Abstract
Objective:
To compare intermediate-term outcomes in adult recipients of expanded criteria (ECD) versus concurrent standard criteria (SCD) deceased donor kidney transplants at a single center using a standardized approach.
Summary Background Data:
Expanded criteria donors (ECDs) are a source of kidneys that increase the donor organ pool, but the value of transplanting these kidneys has been questioned because of concerns regarding diminished survival and predicted poorer intermediate-term outcomes.
Methods:
Over a 47-month period, we performed 244 deceased donor kidney transplants into adult recipients, including 143 from SCDs and 101 from ECDs. Management algorithms were implemented to preserve nephron function, and recipient selection for an ECD kidney transplant was based on low immunologic risk. All patients received depleting antibody induction in combination with tacrolimus and mycophenolate mofetil. A total of 188 patients (77%) had at least a 1-year follow-up.
Results:
ECDs were older, had a higher BMI, had an increased incidence of cerebrovascular brain death and preexisting donor hypertension, and had a lower estimated creatinine clearance (CrCl, all P < 0.01) compared with SCDs. Cold ischemic times were similar between groups, but more ECD kidneys were preserved with pulsatile perfusion (P < 0.01). ECD kidney recipients were older, less sensitized, had a lower BMI, had fewer 0-antigen mismatches, and had a shorter waiting time (all P < 0.01) compared with SCD kidney recipients. Actual patient (93%) and kidney graft (83%) survival rates were similar between groups with a mean follow-up of 24 months. The rates of delayed graft function (DGF), acute rejection, readmissions, operative complications, major infections, and resource utilization were comparable between groups. Renal function followed longitudinally was consistently better in SCD patients (P < 0.05). Black recipients had higher rates of DGF, acute rejection, and graft loss (P < 0.05), but the effects were less pronounced in the ECD group.
Conclusions:
By appropriate donor and recipient profiling and the use of management algorithms to project and protect renal function, excellent intermediate-term outcomes can be achieved with ECD kidney transplants that are comparable to SCD kidney transplants.
A retrospective review was conducted of outcomes in adult recipients of expanded criteria versus standard criteria deceased donor kidney transplants at a single center using a standardized approach. By appropriate donor and recipient profiling and the use of management algorithms to protect renal function, comparable intermediate-term outcomes can be achieved and waiting times can be decreased.
The burgeoning crisis in organ supply challenges the transplant community to maximize and optimize the use of organs from all consented donors. According to United Network for Organ Sharing (UNOS) data, in 2005, more than 60,000 candidates were on the active waiting list for kidney transplantation in the United States, while less than 15,000 kidney transplants were performed in 2004.1 Depending on blood type, median waiting times for a kidney transplant in the United States currently range from 2 to 6 years and continue to increase. Only 25% of active wait-list candidates are transplanted in a given year, and the chance of receiving a deceased donor kidney transplant within 1 year of listing is less than 10%. The waiting list has become a “waiting to die” list, as 6% of patients on the kidney waiting list (10% of diabetic patients) die each year awaiting a potential life-enhancing and life-prolonging transplant.1
The scarcity of available donor kidneys is a pervasive problem and mandates an ongoing reappraisal of the limits of acceptability when accepting kidney offers from deceased donors (DDs). The escalating disparity between organ supply and demand fuels initiatives not only to increase but also to optimize the utilization of available organs. Similar to trends in the overall U.S. general population, there has been an increasing yet disproportionate shift toward increasing numbers of older donors and recipients in kidney transplantation. In the last decade, the proportion of DDs older than 50 years of age has increased from 21% to 31%.2 Cerebrovascular events are now the leading cause of brain death culminating in deceased organ donation.3 Because of the convergence of demographic inevitability and medical advances in the aged, the use of kidneys from older donors has become generally, albeit reluctantly, accepted.4
Expanded criteria deceased donors (ECDs) over age 60 years and those aged 50 to 59 years with additional risk factors accounted for 177 kidney transplants nationally in 1988 and more than 1300 in 2004.1 However, the value of transplanting ECD kidneys has been questioned because of concerns over diminished graft survival and predicted poorer intermediate-term outcomes.4–6 Because it is our contention that ECD kidneys are defined by suboptimal nephron mass, we think that appropriate donor and recipient profiling and selection may maximize and optimize the use of this scarce and controversial resource.7 The purpose of this study was to review retrospectively our intermediate-term single center outcomes in ECD versus concurrent standard criteria deceased donor (SCD) kidney transplantation in adult patients receiving similar immunosuppression and management algorithms implemented to reduce renal injury and preserve nephron function.7
METHODS
Study Design
We conducted a retrospective chart review of all DD kidney transplants performed in adult recipients at our center from October 1, 2001 through August 31, 2005 (minimum 3-month follow-up). Specific exclusions included pediatric recipients (<20 years of age), simultaneous kidney-pancreas transplant recipients, and living donor kidney recipients. A total of 244 DD kidney transplants met the entry criteria and were categorized as either ECD or SCD kidney transplants.7
Definitions
ECDs were classified by the UNOS definition as all DDs over age 60 years and DDs 50 to 59 years of age with any 2 of the following criteria: 1) history of hypertension, 2) cerebrovascular cause of brain death, or 3) terminal serum creatinine (SCr) level >1.5 mg/dL.4,6 For purposes of this study, any DD not meeting the above ECD criteria was defined as an SCD. Recipient outcomes were stratified according to the above DD criteria. Delayed graft function (DGF) was defined as the need for dialysis in the first week posttransplant. Renal allograft loss was defined as death with a functioning graft (DWFG), allograft nephrectomy, resumption of dialysis, or return to the pretransplant SCr level.
Donor Evaluation and Management
No specific DD upper age limit was excluded from consideration, and this series included 16 transplants from DDs ≥70 years of age (oldest 78 years). The Cockcroft-Gault formula was used to estimate donor creatinine clearance (CrCl), using either ideal or adjusted body weight (in donors >30% ideal body weight) to calculate projected donor kidney function and to determine single or dual kidney transplantation (DKT) into a single recipient.8,9 If the estimated donor CrCl was >65 mL/min, then a single kidney transplant was performed, preferably into an older recipient with a body mass index (BMI) <25 kg/m2. If the estimated CrCl was <40 mL/min, then the kidney(s) were not usually transplanted at our center. If the estimated CrCl was 40 to 65 mL/min, then a bilateral DKT was typically performed using a lower midline intraperitoneal or extraperitoneal approach.7 In general, if the terminal SCr was >2.0 mg/dL or the donor SCr was rising, then the kidney(s) were not used. In this series, 21 DDs had a calculated CrCl <50 mL/min, with the lowest being 36 mL/min.
