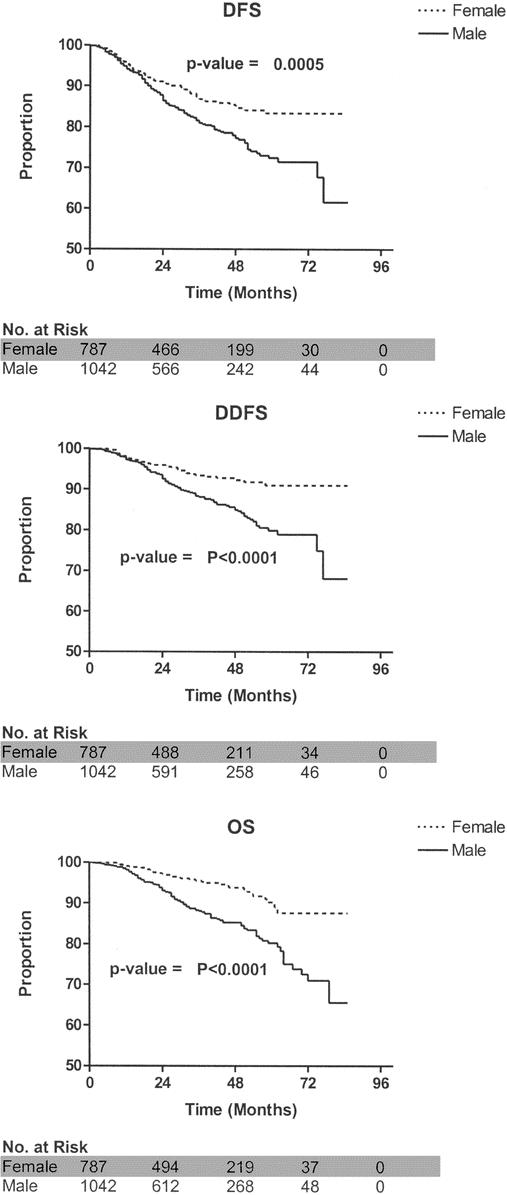
FIGURE 1. Survival comparisons between male and female melanoma patients. DFS, disease-free survival; DDFS, distant disease-free survival; OS, overall survival.

FIGURE 2. Survival comparison between male and female melanoma patients with a negative sentinel lymph node. DFS, disease-free survival; DDFS, distant disease-free survival; OS, overall survival; SLN, sentinel lymph node.
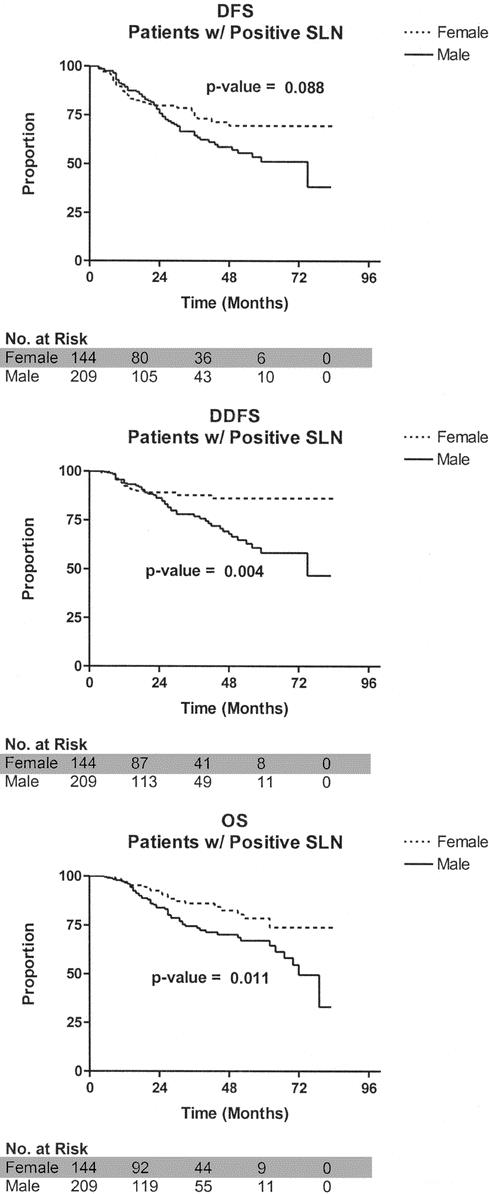
FIGURE 3. Survival comparison between male and female melanoma patients with a positive sentinel lymph node. DFS, disease-free survival; DDFS, distant disease-free survival; OS, overall survival; SLN, sentinel lymph node.
