Abstract
Background:
A recent revision of the American Joint Committee on Cancer (AJCC) staging for gallbladder cancer (6th Edition) involved some major changes. Most notably, T2N0M0 tumors were moved from stage II to stage IB; T3N1M0 disease was moved from stage III to stage IIB; and T4NxM0 (x = any) tumors were moved from stage IVA to stage III.
Methods:
In order to determine if these changes were justified by data, an analysis of the 10,705 cases of gallbladder cancer collected between 1989 and 1996 in the NCDB was performed. All patients had >5 year follow-up.
Results:
The staging according to the 6th Edition provided no discrimination between stage III and IV. Five-year survivals for stage IIA, IIB, III, and IV (6th Edition) were 7%, 9%, 3%, 2% respectively. The data from the National Cancer Database (NCDB) were used to derive a proposed new staging system that builds upon Edition 5 and had improved discrimination of stage groups over previous editions.
Conclusions:
Changes in staging systems should be justified by data. Multicenter databases, including the NCDB, represent important resources for verification of evidence-based staging systems.
Gallbladder cancer staging was evaluated using data from the National Cancer Database (NCDB). Using 10,705 cases of gallbladder cancer collected in the NCDB, a proposed new staging system is suggested that build upon past American Joint Commission on Cancer (AJCC) staging and had improved discrimination of stage groups over previous editions. Multicenter databases, including the NCDB, represent important resources for verification of evidence-based staging systems.
Staging is integral both to selection of cancer treatments as well as to the evaluation of effectiveness of cancer therapies. The prognostic information provided allows distinction of patients at risk for poor outcome to justify toxic therapies. A reasoned and accepted classification system also allows for comparison of results from different institutions and eras. Additionally, staging allows for selection of patients for clinical trials and appropriate stratification. Stage-related assessment of outcome allows confident evaluation of effectiveness of new therapies. In these regards, the American Joint Commission on Cancer (AJCC) staging system has been invaluable in the care and study of many cancer patients.1,2
Gallbladder cancer is a relatively rare cancer that affects only approximately 2500 patients yearly in the United States. This cancer is much more common in Japan,3 India,4 and Chile.5 Much of our knowledge in natural history and, consequently, in staging for this cancer is derived from experiences from these other populations, although it is not yet proven that the genetics or cellular biology of tumors from these other populations are the same as our North American patients. Because of the rarity of this cancer in North America, only a few centers have accumulated sufficient experience6–8 to definitively report on outcome. In the current study, we seek to validate staging for this disease using data from the National Cancer Database (NCDB). This database collects cancer data from approved cancer centers across the nation with the goal of allowing cooperative evaluation of cancer care and outcome. Using data from over 10,000 cases of gallbladder cancer, we critically examined the current staging and propose a new schema justified by these data.
MATERIALS AND METHODS
Case Selection for Gallbladder Cancer
The NCDB is a hospital-based data resource that captured approximately 75% of all newly diagnosed cancer cases. Between 1989 and 1996, 25%–35% of incident gallbladder cancer cases were collected from a total of 1744 hospitals submitted data to the NCDB. The dataset was queried for gallbladder cases (ICD-0-2 topology code C23.9) with no exclusions made based on histology for solid tumors, and the data included both behavior codes 2 (in situ, 3.6%) and 3 (malignant, 96.4%). The cases were limited to male and female cases aged 16 and older. A total of 15,131 patients satisfied the selection criteria. Patients that had complete staging and follow-up information (n = 10,705) available for survival analysis were grouped according to the AJCC, 5th Edition staging schema, 6th Edition staging schema, and a newly proposed staging schema outlined below.
