Abstract
Background:
While incidental masses in certain organs have received particular attention, periampullary and pancreatic incidentalomas (PIs) remain poorly characterized.
Methods:
We reviewed 1944 consecutive pancreaticoduodenectomies (PD) over an 8-year period (April 1997 to October 2005). A total of 118 patients (6% of all PDs) presented with an incidental finding of a periampullary or pancreatic mass. The PI patients were analyzed and compared with the rest of the cohort (NI, nonincidentaloma group, n = 1826).
Results:
Thirty-one percent of the PI patients (n = 37) had malignant disease (versus 76% of the NI patients, P < 0.001), 47% (n = 55) had premalignant disease, and the remaining 22% (n = 26) had little or no risk for malignant progression. The 3 most common diagnoses in the PI group were IPMN without invasive cancer (30%), cystadenoma (17%), and pancreatic ductal adenocarcinoma (10%). The PI group had a higher overall complication rate (55% versus 43%, P = 0.02), due in part to a significantly increased rate of pancreatic fistulas (18.4% PI versus 8.5% NI, P < 0.001). Patients in the PI group with malignant disease had a superior long-term survival (median, 30 months, P = 0.01) compared with patients in the NI group with malignant disease (median, 21 months).
Conclusions:
Incidentally discovered periampullary and pancreatic masses comprise a substantial proportion of patients undergoing PD. Roughly three fourths of these lesions are malignant or premalignant, and amenable to curative resection. Resected malignant PIs have favorable pathologic features as compared with resected malignant NIs, and resection of these early lesions in asymptomatic individuals is associated with improved survival, compared with patients with symptomatic disease.
Periampullary and pancreatic incidentalomas (PIs) have become increasingly common in the past decade due to advances in imaging technology. We present a single institution's experience with 118 resected PIs. Ninety-two lesions in the PI group (78% of the PI lesions) were malignant or premalignant.
Computerized tomography integrates x-ray and computer technology to visualize internal patient anatomy. The foundation for present-day CT scans was begun in the 1960s by Alan Cormack and Godfrey Hounsfield. Although they worked independently, in 1979 the 2 were jointly awarded the Nobel Prize in Medicine for their pioneering work in diagnostic radiography.1 The first CT scans built in the early 1970s took 40 minutes to scan 10 cm and required overnight image reconstruction.2 Multislice CT (MSCT) technology came to market in 1998 and offered faster data acquisition and improved resolution. Since the advent of MSCT, the number of CT scans performed in the United States has increased by roughly 20% each year.3 Today, more than 7000 scanners in the United States perform over 50 million CT scans annually,4 comprising over 13% of all radiologic procedures.
Because of the improvements in CT technology and its widespread use, abnormal findings that represent asymptomatic disease are commonly observed.5 The term “incidentaloma” is used clinically and in the medical literature to refer to an asymptomatic mass detected accidentally by imaging or another diagnostic test. This diagnosis is most often associated with the adrenal gland6,7 but may also be encountered with other organs, including the parathyroid,8,9 thyroid,10–12 pituitary,13 liver,14 heart,15 prostate,16 and kidney.17 The first report of a pancreatic incidentaloma was published in the Russian literature in 2001.18 Since that time, 2 series of incidental pancreatic cysts have been reported.19,20
Although CT is the most common mechanism by which an asymptomatic periampullary mass is detected, other diagnostic tests such as serum transaminase levels and endoscopy may also uncover asymptomatic pathology around the pancreas or periampullary region. We have observed that pancreatic and periampullary incidentalomas (PIs) are being increasingly recognized at our institution, and are perhaps underappreciated in the surgical literature. Therefore, we performed a focused review of our experience with periampullary and pancreatic incidentalomas to better characterize this diagnosis. We hoped to gain insight into the potential survival benefit of early diagnosis and to help shape treatment strategies for PIs.
METHODS
The records of 1944 patients who underwent pancreaticoduodenectomy (PD) between January 1997 and October 2005 at the Johns Hopkins Hospital were reviewed. Data were extracted from the IRB approved Johns Hopkins pancreaticoduodenectomy database and from electronic patient records. Patients were categorized into 2 groups: patients with symptomatic disease attributable to the periampullary region (nonincidentaloma group, NI, n = 1826) and asymptomatic patients diagnosed with a periampullary or pancreatic incidental lesion (periampullary incidentaloma, PI, n = 118). In certain data analyses, the PI group is subcategorized into 3 groups based on the method of early detection. Imaging incidentalomas (IIs) were identified by CT scan or other high-resolution cross-sectional imaging technique, biochemical incidentalomas (BIs) were detected by elevated serum liver or pancreatic enzymes, and endoscopic incidentalomas (EIs) were detected during an endoscopic evaluation. In most of the cases, the finding of a periampullary lesion was an unexpected result of an evaluation for suspected nonpancreatic or periampullary pathology. In 5 cases, the finding of a periampullary lesion was a result of a directed screening protocol in a population of patients with increased risk for pancreatic cancer. Although these lesions were not found entirely “accidentally,” they are grouped with the PIs in this study. All of the patients were asymptomatic with regards to their periampullary or pancreatic pathology at the time of their PD.
