Abstract
Objective:
Laparoscopic myotomy is the preferred treatment of achalasia. Our objectives were to assess the long-term outcome of esophageal myotomy and to identify preoperative factors influencing the outcome.
Methods:
Preoperative and long-term outcome data were collected from patients undergoing laparoscopic myotomy for achalasia at our institution. The primary endpoint of the study was the postoperative change (delta) in dysphagia score. This score was calculated by combining the frequency and the severity of dysphagia. Persistent postoperative dysphagia was defined as 1 standard deviation less than the mean delta score of all patients. Logistic regression was used to identify independent preoperative factors associated with successful myotomy.
Results:
A total of 200 consecutive patients were included in the study. At a mean follow-up of 42.1 months, the mean delta dysphagia score was 7.1 ± 2.6; therefore, the myotomy was considered successful when the delta score was >4.5. According to this definition, 170 (85%) patients achieved excellent dysphagia relief (responders). Responders had higher preoperative low esophageal sphincter (LES) pressure than nonresponders: 42.6 ± 13.1 versus 23.8 ± 7.0 mm Hg (P = 0.001). High preoperative LES pressure remained an independent predictor of excellent response in the multivariate logistic regression model. Patients with LES pressure >35 mm Hg had an odds ratio of 21.3, making more likely to achieve excellent dysphagia relief after myotomy compared with those with LES pressure ≤35 mm Hg (odds ratio, 21.3; 95% confidence interval, 6.1–73.5, P = 0.0001).
Conclusion:
Laparoscopic myotomy can durably relieve symptoms of dysphagia. Elevated preoperative LES pressure represents the strongest positive outcome predictor.
The objectives were to assess the long-term outcome of esophageal myotomy for achalasia and to identify preoperative factors influencing the outcome. A total of 200 patients were included in the study. The multivariate logistic regression model demonstrated that excellent dysphagia relief is more common in achalasia patient with high preoperative lower esophageal sphincter pressure.
In North America, esophageal achalasia is a rare idiopathic motility disorder that affects 1 in 100,000 individuals; yet it is the most commonly diagnosed primary esophageal motility disorder, and second only to gastroesophageal reflux disease (GERD) as the most common functional esophageal disorder.1 The widespread availability of pneumatic dilation, botulinum toxin injections and minimally invasive surgical techniques for myotomy has resulted in contentious therapeutic strategies. In general, all treatments are focused on the reduction of lower esophageal sphincter (LES) resting pressure, resulting in improved esophageal emptying and symptomatic relief of dysphagia. While partially and for the short-term responsive to endoscopic dilatation or botulinum toxin injections, most patients with achalasia eventually require operative management because only surgical treatment has been proven to provide long-term relief of dysphagia.2
Surgical treatment of achalasia has evolved dramatically over the past 13 years. Since the first report of laparoscopic Heller myotomy in 1991 by Cuschieri3 and thoracoscopic Heller myotomy by Pellegrini4 in 1992, minimally invasive surgery has became the gold standard for the treatment of achalasia. More recently, the laparoscopic management of esophageal achalasia has achieved widespread acceptance and is now the first line of therapy for patients with achalasia.2 The satisfactory short-term results of this procedure are well documented in several large series.5–11 In these studies, persistent postoperative dysphagia was observed in 10% to 30% of the patients, and little is known about the preoperative factors that may predict long-term resolution of dysphagia after laparoscopic esophageal myotomy. Therefore, the aim of this study was to identify preoperative patient characteristics, including manometric and clinical findings, that predict long-term successful outcome after laparoscopic esophageal myotomy.
METHODS
Patients
The study, following Institutional Review Board (IRB) approval, was conducted at Vanderbilt University Medical Center in Nashville, Tennessee. Preoperative data, including a structured dysphagia score, were prospectively collected on patients undergoing laparoscopic myotomy for achalasia at our institution.12 All patients underwent preoperative manometry. Patients were tested while off all antisecretory or promotility medications. Standard esophageal manometry was performed using a 6-channel solid-state probe (Sandhill Scientific, Highlands Ranch, CO) with a pull-through technique. The LES pressure was defined as the difference between the end expiratory gastric baseline pressure and the middle end-expiratory pressure just distal to the respiratory inversion point. Clinical diagnosis of achalasia was confirmed manometrically by the presence of simultaneous esophageal body contractions and a nonrelaxing LES. In the few patients who underwent preoperative manometry outside of our facility, manometric data were reanalyzed.