TABLE 3. Univariate Analysis of Clinicopathologic Factors' Influence on Survival Using Log-Rank Tests

TABLE 4. Multivariate Analysis of Factors Influencing Survival Endpoints
Following a positive SLN biopsy, patients underwent CLND. There were no differences in DFS (P = 0.97), DDFS (P = 0.07), or OS (P = 0.08) between males and females who had additional positive nonsentinel nodes upon CLND (data not shown). Furthermore, there were no gender-specific differences in nodal basin recurrences following a negative SLN biopsy (P = 0.27), following a CLND for a positive SLN (P = 0.2), or in a previously unmapped nodal basin (P = 0.36).
DISCUSSION
Gender is widely accepted as an important prognostic factor in predicting outcome for patients with cutaneous melanoma.17–20 The relationship between tumor thickness and gender has been explored in prior studies, yet the findings are somewhat variable. Balch et al1 did not find gender to be an independent prognostic factor in intermediate to thick melanomas. However, in the current study of intermediate and thick melanoma patients, men clearly had a worse prognosis. Others have also reported worse survival for males when stratified by tumor thickness.19 The data presented herein demonstrate that gender is an independent predictor of survival. In addition, male melanoma patients have worse primary tumor characteristics than females.
In addition to thicker tumors, the current study demonstrates that men have a higher incidence of ulceration and axial melanomas. Both of these factors have been shown to independently predict poor outcome for melanoma patients, with tumor thickness and ulceration being the most powerful primary tumor-related factors.1 These findings are similar to those previously reported,21,22 adding further to the notion that men as a group have worse primary tumors than women. It remains unclear whether a delay in presentation to the clinician has any bearing on this difference or whether there is a yet-to-be-determined biologic explanation. It appears that male melanoma patients are older than their female counterparts. Advanced age is well known to adversely affect outcome in this disease.1,23 This effect appears to be independent of nodal status, as older patients have a worse outcome, despite a decreased incidence of nodal metastasis.12,24
Despite the fact that nodal status is the most important predictor of survival, we did not find a significant relationship between gender and SLN metastasis on multivariate analysis. These data confirm the results of previous studies.2,4,13,15,25 Men with positive SLN, however, do have a worse outcome than SLN-positive women. This has been described by others as well.1 In the current study, additional nodal metastases following a positive SLN biopsy did not appear to affect gender-specific survival. The literature contains reports of positive26 and negative27 results on this issue. Additionally, our results confirm that there is no gender-related difference in nodal basin failure rates following a SLN biopsy.28
Investigators have sought a more thorough understanding of the gender-related differences in melanoma patients, with the hopes that this might lead to novel therapeutic approaches. Recognizing the survival difference between genders, investigators have explored the role of hormonal therapy for patients with advanced melanoma. Multiple regimens containing tamoxifen have been studied. Several phase II studies reported increased response rates when tamoxifen was combined with cytotoxic chemotherapy.29–33 These early results, however, have not been replicated by randomized phase III trials34–36 or by a recent meta-analysis.37 To date, no definitive evidence exists to support the use of hormonal therapy for treatment of melanoma. Sex steroids have been suggested as playing a role in melanoma progression in cell culture models;38 however, no definitive in vivo evidence exists to support this notion. The molecular basis for the gender difference in prognosis of melanoma patients remains undefined, and deserves further research.