The PDs in this series were performed by 25 different surgeons, although 4 surgeons performed 91% of the cases (J.L.C., K.D.L., K.A.C., C.J.Y.). Demographic characteristics, past medical history, intraoperative data, pathologic data, perioperative morbidity, and perioperative mortality (30-day or in-hospital death) were compared between the PI and NI groups. Lesions with carcinoma in situ are considered benign in statistical comparisons and excluded from analyses of malignant lesions.
Comparison of continuous variables was performed using the Mann-Whitney rank sum test and comparison of categorical variables was performed using a χ2 test. Long-term survival data were compared using the Kaplan-Meier method. Results are reported as median values, unless indicated otherwise. Statistical significance was accepted for P < 0.05. Data analyses were performed using Intercooled Stata Version 7.0 (Chicago, IL).
RESULTS
There were 1944 PDs performed during the study period: 1826 patients in the NI group and 118 patients in the PI group (6% of the total). PIs were identified by 3 general modalities, including imaging (n = 86), serum enzymes (n = 21), and endoscopy (n = 11). The specific reasons for the radiographic, biochemical, and endoscopic evaluations are given in Tables 1, 2, and 3, respectively.
TABLE 1. Reason for Imaging Study (Imaging Incidentalomas, n = 86)

TABLE 2. Reason for Blood Draw (Biochemical Incidentalomas, n = 21)

TABLE 3. Reason for Endoscopy (Endoscopic Incidentaloma, n = 11)

The majority of imaging incidentalomas (IIs) were detected during a diagnostic workup of symptoms unrelated to the periampullary pathology (55.8% of all IIs, 22.1% of the IIs were discovered during routine surveillance studies for a known chronic disease). Imaging studies were also obtained as part of a preoperative workup (8.1% of IIs), for reasons not clear from the patients’ records (8.1% of IIs), and as part of a “virtual” or “executive” physical examination (5.8% of IIs).
BIs refer to periampullary lesions identified without signs or symptoms of periampullary pathology, but with abnormal biochemical results, such as serum AST, ALT, or amylase (Table 2). In all but one instance, the transaminases served as the red flag. The remaining patient had an elevated serum amylase. Most of the laboratory data were obtained during a routine physical examination (81% of BIs). Less commonly, BIs were detected as part of routine surveillance of a chronic disease (9.5% of BIs) or a workup for signs and symptoms unrelated to the periampullary region (9.5% of BIs).
EIs were detected during the evaluation of a chronic disease (73% of EIs) or due to new signs and symptoms unrelated to the periampullary region (27% of EIs). GERD served as the impetus for 5 (45% of EIs) of the endoscopic evaluations that led to the incidental detection of periampullary pathology (Table 3).
Patient demographics were similar in the PI and NI groups (Table 4). The similarities were observed in each of the PI subgroups as well.
TABLE 4. Demographics
The PI and NI groups had similar medical comorbidities for most parameters. Medical history data are provided in Table 5. There was significantly less chronic pancreatitis (P = 0.009) in the PI group, likely explained by the fact that this disease is most often associated with abdominal pain, which is incompatible with the diagnosis of PI. A significantly lower incidence of tobacco use in the PI group was observed (P = 0.005), as compared with the NI group.
TABLE 5. Past Medical History

Pathologic diagnoses are listed in Table 6. IPMN (including IPMN adenoma, borderline IPMN with dysplasia, and IPMN with carcinoma in situ), cystadenoma, and ductal adenocarcinoma of the pancreas were the 3 most common diagnoses in both the II subgroup, and the entire PI group. Ampullary adenocarcinoma was the most common abnormality in the BI subgroup, and periampullary adenoma was the most common lesion in the EI subgroup. A smaller proportion of the PIs harbored malignant disease as compared with the NI group (31.4% PI versus 75.9% NI, P < 0.001). A total of 37 malignancies were resected from 118 patients diagnosed with a PI. Malignant PIs included ductal adenocarcinoma of the pancreas (n = 12), IPMN with invasive cancer (n = 7), ampullary adenocarcinoma (n = 7), malignant neuroendocrine tumor (n = 4), duodenal adenocarcinoma (n = 3), metastatic renal cell cancer (n = 2), malignant GIST (n = 1), and adenosquamous carcinoma of the pancreas (n = 1).
TABLE 6. Pathologic Diagnoses
Specific pathologic data for the malignant tumors are detailed in Table 7. Cancers that were discovered incidentally tended to have more favorable histologic features. The TNM staging for malignancies in the PI group were significantly lower compared with the NI group (stage I, 34.4% versus 10.4%, P < 0.001), with significantly fewer positive lymph nodes in the PI group (median positive lymph nodes, 1 versus 2, P = 0.01). Other histologic parameters were consistently more favorable in the PI group, although they did not achieve statistical significance. These included, for the PI and NI groups, respectively, margin status (15.6% positive versus 28.1%, P = 0.1), perineural invasion (64.3% versus 76.3%, P = 0.1), vascular (small vessel) invasion (30.8% versus 47.2%, P = 0.1), and histologic grade (well or moderately differentiated, 72.4% versus 56.5%, P = 0.09). In a subgroup analysis of pancreatic ductal adenocarcinomas, cancers in the PI group showed a trend toward earlier stage at diagnosis compared with cancers in the NI group (stage I, 16.7% versus 4.7%, P = 0.06).