Donor kidney biopsy was also used to assist in the evaluation of preexisting and terminal parenchymal pathology. A donor kidney biopsy showing >40% glomerulosclerosis or moderate to severe tubular, interstitial, or vascular changes was a contraindication to kidney utilization.7,10 Whenever possible, ECD kidneys were placed on a pulsatile perfusion apparatus to minimize preservation injury, maintain functional reserve, and provide another means of assessment.11 Within our organ procurement organization (OPO), locally procured kidneys are placed routinely on the perfusion pump at the accepting center's discretion or if the DD is older than 40 years, is hemodynamically unstable or oliguric, has a SCr >1.2 mg/dL, or has a history of hypertension or diabetes. ECD or donation after cardiac death (DCD) donor kidneys are routinely pumped, and imported kidneys meeting these criteria are likewise pumped if time permits. Although pump parameters were not exclusively used to discard kidneys, a flow rate >80 mL/min and a resistance <0.40 mm Hg after a minimum of 6 hours on the perfusion apparatus were considered thresholds for utilization.7,11
Recipient Selection
At our center, no specific upper age limit is an absolute contraindication to kidney transplantation, and this series included 19 recipients ≥70 years of age (oldest 77 years). All patients undergo a comprehensive pretransplant medical, psychosocial, and financial evaluation, with emphasis placed on the cardiovascular system and any other nonrenal organ failure to determine operative risks and physiologic age.7 Patients approved for transplantation are assigned a risk assessment (to aid in waiting list maintenance and follow-up), and a decision is made whether or not to list the patient as willing to accept an ECD kidney. In general, if the patient is younger than 40 years, highly sensitized (panel reactive antibody [PRA] titer >50%), morbidly obese (BMI >30 kg/m2), or a retransplant candidate, then it is recommended that the patient not be listed as willing to accept an ECD kidney. The final arbitration regarding ECD candidacy is made after consultation with the patient and referring physician. If the patient is not yet on dialysis, is doing well on dialysis, has a potential living donor, has a projected short waiting time (blood type AB), or is either hepatitis B or C positive, then we may elect not to list the candidate for an ECD kidney because in our experience these patients will get transplanted successfully with an SCD kidney.
At the time of transplantation, patients are selected on the basis of blood type compatibility, waiting time, human leukocyte antigen (HLA)-matching, a negative flow T and B cell crossmatch, and special listing for ECD (when applicable) in accordance with UNOS guidelines.7 Listing patients for an ECD kidney neither mandates nor restricts them to receiving an ECD kidney, as the decision to accept any kidney, whether from an ECD or SCD, is reaffirmed at the time of the offer with informed consent.
Whenever possible, ECD kidneys are used by matching estimated renal functional mass to recipient nephron need, including the use of DKTs (n = 15).7,9,12 However, recipients of DKTs are typically <60 years old because of the greater anesthetic and surgical risks associated with this longer procedure. Recipient selection for ECD kidneys is based on identifying low immunologic risk and low metabolic need patients such as primary transplant, recipient age >40 years, HLA matching, low PRA titer (usually 0%), BMI <25 kg/m2, and informed consent for either single or DKT.7
Immunosuppression
All DD kidney transplant patients received depleting antibody induction with either rabbit antithymocyte globulin (rATG, Thymoglobulin, Genzyme Corp., Cambridge, MA) at a dose of 1.5 mg/kg (maximum dose, 150 mg) based on actual body weight (n = 226) or alemtuzumab (Campath-1H, Millennium Pharmaceuticals, Cambridge, MA) 30 mg intravenous as a single intraoperative dose (n = 18). The first dose of rATG was given intraoperatively and subsequent rATG infusions were administered at alternate-day intervals for a minimum of 3 and maximum of 7 doses depending on initial graft function. Maintenance immunosuppression consisted of tacrolimus (TAC), mycophenolate mofetil (MMF), and tapering doses of steroids. The administration of TAC was delayed until the patient had exhibited a brisk diuresis and a declining SCr level (<4.0 mg/dL). Target 12-hour TAC trough levels were based on donor quality and recipient immunologic risk but typically ranged from 10 to 12 ng/mL for younger and 6 to 8 ng/mL for older recipients. In patients >60 years of age, the maximum MMF dose was kept at 500 mg twice daily. In the last year, 44 low immunologic risk patients have successfully undergone early steroid elimination. Details regarding our immunosuppressive regimen have been published previously.7
Anti-Infective Prophylaxis
All patients received surgical site prophylaxis with a first-generation cephalosporin for 24 hours, antifungal prophylaxis with low-dose fluconazole or nystatin for 1 to 2 months, and anti-Pneumocystis prophylaxis with sulfamethoxazole/trimethoprim (dapsone if allergic to sulfa) for 12 months. Antiviral prophylaxis consisted of oral valganciclovir for 3 to 6 months, depending on donor and recipient cytomegalovirus (CMV) serologic status. Specifics regarding drug dosing and duration have been published previously.7
Posttransplant Management
When appropriate, patients received aspirin prophylaxis. Treatment of hypertension, hyperlipidemia, anemia, diabetes, and other medical conditions was initiated as indicated, aiming to maintain the blood pressure <140/90 mm Hg, fasting serum cholesterol <200 mg/dL, hematocrit >30%, and fasting blood sugar <126 mg/dL. The diagnosis of renal allograft rejection was suggested by an unexplained rise in SCr level of >0.3 mg/dL or a 25% increase from baseline level and confirmed by ultrasound-guided percutaneous biopsy. Grade I rejection episodes were treated with 3 steroid boluses and/or an oral prednisone recycle. Grade I rejection episodes without biochemical evidence of improvement or unresolved infiltrates on a repeat biopsy within 2 to 3 weeks (persistent or steroid-resistant rejection) were treated with rATG rescue therapy. Grade II and III rejection episodes were also treated with rATG for 5 to 7 doses depending on biochemical and clinical response.7
CMV infection was defined as positive blood cultures (early antigen) or polymerase chain reaction (PCR) assay. Treatment of CMV infection consisted of intravenous ganciclovir or oral valganciclovir for 2 to 4 weeks and selective use of CMV hyperimmune globulin (CytoGam, MedImmune, Inc., Gaithersburg, MD) concomitant with a reduction in immunosuppression.7 Polyomavirus-induced nephropathy (PVN) was diagnosed on the basis of renal allograft biopsy and treated with a reduction in immunosuppression and conversion from MMF to leflunomide.13 Surveillance monitoring of urine cytology for decoy cells, blood PCR for polyomavirus or CMV, and kidney transplant biopsies were not performed unless clinically indicated.
Posttransplant renal allograft function was evaluated by measuring SCr levels as well as calculating glomerular filtration rate (GFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) formula.14
Statistical Analysis
Data were compiled from both prospective and retrospective databases, with confirmation by medical record review in accordance with local Institutional Review Board guidelines and approval. Univariate analysis was performed by the unpaired t test for continuous variables, the χ2 test for categorical variables, and the Fisher exact test when data were sparse. Unadjusted actual patient and graft survival rates were reported. Four-year actuarial patient and graft survival curves were also computed using the Kaplan-Meier method and compared using the log-rank test. Categorical data were summarized as proportions and percentages, and continuous data were summarized as means and standard deviations. A two-tailed P value of <0.05 was considered to be significant.
