Abstract
Objective:
To examine cellular mechanisms by which short-term elevations of glucose or insulin impair leukocyte functions and to assess the occurrence of perioperative hyperglycemia in surgical patients.
Summary Background Data:
A major factor in the contemporary management of the critically ill surgical patient is the progressively exact control of blood glucose. However, the separate role of insulin and underlying immunologic mechanisms are not well understood.
Methods:
Venous blood samples of 20 healthy volunteers were exposed for 24 hours to various glucose and insulin concentrations. Lipopolysaccharide (LPS) was added at 1 ng/mL for up to 16 hours and the monocytes' ability to express CD14 and HLA-DR assessed as an index of the monocyte's capability to present antigen. To evaluate the clinical importance of the observed experimental results, a prospective evaluation of perioperative blood glucose values in 5285 surgical patients in Kentucky was performed.
Results:
Both exposure to high glucose (400 mg/dL) and insulin (100 μU/mL) led to an independent and additive impairment of monocyte HLA-DR expression after 24 hours (P < 0.01). Perioperative blood glucose exceeded 200 mg/dL in 21% of all cardiothoracic patients and in 31% of diabetic patients undergoing common major operations.
Conclusions:
Both short-term hyperglycemia and hyperinsulinemia are associated with significantly decreased monocyte HLA-DR expression, a parameter correlating with infectious complications and patient mortality. This may provide a mechanism by which high glucose and insulin impair innate immunity. It also appears that perioperative maintenance of normoglycemia will become a valid performance measure for practicing surgical specialists.
Perioperative glycemic control was insufficient (≥200 mg/dL) in 31% of diabetic general surgical patients and 21% of all cardiac surgical patients in a prospective analysis of 5285 elective patients. Also, both acute hyperglycemia and hyperinsulinemia are associated with decreased monocyte HLA-DR expression, a parameter correlating with infectious complications and mortality.
The value of strict blood glucose control in all perioperative and critically ill patients, including nondiabetic individuals, has received growingly broad attention in the last few years. A number of well-controlled clinical trials have impressively demonstrated significant survival benefits by strict glycemic control in different subgroups of nondiabetic critically ill patients.1–5 Van den Berghe's original randomized-controlled trial of 1548 ICU patients,5 in which the authors recorded a 34% decrease in overall mortality in the group receiving high doses of insulin, has been followed by other studies which mostly support their findings.1–3 Similarly, hyperglycemia on admission has been shown to serve as an independent predictor of mortality not only in various subgroups of ICU patients,4,6–8 but recently also as a predictor of in-hospital mortality in noncritically ill patients.9 These studies generally agree that the correction of stress-induced hyperglycemia with insulin improves outcome regardless of a previous history of diabetes mellitus. Perioperative hyperglycemia has been demonstrated to increase mortality and the rate of infectious and organ system-specific complications in both diabetic and nondiabetic cardiac surgery patients.10–13 Less evidence exists with respect to the value of perioperative glycemic control in nondiabetic patients undergoing procedures other than cardiac surgery. However, in light of the growing evidence in critically ill, cardiovascular and trauma patients, the avoidance of perioperative hyperglycemia has been proposed to be a valid surrogate for surgeon and critical care physician performance.
A number of different immunologic parameters have been identified by which hyperglycemia impinges on the host susceptibility to infection. Among these are a disturbance in the inflammatory cytokine cascade with increasing levels of early proinflammatory cytokines such as IL-6 or TNF-α in response to hyperglycemia.14,15 High glucose levels are also known to impair the microvasculature's ability to relax in the presence of vasodilating stimuli such as nitric oxide (NO),16,17 and to promote the adherence and sequestration of neutrophils and monocytes into peripheral tissue.18,19 Acute hyperglycemia also inhibits specific cellular functions such as the production of reactive oxygen species, or phagocytosis.20,21 However, most of these studies were performed in either diabetic patients or models of chronic, as opposed to acute, hyperglycemia; a lack of clear understanding therefore exists with respect to the effects of acute, short-term hyperglycemia on various host immune parameters.
The purpose of this study was to investigate cellular mechanisms that could explain the adverse clinical effects of acute hyperglycemia in nondiabetics and the corresponding benefits of insulin therapy. Specifically, we hypothesized that the monocyte's ability to present antigen, indirectly assessed by its capability to express HLA-DR, is compromised during short-term hyperglycemia, thus impairing specific recognition and clearance of foreign antigen. Decreased levels of monocyte HLA-DR have previously been shown to correlate with infectious complications and mortality in critically ill patients,22–24 providing a potential link by which short-term hyperglycemia may exert its adverse effects on the host' immune system.
In a concurrent clinical study, we assessed the frequency of imperfect glycemic control in patients undergoing major operations in Kentucky, to provide an estimate of the potential benefit resulting from stricter perioperative glycemic control in these patients.