TABLE 7. Pathologic Data for Malignant Tumors in Patients Undergoing PD
Analysis of IPMN lesions in the II subgroup demonstrates a shift toward earlier lesions in the asymptomatic II patients (Table 8). Ninety-three percent of the IPMNs in the II subgroup were either benign, stage 0 or stage I cancers as compared with 67% of the IPMNs in the NI group (P = 0.001).
TABLE 8. Stage of IPMNs for Imaging Incidentalomas (II) and Nonincidentalomas (NI)

Postoperative data appear in Table 9. There were no deaths in the PI group, while the mortality rate for the NI group was 1.8% (P = not significant). Perioperative morbidity was significantly higher in the PI group (54.8%) than in the NI group (43.3%, P = 0.02). The higher morbidity in the PI group appears largely due to higher rates of pancreatic fistulas (18.4% versus 8.5%, P < 0.001) and bile leaks (6.1% versus 2.4%, P = 0.01), as well as other complications not appearing in Table 9 (28.9% versus 20.9, P = 0.04). The postoperative lengths of hospital stay were similar in the PI (10 days) and NI groups (9 days).
TABLE 9. Postoperative Mortality and Complications
The actuarial survival of all patients (including all pathologic diagnoses) in the PI and NI groups is plotted in Figure 1. The median survivals are 82 months and 33 months, respectively (P < 0.001), with a better survival rate in the PI group. Long-term survival was similar for patients in the PI and NI groups with benign disease (5 year-survival: 80% PI versus 79% NI, P = not significant). Figure 2 depicts the long-term survival of patients with invasive cancer. The median survivals in the PI and NI groups are 30 months and 21 months, respectively (P = 0.01). The survival data in Figure 3 are restricted to patients undergoing PD for ductal adenocarcinoma of the pancreas. The median survivals in the PI and NI groups are 28 months and 18 months, respectively (P = 0.04). A multivariate Cox regression analysis showed a significant survival advantage in the PI group with ductal adenocarcinoma of the pancreas, after adjusting for demographics, medical comorbidities, intraoperative factors, pathologic features (stage and differentiation), and postoperative complications (P = 0.05).
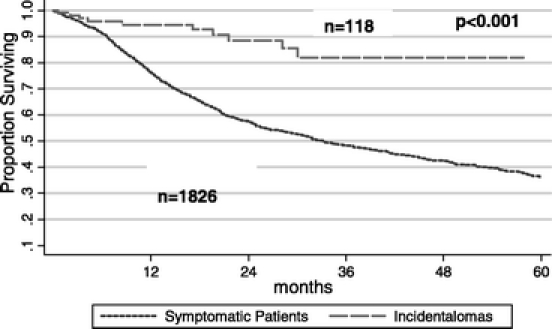
FIGURE 1. Kaplan-Meier survival curves for patients who underwent a PD for a PI, as compared with patients who presented with symptoms (NI). The 1-, 2-, and 5-year survival rates for patients in the PI group were 95%, 88%, and 68%, respectively (median, 82 months, P < 0.001). The 1-, 2-, and 5-year survival rates for patients in the NI group were 76%, 57%, and 36%, respectively (median, 33 months). PI, periampullary or pancreatic incidentaloma; NI, nonincidentaloma.

FIGURE 2. Kaplan-Meier survival curves for patients who underwent a PD for an invasive cancer (in situ lesions are excluded) in the PI group, as compared with patients who presented with symptoms (NI). The 1-, 2-, and 5-year survival rates for patients in the PI group were 93%, 70%, and 50%, respectively (median, 30 months, P = 0.01). The 1-, 2-, and 5-year survival rates for patients in the NI group were 70%, 46%, and 23%, respectively (median, 21 months). PI, periampullary or pancreatic incidentaloma; NI, nonincidentaloma.

FIGURE 3. Kaplan-Meier survival curves for patients who underwent a PD for ductal adenocarcinoma of the pancreas in the PI group, as compared with the patients who presented with symptoms (NI). The 1-, 2-, and 5-year survival rates for patients in the PI group were 100%, 62%, and 50%, respectively (median, 28 months, P = 0.04). The 1-, 2-, and 5-year survival rates for patients in the NI group were 64%, 38%, and 14%, respectively (median, 18 months). PI, periampullary or pancreatic incidentaloma; NI, nonincidentaloma.
DISCUSSION
This series of 118 patients represents the largest reported series of resected pancreatic and PIs to date. The diagnosis of PI is a relatively recent clinical phenomenon, but its incidence will likely continue to increase with future innovations in diagnostic imaging. Prior to MSCT, nearly all patients undergoing a PD at our institution had symptomatic disease. By 2004, roughly 1 of every 10 PDs was performed on asymptomatic patients, with a PI.