All the patients (n = 224) entered in our achalasia database from 1994 to 2004 were mailed a follow-up structured dysphagia score questionnaire or asked to complete one during a follow-up visit. The patients who did not return the questionnaires were allowed to answer the survey over the phone. At the end, 24 patients were excluded from the study because we were unable to obtain a postoperative dysphagia score from them.
Surgical Technique
Our technique for laparoscopic Heller myotomy has been previously described in detail.10 Briefly, after the phrenoesophageal ligament is divided and the fat pad excised exposing the anterior gastroesophageal junction, the myotomy is performed by incising the distal 4 to 6 cm of esophageal musculature. The myotomy is extended 1 to 2 cm onto the gastric cardia using cautery scissors or an ultrasonic scalpel. Intraoperative endoscopy is performed simultaneously to assess the adequacy of the myotomy, to gauge how far to carry the myotomy onto the gastric cardia, and to detect mucosal perforations. We added a Dor anterior hemifundoplication in selected patients early in our experience, those having intraoperative perforation, and more routinely in our most recent experience. In our early experience, we routinely performed a contrast swallow on postoperative day one in all patients to rule out an occult leak. We currently do it selectively for patients who had intraoperative perforation, and those who have postoperative chest pain, tachycardia, or fever. For patients not requiring swallow study, and those with a negative one, a clear liquid diet is started the morning after surgery, and patients are discharged later that day.
Outcome Measures
The primary end-point was postoperative change (delta) in dysphagia score.12 The score (range 0–10) was calculated by combining the frequency of dysphagia (0 = never, 1 = <1 day/wk, 2 = 1 day/wk, 3 = 2–3 days/wk, 4 = 4–6 days/wk, 5 = daily) with the severity (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = moderately severe, 5 = severe). The cutoff point used to dichotomize the outcome after laparoscopic esophageal myotomy was selected at one standard deviation below the mean delta score of the entire cohort. Patient with delta dysphagia score falling below the cutoff point were considered with unsuccessful outcome group. Patients who underwent endoscopic dilation and/or redo myotomy, after the initial myotomy, were also classified into this group.
Statistical Analysis
The data are presented as mean ± SD for continuous variables, and as counts or proportions (%) for categorical variables. Correlations were analyzed by univariate regression analysis (Pearson) and Spearman correlation coefficients.
Binary logistic regression analysis was used in both univariate and multivariate modeling to identify independent preoperative variables associated with long-term relief of dysphagia after Heller myotomy. Independent variables examined included 6 putative preoperative factors: age, gender, LES pressure, history of endoscopic dilation, history of botulinum toxin injection, and ASA class. The following model-building strategy was used. Univariate analysis using logistic regression was applied to identify significant associations with the dependent variable (surgical outcome). Transformed and untransformed data were used in the analysis. All independent variables with associations of P ≤ 0.1 then underwent multivariate analysis by simply entering them together using the backward stepwise method. The following cutoff points were used for the binary logistic regression stepwise methods: P = 0.05 for entry into the model and P = 0.10 for removal from the model. The “best” model for each case definition was based on the strength of the model (Hosmer and Lemeshow goodness-of-fit test), its clinical utility, and the biologic plausibility of the model. All continuous variables included in the final model were then categorized to improve ease of use. Model parameters were estimated by the maximum-likelihood method. From these estimates, odds ratios (OR) with 95% confidence intervals (CI) were computed.
The SPSS statistical software program (version 13.0, SPSS, Chicago, IL) was used for all analyses. Statistical significance was set at P < 0.05.
RESULTS
Follow-up dysphagia scores were available from 200 patients (93 females) who underwent laparoscopic myotomy at Vanderbilt University Medical Center. Patient ages ranged from 13 to 80 years (49.5 ± 1.5 years). The median hospital stay was 1 day (range, 1–3 days) There were 12 intraoperative perforations: 6 in the first 50 procedures and 6 in the last 150. All the esophageal perforations were identified and repaired at the time of surgery and patients had no sequelae. Four conversions to an open procedure occurred within the first 50 patients; there were no conversions in the last 150 patients. Morbidity was limited to 1 case of postoperative aspiration pneumonia. Dor fundoplication was performed in 53 patients. The postoperative symptom questionnaire was completed at a mean follow-up of 42.1 months. As shown in Figure 1, patients undergoing Heller myotomy had a mean decrease (delta) in dysphagia score of 7.1 ± 2.6. Therefore the esophageal myotomy was considered successful when the delta score was ≥5. According to this definition, 170 (85%) patients achieved excellent dysphagia relief (responders).