RESULTS
Over a 47-month period, we performed a total of 244 DD kidney transplants into adult recipients, including 143 (58.6%) from SCDs and 101 (41.4%) from ECDs. The majority of ECD kidneys were refused by multiple other transplant centers, and many were targeted for discard. ECD kidneys were used by matching estimated renal functional mass to recipient metabolic need, including the use of DKTs (n = 15) exclusively into recipients below 60 years of age.
Donor, recipient, and transplant characteristics are depicted in Table 1. Not surprisingly, ECDs were nearly twice as old and had a higher BMI, increased incidence of cerebrovascular brain death and preexisting donor hypertension, and a lower estimated CrCl compared with SCDs (all P < 0.01). Mean donor SCr levels, cold ischemic times, and proportion of DCD donors were similar in both groups. However, ECD kidneys were 2 times more likely to be preserved with pulsatile perfusion (P < 0.01) compared with SCD kidneys.
TABLE 1. Donor, Recipient, and Transplant Characteristics
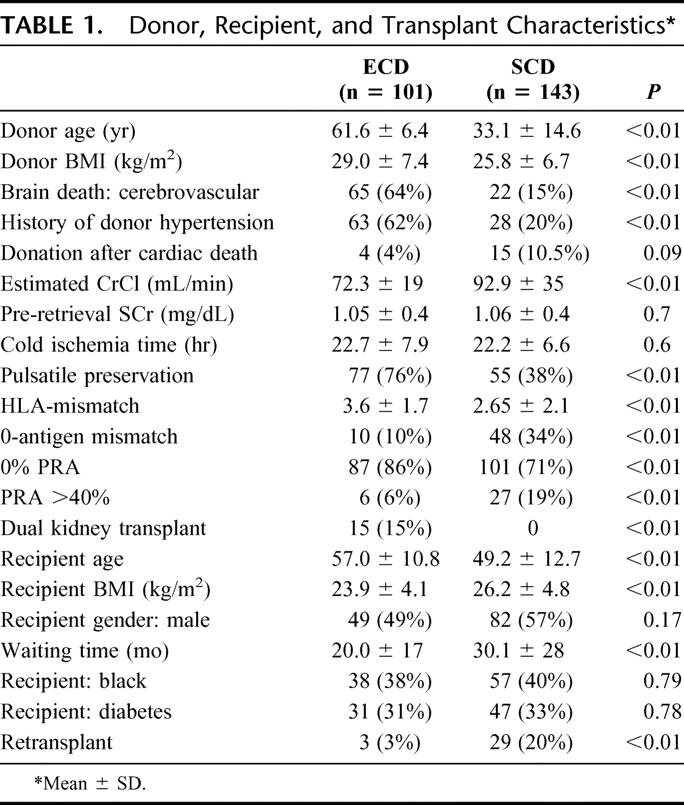
ECD kidney transplant recipients were older by a mean of 8 years, had a lower BMI, had less preexisting allosensitization, had fewer 0-antigen mismatches and less HLA-matching, and had a shorter waiting time (Table 1) compared with SCD kidney recipients (all P <0.01). Other demographic (gender, ethnicity, diabetes) and transplant characteristics were similar between groups except for more retransplants in the SCD group, and all DKTs were performed in the ECD group (both P < 0.01).
Outcomes are shown in Table 2. A total of 188 patients (77%) had at least 1-year follow-up. Actual patient (93%) and kidney graft (83%) survival rates were similar between groups with a mean follow-up of 24 months. Four-year actuarial patient (Fig. 1) and kidney graft (Fig. 2) survival rates were likewise similar. There were a total of 16 deaths in the study including 7 (6.9%) in the ECD and 9 (6.3%) in the SCD groups (P = not significant). Three patients died within 1 month of transplant in the ECD group, whereas there were 2 early deaths in the SCD group; only 1 of these early deaths was related to poor graft function in an ECD recipient. The remaining late deaths occurred at a mean of 24 months in ECD and 16 months in SCD kidney recipients (P = not significant). A total of 42 kidney grafts were lost, including 17 (16.8%) in the ECD and 25 (17.5%) in the SCD groups (P = not significant). There were 7 graft losses within 3 months of transplant in the ECD group compared with 10 early graft losses in the SCD group. The remaining late graft losses occurred at a mean of 20.1 months in ECD and 20.5 months in SCD kidney recipients. DWFG occurred in 4 (4.0%) ECD and 9 (6.3%) SCD kidney recipients.
TABLE 2. Outcomes
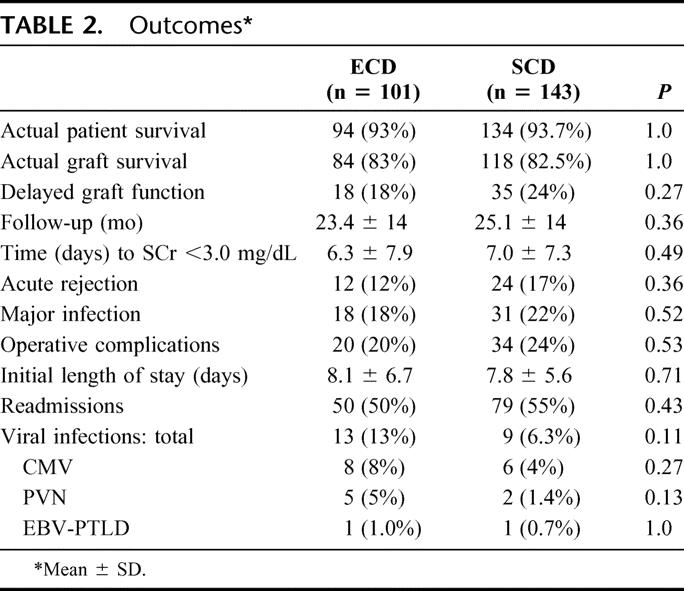
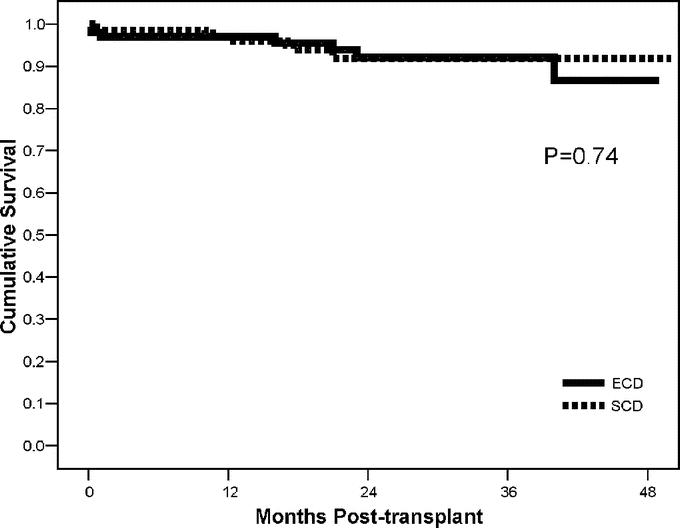
FIGURE 1. Four-year actuarial patient survival rates in ECD versus SCD kidney transplant recipients.