Patients with PIs tended to have similar demographic features and medical comorbidities as compared with patients in the NI group. The long-term survival of all patients in the PI group was significantly greater (P < 0.001) than patients in the NI group (Fig. 1; median, 82 months versus 33 months). Of course, the difference in survival between the 2 groups can be explained in part by the increased incidence of benign disease in the PI group. However, when patients with benign pathology were removed from the analysis, the superior survival of the PI group was preserved (Fig. 2; median, 30 months PI versus 21 months NI, P = 0.01). Furthermore, improved survival was observed in the PI group when the data were further confined to pancreatic ductal adenocarcinoma (Fig. 3; median, 28 months PI versus 18 months NI, P = 0.04).
There are several possible explanations for the improved survival in the PI group as compared with the NI group. First, in the larger analysis including all patients, the survival difference can be attributed in large part to more favorable pathology in the PI group. However, as the subgroup analyses were restricted in a stepwise fashion to include patients with a more homogeneous spectrum of pathology (malignant disease or pancreatic ductal adenocarcinomas), the improved survival in the PI group continued to be observed. Lead-time bias is a second possible explanation for the survival difference. The interval between disease detection and symptom-onset may account for some of the observed survival increase, particularly in the subgroup of patients with only ductal adenocarcinoma of the pancreas, where the improvement in median survival was 10 months. A third explanation for improved survival is that early detection results in resection of earlier, less virulent lesions, in the progression from premalignant to malignant disease. The high proportion of premalignant lesions and early cancers in our series supports this hypothesis.
Approximately 78% of resected lesions in the PI group were for a malignancy or for pathology with malignant potential.21–24 The percentage of PIs that were malignant (31.4%) is high compared with the percentage of malignant incidentalomas found in other organs (eg, 6% for adrenal incidentalomas7 and 9% for thyroid incidentalomas12). The premalignant or potentially premalignant lesions (47% of PIs) included 23 IPMN adenomas or borderline IPMNs with dysplasia, 12 IPMN with carcinoma in situ, 11 tubulovillous adenomas, 7 benign neuroendocrine tumors, and 2 mucinous cystadenomas. Twenty-six lesions (22% of PIs) may be considered to have had no or low malignant potential in retrospect. These lesions included 18 serous cystadenomas, 4 cases of chronic pancreatitis, 2 duodenal diverticulae, 1 benign ampullary stricture, and 1 case of dilated pancreatic ducts consistent with occult cystic fibrosis.
Improved diagnostic imaging and the use of sampling by EUS-FNA have enabled the discrimination of certain benign lesions from more ominous ones. Incidentally discovered lesions thought to have a low-risk of cancer may be managed by serial observation in lieu of resection. The trend at our institution, and others,19 has been to observe selected asymptomatic pancreatic cystic lesions less than 2 cm in diameter. Our recommendation regarding resection versus observation is influenced by several factors, including patient age, comorbidities, family history of pancreatic neoplasia, and patient input. We are more likely to recommend resection for a young, healthy, and anxious individual, with a family history of pancreatic cancer. We would tend to favor observation by serial imaging in an octogenarian with multiple medical comorbidities and little anxiety related to the finding of a small cystic incidentaloma. This series of PIs reflects those asymptomatic lesions selected for resection.
The data presented herein deal with those patients with PIs selected for resection, and resected by pancreaticoduodenectomy. Our data are not sufficiently accurate to identify the total number of patients with incidentalomas located anywhere in the pancreas being screened for possible resection. Further, we do not have the data concerning the numbers of patients undergoing central or distal pancreatectomy for incidentalomas. However, it may be reasonable to extrapolate from other series in the literature. For example, a recent publication from Fernandez-del Castillo and the group at the Massachusetts General Hospital reviewed their 5-year experience with incidental pancreatic cysts.19 Only 22% of their asymptomatic patients were observed, while 78% underwent resection. Incidental lesions tend to be rather evenly distributed throughout the pancreatic parenchyma, with perhaps half being resectable via pancreaticoduodenectomy. By extrapolation, it may be reasonable to estimate that our 118 PIs reflect a 78% resection rate from a total of 151 right-sided PIs, and a total of about 300 patients with PIs anywhere in the pancreas.
Improvements in MSCT during the past decade, and series such as this one may stimulate discussion in the medical literature on the potential role of CT in cancer screening. Although there is no consensus in the medical community, whole-body cancer screening is widely performed in the United States. The concept received national attention in 1999 when Oprah Winfrey discussed her “virtual physical” on her popular television show. Subsequently, private whole-body imaging centers have marketed aggressively to healthy consumers. There are now hundreds of centers performing millions of whole-body scans each year in the United States. In addition, some academic centers, including ours, offer the service. The protocol at Johns Hopkins requires a physician referral and involves CT imaging from the base of the skull to the pubic symphysis with IV contrast on a 64-slice Siemens Sensation scanner (Siemens Medical Solutions USA, Malvern, PA).25 The images are reconstructed in 3 dimensions at 0.5-mm intervals. At least 5 of the IIs in our series were discovered during such a whole-body CT examination.