CONCLUSION
The present study demonstrates that, although gender is associated with melanoma thickness, ulceration, tumor location, and age, gender itself is an independent factor associated with lower survival. Gender-specific differences in survival appear to be independent of nodal status. Further research into the survival advantage experienced by female melanoma patients may lead to the generation of novel therapies for this disease.
ACKNOWLEDGMENTS
The authors thank Deborah Hulsewede, Sherri Matthews, Pam Harlan, Ivan Deyahs, and the coordinators of the Sunbelt Melanoma Trial for their dedication and hard work in managing the study as well as the Sunbelt Melanoma Trial Study Group for participation in the study, and Advertek, Inc. for statistical analysis.
Discussions
Dr. Douglas R. Murray (Atlanta, Georgia): At the Society of the Surgical Oncology meeting in 2003, Dr. Celia Chao and her colleagues at the University of Louisville, Kentucky, upon review of 3076 patients, reported that older patients with melanoma are more likely than their younger counterparts to have thicker melanomas, deeper ulcerations, and experienced increased incidents of regression. However, they were significantly less likely to experience metastases to the sentinel lymph nodes, 14% versus 22%. It was not known then whether the lower frequency of sentinel metastases in older patients represented a decreased sensitivity of the sentinel lymph node procedure in this group of patients or a different biologic behavior of melanoma in older individuals.
Dr. Scoggins and his colleagues now present a growing concept that gender plays an independent role in the outcome of disease. Interest in gender has certainly picked up in the literature, and my colleague, Dr. David Lawson, recently reminded me that the MedLine database in 1950 to 1965 recorded zero references regarding gender in prognosis, gender in melanoma, and gender in prognosis and cancer. There are now 5000 references addressed to this issue.
In the current Sunbelt study, 3324 patients are reviewed. And as we saw in the multivariate analysis, thickness, ulceration, age, and primary site remained, showing a difference between males and females, and the sentinel lymph node status drops out. This seems to be in agreement with some series but not all. Review of our Emory database by my colleagues Dr. Grant Carlson and Dr. Keith Delman confirms that in older patients often males have thicker tumors, more ulceration, more truncal lesions, leading to poorer outcomes seemingly driven by the advanced primary tumor stage. However, we differ in our series of increased percentage of positive sentinel lymph nodes in males. Rate of sentinel lymph node involvement in older patients does diminish, however.
As noted by the authors, it is of interest to return to Dr. Charles Balch's paper in the Journal of Clinical Oncology 2001, in which gender was found to be the sixth most important predictor of survival after thickness, ulceration, age, site, and level. The Sunbelt trial contributed heavily to that series. No significant survival differences between genders were noted, as is my understanding at that time.
I have a series of questions, Dr. Scoggins. Due to the lateness of the hour I realize you won't be able to address them all and I leave it to your discretion.
What are the possible reasons for the differences with the clinical oncology series? Is it because all the patients in the current study are staged by sentinel lymph biopsy whereas some of the patients in the 2001 paper are staged by elective lymph node dissection? The other reason, of course, might be that the JCO paper has a much larger database.
In looking for an explanation of the findings in this fine study, the authors did examine a number of patients with additional positive nodes on completion lymph node dissection and found no gender differences. However, was there a difference in total number of positive nodes between men and women? The survival differences in the figures are quite striking. Why then do the males do poorly?
Your paper has offered several explanations, noting that age contributes to worse prognosis and males in the series tend to be older; and the authors have explored the role that hormones may play and cite the tamoxifen experience which ultimately has failed to improve outcome in randomized trials. What was the pattern of failure in the male group if it was not in the regional beds? Our older patients tend to recur at distant sites. Does this imply then an increased rate of hematogenous spread? Survival analyses were done on 1829 patients. Do we know what criteria the patients had to meet to go on to randomization?