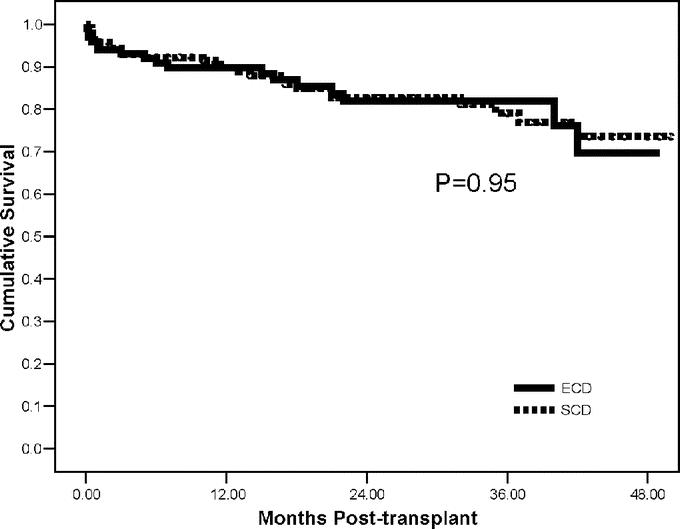
FIGURE 2. Four-year actuarial kidney graft survival rates in ECD versus SCD kidney transplant recipients.
The incidences of DGF, acute rejection, operative complications, and major infections were similar among groups (Table 2). Preserving ECD kidneys by pulsatile perfusion lowered the rate of DGF from 33% to 13% (P = 0.03). The presence of DGF was not a significant risk factor for subsequent acute rejection in either group but did have a significant impact on graft survival in both groups combined (38.5% incidence of graft loss in patients with DGF versus 11.5% without, P < 0.001). The magnitude of this effect was similar in each group. In addition, the presence of acute rejection was a significant risk factor for graft loss in both groups combined (50% incidence of graft loss in patients with acute rejection versus 11.5% without, P < 0.001). However, the impact of this detrimental effect was less pronounced in the ECD group (P = 0.11). The majority of operative complications in both groups were wound-related issues (bleeding, fluid collections, dehiscence, or infection).
Initial graft function (the time to achieve a SCr level <3.0 mg/dL), length of initial hospital stay, readmissions, and resource utilization were comparable between groups (P = not significant). However, the incidence of viral infections (CMV, PVN, Epstein-Barr virus [EBV]) was twice as high in ECD recipients (12.9% ECD versus 6.3% SCD, P = 0.11). Of the 14 CMV infections, 5 occurred in patients with primary CMV exposure and 8 in the setting of both donor and recipient seropositive for CMV. Of the 7 cases of PVN, 2 (both ECD recipients) resulted in graft loss. There was 1 case of EBV-associated posttransplant lymphoproliferative disease (PTLD) in each group.
Both groups initially stabilized renal function at approximately 60% of the estimated donor CrCl, so that renal function followed longitudinally was consistently better in SCD recipients (P < 0.05). Six-, 12-, 24-, and 36-month SCr levels and MDRD-calculated GFRs are displayed in Figures 3 and 4, respectively.
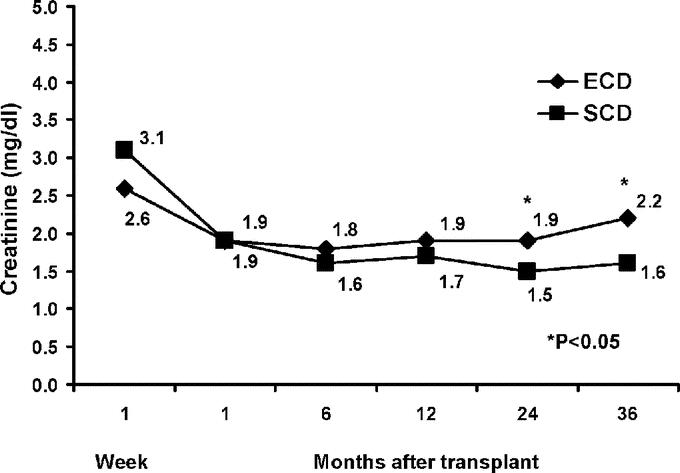
FIGURE 3. Renal allograft function up to 36 months posttransplant as measured by mean serum creatinine levels in ECD versus SCD kidney transplant recipients.
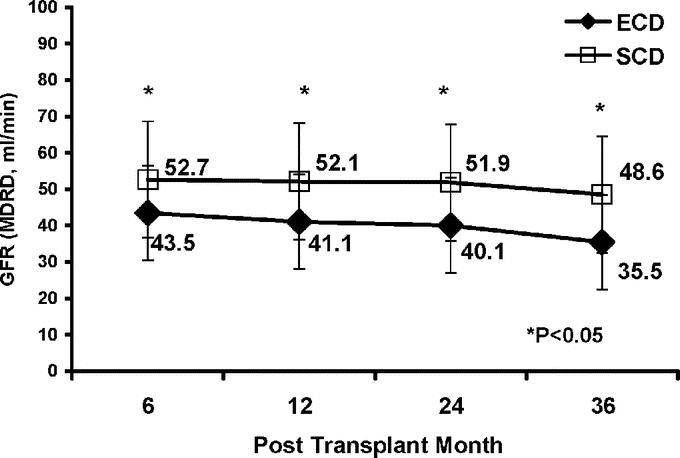
FIGURE 4. Renal allograft function up to 36 months posttransplant, by mean calculated glomerular filtration rate (GFR) using the abbreviated MDRD formula, in ECD versus SCD kidney transplant recipients.
Approximately 40% of patients in both groups were black. The incidence of DGF was higher in black recipients in both groups combined (32.6% black versus 14.1% non-black, P < 0.001), but the magnitude of this effect was actually less in ECD recipients (26% black versus 13% non-black, P = 0.11). Black recipients also had a much higher rate of acute rejection (26.3% versus 10.5% in non-black recipients, P = 0.02) in the SCD group. Conversely, black recipients of ECD kidneys actually had a lower rate of acute rejection (7.9% black versus 14.3% non-black, P = 0.53). There were no differences in patient survival, 36-month MDRD-calculated GFR, or viral infections in black compared with non-black recipients. However, the rate of kidney graft loss was higher in black recipients in both groups combined (26.3% graft loss in black versus 11.4% in non-black recipients, P = 0.003), although again the magnitude of this effect was less pronounced in the ECD group (24% black versus 13% non-black, P = 0.18). Four-year actuarial kidney graft survival rates for black versus non-black recipients according to donor group are shown in Figure 5.