Few studies have been performed to evaluate whole-body CT screening. A prospective-randomized trial to determine survival benefit would be costly and impractical. In one retrospectively analyzed series of 1500 whole-body CT scans, asymptomatic cancers were discovered in 1% of cases.26 A much greater proportion of such scans detected another significant abnormality such as advanced coronary artery or aortic disease.5,25 Critics of whole-body imaging point out that most studies on the subject, including the present one, lack data on disease-specific mortality. Additionally, whole-body CT is expensive (up to $2513 per patient including follow-up tests) and carries risks such as radiation exposure and false-positive results that lead to additional tests or procedures.27,28
Although cost-benefit analysis may not justify whole-body cancer screening for the general population at the present time, certain high-risk populations may benefit from focused surveillance. Brentnall et al performed ERCP and EUS on 14 patients from 3 kindreds with familial pancreatic cancer and identified evidence of dysplasia in half of the study group.29 The establishment of large familial pancreatic cancer registries can help identify patients who may benefit from routine endoscopy, CT scans, and laboratory studies.30
Currently, accidentally discovered periampullary and pancreatic masses (termed pancreatic incidentalomas herein) comprise a substantial proportion of patients undergoing pancreaticoduodenectomy. Resected malignant PIs have favorable pathologic features and improved patient survival, compared with patients with symptomatic disease (termed nonincidentalomas herein). These data highlight the need for future studies, with larger numbers of patients, to better understand this increasingly common clinical diagnosis and encourage clinicians to take part in the important debate on cancer screening.
Discussions
Dr. Andrew L. Warshaw (Boston, Massachusetts): This is a beautifully written and presented examination of pancreatic and periampullary neoplasms that were found accidentally during studies for other purposes.
In 92 of 118 patients, the unsuspected lesion was malignant or premalignant, including 10% being ductal adenocarcinomas and 40% other malignancies such as intraductal papillary mucinous neoplasms, mucinous cystic neoplasms, and neuroendocrine tumors which also have malignant potential. These were treated effectively with no mortality, albeit with an increased fistula rate, perhaps due to a soft pancreas. Notably, there was comparatively a superior long-term survival in this group of patients, probably a direct consequence of a lower AJCC and TNM stage.
Most pancreatic neoplasms are cancer-prone and have a bad reputation for cancer-related survival. Even among the cystic neoplasms, mucinous tumors, IPMNs and MCNs, all of these are considered at least premalignant; only serous cystic tumors can generally be dismissed as benign. In our recent experience, 37% of 212 patients with cystic neoplasms were asymptomatic and were diagnosed, as in this study, by chance during imaging for unrelated diagnoses.
Incidental cystic tumors were smaller and more likely to be serous, but 60% were mucinous neoplasms or adenocarcinomas, and 17% of the asymptomatic cysts harbored some degree of cancer. Another 42% contained premalignant epithelium. When the tumors were smaller than 2 cm, only 3.5% of asymptomatic cystic tumors contained cancer, in contrast to 26% of lesions larger than 2 cm. Premalignant epithelium was found in 40%, regardless of size.
These studies validate the concept that earlier diagnosis improves survival and cure of pancreatic tumors. They illustrate the importance of modern imaging in case-finding, as well as the value of modern expert surgical treatment. They also indicate the potential value, if we had one, of a true screening test, which would have to be both accurate and applicable. Current imaging techniques and endoscopy do not meet these criteria.
This latter point then leads to my questions: What high-risk groups are currently worth screening and with what current methods? What is the value of endoscopic ultrasonography, CA-19-9, or other tumor markers in case-finding? Can you anticipate that proteomic or genomic characteristics, not yet really established, might lead to a focused study of a high-risk population? If a cystic lesion is found, when is a tissue diagnosis needed or appropriate? Or conversely, when is it simpler and safer just to take it out, perhaps even by total pancreatectomy, if you can't identify a specific lesion but there is a worrisome genetic/proteomic abnormality?
Dr. Jordan M. Winter (Baltimore, Maryland): Your question as to which patients might be relevant to be integrated into a protocol to screen with present technology; it has been shown by multiple institutions that there are families with increased risk for pancreatic cancer. Certainly, there are familial syndromes such as Peutz-Jeghers syndrome where the lifetime risk of pancreatic cancer approaches 30%, and there is a 130-fold risk above the general population. And it has been shown by Dr. Ralph Hruban from our institution that there are kindreds with 3 or more first-degree relatives with pancreatic cancer, where there is an increased risk upwards around 57-fold over the general population of developing a pancreatic cancer. Certainly, these patients could be identified through familial registries as being worthwhile for pancreatic cancer screening using imaging protocols, endoscopy, and biochemical markers.
The question as to whether there are biochemical markers that could be useful in trying to distinguish benign from malignant neoplasms that could help reduce the number of benign neoplasms that are resected and try and improve the diagnostic yield on preoperative workup; certainly, from your institution you have demonstrated that by looking at cystic neoplasms of the pancreas and analyzing the cyst fluid, cyst fluid CEA is a marker that could yield a diagnostic accuracy of 75%, and 90% in some series, as reported by a group at Northwestern, using a threshold of cyst CEA level of between 150 and 200.