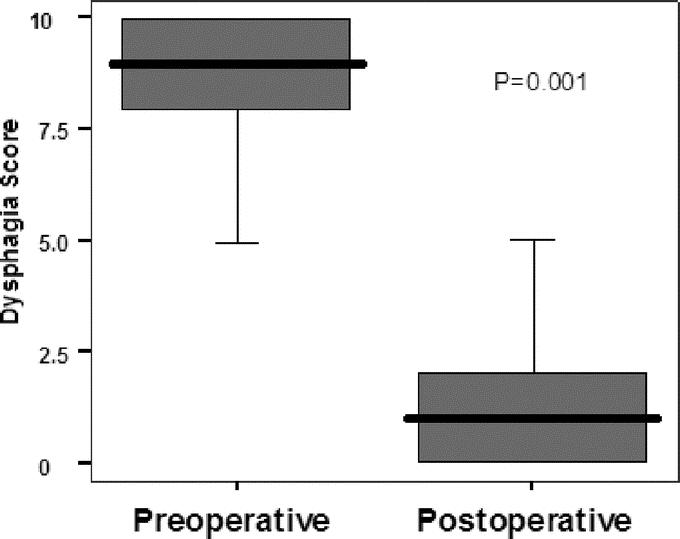
FIGURE 1. Preoperative and postoperative dysphagia scores. Data are shown as median (horizontal line), interquartile range (box), and 5th to 95th percentile (vertical line).
Sixteen patients required one or more postoperative procedures for continued symptoms of dysphagia after myotomy. Eight patients underwent bougie dilation, 3 had pneumatic dilation, 3 had Botox injections, and 4 had redo myotomies. Esophagectomy was ultimately undertaken in 3 patients. All these patients had a mean delta dysphagia score falling below the cutoff point and were included in the nonresponder group (n = 30).
Demographics and characteristics of the responder group compared with the nonresponder group are presented in Table 1. The 2 groups had similar preoperative demographic, past medical history, and clinical characteristics. Outcome comparison of patients with and without Dor fundoplication showed no significant difference. There were no learning curve issues with regard to dysphagia relief. As shown in Figure 2, the patients early in our series had as effective dysphagia relief as those later in the series (P = 0.37).
TABLE 1. Preoperative Demographic and Clinical Findings in the 2 Groups
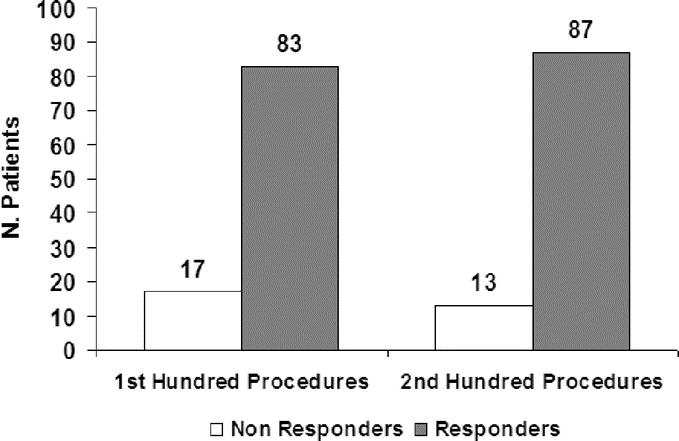
FIGURE 2. Distribution of responder and nonresponders according to the learning curve.
As shown in Figure 3, on univariate analysis, preoperative LES pressure was the only independent variable predictive of adequate relief of dysphagia. The responder group had higher preoperative LES pressure than nonresponders: 42.6 ± 13.1 versus 23.8 ± 7.0 mm Hg (P = 0.001).