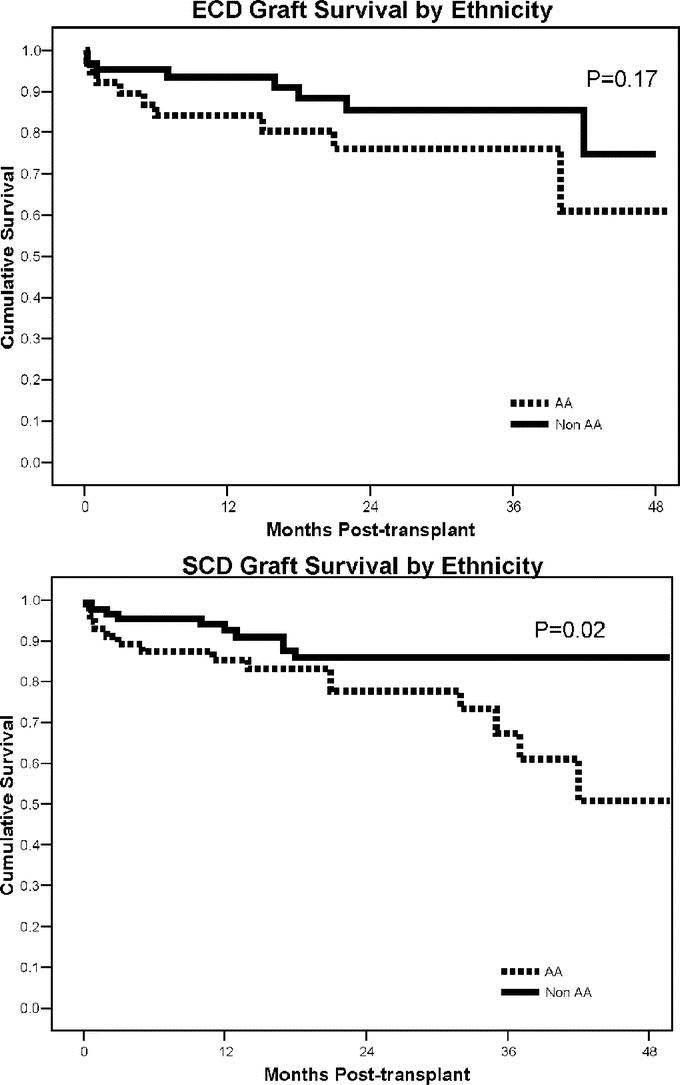
FIGURE 5. Four-year actuarial kidney graft survival rates in black versus non-black recipients according to ECD versus SCD study groups.
DISCUSSION
The annual incidence of new cases of kidney failure is approximately 340 per million individuals and >50% of these 100,000 incident patients are 65 years of age and older.15 At present, there are more than 350,000 patients on dialysis in the United States. The risk of end-stage renal disease increases disproportionately with age, and more than 150,000 patients in the United States over 65 years of age currently receive renal replacement therapy.15 Fewer than 20% of the dialysis population are actually listed as candidates for kidney transplantation, and it is not unrealistic to presume that many other patients currently on dialysis could benefit from kidney transplantation. The loss of quality and quantity of life by those on the waiting list remains a staggering and sobering reality. Among the treatment modalities that are available for patients with kidney failure, kidney transplantation is preferred because it is associated with an improved life expectancy, better quality of life, and is cost-effective both for patients and payers.5,16
In response to the increasing disparity between organ supply and demand, a number of new initiatives have been undertaken, such as liberalizing criteria for living and deceased donors, performing DKTs from donors at the extremes of age, increasing organ yield from ECDs and DCDs, using incompatible living donors, considering anonymous living donors and matching donor websites, and arranging paired kidney exchanges.4–7,9–12,17–20 In the recent past, ECDs were referred to as marginal, nonideal, older, unsuitable, inferior, suboptimal, damaged, high-risk, or extended (criteria) donors.4–7,9,12,21–23 Many kidneys from these donors, because of limited nephron mass, were discarded or transplanted by some centers either as DKTs or as “the kidneys that nobody wanted.”24
In an attempt to study, standardize, and promote the use of ECD kidneys, a retrospective analysis of primary DD adult kidney transplants was performed by the Scientific Registry of Transplant Recipients, and a consensus definition of an ECD kidney was developed according to basic donor characteristics.4,6 Based on analysis of the data, ECD kidneys were defined as having a 70% greater risk of graft failure when compared with a reference group of nonhypertensive donors aged 10 to 39 years whose cause of brain death was not cerebrovascular and whose terminal SCr level was ≤1.5 mg/dL. During the study period spanning 1995 to 2000, 14.8% of transplanted kidneys had a relative risk of graft loss >1.7 and 39% of these procured kidneys were ultimately discarded.4,6 In October 2002, UNOS introduced new policy that not only defined but addressed special allocation issues pertaining to ECDs.25
The value of using ECD kidneys has been questioned because there are data to suggest that these kidneys have a higher rate of primary nonfunction, DGF, acute rejection, and a greater susceptibility to preservation injury, drug toxicity, and posttransplant hypertension.4–7, 9, 21–24 In addition, ECD kidneys appear to be more resource-intensive, costly, and not appropriate for retransplantation.23,26–28 Moreover, the longevity of an ECD kidney is thought to be much shorter, with the half-life estimated to be 6 to 8 years compared with 10 to 12 years with an SCD kidney from a DD.4–7,23 By definition, the durability of an ECD kidney is limited, and the majority of these kidneys are doomed to premature obsolescence compared with SCD kidneys if they are transplanted into unselected recipients.
For these reasons, guidelines have been promulgated regarding the appropriate use of ECD kidneys to include recipients older than 60 years, diabetic patients older than 40 years, patients doing poorly on dialysis or with dialysis access failure, or patients with limited life expectancy.4–6,23 These guidelines are based on the principle of improving access to transplantation for patients whose life expectancy is less than their predicted waiting time for a kidney. Transplantation has become the practice of rationing, with transplant centers functioning as gatekeepers rather than providers. The ethics of transplantation mandate that a balance is reached between medical utility and justice. The optimal use of ECD kidneys remains poorly defined because of the broad spectrum of quality that the current definition encompasses and the looming specter of predicted poorer intermediate-term outcomes.29,30
It is our contention that ECD kidneys are defined by suboptimal nephron mass, so that it may be inappropriate to place an ECD kidney into a high medical (or immunologic) risk patient unless that recipient is matched according to nephron (or metabolic) need. If one places a “high-risk” DD kidney into a “high-risk” recipient, then it is not unexpected that the clinical outcomes will be inferior and graft durability will be compromised. Since donor and recipient risk factors for graft survival and longevity are cumulative, it may be more appropriate to transplant an ECD kidney into a low-risk (and low functional need) recipient.7 Consequently, whenever possible, we attempted to profile the potential recipient based on the estimated need for nephron mass, using criteria such as older age (>40 years), low BMI (<25 kg/m2), low immunologic risk (primary transplant, 0% PRA, HLA matching), and informed consent, rather than automatically relegating the ECD kidney to the next patient on the top of the waiting list. Since many of these kidneys were refused by all other centers, we were often afforded the opportunity of selecting an appropriate recipient matched to the estimated nephron mass of the donor.