We are certainly not there yet where we can totally rely on preoperative tissue sampling independently as a method to determine whether or not a lesion is malignant or benign. Clinical assessment also plays a role. Those are all the factors that are important in going into the evaluation of a patient and determining whether or not a patient is appropriate for resection.
We tend to follow a general guideline which others have described where cystic neoplasms of the pancreas that are small, generally less than 2 cm in size, are appropriate for follow-up, if the patient is a patient that is amenable to close surveillance, their anxiety level is amenable to such an approach, and these are lesions that have a low risk of malignancy (although there is still a significant risk of premalignancy in that general population).
And certainly for the future, as you alluded to, I think that proteomic or genomic analysis is going to become a very important element in the preoperative workup of pancreatic and periampullary incidentalomas. I think there is hope that molecular markers can improve the diagnostic yield beyond cyst fluid CEA and certainly be applied even for solid tumors of the pancreas. And it has been demonstrated that there are potential benefits to looking at K-ras mutations and loss of heterozygosity and microsatellite markers.
Dr. Kelly M. McMasters (Louisville, Kentucky): You reported the results of 118 patients with incidentalomas out of 1944 consecutive patients who underwent a Whipple operation; 42% of the incidentaloma patients had cancer, and another 36% had premalignant lesions. This is obviously a highly selected group, as you had a fairly high yield of malignant or premalignant lesions. Yet these are the patients upon which you chose to operate. Do you have any idea how many patients you evaluated with pancreatic incidentalomas and chose to observe? While this paper tells us about the patients that went on to operation, it doesn't tell us about the fate of the patients that you chose to observe. Obviously, the experienced pancreatic surgeons at John Hopkins used a lot of judgment in deciding not to operate on some patients with incidentalomas. I think there is probably a lot for the rest of us to learn about this population as well. Clearly, the worst outcome is when we perform a Whipple operation for an incidentaloma, the patient has a pancreatic leak or some other complication that leads to a prolonged hospital course, and they have a serous cystadenoma that is benign. As you have shown, the incidence of pancreatic leak and other complications is higher among these patients.
In this regard, and Dr. Warshaw touched upon some of this, which factors do you use to determine which incidentaloma patients should go to surgery? When is it safe to watch one of these, and if you do, how often do you follow these patients? Do elevated tumor markers CA-19-9 and CEA sway you one way or the other? Do atypical cells on ERCP brushings and washings mean anything? Do you use EUS-guided FNA for diagnosis? How is your approach different for cystic versus solid tumors? And again, cystic fluid, how do you use that? And do you use any markers from the cyst fluid to help make a determination?
Finally, you present your results for patients who underwent a Whipple operation for pancreatic incidentaloma. It seems to me that incidentalomas in the body and tail of the pancreas are also quite common. Are they found as commonly as pancreatic head lesions? Is there anything to be learned from studying this population as well?
I commend the authors for yet another important contribution which will become the classic reference on this subject.
Dr. Jordan M. Winter (Baltimore, Maryland): First, I will try and address the best I can the question of the denominator in our study, which we didn't describe. This also may get to the question that you addressed at the end of your questions as to the importance or the potential for investigation of incidentalomas in other regions of the pancreas.
In order to determine the incidence of periampullary and pancreatic incidentalomas, this level of detail isn't typically found in retrospective databases and requires the accumulation of these data in a prospective manner. At the present time, our data on the proximal, the right pancreas, has this level of detail. I can't give you an exact number as to the incidence of these lesions in the body and tail of the pancreas, except to extrapolate from other data in the literature for cystic neoplasms (which out of our 118 incidentalomas in this study, about 75% of them can be considered cystic neoplasms). And that is that they tend to be distributed (incidental neoplasms) a third, a third, a third from right to left in the different regions of the pancreas.
And on the same subject, the number of incidentalomas that were cautiously observed in the studied cystic neoplasms from the Massachusetts General series was 20% in the incidental group of pancreatic cysts. So I think the denominator for our study is between 140 and 150 incidentalomas as a conservative estimate, observed or were evaluated by our surgeons ultimately, with about three fourths of them going to resection.
In terms of preoperative tissue sampling, this is a subject which we are beginning to evaluate in our series of patients. While we haven't fully analyzed the data, I can share some preliminary numbers to help address that question. Of the 2800 patients in our Whipple database, about 1000 of them have had some sort of preoperative biopsy or tissue sampling. So about 40% of patients. And these are biopsied either through brushings, endoscopic, or more commonly about a decade ago percutaneous biopsies, or more recently, endoscopic ultrasound-guided fine needle aspirations.
In the last calendar year, the percentage of patients who have had FNAs that come to our surgery clinic are about 15% of patients. And when I looked at the incidentaloma group, those proportions were consistent. 40% of the incidentaloma patients had preoperative tissue sampling, and 15% had endoscopic ultrasounds.
These patients generally come to the office with their preoperative biopsy results in hand. So we generally do not as part of our workup have to ask for a preoperative FNA or a cyst fluid sample to be assessed for CEA. As I alluded to earlier, that can be helpful, but certainly should not be an independent guide for use in the determination of whether these patients are appropriate for a resection.