Analysis
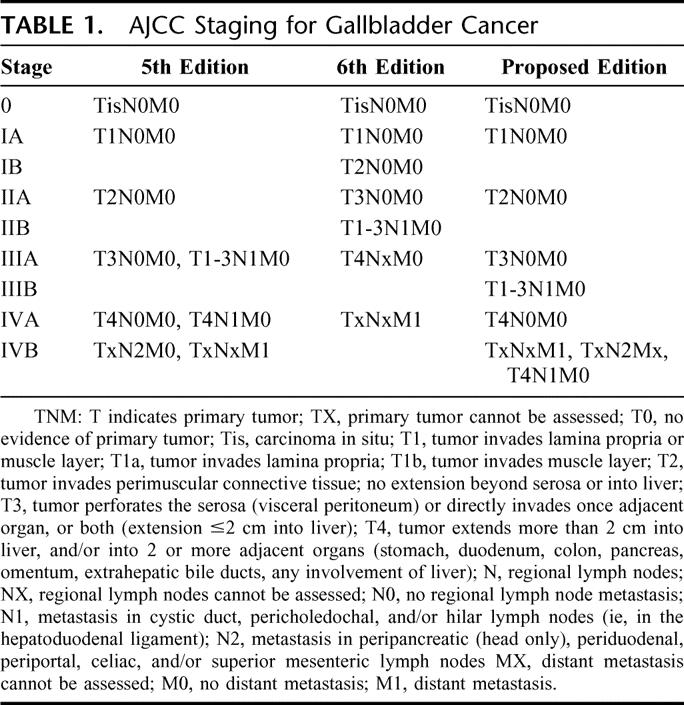
The data were analyzed according to staging by the AJCC, 5th Edition staging1 as well as by the 6th Edition2 (Table 1). Major changes were made in this most recent revision of the AJCC staging for gallbladder cancer. In particular, tumors with more than 2 cm invasion into the liver were reclassified from stage T4 to stage T3. A new T4 classification was instituted defined as invasion into the portal vein, hepatic artery, or multiple extrahepatic organs. Furthermore, T2N0M0 tumors were moved from stage II to stage IB. T3N0M0 and T1-3N1M0 tumors were both moved from stage III to stage II. Finally, T4NxM0 tumors were moved from stage IV to stage III. After examining the data, a new, proposed staging system was also devised and is delineated in Table 1.
TABLE 1. AJCC Staging for Gallbladder Cancer
Survival was plotted by the method of Kaplan-Meier9 and illustrated in Figures 1 to 3. Individual survival curves were compared by Cox regression.10
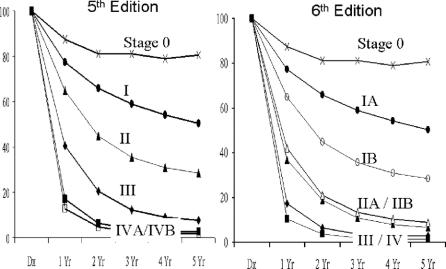
FIGURE 1. Survival curves for all gallbladder cancer patients (n = 10,705) staged according to AJCC, 5th Edition or 6th Edition.
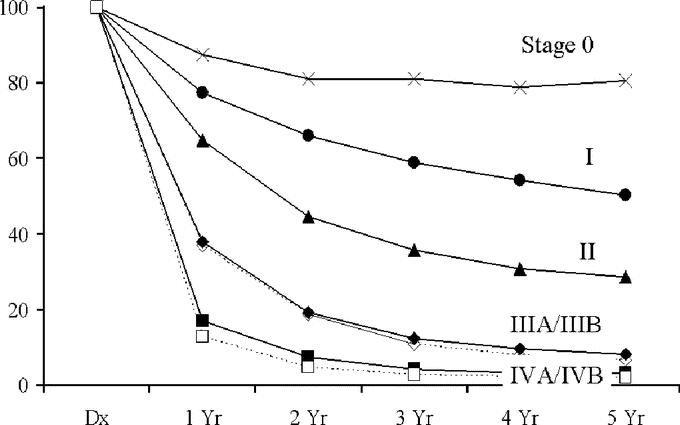
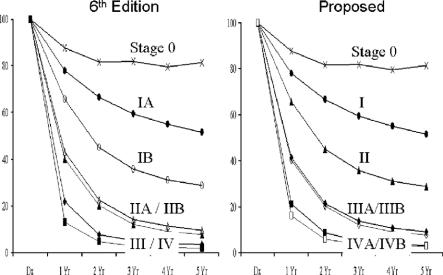
FIGURE 2. Survival curves for all gallbladder cancer patients staged according to the proposed staging schema.
FIGURE 3. Survival curves for gallbladder cancer patients subjected to potentially curative surgery (n = 7462) staged according to AJCC, 6th Edition or the proposed staging schema.
The discriminatory power of the staging systems was evaluated using the concordance probability estimate.11 Concordance probability is a measure of the amount of agreement between the staging system and the actual outcome. A value of 1 is an indicator of perfect agreement and a value of 0.5 is an indicator of agreement by chance alone.