Our management protocol for the ECD kidney was based on a number of potential nephron-sparing maneuvers, including minimizing cold ischemia time, pulsatile perfusion preservation, front-loaded immunosuppression with depleting antibody induction to minimize preservation injury and acute rejection, delayed administration of a calcineurin inhibitor, and targeting lower TAC levels long-term to minimize drug toxicity.7 Since many of the ECD recipients were profiled based on low immunologic risk and had a negative flow T- and B-cell crossmatch, we felt comfortable with targeting lower TAC levels to achieve a balance between efficacy and toxicity.
In actuality, donor age is a surrogate for nephron mass, whereas recipient age is a surrogate for generalized atherosclerosis.31 Older or ECD kidneys have decreased renal blood flow and intrinsic GFR, and are accompanied by a number of histopathologic changes such as glomerulosclerosis, tubular atrophy, vascular changes, and interstitial fibrosis.7,10,31 In addition, older donor kidneys lose the ability to undergo compensatory hypertrophy and are more susceptible to nephrotoxicity, hypertension, and immune-mediated damage. Consequently, the concept of age matching has become popular as a method of optimizing utilization of ECD kidneys.31,32 The results of kidney transplantation in the elderly are indeed quite similar to those in younger recipients, especially when considering death-censored analyses. Conversely, a number of studies have demonstrated that the use of older donor kidneys transplanted into younger recipients results in inferior outcomes, suggesting that donor age is a more important risk factor than recipient age.31,32
In this study, ECDs were nearly twice as old and had significantly more comorbid conditions, resulting in an estimated renal functional reserve that was only 77% of SCDs. Donor BMI was significantly higher in the ECD group. Implementation of the UNOS policy had no discernible effect on minimizing preservation time, which was greater than 22 hours in both groups and reflects the fact that many of the kidneys in both groups (ECDs and 0-antigen mismatch SCDs) were imported from other OPOs. Despite these drawbacks, the use of pulsatile perfusion preservation in the majority of ECD kidneys remarkably led to a DGF rate that was numerically (but not statistically, P = 0.34) lower than SCD kidneys. It is interesting to note that the cited national incidence of DGF with ECD kidneys is 33%, which is exactly our rate of DGF in ECD kidneys that were not preserved by pulsatile perfusion.
Although ECD kidney transplant recipients were on average 8 years older and had a lower mean BMI compared with SCD kidney recipients, fewer than half (43%) were ≥60 years of age and only 31% were diabetic. In the SCD group, 34% of patients received 0-antigen mismatched kidneys through the national sharing system, but more patients in this group were sensitized or received retransplants. Time on the waiting list was significantly shorter for ECD kidney recipients by 10 months. It is tempting to speculate that at least some of these patients may not have survived long enough to receive a kidney through conventional allocation methods, particularly since 19 were ≥70 years of age. None of the ECD kidney recipients was selected based on either medical urgency or dialysis access failure. Importantly, donor BMI in the ECD group was much higher than recipient BMI, whereas in the SCD group, donor and recipient BMIs were similar. In essence, a higher BMI in an older donor translates into a predicted CrCl by the Cockcroft-Gault formula that is “matched” to an adult recipient with a lower BMI and predicted lower nephron need.
Approximately 40% of recipients in both groups were black. We analyzed a number of established risk factors known to adversely influence kidney graft survival rates such as ethnicity, DGF, and acute rejection in both ECD and SCD groups. Although similar risk factors for graft loss were present in both groups, somewhat surprisingly the magnitude of the effect was less pronounced in black recipients of ECD compared with SCD kidneys. In other words, although black recipients have higher rates of DGF, acute rejection, and graft loss compared with non-black recipients, transplanting an ECD kidney into a black recipient does not appear to provide any additional jeopardy when compared with an SCD kidney transplanted into an black recipient.
Consequently, by avoiding high BMI, young, and presensitized patients, we were able to achieve acceptable intermediate-term outcomes with ECD kidneys that were comparable to concurrently transplanted SCD kidneys. A number of clinical outcomes and parameters of morbidity and resource utilization were similar between groups. We noted neither an increased susceptibility to DGF nor an increased risk of acute rejection in the ECD group. Not surprisingly, intermediate-term renal function followed longitudinally was consistently better in SCD kidney recipients. Interestingly, the main adverse effect that was observed in our study was a somewhat greater propensity (P = 0.11) to viral infections (CMV, PVN, or EBV) in the ECD kidney recipients, suggesting either over-immunosuppression (in an older recipient population) or perhaps preexisting injury in the allograft contributing to subsequent viral activation.
Although ECD kidney transplants currently comprise 15% of national DD activity, more than 40% of DD kidney transplants at our center are performed from ECDs. Based on this experience, we do not think it is necessary to match the life expectancy of the recipient with the kidney, nor do we think that the use of ECD kidneys can be optimized only if they significantly decrease waiting time. Moreover, we remain unconvinced that ECD kidneys represent an inferior resource and believe that appropriate donor and recipient profiling may offset some of the intrinsic difficulties heretofore associated with ECD kidneys. By incorporating nephron mass matching and nephron-sparing measures into the allocation and management algorithms, we think that one can achieve excellent intermediate-term outcomes with ECD kidneys that rival those currently being attained with SCD kidneys. Moreover, because physiologic age is more important than chronologic age, there exists a cohort of low risk elderly recipients who predictably will do well with an ECD kidney. Age-matching provides both a physiologic match (nephron mass demand and supply) and an immunologic match for some patients (for example, low BMI and low PRA recipients). However, age by itself is not an adequate predictor of overall risk, so understanding the factors that determine outcome and longer-term follow-up are necessary to fully delineate the risks and benefits of transplanting ECD kidneys.
Discussions
Dr. Ronald W. Busuttil (Los Angeles, California): As I am going to show you in the next presentation, what works for the kidney works for the liver. I would like Dr. Stratta, if he would, to comment a little bit more on the matching of the high-risk donor for kidney transplantation with an appropriate recipient risk patient and how he feels that impacts on the outcome.
Dr. Robert J. Stratta (Winston-Salem, North Carolina): This is really one of the basic principles of our paper and our experience. These kidneys by definition are high-risk kidneys, and if you place these kidneys into high-risk recipients, you are going to achieve inferior outcomes, certainly intermediate term.
So our definition, if you will, of an expanded criteria recipient would be someone who is older rather than younger, someone with a lower rather than a higher BMI, because of the nephron mass matching that we would like to achieve. We would prefer HLA matching, if possible. We would also prefer someone who is a primary transplant, who has a low panel reactive antibody titer (PRA), and, of course, this needs to be done with informed consent.
Dr. James J. Wynn (Augusta, Georgia): Dr. Stratta has clearly outlined for us what may be the central problem facing transplantation today: we simply have too few organs. As a result, we are faced with the need to utilize organs for transplantation that we would not have considered only a few years ago, and the imperative to utilize these less-than-ideal organs wisely and effectively.
Dr. Stratta has presented outstanding outcomes from kidney transplants using so-called “expanded criteria donor” kidneys. His group's graft and patient survivals with ECD kidneys equal that with ideal kidneys, in a situation where, by definition, the failure rates would be expected to be at least 70% greater.