METHODS
Monocyte HLA-DR Analysis: Diluted Whole Blood Incubation Preparation
Following approval of the University of Louisville Institutional Review Board and written, informed consent, venous blood samples were collected from 20 healthy, nondiabetic volunteers in EDTA Vacutainers (Becton Dickinson, Franklin Lakes, NJ). Exclusion criteria included any acute illness, history of immunosuppressive disorders, diabetes mellitus, or any chronic medication. The average age of the enrolled subjects was 28.7 years. Immediately following venipuncture, blood glucose levels were determined by a Glucometer Elite (Bayer Corporation, Elkhart, IN). Whole blood was diluted 1:10 with RPMI-1640 (no glucose; ICN Biochemical Inc., Aurora, OH) supplemented with 10% heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, MO), 200 mmol/L l-glutamine (Sigma Chemical Co., St. Louis, MO), 1% antibiotic/antimycotic solution (penicillin, streptomycin, and amphotericin B; Sigma Chemical Co.) in Falcon 5-mL polypropylene culture tubes (Fisher Scientific). All samples were cultured either with or without 1 ng/mL lipopolysaccharide (LPS [from Escherichia coli 0111:B4; Sigma Chemical Co.]) for different lengths (0, 2, 4, 8, and 16 hours of LPS) at the end of the incubation period, to examine HLA-DR expression of monocytes at different levels of cellular activation.
Adjustment of Glucose, Insulin, and Sodium Chloride Concentrations
Following the 1:10 dilution with RPMI, glucose concentrations were titrated to 100 or 400 mg/dL using 5% Dextrose (Baxter Healthcare Corporation, Deerfield, IL). Insulin was titrated up to a concentration of 10 μU/mL (controls) or 100 μU/mL to simulate iatrogenic hyperinsulinemia, both at normal (100 mg/dL) and high glucose (400 mg/dL) concentrations. Reference values for fasting plasma insulin concentration in most laboratories are 5 to 25 μU/mL.25 To control for increased osmolarity caused by high glucose concentrations (400 mg/dL glucose = 300 mg/dL or 16.5 mm/L above normal), appropriate samples from all subjects were incubated at 100 mg/dL glucose with 8.25 mmol/L NaCl (equals 16.5 mOsm/L), which then simulates the increased osmolarity caused by 400 mg/dL glucose.
Monocyte Extraction
Following 24 hours of incubation, all blood cultures were mixed and layered onto an equal volume (2 mL) of Histopaque 1.077 (Sigma Chemical Co.). The gradients were centrifuged at room temperature for 30 minutes at 400g. The mononuclear layer was removed, and remaining RBCs were lysed with ice-cold ammonium chloride, potassium bicarbonate, and EDTA (Sigma Chemical Co.). The monocytes were pelleted by centrifugation, and washed once with Dulbecco Phosphate Buffered Saline (DPBS; Sigma Chemical Co.).
Monocyte CD14/HLA-DR Staining and Flow Cytometric Analysis
Monocyte HLA-DR expression was determined by staining 100 μL of each sample with FITC-labeled antihuman CD14 and PE-labeled anti-HLA-DR antibodies (BD Biosciences, La Jolla, CA) according to the manufacturer's instructions. Appropriately matched isotype controls were used to determine the threshold for nonspecific binding. Briefly, 100 μL of each culture were stained with appropriate concentrations of each antibody for 25 minutes at 4°C. The final cell suspension was fixed in a 1% paraformaldehyde solution. Flow cytometric analysis of the percentages of cells binding to CD14/HLA-DR was performed within 1 hour of staining on a FACScan flow cytometer (Becton Dickinson, San Diego, CA); 10,000 events were acquired and mean channel HLA-DR fluorescence (MCF) analyzed in CD14+ monocytes using Cell Quest software (Becton Dickinson, San Diego).
Statistics
One-way repeated measures analysis of variance was used to detect differences following multiple interventions (different doses of LPS, glucose, insulin or NaCl), followed by the Holm-Sidak test to isolate the group or groups that differ from the others. The paired t test was used to identify significant changes following only one intervention (changes in glucose or insulin concentrations) in corresponding groups. For data that were not normally distributed, the Wilcoxon rank sum test (single intervention) or Friedman statistic (multiple interventions) was applied. Data are presented as mean ± SEM, and statistical analysis was performed using SigmaStat 3.0 software (Systat Software, Inc., Richmond, CA). Results were considered significant at P < 0.05.