RESULTS
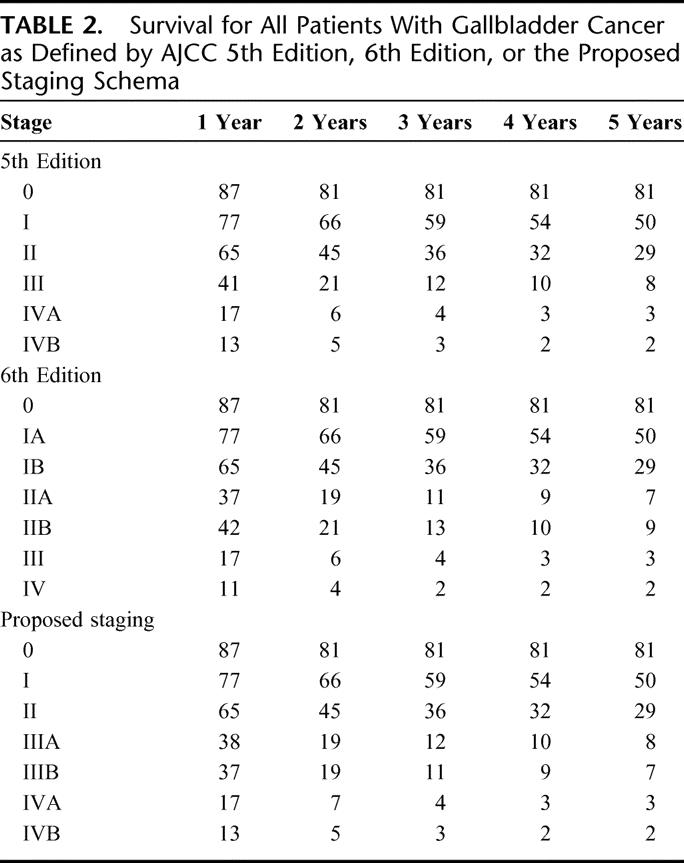
Figure 1 compares staging for all of the patients according to definitions in the AJCC, 5th Edition and the 6th Edition. It is clear that the staging according to the 6th Edition provided no discrimination between stage III and IV. By Cox regression, these 2 curves were not significantly different. Indeed, there was little discrimination in long-term survival for any stage above stage I (Table 2). Three-year survivals for stage IIA, IIB, III, and IV were 11%, 13%, 4%, and 2%, respectively. Four-year survivals were 8%, 10%, 3%, and 2%, respectively. Five-year survivals for stage IIA, IIB, III, and IV were 7%, 9%, 3%, and 2%, respectively. These data would indicate that the 6th Edition AJCC staging system provides poor representation of gallbladder cancer. Furthermore, even in early stage disease according to this staging schema, patients had poor outcome. Median survival for stage IB is less than 2 years. Median survival for stage II is less than 1 year. Less than 20% of patients with disease more advanced than stage I are alive at 2 years. Such a staging system cannot be used in discriminating among good, moderate, and poor prognosis patients.
TABLE 2. Survival for All Patients With Gallbladder Cancer as Defined by AJCC 5th Edition, 6th Edition, or the Proposed Staging Schema
We therefore examined the data and derived a proposed staging system for future revisions (Table 1). In this proposed system, stages 0, I, and II are as it was in the 5th Edition. We have segregated out stage III into IIIA for the T3N0M0 patients, and a stage IIIB consisting of T1-3N1M0 patients. The rationale for this is that lymph node metastases represent a different cancer biology.12 Nodal metastases may also dictate specific therapeutic strategies such as local-regional radiotherapy.13 Having these patients segregated into a different stage may allow easier sorting of results in studies of such treatment modalities. Stage IVA and IVB has also been regrouped as T4N0M0 for IVA. Patients with nodal metastases have been moved to IVB. The survival curves for the patients in the current study segregated by the proposed staging system are shown in Figure 2. There appears to be improvement for stage discrimination compared with the 6th Edition.
Analysis was also performed for the subset of 7462 patients who were subjected to potentially curative surgical therapy. The comparison of survival according to the 6th Edition and the proposed system are shown in Figure 3. The trends are identical to those for the entire group of patients. Thus, the proposed system would appear to be a superior classification of all patients with gallbladder cancer, as well as for those treated by curative resection.