The locoregional failure rate, false-negative regional bed failure, and evidently in-transit bound disease rate was not elevated over that of women despite the increased rate of these adverse primary tumor factors. Do we have an explanation for that? Is detection delayed by failure to identify a lesion on the back in the hirsute male or is it just an inattentive spouse or is it significant at all in that early symptoms may be disregarded?
It is of interest that the survival groups do not seem to separate for about 12 to 24 months. This is most evident in the positive sentinel lymph node cohort where for a short time disease-free survival and distant disease-free survival curves for women are actually a little inferior to those of men. Do we know why that should be? Are there host factors as well as tumor factors, for instance, compliance in the adjuvant care? Females seem to take on interferon more willingly.
Finally, recent molecular advances in melanoma research have highlighted linkages between oncogenic transformation and melanocytic transformation and melanocytic development. In the body of the manuscript, the authors do mention molecular basis for gender difference in prognosis may be most relevant, but factors remain to be determined. Would you wish to amplify on that possibility?
Dr. Charles R. Scoggins (Louisville, Kentucky): You asked were there any differences in number of positive sentinel lymph nodes recovered in men versus women? We found no differences in those numbers between the sexes.
Additionally, you touched on the patterns of failure and that it appears that men may have more hematogenous metastases than women. We don't exactly know why that is. We don't have a good marker for that other than following the patients until they do fail. You are right that there is a separation that occurs at about 12 months for patients with nodal metastases. That again may be a function of the distant failure rate in that in fact it takes time for the patient to manifest disease that can be detected by the clinician.
Finally, you touched on the possibility that there is a difference between the sexes in the willingness to take adjuvant therapy. I don't have those data for you. I did not analyze those data for that possibility.
And then, what are the molecular factors that may be driving this difference? I don't know that certainly does deserve further research. Sex steroids have been investigated, and currently there is no in vivo evidence that they play any role.
Dr. Craig L. Slingluff, Jr. (Charlottesville, Virginia): This paper addresses the long-observed but enigmatic survival advantage for women over men with melanoma. The database used for this study is unique, as it was developed prospectively and represents input from over 75 medical centers and over 3000 patients. This is not the largest melanoma database, but it may be the largest multicenter database collected as part of a large therapeutic clinical trial for melanoma. As a result, findings from this data set are probably of greater quality and greater applicability than most clinical database studies, and therefore deserve particular attention.
As a profession, we have a long way to go in creation of new knowledge by prospective randomized trials. Dr. McMasters and his colleagues should be congratulated for their tireless work on the Sunbelt melanoma trial because of its value both to the profession and to our patients, and I think it is important for us to follow their lead and to try to push this sort of investigation further in the future.
With regard to the findings in this paper, the improved clinical outcomes of women appear in all subsets of patients, which has already been mentioned by Dr. Murray, is consistent with what has been generally reported in other series.
It has been alluded to that the contribution of the paper is the finding that gender is not an independent predictor of sentinel node positivity but is an independent predictor of overall survival and distant disease-free survival. Thus, it really does appear to me from these data that gender is associated with a higher risk of systemic or hematogenous spread rather than lymphatic spread.
I have three specific questions related to this:
Just for the sake of completeness, it is worth discussing how sentinel node positivity was defined. This analysis was based on sentinel node positivity defined as standard histologic assessment and immunohistochemistry. Obviously, this trial is based in particular looking at PCR positivity, and I wonder whether other definitions of node positivity such as PCR positivity could explain some gender-related differences?
Secondly, if we accept the findings of this paper, should gender be added to the next revision of the AJCC staging system for melanoma given its high prognostic relevance?
Third, should clinical follow-up or treatment of patients with melanoma be more aggressive for men as opposed to women?
Dr. Charles R. Scoggins (Louisville, Kentucky): You asked us how we define sentinel node positivity. For the purposes of the Sunbelt melanoma trial, the protocol defines a positive sentinel lymph node as histologic evidence by H&E and/or immunochemical analysis either with S-100 protein, and some centers also analyzed HMB-45. For this analysis, we did not consider PCR-only positive nodes.