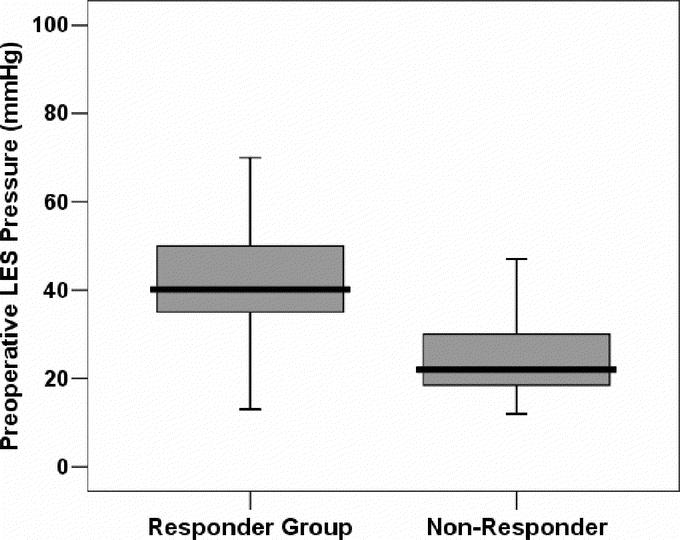
FIGURE 3. Preoperative LES pressure in the 2 groups. Data are shown as median (horizontal line), interquartile range (box), and 5th to 95th percentile (vertical line).
High preoperative LES pressure remained an independent predictor of excellent response in the multivariate logistic regression model after adjusting for covariates. To further simplify the model so that it could be easily used in a clinical setting, LES pressure was dichotomized using a receiver operating characteristic curves. The cutoff value was set at 35 mm Hg for the LES pressure. Patients with LES pressure >35 mm Hg had more than 21 times the likelihood to achieve excellent dysphagia relief after myotomy compared with those with LES pressure ≤35 mm Hg (Table 2).
TABLE 2. Results of Binary Logistic Regression Analysis
There was no correlation between delta dysphagia scores and postoperative LES pressure (r = 0.09; P = 0.4). Postoperative LES pressure, available from 75 patients, was similar in the 2 groups (responders, 14.4 ± 6.3; versus nonresponders, 13.2 ± 5.3 mm Hg, P = 0.3). However, the postoperative magnitude of change in LES pressure had a significant direct correlation (r = 0.52, P = 0.01) with delta dysphagia scores (Fig. 4).
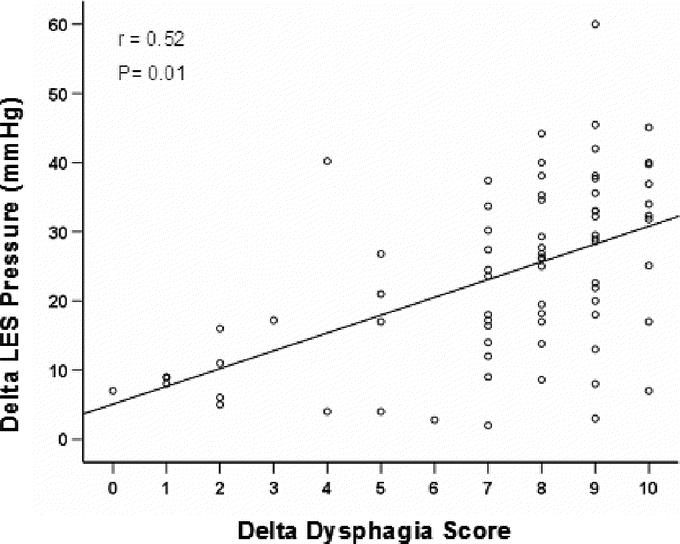
FIGURE 4. Delta LES pressure correlate positively with delta dysphagia score after esophageal myotomy. The line represents the linear regression fitting.