Concordance Analysis
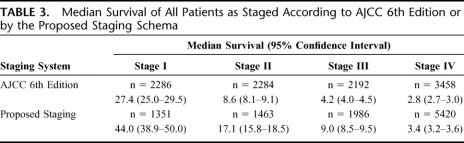
To facilitate visual and statistical comparison, we compared stages I through IV for the 6th Edition and the proposed staging system. Survival curves for these 4 stages by both systems are given in Figures 1 and 2, and median survivals are reported in Table 3. Concordance probabilities are estimated to be 0.636 and 0.651 for the 6th Edition and for the proposed staging, respectively, indicating an improvement in the discriminatory power through the use of proposed system.
TABLE 3. Median Survival of All Patients as Staged According to AJCC 6th Edition or by the Proposed Staging Schema
Stage II of the 6th Edition is almost identical to stage III of the proposed staging, so there are only 2 possible sources for this improvement in discriminatory power: 1) stage I of the 6th Edition is represented by stages I and II in the proposed staging; or 2) stage IV of the proposed staging is represented by stages III and IV in the 6th Edition. The concordance probability of stages I and II of the proposed staging is 0.64 while the concordance probability of stages III and IV of the 6th Edition is 0.58. While separating T4NxM0 tumors from metastatic disease provides the 6th Edition with some predictive power, it is not enough to overcome the loss that stems from grouping T1 and T2 tumors together in stage I.
In addition, there is also a difference in discrimination of stages III and IV as defined by 6th Edition and by the proposed system. Stage IV patients, as defined by the 6th Edition, are 1.33 times more likely to die than stage III patients (95% confidence interval, 1.26–1.40). Stage IV patients, as defined by the proposed staging, are twice as more likely to die than stage III patients (95% confidence interval, 1.89–2.11). While a direct comparison of these 2 relative risks is elusive, nonoverlapping confidence intervals provides evidence that the proposed staging system provides much better separation between stage III and IV patients.
DISCUSSION
Multiple staging systems have been described for gallbladder cancer. The various staging schema take into account the pathologic and clinical characteristics most often found to have prognostic significance, namely, size of tumor, depth of penetration through the gallbladder wall, invasion into adjacent organs, lymphatic metastases, peritoneal metastases, and hematogenous metastases.3,6,7,14,15 Two of the most accepted classification systems are the modified Nevin system8,14 and the Japanese Biliary Surgical Society system.15 The Japanese Biliary Surgical Society staging system separates tumors into 4 stages: stage 1 = cancers confined to gallbladder capsule; stage 2 = cancers with positive N1 lymph nodes and/or minimal liver/bile duct invasion; stage 3 = positive N2 lymph nodes and/or marked liver/bile duct invasion; and stage 4 = distant metastasis. This system is not commonly used outside of Japan since few Western reports have found a long-term disease-free survivor who had lymphatic metastases. Thus, classifying these patients with nodal metastases as stage 2 disease is not supported by the experience from Europe or the Americas.