Secondly, whether or not gender should be included in the AJCC staging manual? Gender is not included as a criterion for any other cancer; I doubt it should be for melanoma. Not every prognostic factor found to be significant on multivariate analysis needs to be in the staging system.
Third, whether or not there should be an adjustment in the clinical follow-up and/or therapy between the sexes? I think that currently there are limited options for adjuvant therapy for patients with melanoma, and certainly none of the phase 3 trials demonstrated any effect for specific sex-related therapies such as tamoxifen. So I guess right now, I wouldn't adjust how I follow a man versus a woman. Perhaps if there is some progress made on a molecular level to identify potential therapy targets, that may change.
Footnotes
∥∥For a list of participating investigators, see McMasters KM, Noyes RD, Reintgen DS, et al. Lessons learned from the Sunbelt Melanoma Trial. J Surg Oncol. 2004; 86:212–223.
Supported by a grant from Schering Oncology-Biotech and the Center for Advanced Surgical Technologies of Norton Hospital, Louisville, KY.
Reprints: Kelly M. McMasters, MD, PhD, Department of Surgery, University of Louisville, Louisville, KY 40292. E-mail: mcmasters@louisville.edu.
REFERENCES
- 1.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. [DOI] [PubMed] [Google Scholar]
- 2.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–983. [DOI] [PubMed] [Google Scholar]
- 3.Gershenwald JE, Mansfield PF, Lee JE, et al. Role for lymphatic mapping and sentinel lymph node biopsy in patients with thick (> or = 4 mm) primary melanoma. Ann Surg Oncol. 2000;7:160–165. [DOI] [PubMed] [Google Scholar]
- 4.Clary BM, Brady MS, Lewis JJ, et al. Sentinel lymph node biopsy in the management of patients with primary cutaneous melanoma: review of a large single-institutional experience with an emphasis on recurrence. Ann Surg. 2001;233:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson GW, Murray DR, Hestley A, et al. Sentinel lymph node mapping for thick (> or = 4-mm) melanoma: should we be doing it? Ann Surg Oncol. 2003;10:408–415. [DOI] [PubMed] [Google Scholar]
- 6.Doubrovsky A, de Wilt JH, Scolyer RA, et al. Sentinel node biopsy provides more accurate staging than elective lymph node dissection in patients with cutaneous melanoma. Ann Surg Oncol. 2004;11:829–836. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Murad TM, Soong SJ, et al. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978;188:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma: II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979;86:343–351. [PubMed] [Google Scholar]
- 9.Balch CM, Murad TM, Soong SJ, et al. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979;43:883–888. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Wilkerson JA, Murad TM, et al. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45:3012–3017. [DOI] [PubMed] [Google Scholar]
- 11.Balch CM, Soong SJ, Milton GW, et al. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982;196:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao C, Martin RC, Ross MI, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004;11:259–264. [DOI] [PubMed] [Google Scholar]
- 13.McMasters KM, Wong SL, Edwards MJ, et al. Factors that predict the presence of sentinel lymph node metastasis in patients with melanoma. Surgery. 2001;130:151–156. [DOI] [PubMed] [Google Scholar]
- 14.McMasters KM, Noyes RD, Reintgen DS, et al. Lessons learned from the Sunbelt Melanoma Trial. J Surg Oncol. 2004;86:212–223. [DOI] [PubMed] [Google Scholar]
- 15.Essner R, Conforti A, Kelley MC, et al. Efficacy of lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. Ann Surg Oncol. 1999;6:442–449. [DOI] [PubMed] [Google Scholar]
- 16.McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol. 2001;8:192–197. [DOI] [PubMed] [Google Scholar]