Several key factors have, I think, contributed importantly to their outstanding results. Careful assessment of donor organ structure and function guided their organ acceptance practices. Organ function was supported through the use of pump preservation. Immunosuppressive therapy was tailored to the need of the individual patient, with the use of less intense calcineurin inhibitor therapy in recipients of ECD kidneys. Perhaps most importantly, good surgical judgment was used to pick the right recipients for their ECD kidneys, avoiding their use in patients requiring large nephron mass.
Bob, I do have a few of questions: 1) Your high utilization of ECD kidneys has been dependent on the use of a large number of imported kidneys. As a result, your ECD cold ischemia times are no shorter than that seen with SCD kidneys. Did you see any beneficial effect of shorter cold ischemia with locally recovered ECD kidneys or with locally recovered kidneys compared to imports? 2) What percentage of your locally recovered kidneys qualify as ECD kidneys: What percent of locally recovered kidneys were you forced to discard? 3) Your manuscript outlines pump and biopsy characteristics that would lead you to not utilize kidneys. Are there clinical criteria that would similarly lead you to judge that a kidney is simply untransplantable?
Dr. Robert J. Stratta (Winston-Salem, North Carolina): With regard to the imported versus exported kidneys, that is really an important concept. Where the allocation system tends to fall down is if we receive an organ offer from outside our donor service area, certainly outside our local OPO, but even if it is outside our region we will more times than not turn those kidneys down because of logistical issues. Those kidneys typically are not being pumped, and we are looking at getting them to our facility and transplanting them with cold ischemia times in excess of 30 hours. So actually, the location of the donor plays into the decision as to whether or not to accept an ECD kidney from a given donor.
We are in layers of sharing, and even with our locally procured kidneys, more times than not our ischemia times are beyond 24 hours. One of the pivotal ideas behind the whole ECD concept was to reduce cold ischemia time, but at least in our hands, we have not been able to reduce cold ischemia time, again, because many other centers have to turn these kidneys down before they eventually come to us.
When we do import kidneys, we try to pump them for a period of time, at least a minimum of 6 hours. So even though the majority of their ischemic time is cold storage, I think that pumping them even for brief periods of time can have a salutary benefit.
We certainly have to do better, though, in terms of shortening cold ischemic times. I think that, if we can get these kidneys transplanted within 12 hours, then we would not see the benefit of perfusion preservation that we are seeing in our experience. Locally, our organ procurement organization has about 15% to 20% ECDs. We are using the vast majority of them. But we are also getting ECD kidneys from a number of neighboring OPOs as well.
It is a gestalt in terms of determining whether or not to use the kidney from a given donor. A creatinine of 1.2 in a 65-year-old 110-pound woman is a lot different than a creatinine of 1.2 in a 65-year-old 225-pound man. So we look at donor size, kidney size, kidney biopsy, estimated creatinine clearance, and gross anatomy. If there are 3 or 4 renal arteries, we may turn it down, especially if it is going to be a longer cold ischemia. Unfortunately, we learn from our mistakes and our experience.
At our center, although we are using an inordinate number of these kidneys, our organ nonacceptance rate is still 50%. Now that is lower than the majority of other transplant centers. But at least half the time we are saying no to many of these offers. So I guess the next step is to analyze the characteristics of donor offers that we do not accept. I believe that the federal government will become involved with nonacceptance rates as well because it will be one of the quality indicators that transplant centers will be measured by in the future.
Dr. Paul C. Kuo (Durham, North Carolina): I think this paper is especially timely given the recent emphasis by governmental agencies looking not only at organ donation rates and organ procurement rates, but now organ acceptance rates. So transplant centers are going to be graded on the percentage of organs they accept. But with respect to the paper itself, his acceptance and utilization criteria are very well delineated and actually will serve as a nice recipe for those of us who wish to follow his path.
In the interest of time, I will just ask a philosophical question. It arises from a statement in the paper, and I think something that Dr. Stratta alluded to in his presentation, being that “we do not believe that it is necessary to match the life expectancy of the recipient with the kidney.”
If that is the case, and certainly there is no right answer for what I am about to pose to you, but what I would ask of you is, what should the programmatic goal for kidney transplantation be, what do recipients expect, and what should they expect from kidney transplantation when they come to a kidney transplant center?
Dr. Robert J. Stratta (Winston-Salem, North Carolina): Recipients expect perfection. They expect 100% success and the kidney to last a lifetime, which, as you know, is an unrealistic goal, but it is still their expectation. I don't think that will ever change, despite education.
You raise a very important point because there is this statement that we don't match the life expectancy of the recipient with the kidney. This statement can be interpreted one of two ways. Actually, the programmatic goal of kidney transplantation is for every patient to die with a functioning graft. Death with a functioning graft, as long as that death is not premature and not transplant related, is a success in kidney transplantation. So one might take that argument and say then, “Well, we should only use these kidneys in older donors and try to match the life expectancy of the kidney with the recipient.”
The point of that statement and a second way it could be interpreted is that, more times than not, that refers to a recipient who is high risk and has other medical conditions, a lot of comorbidities. I believe that trying to place these kidneys into high-risk medical recipients, as I mentioned, results in a high-risk transplant outcomes.
Moreover, we have already proved that point. We have national data to show that these kidneys by definition do less well when they are indiscriminately placed into recipients. If we discriminately place these kidneys into the appropriate recipients, I am not saying that the results are going to be identical to the standard criteria donors, but I think that we can do better than heretofore has been seen historically.
Dr. Ralph R. Bollinger (Durham, North Carolina): I congratulate you and your colleagues, Dr. Stratta, for using a lot of kidneys that up until now would have been wasted. But the cat is out of the bag. You have told everybody that with proper selection of recipients and good maintenance of donors, we should be using those kidneys. So my question has to do with the way that you determine the priority for your recipient.
We have already seen that in a national sharing system, say based upon HLA, that it isn't always outcome that determines how organs become shared. For example, in HLA, B and DR both improve survival when they are matched. But B has been eliminated because the untoward effect on equity and justice overwhelmed the good graft survival effect of the B locus. So only DR is matched.
How do you apply this new expanded donor approach where recipients are selected according to special criteria and still maintain the justice and equity of the allocation system?
Dr. Robert J. Stratta (Winston-Salem, North Carolina): That is a key question. It is one reason why I focused on the results of the blacks in this particular presentation. We wanted to ask ourselves the question: are we disadvantaging minority populations by using the kidneys the way that we are, or are we unknowingly creating a disadvantage? Interestingly, the black recipients were equal in both groups, 40%, and the outcomes were actually slightly better in the ECD recipients versus the SCD recipients, which is somewhat counterintuitive. But you are right, this is an outcomes-based allocation. It is based more on utility than it is justice.
But with that thought in mind, we are still trying to follow the spirit of the UNOS policy. We look at waiting time. We look at HLA matching. In addition, it is important to emphasize that most of these kidneys have been completely refused by other transplant centers. So each of those centers has made an intentional decision not to use these kidneys on any of their recipients.