DISCUSSION
The most important outcome for the patient with achalasia is relief of dysphagia. Our study findings suggest that laparoscopic esophageal myotomy can durably relieve symptoms of dysphagia and a high preoperative LES pressure is predictor of long-term successful outcome. Patients with LES pressure > 35 mm Hg had more than 21 times the likelihood to achieve long term excellent relief of dysphagia after myotomy compared with those with LES pressure ≤ 35 mm Hg. This finding probably means that patients with a low preoperative LES pressure did not get as much relief because their relative decrease in outflow obstruction was less. This explanation is also corroborated by the significant direct correlation that we found between the magnitude of decrease in postoperative LES pressure and the degree of dysphagia relief. This significant correlation was also pointed out in our first report that encompassed 100 patients.10
Similarly, DeMeester et al, in a smaller cohort, have already pointed out that the preoperative LES sphincter pressure can affect the short-term outcome of laparoscopic esophageal myotomy for achalasia.13 On their multivariate analysis of preoperative variable, only a high resting LES pressure prior to surgery was a predictor of resolution of dysphagia after Heller myotomy. Divergent findings were published by Patti et al.6 In their study, preoperative low LES pressure had little influence on the outcome of laparoscopic Heller myotomy for achalasia. Our findings and those of Demeester were recently corroborated by a study from Guardino et al suggesting that pneumatic dilation is more effective in older patients with a higher basal LES pressure than in their younger counterparts with a lower LES pressure.14
We did not find that previous treatment with Botox or endoscopic dilation had any effect on the degree of dysphagia relief. Similarly to Rosemurgy et al, we found out that excellent dysphagia relief can be achieved even after failures of dilation and/or botulinum toxin injection therapy.11 Several authors have commented on the increased difficulty in performing a surgical myotomy in a patient who has had previous nonoperative treatment of achalasia, such as Botox injection or pneumatic dilation.15–17 Raftopoulos et al found that there was a trend toward a higher incidence of intraoperative esophageal perforation and recurrent dysphagia in patients with prior nonoperative treatment.17 Pneumatic dilation has been identified as a specific risk factor for complication with laparoscopic Heller myotomy in a previous report.15 In that report, 28% of patients with previous dilation suffered an intraoperative mucosal perforation, whereas none of the patients not previously treated had perforations. In our series of 200 patients, 12 perforations occurred but no statistically significant correlation was found with previous nonsurgical procedures.
There were no learning curve issues with regard to dysphagia relief. The patients early in our series had an effective relief as those later in the cohort. Gender, age, duration of the disease, and ASA class were not found to be predictor of surgical outcome.
The need for an antireflux procedure after myotomy has been one of the most controversial issues surrounding esophageal myotomy.18–20 We have been a vocal proponent of laparoscopic Heller myotomy without antireflux procedure during the last decade prior to our recently published prospective randomized study.12 The trial has finally answered this surgical dilemma showing that the addition of an anterior partial fundoplication significantly decreased the incidence of postoperative gastroesophageal reflux, when compared with no fundoplication. Since the end of the trial, routine application of Dor fundoplication is our current standard treatment. Equally important, the randomized study did not show any difference in terms of postoperative dysphagia scores among the 2 groups of patients. In this larger retrospective cohort, we confirmed that the addition of a Dor fundoplication does not affect the postoperative functional outcome of an esophageal myotomy. Similar findings were also demonstrated by Oelschlager et al with a different type of fundoplication.7 In their cohort of patients, the esophageal myotomy (extended 3 cm down from the external gastroesophageal junction) achieved low postoperative LES pressure despite of the addition of a Toupet fundoplication.
CONCLUSION
Our data suggest that laparoscopic esophageal myotomy can durably relieve symptoms of dysphagia. Excellent dysphagia relief is more common in achalasia patients with high preoperative LES pressure. Probability estimates based on our logistic regression model using this preoperative factor may help surgeons to identify patients who would most likely benefit from the esophageal myotomy.
ACKNOWLEDGMENTS
The authors thank Joan Kaiser, RN, MA, for her help in the follow-up of these patients.
Discussions
Dr. Bruce D. Schirmer (Charlottesville, Virginia): This paper by Drs. Torquati, Sharp, Richards, and Holzman at Vanderbilt represents the latest insight from their large experience of Heller myotomy which they have previously both shared with us at this Society's meeting as well as published.
The manuscript details the fact that preoperative manometry pressure is the only tested variable that correlates with postoperative symptom improvement after myotomy. In other words, the more you lower the LES pressure with myotomy, the better the results. The manuscript is succinct and well written, and I wish to thank the authors for sending it to me and asking me to comment.
I do have several questions for the authors.