Glycemic Control in Patients Undergoing Major Surgical Operations
A total of 5285 consecutive patients admitted to Kentucky hospitals who underwent any of the surgical procedures listed in Table 1 were enrolled into a database generated by SCIP (Surgical Care Improvement Project), a joint project of the Centers for Medicare and Medicaid Services, the Center for Disease Control and Prevention (CDC), the American College of Surgeons (ACS), and the Department of Veterans Affairs (VA).26–28 The purpose of this study was to identify and test a number of process measures by which to improve surgical practice, including the perioperative control of blood glucose. Perioperative hyperglycemia was defined as any serum glucose value >200 mg/dL within 24 hours prior to and 48 hours after the operation. In cardiac surgery patients, both diabetic and nondiabetic patients were analyzed. In noncardiac patients, only those with a preoperative diagnosis of diabetes mellitus were included. This was done because the current literature provides less evidence on perioperative glycemic control in nondiabetic, noncritically ill patients undergoing major operations other than cardiac procedures. Univariate statistical analysis was performed to determine whether inadequate glycemic control correlated with 30-day postoperative mortality and a number of specific adverse outcome measures in both groups. These measures included wound infections, acute myocardial infarction, cardiac arrest, pulmonary embolism, deep venous thrombosis, ventilator-associated pneumonia, and 30-day readmission rates. While these data are derived from a Kentucky pilot study to assess a broad variety of process and outcome measures in surgical patients in the state of Kentucky, we have chosen to analyze and report physician performance in perioperative blood glucose control in this separate report.
TABLE 1. Perioperative Glycemic Control and Its Effect on Outcome by SCIP in Calendar Year 2004

RESULTS
Monocyte HLA-DR as a Function of High Glucose and Insulin Concentrations
Following 24 hours of incubation in a normoglycemic medium, monocytes from healthy nondiabetic volunteers had an average of 2260 ± 215 mean channel fluorescence (MCF) for the expression of HLA-DR (Fig. 1). Increasing lengths of LPS stimulation enhanced monocytic MCF up to a level of 3040 ± 220 MCF at 8 hours of LPS. At 16 hours of LPS, monocyte HLA-DR expression declines, and an increasing percentage of cells become necrotic, presumably through LPS overactivation.

FIGURE 1. Monocyte HLA-DR expression as a function of increasing lengths of LPS stimulation at different glucose and insulin concentrations (n = 20). *All P < 0.05 (400 mg/dL vs. 100 mg/dL glucose). ‡All P < 0.01 (400 mg/dL glucose + 100 μU/mL insulin vs. 100 mg/dL glucose without insulin).
Monocytes from samples incubated at higher glucose concentrations (400 mg/dL) showed a decrease in their ability to express HLA-DR at all lengths of LPS stimulation as compared with normoglycemic control groups (Figs. 1, 2A). These changes were highly reproducible and consistent (P < 0.05 as compared with normoglycemic controls). Groups that were simultaneously incubated with high glucose and high insulin concentrations (100 μU/mL) showed further decreases in monocyte HLA-DR, which on average remained 26% below the normoglycemic control groups (Fig. 1, all P < 0.01 compared with normoglycemic controls [100 mg/dL glucose]).
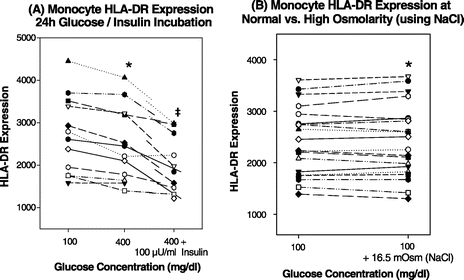
FIGURE 2. A, Monocyte HLA-DR expression after 24 hours of incubation at 100 mg/dL versus 400 mg/dL glucose and 400 mg/dL glucose with high insulin (100 μU/mL). 2 hours LPS, n = 12. *P = 0.005 (400 mg/dL vs. 100 mg/dL glucose). ‡P = 0.002 (400 mg/dL glucose + 100 μU/mL insulin vs. 100 mg/dL glucose). B, Control experiments for increased osmolarity: Monocyte HLA-DR expression in the presence of 8.25 mm/L (≈16.5 mOsm/L) NaCl and 100 mg/dL (5.5 mmol/L) glucose (≈5.5 mOsm) as compared with normoglycemic control samples (*P = 0.74). n = 20.
Individual values after 24 hours of incubation, including 2 hours of LPS, are shown in Figure 2A. There was a consistent decrease in HLA-DR expression with 400 mg/dL glucose and a further decline in the presence of 100 μU/mL insulin. In experiments with NaCl as an osmolar control (Fig. 2B), no differences were observed between the hyperosmolar (+16.5 mOsm/L) group simulating the level of hyperosmolarity seen in 400 mg/dL glucose and the control group (100 mg/dL glucose, P = 0.74). This indicates that the decrease in monocyte HLA-DR was caused by a specific effect of glucose, not the increase in plasma osmolarity caused by high glucose concentrations.
Isolated Effects of Insulin on Monocyte HLA-DR Expression
Twenty-four hours of whole blood incubation with 100 μU/mL insulin at normal glucose concentrations (100 mg/dL) were associated with a small decrease in monocyte HLA-DR expression at any level of LPS stimulation (Fig. 3A). The observed differences were significant in all treatment groups as compared with normoinsulinemic controls. In a separate analysis for insulin, we found a dose-dependent effect of insulin to decrease monocyte HLA-DR in a hyperglycemic environment (400 mg/dL), but only concentrations of 100 μU/mL insulin or greater led to significant differences (Fig. 3B) after 24 hours of incubation.