- 17.Shaw HM, McGovern VJ, Milton GW, et al. Histologic features of tumors and the female superiority in survival from malignant melanoma. Cancer. 1980;45:1604–1608. [DOI] [PubMed] [Google Scholar]
- 18.Clark WH Jr, Elder DE, Guerry D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. [DOI] [PubMed] [Google Scholar]
- 19.Karakousis CP, Emrich LJ, Rao U. Tumor thickness and prognosis in clinical stage I malignant melanoma. Cancer. 1989;64:1432–1436. [DOI] [PubMed] [Google Scholar]
- 20.Garbe C, Buttner P, Bertz J, et al. Primary cutaneous melanoma: identification of prognostic groups and estimation of individual prognosis for 5093 patients. Cancer. 1995;75:2484–2491. [DOI] [PubMed] [Google Scholar]
- 21.Balch CM. Cutaneous melanoma: prognosis and treatment results worldwide. Semin Surg Oncol. 1992;8:400–414. [DOI] [PubMed] [Google Scholar]
- 22.Evans GR, Manson PN. Review and current perspectives of cutaneous malignant melanoma. J Am Coll Surg. 1994;178:523–540. [PubMed] [Google Scholar]
- 23.Austin PF, Cruse CW, Lyman G, et al. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994;1:487–494. [DOI] [PubMed] [Google Scholar]
- 24.Statius Muller MG, van Leeuwen PA, de Lange-De Klerk ES, et al. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with Stage I or II cutaneous melanoma. Cancer. 2001;91:2401–2408. [PubMed] [Google Scholar]
- 25.Chao C, Wong SL, Ross MI, et al. Patterns of early recurrence after sentinel lymph node biopsy for melanoma. Am J Surg. 2002;184:520–524. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Essner R, Torisu-Itakura H, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22:3677–3684. [DOI] [PubMed] [Google Scholar]
- 27.Sabel MS, Griffith K, Sondak VK, et al. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005;201:37–47. [DOI] [PubMed] [Google Scholar]
- 28.Gershenwald JE, Berman RS, Porter G, et al. Regional nodal basin control is not compromised by previous sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol. 2000;7:226–231. [DOI] [PubMed] [Google Scholar]
- 29.Rumke P, Kleeberg UR, MacKie RM, et al. Tamoxifen as a single agent for advanced melanoma in postmenopausal women: a phase II study of the EORTC Malignant Melanoma Cooperative Group. Melanoma Res. 1992;2:153–156. [DOI] [PubMed] [Google Scholar]
- 30.Cocconi G, Bella M, Calabresi F, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N Engl J Med. 1992;327:516–523. [DOI] [PubMed] [Google Scholar]
- 31.Flaherty LE, Liu PY, Mitchell MS, et al. The addition of tamoxifen to dacarbazine and cisplatin in metastatic malignant melanoma: a phase II trial of the Southwest Oncology Group (SWOG-8921). Am J Clin Oncol. 1996;19:108–113. [DOI] [PubMed] [Google Scholar]
- 32.McClay EF, McClay ME, Monroe L, et al. The effect of tamoxifen and cisplatin on the disease-free and overall survival of patients with high risk malignant melanoma. Br J Cancer. 2000;83:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiarion SV, Nortilli R, Aversa SM, et al. Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients. Melanoma Res. 2001;11:189–196. [DOI] [PubMed] [Google Scholar]
- 34.Agarwala SS, Ferri W, Gooding W, et al. A phase III randomized trial of dacarbazine and carboplatin with and without tamoxifen in the treatment of patients with metastatic melanoma. Cancer. 1999;85:1979–1984. [PubMed] [Google Scholar]
- 35.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. [DOI] [PubMed] [Google Scholar]
- 36.Middleton MR, Lorigan P, Owen J, et al. A randomized phase III study comparing dacarbazine, BCNU, cisplatin and tamoxifen with dacarbazine and interferon in advanced melanoma. Br J Cancer. 2000;82:1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lens MB, Reiman T, Husain AF. Use of tamoxifen in the treatment of malignant melanoma. Cancer. 2003;98:1355–1361. [DOI] [PubMed] [Google Scholar]
- 38.Richardson B, Price A, Wagner M, et al. Investigation of female survival benefit in metastatic melanoma. Br J Cancer. 1999;80:2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]