What was the lowest preoperative dysphagia score? It seems from the manuscript it must have been above 5. Were any patients seen and evaluated, found to have a manometry consistent with achalasia, but a symptom score less than 5? How were they treated? Similarly, how would you treat such a patient hypothetically or do you think this situation does not exist?
You conclude your manuscript by saying that these data may serve as a guide for surgeons evaluating patients to determine who is an appropriate candidate for a myotomy. How should we treat the patient with a dysphagia score of 9 or 10 and a preoperative LES pressure of 30?
Finally, in view of the paramount importance of lowering LES pressure to symptomatic success of the operation, do the authors advocate exploration of development of a means of easily measuring intraoperative sphincter pressures? If so, I would like to hear their thoughts on the process.
Dr. Alfonso Torquati (Nashville, Tennessee): The first question is about what is the lowest preoperative dysphagia score in our series. It was 5, and only four patients had a score less than 6. Indeed, most of our patients cluster between 6 and 10 with a median of 9. We found a good correlation between the surgical outcome and the severity of residual achalasia reported by the patient. All patients who underwent redo myotomy for high residual LES pressure had also a very high postoperative dysphagia score. We know that there is not a good surrogate endpoint to define surgical outcome after myotomy for achalasia. But we think that the dysphagia score is still the best available right now.
The second question: what to do in the patient with a preoperative dysphagia score of 9 to 10 and LES pressure of 30. I think still this patient is a good candidate for Heller myotomy. However, in the preoperative counseling, we have to tell this patient that his/her chance of good dysphagia relief is lower than that observed in patients with higher LES pressure. But still I think some patients can defeat the odds. Our Vanderbilt football team finally defeated the University of Tennessee team after 23 years. Again, in general, most of the patients with high LES pressure do well after myotomy. However, some of the patients with low LES pressure, in our experience, they do equally well. It is our opinion that every achalasia patient with a life expectancy of more than 6 months is a suitable candidate for a Heller myotomy.
What about intraoperative manometry? This is a good question. We are not doing intraoperative manometry. We don't have the technology to do that. We have great results with endoscopy. Endoscopy has great advantages: helps in sizing the, myotomy also can tell you if you have a perforation. In fact, using intraoperative endoscopy, we found 12 perforations. Endoscopy is easier, it is more available in different centers, and it can be less technically demanding than intraoperative manometry.
Dr. Mark A. Talamini (San Diego, California): I would also like to thank the authors for an excellent presentation and an excellent study regarding factors that had a successful result following laparoscopic Heller myotomy. I also thank you for the manuscript ahead of time.
As is well known, achalasia patients tend to be a very optimistic patient group. Were you able to correlate your postoperative procedure scores with any other studies, such as contrast radiography, endoscopy, or postop manometry, to confirm the findings that you have found with regard to your preoperative manometry?
Also, it looks as if we may have found a procedure which at least in this excellent group's hands doesn't appear to have a learning curve. Perhaps you could comment on why you think that may be the case.
Your findings with regard to the preoperative therapeutic procedures, as has already been mentioned, are at odds with the last paper. Could this be a numbers effect? Or do you have any additional thoughts on why there are differences between those two studies?
Finally—perhaps you may have already answered this in response to Dr. Schirmer's question—based upon this work, can you offer a rational protocol for patients with achalasia in terms of when they should have perhaps a preoperative gastroenterologic procedure, if at all, and when in the course of their manometry pressures they should actually undergo an operation?
One last thing. Another difference between the last paper and this is the performance of the Dor procedure. Many groups across the country have found that to be, I guess I would charitably use the word “tricky.” So perhaps you could share with us some of the technical insights that you found in doing that operation successfully.
Dr. Alfonso Torquati (Nashville, Tennessee): Your first point is a well-taken point. We don't have any postoperative study other than manometry. In our experience, it has been very hard for us to bring back these patients for postoperative studies. It is much easier to send a questionnaire, to ask them over the phone. We had a hard time for our randomized trial to get these patients back for a postop evaluation. Again, the dysphagia score is not perfect. But I think it is a good surrogate point for surgical outcome after myotomy for achalasia.