FIGURE 3. A, Comparison of monocyte HLA-DR expression at normal (10 μU/mL) versus high (100 μU/mL) insulin concentrations after 24 hours of incubation and different lengths of LPS (1 ng/mL). n = 20. B, Effect of increasing concentrations of insulin on monocyte HLA-DR expression in a hyperglycemic (400 mg/dL glucose) environment (*P < 0.05). n = 12.
Glycemic Control in Patients Undergoing Major Surgical Operations in 2004
The distribution of surgical procedures performed in the 5285 patients enrolled in this study and the level of perioperative glycemic control are shown in Table 1. From the total of 4862 noncardiac patients, 658 patients were recorded as patients with diabetes. In this combined group, an average of 31% had perioperative blood glucose values exceed 200 mg/dL. Highest percentages of hyperglycemic patients were recorded in colorectal resection patients (42%). In the 423 cardiac surgery patients, an average 21% percent of both diabetic and nondiabetic patients exceeded 200 mg/dL; 32 of 5285 patients died (0.6%), and less than 5% of the total were readmitted and reoperated upon, some of the latter being scheduled reoperations.
Following univariate analysis, a significant correlation existed between perioperative hyperglycemia and in-hospital mortality in cardiac surgery patients (P = 0.045), whereas no such correlation existed between perioperative hyperglycemia and mortality in noncardiac patients (P = 0.6). Of the 423 cardiac surgical patients, 2 died. However, the presence of perioperative hyperglycemia was a significant overall predictor of perioperative morbidity in both groups, including both infectious and noninfectious complications (P < 0.05).
DISCUSSION
Aggressive inpatient blood glucose control has been documented to lower mortality in both diabetic and nondiabetic ICU patients. It reduced the rate of organ failure and septic complications, and shortened the length of hospital stay in a number of well-conducted clinical trials.1–5 Similarly, the initial level of hyperglycemia upon admission to the surgical ICU has been shown to directly correlate with mortality and serve as a predictor of negative outcome in both medical and surgical ICU patients.4,6–8
While most would agree on the clinical value of strict glycemic control in ICU patients, the potential immunologic or other mechanisms behind this phenomenon are not at all understood. Clearly, acute hyperglycemia negatively affects the innate immune system in a number of ways,13,29 but the precise mechanisms by which short-term blood glucose elevations impair the host immune system are only beginning to be revealed.
Our previous work has shown that the apoptotic turnover of neutrophils, the first phagocytic cells to arrive at a site of infection, withstands short-term elevations of glucose and insulin concentrations without affecting the number of viable neutrophils after 24 hours.30 Early proinflammatory cytokines such as TNF-α or IL-6 were shown by others to be increased in the presence of high levels of glucose, with insulin reportedly decreasing these levels and promoting the more anti-inflammatory IL-4 and IL-10, but significant discrepancies exist between these studies.29 In previous experiments done in our laboratory, we found that the technique by which to measure cytokines (ie, conventional ELISA vs. modern, protein-array based systems) greatly affects the results of such experiments and may, in part, be responsible for this divergence, as well as our inability to identify consistent effects of hyperglycemia on such cytokines.
Our current experiments demonstrate that the monocyte's ability to present antigen, indirectly assessed by its level of surface HLA-DR expression, is significantly depressed following only 24 hours in a hyperglycemic environment (Figs. 1, 2A). This observation is compatible with prior work from our unit and numerous others that indicate a central role for monocyte antigen presentation in infection related to trauma and major surgery.23,24 These studies had shown that a correlation exists between the extent of monocyte HLA-DR depression in critically ill patients and the subsequent rate of major infections and mortality. Wasmuth et al had previously recorded lower levels of monocyte HLA-DR in their study population of hyperglycemic medical ICU patients, but this effect did not withstand multivariate testing, whereas the authors recorded altered levels of interleukin (IL)-6 and TNF-α levels in these patients.31
More surprising to us, however, is the observation that insulin, which from a clinical perspective is used most commonly to correct the adverse effects of hyperglycemia, was associated with even more depressed levels of monocyte HLA-DR expression (Figs. 1, 2A, 3). It is known that the monocyte membrane contains a high number of insulin receptors and that insulin upon binding to its receptor facilitates glucose uptake via externalization of glucose-specific GLUT3/4 transmembrane channel proteins.32 In one study, 100 μU/mL of insulin were shown to increase intracellular glucose concentrations in monocytes more than 3-fold,32 which may explain the seemingly additive effect of combined hyperinsulinemia on the already existing depression of monocyte HLA-DR during hyperglycemia. Potentially, the additive decrease of HLA-DR by insulin may also be due to its ability to suppress the proinflammatory intracellular nuclear factor kappa B (NF-κB) pathway,33 which has been shown to be essential for an adequate HLA-DR response to LPS on antigen-presenting cells.34 From a more clinical point of view, Kappel et al had previously reported decreased levels of monocyte HLA-DR in response to a 2-hour normoglycemic hyperinsulinemic clamp in healthy nondiabetic males.35 However, they had recorded this effect only at extremely high concentrations of insulin (>1000 μU/mL) after 2 hours, whereas no effect was seen at high but more physiologic levels of insulin (50 μU/mL). We have chosen to investigate the effects of insulin at doses between 10 and 200 μU/mL (Fig. 3B), which more closely represent those seen in patients under insulin therapy and accompanying insulin resistance of critical illness. Our data indicate that 24 hours exposure to elevated levels of glucose and insulin is sufficient to significantly impair monocytic HLA-DR expression, and that insulin at high concentrations of glucose lessens monocyte HLA-DR fluorescence in a dose-dependent manner. Also, the extent of the reduction of monocytic HLA-DR in our model of hyperglycemia/hyperinsulinemia correlates with the depression of HLA-DR in severely injured patients who develop major sepsis. In these patients, a loss of 20% to 40% of monocyte HLA-DR expression has been described at 24 to 48 hours after injury.36 Prior publications suggest that this level of suppression may be clinically important.