Dr. Kenneth W. Sharp (Nashville, Tennessee): I noted in the manuscript that 10% of your pancreaticoduodenectomies are now being done for incidentalomas. That is now about 25 to 30 patients a year in your numbers. This is a truly growing problem. But the one group that I was interested in were the 5 patients who had a pancreaticoduodenectomy done for findings on an executive physical or a virtual CT physical exam. And in the manuscript you mention that at Hopkins you must have a physician who orders a virtual CT physical exam. Our internists just do virtual CT, they don't examine patients.
Do you have a sense of how many executive physicals or virtual physicals that this population came from? Were these all from Hopkins or did some go out from the trailers going around the country with CT scanners in them? Because if you have 5 patients out of the Hopkins virtual CT, you might have some incidence of this true entity here, too. My final question is: how about those patients with the virtual physical exams?
Dr. Jordan M. Winter (Baltimore, Maryland): I think the concept of a virtual physical is something that is becoming much more popular and commonplace and is something that we are all going to see much more of. So I suspect that 5% will turn into a much greater number over the next couple of years.
I don't know whether the patients had their virtual physicals in the Hopkins system or whether they were done elsewhere. But I think it is an important point to make ānd that is something that I can very easily look up t̄shat with the concept of virtual physicals, again, there are no prospective randomized studies that demonstrate the benefit of them, nor will there be in the near future. It would be a very impractical study. It would require many years of follow-up and be very expensive.
But the problem with it now is that there is no quality control on this type of diagnostic test. At Johns Hopkins, which I believe is one of the few academic centers at this point that is actually performing this type of service, the test is done on a 64-slice CAT scan. 3-D imaging is done on every patient. The slices are 0.75 mm thick. And patients have the option and are recommended to have the diagnostic test done with intravenous contrast.
All those things that I just elaborated are totally variable and often are not done in the commercial setting out in the community where these for-profit virtual physical CT centers are popping up. The thickness of the CT scan slices is quite variable, the expense of the radiologist is variable. So where they are done, in large part, will result in the sensitivity and the frequency with which lesions in the periampullary region and pancreas are discovered. I think it would be very difficult to find these small asymptomatic lesions in a lot of the CAT scans done in the community.
Dr. Michael J. Edwards (Little Rock, Arkansas): We are seeing CT findings today suggestive of neuroendocrine tumors more and more often. And clearly, there are some of these patients, at least we believe, that should be followed and not operated.
Autopsy studies show that there is a prevalence, albeit low, of people who die with an asymptomatic neuroendocrine tumor. What are your guidelines for recommending a resection of something that appears consistent with a neuroendocrine tumor as opposed to following that lesion?
Dr. Jordan M. Winter (Baltimore, Maryland): Let me just reemphasize or summarize our results with neuroendocrine tumors. In the incidentaloma group, about 10% of the 118 patients had neuroendocrine tumors, and three fourths of those were benign. A few of them were malignant endocrine tumors.
Our general approach at this point is to recommend resection, if we believe that the patient has a neuroendocrine tumor, although again this is individualized for the patient. If we have a patient who is in their 80s with significant medical comorbidities and likely has a small nonfunctional benign neuroendocrine tumor, a very hypervascular region in the head of the pancreas, we might be willing to follow them with serial imaging. But a younger patient, still we would respect that potential to develop into a malignant lesion over time and would favor resection.
Dr. Alan S. Livingstone (Miami, Florida): You made the point that these were lower-stage and lower-grade tumors. The final conclusion, if I recall, was that they also have a better prognosis. My question is: do they? Or is this basically lead time bias? Did you compare them stage for stage and was there a better survival, or were you just operating on earlier tumors?
Dr. Jordan M. Winter (Baltimore, Maryland): That is a good question and a very difficult one to answer. I can't say for certain that lead time bias did not play a role in the survival benefit that we observed and I can't quantitate the extent of that lead time bias factored in. Certainly, in any screening procedure when you are talking about cancer, lead time bias is going to play a role.
As a reminder, the survival benefit in ductal adenocarcinomas in our series was from 28 months as a median survival in our patients who had an incidentaloma, to 18 months in patients who had symptoms. I am talking about the cohort with ductal adenocarcinoma. So could those 10 months be accounted for by a lead time bias? I guess that is a possibility. However, we did do a multivariate analysis and adjusted for stage, and in doing that, there was still survival benefit in the incidentaloma group, with a P value of 0.05.
The other point that I think is worth mentioning in addressing lead time bias is that perhaps we are underestimating the survival benefit in our incidentaloma group. If you assume that all of the patients with ductal adenocarcinoma of the pancreas that are asymptomatic and diagnosed incidentally have resectable disease ānd I don't know this to be true, but I believe that that assumption can be approximated t̄hen the control group ought not to be the preselected group that we have graphed for you (that is patients who already have a favorable prognosis because they have resectable disease) but ought to include all patients with symptomatic pancreatic cancer, including the 85% of the patients that are not resectable. In this case, you would be comparing a median survival of 28 months to a median survival of 7 or 8 months. I believe, in that group, lead time bias is intuitively not going to explain all of the survival difference.
I would like to thank the Southern Surgical Association for the opportunity to answer these questions and close the discussion.