Regarding the second question, I think we had a learning curve. We had six perforations in the first 50 patients, and then six in the last 150. Definitely, there was a learning curve in terms of perforation. Also, we had four conversions in our first 50 patients and zero conversions in the last 150. That is definitely a learning curve in terms of conversion and perforation. We did not have a learning curve in terms of dysphasia relief. We believe all our patients had a common denominator. All were done under endoscopy guidance. We feel that you can achieve a good relief of dysphagia if you use endoscopic visualization.
In terms of difference between our study and the Emory study, their primary endpoint was to look how preop endoscopy treatment affects outcome. Our primary endpoint was to find a preoperative variable that can predict the outcome. We considered preop endoscopy treatments, Botox injection, and dilation among the variables, and we did not find any predictive value. But again, the most striking difference was that less than 50% of our patient population underwent preoperative endoscopic procedures. In the Emory study, I think more than 70% of the patients underwent preoperative endoscopic procedures.
The last question is about our protocol for achalasia. I have partially answered that in my reply to Dr. Schirmer, but I would like to stop a second to explain better what our current algorithm is. Definitely, the majority of patients with achalasia are going to be good candidates for Heller myotomy as first approach because it is their best chance for relief of dysphasia. No other treatment is as efficacious, like surgery.
When you have a patient with failed myotomy, you have to evaluate the postop LES pressure. If the LES pressure is above 20, they should undergo redo myotomy. If the pressure is below 20 but above 12, we prefer to do a trial with a Botox injection. If the patient responds to Botox injection, that is an indication that could be a good candidate for redo myotomy. Patients with a LES pressure of less than 12 mm Hg are poor candidates for a redo myotomy, and in fact, we found that most of them end up having an esophagectomy.
How we perform our Dor fundoplication. I would like to invite everybody to look to our paper published in the Annals of Surgery in 2004. The paper has great illustration about our technique. It is very important to perform the fundoplication with four rows of sutures. It is critical.
Dr. J. Patrick O'Leary (New Orleans, Louisiana): You showed a wonderful slide of sigmoid dilatation of the esophagus, and then you went on to tell us that patients with higher LES pressures did better than patients with lower pressures. Was there a relationship between the degree of dilatation of the esophagus and the LES pressure? If I had a patient with that degree of dilatation of the esophagus, could I, with confidence, do the Heller myotomy and expect a good outcome or would an esophagectomy be a better choice?
Dr. Alfonso Torquati (Nashville, Tennessee): That is a very good point. We did not include that barium swallows data in our statistical analysis because we do not have complete data. To do an effective multivariate analysis, you have to have complete data, and we have a lot of missing barium swallows in our series. But I can tell you that there was a good correlation between the marked dilation of the esophagus on barium swallow and low preoperative LES pressure. And, we found that some of our patients that failed myotomy, they all had a dilated esophagus. Another problem is how you define dilation. Sometimes it is pretty tricky and subjective how you measure dilation on the swallow study. But definitely, we found some correlation between the esophageal dilation and the low LES pressure and also failure after myotomy.
Dr. Robert V. Rege (Dallas, Texas): I actually had the same question about the sigmoid esophagus. I think many of us think that mystery has a much worse outcome in these patients. I would like to you repeat this because you said there was a good correlation. Are you just measuring the dilated burned-out esophagus with your lower pressure? Or are there patients that had nondilated esophagus with low pressure who did poorly also?
Dr. Alfonso Torquati (Nashville, Tennessee): Most of the patients with dilated esophagus have very low LES pressure and usually very high dysphagia score, and definitely, those are predictors of a poor outcome after a Heller myotomy. Most of these patients end up pretty much having an esophagectomy. You can try to do a Heller myotomy and see if it works. Heller myotomy is a very low morbidity and low mortality operation, and it can be offered in some of these patients. But you have to sit down with the patient and discuss the possibly that it is not going to work for them and they have to consider an esophagectomy.
Footnotes
Dr. Torquati is supported by the Vanderbilt Clinical Research Scholar Award (NIH-K12RR017697-03).
Reprints: Alfonso Torquati, MD, Department of Surgery, Vanderbilt University Medical Center, D-5219 MCN, Nashville, TN 37232. E-mail: alfonso.torquati@vanderbilt.edu.