Insulin itself has previously been ascribed specific immunosuppressive effects, such as lowering CRP levels,37 up-regulating the anti-inflammatory cytokines IL-4 and IL-10,38 or inhibiting the NF-κB pathway in monocytes.33 In this sense, the ability of insulin to decrease monocyte HLA-DR may further promote a state of relative immunosuppression, which might counterbalance some of the immuno-activating effects of hyperglycemia. Other investigators have actually found a positive correlation between the amount of insulin administered to control stress-induced hyperglycemia, and overall ICU mortality,1 and have then suggested that the avoidance of overt hyperglycemia rather than its correction with insulin benefits critically ill patients.
Recent trials have also found a positive correlation between intraoperative blood glucose levels and outcome in cardiac surgery patients, emphasizing strict glycemic control even in the intraoperative period regardless of the presence or absence of known diabetes mellitus.10,12 While data on intraoperative or perioperative glycemic control in a more heterogeneous, noncritically ill surgical patient population are presently missing, the growing evidence in critically ill, trauma, and cardiovascular patients is likely to make perioperative blood glucose control a measure of contemporary physician performance in coming years. One of the aims of our clinical study, therefore, was to assess the current performance to control perioperative glucose levels in diabetic general surgical patients and all cardiac surgery patients and its impact on a number of outcome measures in the state of Kentucky. We have separately analyzed data from the SCIP pilot study in Kentucky, which evaluated a number of performance and outcome measures in a heterogeneous population of surgical patients in calendar year 2004.26–28 We found that, on average, one third of diabetic general surgical patients and one fifth of all cardiac surgery patients exceeded an admittedly high perioperative threshold of 200 mg/dL blood glucose. Interestingly, these results precisely match those of Latham et al, who found that 21% of his patients undergoing cardiac procedures had postoperative blood glucose values above 200 mg/dL, which correlated with the incidence of surgical site infections in his study population.13 In our study, perioperative hyperglycemia was found to be a significant predictor of 30-day mortality in cardiac patients, and it also correlated with postoperative complications in diabetic noncardiac patients in the data currently available from this pilot study. However, these early findings are presently based on univariate analysis, and must therefore be considered with caution, as a number of potential confounders may likely affect the correlation between perioperative hyperglycemia and the outcome measures analyzed in the future in a subsequent, nationwide multivariate analysis such as SCIP.
Although the reasons behind the modest performance in control of perioperative blood glucose are speculative today, we feel that a current lack of surgeon awareness in recognizing and aggressively treating this condition accounts for the majority of these cases. Even though strict glycemic control has received widespread attention in recent literature, especially among European intensive care research groups, there may be a “lag phase” among practicing surgical specialists to implement this new concept into their broader current practice. It is likely that these measures will be part of a further national study on improvement in surgical care to be sponsored by Centers for Medicare and Medicaid Services.
CONCLUSION
We found that both short-term hyperglycemia and hyperinsulinemia are associated with significantly decreased monocyte HLA-DR expression, a parameter that closely correlates with the rate of infectious complications and mortality in critically ill patients. This may provide a mechanism by which high glucose and insulin impair innate immunity, and it underlines the need for early avoidance of overt hyperglycemia in surgical patients, which in turn probably should be more broadly implemented into surgical specialty practice. The overall control of hyperglycemia in surgical patients in Kentucky in the year 2004 was less than optimal. This emphasizes the need for wider dissemination of the knowledge obtained from laboratory experiments and large-scale clinical trials investigating the benefits of strict glycemic control, especially in nondiabetic patients undergoing major operations other than cardiac procedures.