We are making an intentional decision to use these kidneys selectively into some of our recipients. But as long as we are still trying to follow the spirit of the UNOS policy with waiting time, HLA matching, at least at this point in time, we do not appear to be creating a disadvantage with our minority population, which, as you know is a timely and key issue.
Dr. Ralph R. Bollinger (Durham, North Carolina): Going forward, when you are not the only institution using these criteria, all of the good recipients will tend to be pushed to this category. And that affects a large number of recipients in many centers, not just Winston-Salem.
Dr. Robert J. Stratta (Winston-Salem, North Carolina): This system works well when everyone else has said no to the kidneys, which for the majority of these circumstances is what occurred. But now that more centers are getting involved, it is like anything else, things get a lot trickier with regard to allocation. Although we list many of our patients for ECD kidneys, the purpose of the title, “A Spectrum or Specter of Quality,” is not that all ECD kidneys are created equal, but that some are better than others, and that is where judgment has to come in.
Footnotes
Reprints: Robert J. Stratta, MD, Department of General Surgery, Wake Forest University Baptist Medical Center, Medical Center Blvd., Winston-Salem, NC 27157-1095. E-mail: rstratta@wfubmc.edu.
REFERENCES
- 1.United Network for Organ Sharing. National data. Available at: http://www.unos.org/data. Accessed November 17, 2005.
- 2.Rosendale JD. Organ donation in the United States: 1988–2002. In: Cecka JM, Terasaki PI, eds. Clinical Transplantation 2003. Los Angeles: UCLA Immunogenetics Center, 2004:65–76. [Google Scholar]
- 3.Nathan HM, Conrad SL, Held PJ, et al. Organ donation in the United States. Am J Transplant. 2003;3(suppl. 4):29–40. [DOI] [PubMed] [Google Scholar]
- 4.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(suppl 4):114–125. [DOI] [PubMed] [Google Scholar]
- 5.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589–597. [DOI] [PubMed] [Google Scholar]
- 6.Port FK, Bragg JL, Metzger RA, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–1286. [DOI] [PubMed] [Google Scholar]
- 7.Stratta RJ, Rohr MS, Sundberg AK, et al. Increased kidney transplantation utilizing expanded criteria deceased organ donors with results comparable to standard criteria donor transplant. Ann Surg. 2004;239:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 9.Alfrey EJ, Lee CM, Scandling JD, et al. When should expanded criteria donor kidneys be used for single versus dual kidney transplants? Transplantation. 1997;64:1142–1146. [DOI] [PubMed] [Google Scholar]
- 10.Escofet X, Osman H, Griffiths DFR, et al. The presence of glomerular sclerosis at time zero has a significant impact on function after cadaveric renal transplantation. Transplantation. 2003;75:344–346. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenday CJ, Cooper M, Kraus E, et al. The hazards of basing acceptance of cadaveric renal allografts on pulsatile perfusion parameters alone. Transplantation. 2003;75:2029–2033. [DOI] [PubMed] [Google Scholar]
- 12.Tan JC, Alfrey EJ, Dafoe DC, et al. Dual-kidney transplantation with organs from expanded criteria donors: a long-term follow-up. Transplantation. 2004;78:692–696. [DOI] [PubMed] [Google Scholar]
- 13.Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352:1157–1158. [DOI] [PubMed] [Google Scholar]
- 14.Poge U, Gerhardt T, Palmedo H, et al. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005;5:1306–1311. [DOI] [PubMed] [Google Scholar]
- 15.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2004 Annual Data Report. Am J Kidney Dis. 2005;45(suppl 1):16–28. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 17.Ugarte R, Kraus E, Montgomery RA, et al. Excellent outcomes after transplantation of deceased donor kidneys with high terminal creatinine and mild pathologic lesions. Transplantation. 2005;80:794–800. [DOI] [PubMed] [Google Scholar]
- 18.Dharnidharka VR, Stevens G, Howard RJ. En-bloc kidney transplantation in the United States: an analysis of United Network of Organ Sharing (UNOS) data from 1987 to 2003. Am J Transplant. 2005;5:1513–1517. [DOI] [PubMed] [Google Scholar]
- 19.Lee CY, Tsai MK, Ko WJ, et al. Expanding the donor pool: use of renal transplants from non-heart-beating donors supported with extracorporeal membrane oxygenation. Clin Transplant. 2005;19:383–390. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery RA, Zachary AA, Ratner LE, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA. 2005;294:1655–1663. [DOI] [PubMed] [Google Scholar]
- 21.Alexander JW, Zola JC. Expanding the donor pool: use of marginal donors for solid organ transplantation. Clin Transplant. 1996;10:1–19. [PubMed] [Google Scholar]
- 22.Ratner LE, Kraus E, Magnuson T, et al. Transplantation of kidneys from expanded criteria donors. Surgery. 1996;119:372–377. [DOI] [PubMed] [Google Scholar]
- 23.Schnitzler MA, Whiting JF, Brennan DC, et al. The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation. 2003;75:1940–1945. [DOI] [PubMed] [Google Scholar]
- 24.Lee CM, Scandling JD, Shen GK, et al. The kidneys that nobody wanted: support for the utilization of expanded criteria donors. Transplantation. 1996;62:1832–1841. [DOI] [PubMed] [Google Scholar]
- 25.UNOS Policy 3.5.1. Expanded Criteria Donor Definition and Point System. Richmond, VA: United Network for Organ Sharing, 2002. [Google Scholar]
- 26.Jacobbi LM, McBride VA, Etheredge EE, et al. The risks, benefits and costs of expanding donor criteria. Transplantation. 1995;60:1491–1496. [DOI] [PubMed] [Google Scholar]
- 27.Whiting JF, Zavala EY, Cohen DS, et al. Economic costs of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments. Transplantation. 2000;70:755–760. [DOI] [PubMed] [Google Scholar]
- 28.Sellers MT, Velidedeoglu E, Bloom RD, et al. Expanded-criteria donor kidneys: a single-center clinical and short-term financial analysis cause for concern in retransplantation. Transplantation. 2004;78:1670–1675. [DOI] [PubMed] [Google Scholar]
- 29.Schold JD, Kaplan B, Baliga RS, et al. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5:757–765. [DOI] [PubMed] [Google Scholar]
- 30.Baskin-Bey ES, Kremers W, Stegall MD, et al. United Network for Organ Sharing's expanded criteria donors: is stratification useful? Clin Transplant. 2005;19:406–412. [DOI] [PubMed] [Google Scholar]
- 31.Stratta RJ, Sundberg AK, Rohr MS, et al. Optimal utilization of older donors and recipients in kidney transplantation. Surgery. 2006 (in press). [DOI] [PubMed]
- 32.Meier-Kriesche HU, Schold JD, Gaston RS, et al. Kidneys from deceased donors: maximizing the value of a scarce resource. Am J Transplant. 2005;5:1725–1730. [DOI] [PubMed] [Google Scholar]