Footnotes
Reprints: Charles J. Yeo, MD, Department of Surgery, Thomas Jefferson University, 1015 Walnut St, Suite 620, Philadelphia, PA 19107. E-mail: charles.yeo@jefferson.edu.
REFERENCES
- 1.emedicine CH. CT Scan Introduction. CT Scan. 2005 August 10, 2005 [cited 2005 October 14, 2005]; Available from: http://www.emedicinehealth.com/articles/11618–1.asp.
- 2.Indrajit IK, D'souza JD. Multislice CT: a quantum leap in whole body imaging. Indian J Radiol Imaging. 2004 2004;14:209–216.
- 3.Davis W. Multi-Slice CT: 64 and Counting. Med Imaging. 2005. [Google Scholar]
- 4.Linton OW, Mettler FA Jr. National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol. 2003;181:321–329. [DOI] [PubMed] [Google Scholar]
- 5.Brant-Zawadzki M. CT screening: why I do it. AJR Am J Roentgenol. 2002;179:319–326. [DOI] [PubMed] [Google Scholar]
- 6.Udelsman R, Fishman EK. Radiology of the adrenal. Endocrinol Metab Clin North Am. 2000;29:27–42. [DOI] [PubMed] [Google Scholar]
- 7.Thompson GB, Young WF Jr. Adrenal incidentaloma. Curr Opin Oncol. 2003;15:84–90. [DOI] [PubMed] [Google Scholar]
- 8.Whineray Kelly EL, Braatvedt G, Harman R. A parathyroid incidentaloma. Aust NZ J Surg. 2005;75:367. [DOI] [PubMed] [Google Scholar]
- 9.Aron DC, Howlett TA. Pituitary incidentalomas. Endocrinol Metab Clin North Am. 2000;29:205–221. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell J, Parangi S. The thyroid incidentaloma: an increasingly frequent consequence of radiologic imaging. Semin Ultrasound CT MR. 2005;26:37–46. [DOI] [PubMed] [Google Scholar]
- 11.Silver RJ, Parangi S. Management of thyroid incidentalomas. Surg Clin North Am. 2004;84:907–919. [DOI] [PubMed] [Google Scholar]
- 12.Chen YK, Ding HJ, Chen KT, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421–1426. [PubMed] [Google Scholar]
- 13.Schneider HJ, Stalla GK. 10-minute consultation: incidentaloma of the hypophysis. MMW Fortschr Med. 2004;146:67–68. [PubMed] [Google Scholar]
- 14.Liu CL, Fan ST, Lo CM, et al. Hepatic resection for incidentaloma. J Gastrointest Surg. 2004;8:785–793. [DOI] [PubMed] [Google Scholar]
- 15.Vancollie O, Rombaut E, Donckier J. Cardiac incidentaloma. Ann Cardiol Angeiol (Paris). 2001;50:316–318. [DOI] [PubMed] [Google Scholar]
- 16.Picurelli Oltra L, Sendra Torres A′, Fernandez Rodriguez A, et al. Incidental prostatic adenocarcinoma in the ere of the PSA. Actas Urol Esp. 1997;21:354–356. [PubMed] [Google Scholar]
- 17.Pobil Moreno JL, Martinez Rodriguez J, Maestro Duran JL, et al. Renal incidentaloma and pregnancy. Arch Esp Urol. 1996;49:755–757. [PubMed] [Google Scholar]
- 18.Kostiuk TS. Observation of pancreatic incidentaloma. Klin Khir. 2001;9:62–63. [PubMed] [Google Scholar]
- 19.Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–423; discussion 433–434. [DOI] [PMC free article] [PubMed]
- 20.Handrich SJ, Hough DM, Fletcher JG, et al. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol. 2005;184:20–23. [DOI] [PubMed] [Google Scholar]
- 21.Ahrendt SA, Komorowski RA, Demeure MJ, et al. Cystic pancreatic neuroendocrine tumors: is preoperative diagnosis possible? J Gastrointest Surg. 2002;6:66–74. [DOI] [PubMed] [Google Scholar]
- 22.Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797; discussion 97–99. [DOI] [PMC free article] [PubMed]
- 24.Treitschke F, Beger HG. Local resection of benign periampullary tumors. Ann Oncol. 1999;10(suppl 4):212–214. [PubMed] [Google Scholar]
- 25.Horton KM. Whole-body CT screening. Appl Radiol online. 2005;34:30–38. [Google Scholar]
- 26.Brant-Zawadzki MN. Screening CT: rationale. Radiographics. 2002;22:1532–1536; discussion 1536–1539. [DOI] [PubMed]
- 27.Beinfeld MT, Wittenberg E, Gazelle GS. Cost-effectiveness of whole-body CT screening. Radiology. 2005;234:415–422. [DOI] [PubMed] [Google Scholar]
- 28.Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232:735–738. [DOI] [PubMed] [Google Scholar]
- 29.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–255. [DOI] [PubMed] [Google Scholar]
- 30.Tersmette AC, Petersen GM, Offerhaus GJ, et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738–744. [PubMed] [Google Scholar]