Discussions
Dr. Lewis M. Flint, Jr. (Tampa, Florida): Dr. Turina and his colleagues have chosen to tackle a very challenging clinical problem, and their effort clarifies the cellular mechanisms of immune suppression associated with hyperglycemia. They go further and document that one third of diabetic patients and more than 20% of nondiabetic patients undergoing elective operations in Kentucky have excessive levels of blood sugar in their perioperative periods. Excessive blood glucose is defined, in this report, as more than 200 mg/dL. Hyperglycemia was associated, in this analysis, with both mortality and morbidity. This work is an extension of more than three decades of research from the Price Institute of Surgical Research, which has sought to improve our understanding of the mechanisms of surgical infection. The clinical portion of the report is an outgrowth of the surgical quality improvement initiative led by Dr. Polk and undertaken by Kentucky surgeons to show that quality performance can be measured and standardized.
This report is timely because of the recent upswing in interest in hyperglycemia and its relationship to outcomes. Hyperglycemia has been associated with increased risk of mortality following cardiopulmonary bypass, septic shock, and traumatic brain injury. Rigid control of blood sugar levels has been associated with decreased mortality in critically ill patients and in patients who have required cardiopulmonary bypass. Most of the studies available have focused on all-cause mortality. Series of patients who have undergone cardiopulmonary bypass and have developed hyperglycemia have been shown to be at increased risk for cardiac events and strokes leading to mortality. There has been, seemingly, little interest in the immune-suppressive aspects of the hyperglycemia and few studies which have specifically evaluated infection risk. Only in transplant patients has hyperglycemia been clearly associated with an increased risk of infection.
Tight control of blood glucose levels has been controversial. In fact, the American Society of Anesthesiologists objected to the recommendation that euglycemia be the goal for managing blood glucose levels intraoperatively in patients who require cardiopulmonary bypass. This Society successfully lobbied for the adjustment of the blood sugar goal to 200 mg/dL level rather than normal blood sugar levels. This may be the reason that the 200 mg/dL level was chosen by Dr. Turina.
The main concern has been the fact that glucose control is a labor-intensive activity and that dangerous levels of hypoglycemia occur in 20% of patients who are subjected to a protocol of tight blood glucose control. When the blood glucose level is adjusted, however, control becomes easier.
For example, 1 year ago, we instituted a clinical pathway in our intensive care unit for control of blood glucose and the goal level was 200 mg/dL or less. We set another goal of less than 2% of patients experiencing a hypoglycemic episode. To date, we have had a 96% compliance with the hyperglycemia goal and an incidence of hypoglycemia of only 0.4%. Realistic glucose control is therefore achievable.
The remaining question concerns the efficacy of this effort in a broad range of patients. Determining the role of infection as a cause of mortality or morbidity in critically ill patients has been a challenge. Because of the techniques of antibiotic use and infection management in surgical patients, many of these, parenthetically, developed and defined by Dr. Polk, surgical patients who die in the ICU more frequently die with infection rather than from infection.
All of these ruminations lead me to the following questions for Dr. Turina.
In the experimental study, insulin and glucose levels were held steady. In real life, insulin and glucose levels fluctuate. Could you tell us if the monocytes can recover if glucose and insulin levels fall? If recovery is possible, what is the time course of recovery? In your clinical analysis, was there evidence that hyperglycemia was discovered in time to stimulate an intervention? Finally, have you instituted a clinical pathway to detect and treat perioperative hyperglycemia?
Dr. Hiram C. Polk, Jr. (Louisville, Kentucky): First of all, I, like most people in this audience, I have spent my life trying to avoid hypoglycemia; monitoring blood sugar is something an intern can take care of and we always have allocated that to the least skilled and least experienced person. It is a fair comment to say for my generation we have been on the other side of these curves, it's not very important, don't worry about it, just don't let the blood sugar get too low, and nothing bad will happen!
What we have observed here, though, is an opposite point and there now is a huge weight, largely of ICU literature, but a great weight of data that suggests that even modest amounts of hyperglycemia are harmful. I don't think you can avoid that whether you count papers by the pound or count by the patients.
The clinical application of this is going to be important because, as most of you know, that there are measures moving towards so-called report cards or quality performance indices for surgeons everywhere. This is going to be one of the parameters that stick. So better and better blood glucose control is going to be part of your practice. It is important that we get on board and begin to do this in the clinical scenario. I think we will come to pulse oximetry for glucose, and very shortly, we will almost certainly end up with a feedback mechanism that works much like an artificial pancreas in which elevated blood sugar as defined by the oximetry-like device will lead to the infusion of insulin or something else.
I think the thing that surprised us most, as Dr. Turina said very nicely in his presentation, is that insulin worsens this process on the laboratory bench-top. Based on our in vitro experiments, it is very interesting because it clearly tells you it is better to avoid hyperglycemia than it is to correct it with insulin. Yet another problem with the emphasis on hyperalimentation is that we need to avoid this rather than try to correct it with insulin.
Now to the questions that Dr. Flint asked: Number 1, we didn't think about his first question about what happens when you return the blood sugar to normal. We would need to move back into that 24-hour clamp period in order to modify that. Because towards the end of this 18-hour test, as you can tell from Dr. Turina's data, the monocytes are dying. To ask that question, we will need to push it back about 12 hours into the process?