The Nevin classification was therefore much more accepted among Western investigators. Nevin et al16 originally classified patients into 5 stages: stage 1 = in situ cancer; stage 2 = cancer not yet transmural; stage 3 = transmural direct liver invasion; stage 4 = lymph node metastases; and stage 5 = distant metastasis. Donahue et al8 modified the Nevin system to include tumors with contiguous liver invasion as stage 3 and noncontiguous liver involvement as stage 5. This classification is very similar to the AJCC, 5th Edition, except that the AJCC is classified as stages 0 to 4 rather than 1 to 5. The major conceptual differences are that the Nevin classification did not differentiate tumors that invade through muscle without invading the liver, or discriminate according to size of tumor. These 2 factors seem to have significant prognostic significance. Tumor penetration into or through the muscularis has prognostic implications because the lymphatic drainage of the gallbladder lies in the layer between the muscle and the serosa. Also, most simple cholecystectomies for gallstone leave the serosa on the liver side because the subserosal plane is the easiest for dissection. Thus, simple cholecystectomies performed for unsuspected gallbladder cancer is likely to leave a positive margin for any tumor that penetrates the muscle layer. The Nevin classification and the recognition of the prognostic implications of the size of tumor and muscle invasion produced the 4 iterations of the AJCC staging system that evolved to the 5th Edition.17
In 2002, the 6th Edition of the AJCC system was devised which greatly changed staging. Therefore, we sought to examine these changes using a large multicenter data set. The results of the current study demonstrate that the 6th Edition was not an improvement over the 5th Edition. In addition, the availability of this large data set allowed further refinement of the staging schema and led to the proposed staging system. The proposed staging system was compared with the 6th Edition and provided for improved prognostic discrimination of patients according to outcome. We have also confirmed the superiority of this proposed staging system by using a widely accepted measure of predictive ability, namely, the concordance probability.18
Two major changes in tumor staging are likely to occur in this decade, namely, the inclusion of molecular prognostic variables and a shift to staging by imaging rather than pathology. Already, there are many molecular and cellular characteristics that are thought to be prognostic for biliary cancer.19 However, such molecular analysis will not be widely used until they can be performed at most hospitals and on small needle biopsy specimens, which are the only tissues available for nonresected cases. Wide acceptance of molecular variables is also hindered by the cost. Moving to imaging rather than pathologic staging has major advantages and challenges. It would allow for complete staging of patients even when resection has not been performed. It would also allow staging information to be used not only for selection of patients for postoperative adjuvant therapy but also for selection of patients for neoadjuvant therapy and for selection of patients for surgical therapies. The results of the current study would indicate that clinical/pathologic staging of gallbladder cancer is well defined. The proposed staging changes are only minor modifications of the 5th Edition staging. These changes should be adopted. Attention in staging for this disease should then be directed to studies to determine what, if any, molecular prognostic variable adds significantly to the prognostic value of the standard clinical or pathologic parameters. Studies should also be conducted comparing staging by preoperative imaging to pathologic findings and clinical outcome, to validate imaging for staging of gallbladder cancer.
The proposed staging schema is a very practical one and conforms to the usual expected outcomes for each stage. Most AJCC staging systems have been set up with stage I and II as early disease, and generally disease requiring no adjuvant therapy. Stage III usually includes large tumors or regional nodes, and are patients considered potentially curable but at high risk for recurrence. These are the prime subjects for adjuvant therapies, or studies of novel agents in the adjuvant setting. Stage IV is generally unresectable and incurable. The proposed schema uses easily attainable clinical and pathologic information to segregate patients into these subgroups.
Each year approximately million case records are gathered for the NCDB. The current study demonstrates one use of these data that is immediate and relevant. Many other cancer-related databases exists in the United States and worldwide. Robust data sets exist within the Veterans Administration,20 the National Medicare Database,21 within most states,22 and within the Social Security Administration.23 These databases could allow validation of the current results and allow for assessment of staging for many other cancers. Comparing outcome results of these North American Databases to well-established National databases of other countries, such as those in Sweden24 or Norway,25 would allow comparison of cancer outcome across ethnic lines. The potential benefits of these and other multidatabase analyses encourage investment of effort to overcome the administrative and regulatory barriers to integration of these databases into a coordinated network for systematic studies.
Discussions
Dr. Keith D. Lillemoe (Indianapolis, Indiana): Although this group of investigators represents a number of institutions, the work follows a pattern that we have seen recently from Dr. Fong and his colleagues at Memorial. First, making an effort to improve the staging of hepatopancreaticobiliary cancers for all the important reasons that he outlined in his talk, that is to allow comparison of surgical results between groups and over time periods, and for interpretation of the results of clinical trials. They have done this previously with cholangiocarcinoma and now I believe they have done it with gallbladder cancer. Secondly, although gallbladder cancer remains a disease with a dismal prognosis, the Memorial group has shown that with aggressive surgical therapy some improvement in clinical outcomes can be seen.
In this review, Dr. Fong and his colleagues have not used their own substantial database but, rather, the National Cancer Database to compare the two most recent AJCC staging systems, but also to generate their own, and what I would believe better, staging system, based on their analysis.
Your data clearly support your conclusion; therefore, I have no real questions about that, but, rather, I would like to ask some questions of you based on the interpretation of this data and its clinical application.
Based on your clinical experience, would you predict that the performance of a previous laparoscopic cholecystectomy without recognition of an underlying cancer might affect gallbladder staging? If so, how much? Therefore, should there be an asterisk or an upstaging related to a previous lap chole?