REFERENCES
- 1.Mayberry JF. Epidemiology and demographics of achalasia. Gastrointest Endosc Clin North Am. 2001;11:235–248. [PubMed] [Google Scholar]
- 2.Richards WO, Torquati A, Lutfi R. The current treatment of achalasia. Adv Surg. 2005;39:285–314. [DOI] [PubMed] [Google Scholar]
- 3.Shimi S, Nathanson LK, Cuschieri A. Laparoscopic cardiomyotomy for achalasia. J R Coll Surg Edinb. 1991;36:152–154. [PubMed] [Google Scholar]
- 4.Pellegrini C, Wetter LA, Patti M, et al. Thoracoscopic esophagomyotomy: initial experience with a new approach for the treatment of achalasia. Ann Surg. 1992;216:291–296; discussion 296–299. [DOI] [PMC free article] [PubMed]
- 5.Zaninotto G, Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic Heller myotomy for esophageal achalasia. Ann Surg. 2004;239:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorodner MV, Galvani C, Fisichella PM, et al. Preoperative lower esophageal sphincter pressure has little influence on the outcome of laparoscopic Heller myotomy for achalasia. Surg Endosc. 2004;18:774–778. [DOI] [PubMed] [Google Scholar]
- 7.Oelschlager BK, Chang L, Pellegrini CA. Improved outcome after extended gastric myotomy for achalasia. Arch Surg. 2003;138:490–495; discussion 495–497. [DOI] [PubMed]
- 8.Swanstrom LL. Laparoscopic esophagomyotomy for achalasia. Surg Endosc. 1999;9:286–292. [DOI] [PubMed] [Google Scholar]
- 9.Hunter JG, Trus TL, Branum GD, et al. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg. 1997;225:655–664; discussion 664–665. [DOI] [PMC free article] [PubMed]
- 10.Sharp KW, Khaitan L, Scholz S, et al. 100 consecutive minimally invasive Heller myotomies: lessons learned. Ann Surg. 2002;235:631–638; discussion 638–639. [DOI] [PMC free article] [PubMed]
- 11.Rosemurgy A, Villadolid D, Thometz D, et al. Laparoscopic Heller myotomy provides durable relief from achalasia and salvages failures after botox or dilation. Ann Surg. 2005;241:725–733; discussion 733–735. [DOI] [PMC free article] [PubMed]
- 12.Richards WO, Torquati A, Holzman MD, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405–412; discussion 412–415. [DOI] [PMC free article] [PubMed]
- 13.Arain MA, Peters JH, Tamhankar AP, et al. Preoperative lower esophageal sphincter pressure affects outcome of laparoscopic esophageal myotomy for achalasia. J Gastrointest Surg. 2004;8:328–334. [DOI] [PubMed] [Google Scholar]
- 14.Guardino JM, Vela MF, Connor JT, et al. Pneumatic dilation for the treatment of achalasia in untreated patients and patients with failed Heller myotomy. J Clin Gastroenterol. 2004;38:855–860. [DOI] [PubMed] [Google Scholar]
- 15.Morino M, Rebecchi F, Festa V, et al. Preoperative pneumatic dilatation represents a risk factor for laparoscopic Heller myotomy. Surg Endosc. 1997;11:359–361. [DOI] [PubMed] [Google Scholar]
- 16.Diener U, Patti MG, Molena D, et al. Laparoscopic Heller myotomy relieves dysphagia in patients with achalasia and low LES pressure following pneumatic dilatation. Surg Endosc. 2001;15:687–690. [DOI] [PubMed] [Google Scholar]
- 17.Raftopoulos Y, Landreneau RJ, Hayetian F, et al. Factors affecting quality of life after minimally invasive Heller myotomy for achalasia. J Gastrointest Surg. 2004;8:233–239. [DOI] [PubMed] [Google Scholar]
- 18.Lyass S, Thoman D, Steiner JP, et al. Current status of an antireflux procedure in laparoscopic Heller myotomy. Surg Endosc. 2003;17:554–558. [DOI] [PubMed] [Google Scholar]
- 19.Richards WO, Sharp KW, Holzman MD. An antireflux procedure should not routinely be added to a Heller myotomy. J Gastrointest Surg. 2001;5:13–16. [DOI] [PubMed] [Google Scholar]
- 20.Peters JH. An antireflux procedure is critical to the long-term outcome of esophageal myotomy for achalasia. J Gastrointest Surg. 2001;5:17–20. [DOI] [PubMed] [Google Scholar]