As any of you who have done work for the Centers for Medicare and Medicaid Services find out, they are interested only in what they are interested in and we were not allowed to collect data on interventions. I think most of us were surprised that we had nearly a third of patients of diabetics and 20% of cardiac patients, with grossly abnormal blood sugars. Furthermore, 200 is not close to normal. If you read the literature, notwithstanding anesthesia right now, that desired number is going to be near 150. Surgeons probably need to take the lead in trying to drive that down.
You asked about protocols in our ICU, which, as a matter of fact, Dr. Flint invented, created, and chaired forever. The answer is yes, we have begun to do that. I think that paying attention to this (whether it is similar to multisystem organ failure, stress, prophylaxis, or indeed hypoxia) is going to shed the light of day on it and make blood sugar control a lot better.
I think that settling the issue with anesthesia will actually be easy because we are going to move towards not only glucose monitoring but temperature monitoring and other things that for high-risk patients begin in the OR as a joint anesthesiology-surgery protocol.
Dr. Timothy L. Pruett (Charlottesville, Virginia): You have very nice data, and I know the answer is going to be no to this but there is very nice data to show the diversity of glucose response in our patients. The question, of course, is: what is the corresponding insulin level with this and when we start modeling ourselves in laboratory events where should we be putting our parameters and to what extent?
The other issue, of course, is that insulin is never released endogenously in a continuous fashion. There is a pulsatile release and you have a variety of different responses. Should our modeling be in a pulsatile fashion versus the way we administer it for therapeutic regimens, which is oftentimes either continuous infusion or in a continuous flow release through the subcutaneous administration and then subsequent degradation?
When I look specifically to the modeling, I am intrigued by notion of DR expression as a model for infectivity. It was mentioned that, certainly with infection, there is increased MHC expression on cells. It is always a question as to whether that is a response to the event or a primary cause. There have been previous studies showing that high insulin levels will decrease the response in terms of TNF secretion and superoxide and has been shown to be sort of anti-inflammatory agent. The question, of course, is: where does this fit in our whole panoply of discussion regarding innate immunity?
Clearly, our cells do more than just express MHC, they release cytokines which are shown IL-6 response. They also adhere and they phagocytose. Do you have any other measures of function associated with the combination of hyperglycemia and hyperinsulinemia to look at that? And again, I would like to re-echo Dr. Flint's comments: is there an adaptation time with the amount of duration and is there a dyssynchrony between, let's say, the MHC expression and subsequent function that you see?
Dr. Hiram C. Polk, Jr. (Louisville, Kentucky): The question about glucose is really a critical question. I thought this was surprising to me that there was so little impact of such high blood sugar on cellular function. While it met all the goals of statistical significance, the changes are still very modest but consistent changes. I would have thought it would have been more. Therefore, one would assume, rightly or wrongly, that blood sugars of 200 in the in vitro scenario might even have less of an impact. So where this works out is hard to tell.
I think the idea of the pulsatility or the pulsatile nature of what happens, both the blood glucose and the way we give insulin, is going to be both a hard thing to monitor or to create in the laboratory. It has to become a continuous variable and is one of several things that we have said we would like to do.
I think that there is no clear idea of what we must do about this today, except to say that in your heart and in your mind you need to know that blood sugar control is going to be something that is going to be monitored, we are going to pay more attention to this, especially in the perioperative period. Joining with our anesthesia colleagues, I would hope we can get them to decide that 150 is a much better number than 200.
As you noted, and Dr. Turina made clear in his presentation, we looked at a number of other parameters, and there is no consistent impact. So monocyte antigen presentation is one of several things that we studied that showed some abnormality and may begin to explain what is going on with this. I think this is one very early step on the surgical aspect of something we will hear a lot about for the rest of the next decade.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Protein glycosylation begins at glucose levels of 150 mg/dL and glycosylation has been implicated in the deleterious effects of hyperglycemia. Why did you select a glucose level of 200 mg/dL that would permit ongoing protein glycosylation?
Secondly, do you have an animal model in which you can change circulating glucose and insulin levels to let us know what the clinical significance of these changes you are reporting might be?
Dr. Hiram C. Polk, Jr. (Louisville, Kentucky): As most of you know, the SCIP project was predicated on a series of so-called “evidence-based” parameters that could be defined and applied to the surgical setting. It is purely happenstance that 200 blood sugar was chosen. That is why we asked the question.
That was not a variable that we chose; it was one that Centers for Medicare and Medicaid Services wanted to study because they felt the best evidence in the clinical literature existed for that number. I appreciate very much the suggestion about a clinical model where you could test this over time. That would obviously be a further study for us to do.
Footnotes
Reprints: Hiram C. Polk, MD, University of Louisville School of Medicine, 550 South Jackson Street, Louisville, KY 40294. E-mail: hcpolk01@louisville.edu.