Second, along the same lines, where does port site recurrence fit in the staging of gallbladder cancer? Should it be viewed as evidence of disseminated disease or, rather, local occurrence? In a related question, how do you deal with this finding in your own management for the reexploration for gallbladder cancer?
Finally, in your manuscript you speculate on the role of imaging prior to staging. Would you define how you would predict that imaging would be used to generate this sort of staging criteria? Do we need to perform MRI, EUS, CT scan? If so, how can image-based staging be applied to your system?
I would like to finally close with a comment and congratulations to Dr. Fong and his colleagues as he showed in his last slide for mining this valuable national database. I think it shows the value of this database as a resource to answer questions which cannot be answered by a single institution, even one as substantial as Memorial Sloan-Kettering. I think this is a great example of important clinical research that can be a stimulus for young investigators at any institution to answer their own clinical questions.
Dr. Yuman Fong (New York, New York): In response to your question about laparoscopy, it certainly has changed this disease and how we treat it. The next paper on the program will specifically address those issues, and our group has certainly written on it.
We all believe that laparoscopy increases the chance that gallbladder cancer may be disseminated. But if someone undergoes a definitive therapy for gallbladder cancer after adequate staging, most of the studies have now shown that the patients after prior laparoscopic cholecystectomy will still do about the same, stage for stage, in terms of outcome. It is just that many more patients after a previous laparoscopic cholecystectomy may no longer be a surgical candidate simply because of peritoneal dissemination or port site dissemination.
Coming to the question about port site dissemination and how that should be handled, I believe now that port site dissemination is M1 disease. That is because those patients who have had laparoscopic cholecystectomy and had an incidental discovery of gallbladder cancer will have about a 5% chance of port site recurrence. I have kept track of this, and most of those patients in my practice with port site recurrence die of their disease. Therefore, I look at dealing with the port site and biopsies of the port site and excising the port site as a staging procedure. It tells us about the extent of disease.
Lastly, in terms of staging using imaging. In the paper, we speculate on the future of staging. And clearly, the two directions it is moving are molecular staging and how we incorporate it into our entire staging system, and whether we should change to imaging as staging rather than TNM simply because then we could have all of the patients, whether they are operated on or not. I don't think we are quite there yet. I don't think the cross-section imaging, even though it is very, very good at most centers, it is not uniform enough. Our biologic imaging capabilities are currently too expensive and not well enough disseminated in all of our centers to currently advocate using imaging as the sole way of staging patients. But I envision that at some time in the future that is going to happen.
Dr. William C. Chapman (St. Louis, Missouri): Based on the results of the data analysis, as we have heard, the authors propose rejection of the most recently instituted changes and instead suggest additional modifications to the 5th Edition scheme that I presume would become the basis for the 7th Edition.
So what was the basis for the changes incorporated in the 6th Edition? I would like to briefly reiterate. Basically for the most recent version, changes were made in overall staging to shift node positive cases to stage II, and stage III was changed to signify locally advanced and unresectable disease. Stage IV was designated as metastatic disease.
In this regard, changes to the 6th Edition went against the standard approach for stratification of stage III disease in most cases, and certainly for most GI cancers, which generally represent node-positive but resectable disease, with stage IV representing metastatic, unresectable patients. In addition to the atypical grouping that this change introduced, it also appears this change does not allow discrimination of outcome for patients with stage II, III, and IV disease on the basis of tumor stage, irrespective of treatment.
On the basis of these 2 major factors, it does appear that the implemented 6th Edition changes are not beneficial and should be abandoned. I have three questions for the authors:
I think the argument you make for modifying any existing staging system is compelling, in other words, basing a decision on outcome data for the proposed staging system changes. I guess I would have thought this approach would have already been a standard one. So I raise the question whether you might know if other data were considered by the committee that recommended the modifications implemented in the sixth Edition perhaps from another data set that supported the implemented changes and may have been countercurrent to your results.
Since the changes you proposed were both derived and tested from the same database, should your proposed changes be tested against one of the other data sets that you illustrated for validity before implementation?
I would like to touch on just a slightly different clinical scenario to one that you just discussed, and really it has to do with some of the dismal results that you outlined for those patients with positive regional nodes, even with only a single N1 site of disease. What would the authors recommend for those patients found with an incidental gallbladder cancer at the time of a laparoscopic cholecystectomy?
For the sake of discussion, let's assume the patient has a margin negative T2 tumor. Should this patient undergo reexploration with regional surgical therapy? At many, if not most, centers, this patient would be subjected to a nonanatomic liver resection along with skeletonization of the portal vein, hepatic artery, with regional lymphadenectomy. And what about a similar patient with a single small metastatic focus on the right lobe of the liver? Tell us what would be done with such a patient at your institution by you and your colleagues. The data you shared with us from this very large database suggests that there are essentially no long-term survivors in either case, even with aggressive surgical resection. So I wonder if we are actually providing benefit in either setting.
In conclusion, the authors have highlighted important details for consideration in any staging system changes and make compelling arguments in favor of modification of the most recent gallbladder cancer staging.
Dr. Yuman Fong (New York, New York): First of all, in terms of how we came about Edition 6, I have to take partial credit. I was actually on the committee that looked at what the staging is. Some of us just didn't speak up loudly enough. And it was dominated by data from the Japanese that have shown that they can have node-positive patients and have potentially curative outcome. No Western paper has ever shown any long-term survivor in the same setting.
So the question is whether the Japanese patient is actually a different patient biologically or whether the operations they do are actually different operations than what we do. I lean towards the first and not the second because there are many centers now doing very radical operations for node-positive disease.
Second, in terms of confirming with other databases, I think that was the problem with Edition 6: we didn't go in and take one of the American databases and validate these very radical changes before we actually put it in the book. I think that it would be a very good thing for us to consider doing that anytime we decide to go and do very radical changes in our staging systems.
In terms of going forward with Edition 7, the groups are currently being formed. I think it would be great to go in and validate the data further either in large single institutional data sets or in other national databases. I think the very next paper from Johns Hopkins on this program will show you exactly some of the same data I just showed you from the national database. From a single institutional standpoint, I looked at our own data from Sloan-Kettering in terms of the various staging, and it confirms what we see in this national analysis.
Coming back to what we do in terms of somebody who has nodal metastases if it turns out all these patients do very poorly. It is not that there are no long-term survivors, there are no long-term disease-free survivors. When a patient is incidentally found to have a gallbladder cancer at the time of surgery with a T2 tumor, the reason that we advocate going back to the operating room to go and do a re-resection is because those patients have tumor that has now gone through the muscularis. Underneath the muscularis layer is where all the lymphatics of the gallbladder live. Therefore, the likelihood of nodal metastases in a D2 tumor is about 35%.
The other reason that we go back and advocate a re-resection is not just for a staging. Obviously for nodal metastases I believe it is staging; it is finding those patients for protocols to study novel ways of treating gallbladder cancer to see whether we will make a difference in the future.
When we do a laparoscopic cholecystectomy or simple open cholecystectomy, we usually leave the cystic plate. The cystic plate is the serosa on the backside of the gallbladder. We are actually operating in the layer right between the muscularis and the serosa. We are leaving the serosa lying right there on the liver. So if the tumor is on that side and it is a T2 tumor, the likelihood we will leave microscopic disease is fair. That is also why we go back and do a re-resection. So I believe for staging reasons as well as for local control in terms of the liver bed, it is worthwhile to go back and do a re-resection. Discontiguous liver metastases is a hematogenous metastasis. I believe all those patients are stage IV. I don't operate on those patients.
Dr. Frederick L. Greene (Charlotte, North Carolina): As editor of the 6th Edition of the AJCC Cancer Staging Manual and as chair of the Commission on Cancer, it is a pleasure for me to add to this discussion. I want to point out a couple of things.
First of all, this is a worldwide system of cancer. In developing the 6th Edition, we had to go in front of all of the other organizations throughout the world who have data sets. Dr. Fong has already alluded to the implication of this when you start talking about hepatobiliary disease.
We are getting ready for the 7th Edition coming out in 2009, and we hope to use all of these data sets, especially the 18.5 million cases in the National Cancer Data Base. I want to salute your coauthors, who are wonderful members of our staff and work with the American College of Surgeons.
My one question is: TNM is a dual system. It is clinical and pathologic. We talked about the pathologic part of it. What recommendations would you have for the clinical part of TNM for gallbladder cancer? I am very interested in starting to mix and match clinical and pathologic TNM, and I would hope that you would suggest that this be done in the future.