REFERENCES
- 1.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. [DOI] [PubMed] [Google Scholar]
- 2.Grey NJ, Perdrizet GA. Reduction of nosocomial infections in the surgical intensive-care unit by strict glycemic control. Endocr Pract. 2004;10(suppl 2):46–52. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. [DOI] [PubMed] [Google Scholar]
- 4.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 6.Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–924. [DOI] [PubMed] [Google Scholar]
- 7.Laird AM, Miller PR, Kilgo PD, et al. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–1062. [DOI] [PubMed] [Google Scholar]
- 8.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–38. [DOI] [PubMed] [Google Scholar]
- 9.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. [DOI] [PubMed] [Google Scholar]
- 10.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144. [DOI] [PubMed] [Google Scholar]
- 11.Estrada CA, Young JA, Nifong LW, et al. Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;75:1392–1399. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80:862–866. [DOI] [PubMed] [Google Scholar]
- 13.Latham R, Lancaster AD, Covington JF, et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607–612. [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 15.Krogh-Madsen R, Moller K, Dela F, et al. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2004;286:E766–E772. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Park KW, Kim YS, et al. Effects of acute hyperglycemia on endothelium-dependent vasodilation in patients with diabetes mellitus or impaired glucose metabolism. Endothelium. 2003;10:65–70. [DOI] [PubMed] [Google Scholar]
- 17.Schiekofer S, Balletshofer B, Andrassy M, et al. Endothelial dysfunction in diabetes mellitus. Semin Thromb Hemost. 2000;26:503–511. [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, Quagliaro L, Piconi L, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701–710. [DOI] [PubMed] [Google Scholar]
- 19.Sampson MJ, Davies IR, Brown JC, et al. Monocyte and neutrophil adhesion molecule expression during acute hyperglycemia and after antioxidant treatment in type 2 diabetes and control patients. Arterioscler Thromb Vasc Biol. 2002;22:1187–1193. [DOI] [PubMed] [Google Scholar]
- 20.Nielson CP, Hindson DA. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes. 1989;38:1031–1035. [DOI] [PubMed] [Google Scholar]
- 21.Weekers F, Giulietti AP, Michalaki M, et al. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144:5329–5338. [DOI] [PubMed] [Google Scholar]
- 22.Polk HC Jr, George CD, Wellhausen SR, et al. A systematic study of host defense processes in badly injured patients. Ann Surg. 1986;204:282–299. [PMC free article] [PubMed] [Google Scholar]
- 23.Ditschkowski M, Kreuzfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livingston DH, Appel SH, Wellhausen SR, et al. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–1312. [DOI] [PubMed] [Google Scholar]
- 25.Foster KJ, Alberti KG, Hinks L, et al. Blood intermediary metabolite and insulin concentrations after an overnight fast: reference ranges for adults, and interrelations. Clin Chem. 1978;24:1568–1572. [PubMed] [Google Scholar]
- 26.Polk HC Jr. Quality, safety, and transparency. Ann Surg. 2005;242:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polk HC Jr, Lewis J, Garrison RN, et al. Process and outcome measures in specialty surgery: early steps in defining quality. Bull Am Coll Surg. 2005;90:9–15. [PubMed] [Google Scholar]
- 28.Shively EH, Heine MJ, Schell RH, et al. Practicing surgeons lead in quality care, safety, and cost control. Ann Surg. 2004;239:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–1633. [DOI] [PubMed] [Google Scholar]
- 30.Turina M, Miller F, Tucker C, et al. The effects of hyperglycemia, hyperinsulinemia, and hyperosmolarity on neutrophil apoptosis. Surg Infect (Larchmt). 2006 (in press). [DOI] [PubMed]
- 31.Wasmuth HE, Kunz D, Graf J, et al. Hyperglycemia at admission to the intensive care unit is associated with elevated serum concentrations of interleukin-6 and reduced ex vivo secretion of tumor necrosis factor-alpha. Crit Care Med. 2004;32:1109–1114. [DOI] [PubMed] [Google Scholar]
- 32.Dimitriadis G, Maratou E, Boutati E, et al. Evaluation of glucose transport and its regulation by insulin in human monocytes using flow cytometry. Cytometry A. 2005;64:27–33. [DOI] [PubMed] [Google Scholar]
- 33.Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. [DOI] [PubMed] [Google Scholar]
- 34.Ardeshna KM, Pizzey AR, Devereux S, et al. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 35.Kappel M, Dela F, Barington T, et al. Immunological effects of a hyperinsulinaemic euglycaemic insulin clamp in healthy males. Scand J Immunol. 1998;47:363–368. [DOI] [PubMed] [Google Scholar]
- 36.Hershman MJ, Cheadle WG, Wellhausen SR, et al. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. [DOI] [PubMed] [Google Scholar]
- 37.Hansen TK, Thiel S, Wouters PJ, et al. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–1088. [DOI] [PubMed] [Google Scholar]
- 38.Jeschke MG, Einspanier R, Klein D, et al. Insulin attenuates the systemic inflammatory response to thermal trauma. Mol Med. 2002;8:443–450. [PMC free article] [PubMed] [Google Scholar]