Dr. Yuman Fong (New York, New York): The AJCC is an enormously important project, as well as the Commission on Cancer, and I think that every now and then stopping and reanalyzing, combining the resources from both groups including data, allows us to move forward with a more reasonable approach.
In terms of the resected patients, TNM is actually pretty good in terms of most cancers in terms of looking at outcome. But many of our patients don't get a resection, and assessment is actually based on mostly radiologic or clinical assessment. And how we put radiology into the mix in terms of staging of these patients will be very important.
But other prognostic factors should be considered. For example, in gallbladder cancer, jaundice has been now seen by multiple groups as an indication of interability for gallbladder cancer. So how do we put that in as a factor? Those are the challenges. I think these are the things that we need to talk about for the next Edition, and hopefully this will be an even better Edition than before.
Footnotes
Requests: Yuman Fong, MD, Murray F. Brennan Chair in Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY 10021. E-mail: FongY@mskcc.org.
REFERENCES
- 1.Fleming ID, Cooper JS, Henson DE, et al. AJCC Cancer Staging Manual, 5th ed. New York: Springer, 1997. [Google Scholar]
- 2.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual, 6th ed. New York: Springer, 2002. [Google Scholar]
- 3.Frena A, La GG, Martin F. Outcome of radical surgery for carcinoma of the gallbladder according to the tumor node metastasis and Japanese Society of Biliary Surgery stages. J Gastrointest Surg. 2004;8:580–590. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor VK, Benjamin IS. Resectional surgery for gallbladder cancer. Br J Surg. 1998;85:145–146. [DOI] [PubMed] [Google Scholar]
- 5.Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon E, Vollmer CM Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue JH, Nagorney DM, Grant CS, et al. Carcinoma of the gallbladder: does radical resection improve outcome? Arch Surg. 1990;125:237–241. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 10.Barlow WE, Sun WH. Bootstrapped confidence intervals for the Cox model using a linear relative risk form. Statist Med. 1989;8:927–935. [DOI] [PubMed] [Google Scholar]
- 11.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–970. [Google Scholar]
- 12.Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer: implications for staging and management. Ann Surg. 1996;224:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kresl JJ, Schild SE, Henning GT, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Intl J Radiat Oncol Biol Phys. 2002;52:167–175. [DOI] [PubMed] [Google Scholar]
- 14.Nevin JE, Moran TJ, Kay S, et al. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer. 1976;37:141–148. [DOI] [PubMed] [Google Scholar]
- 15.Onoyama H, Yamamoto M, Tseng A, et al. Extended cholecystectomy for carcinoma of the gallbladder. World J Surg. 1995;19:758–763. [DOI] [PubMed] [Google Scholar]
- 16.Peters JH, Gibbons GD, Innes JT, et al. Complications of laparoscopic cholecystectomy. Surgery. 1991;110:769–777. [PubMed] [Google Scholar]
- 17.Beahrs OH, Myers MH. Manual for Staging of Cancer. Philadelphia: Lippincott, 1983. [Google Scholar]
- 18.Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 19.Abbruzzese JL. Molecular diagnosis of pancreatic and biliary cancer: ready for broad implementation? Cancer J. 2000;6:282–284. [PubMed] [Google Scholar]
- 20.Dominitz JA, Maynard C, Boyko EJ. Assessment of vital status in Department of Veterans Affairs national databases: comparison with state death certificates. Ann Epidemiol. 2001;11:286–291. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JT, Moncrief WH Jr, Ross JE. Uses of the peer review organization Medicare database. Qual Assurance Utilization Rev. 1988;3:53–56. [DOI] [PubMed] [Google Scholar]
- 22.Choti MA, Bowman HM, Pitt HA, et al. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg. 1998;2:11–20. [DOI] [PubMed] [Google Scholar]
- 23.Watson PJ, Main CJ, Waddell G, et al. Medically certified work loss, recurrence and costs of wage compensation for back pain: a follow-up study of the working population of Jersey. Br J Rheumatol. 1998;37:82–86. [DOI] [PubMed] [Google Scholar]
- 24.Hemminki K, Vaittinen P. National database of familial cancer in Sweden. Genet Epidemiol. 1998;15:225–236. [DOI] [PubMed] [Google Scholar]
- 25.Wibe A, Eriksen MT, Syse A, et al. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg. 2005;92:217–224. [DOI] [PubMed] [Google Scholar]