Abstract
Objective:
To describe a single-center experience of pediatric intestinal transplantation (Itx) and to provide an overview of the children who underwent this procedure along with their outcomes.
Summary Background Data:
Pediatric Itx presents multiple challenges because of the very young ages at which patients require transplantation and their higher susceptibility to infectious complications.
Methods:
We have performed 141 Itx in 123 children with a median age of 1.37 years. Primary grafts included isolated intestine (n = 28), liver and intestine (n = 27), multivisceral (n = 61), and multivisceral without the liver (n = 7). Two protocol modifications were introduced in 1998: daclizumab induction and frequent rejection surveillance. In 2001, indications for multivisceral transplantation were expanded, and induction with Campath-1H was introduced.
Results:
Actuarial patient survival at 1 and 3 years for group 1 (January 1994 to December 1997, n = 25), group 2 (January 1998 to March 2001, n = 29), group 3a (April 2001 to present, daclizumab, n = 51), and group 3b (April 2001 to present, Campath-1H, n = 18) was 44%/32%, 52%/38%, 83%/60%, and 44%/44%, respectively (P = 0.0003 in favor of group 3a). Severe rejection implied a dismal prognosis (65% mortality at 6 months). Observed incidence of severe rejection in groups 1, 2, 3a, and 3b was 32%, 24%, 14%, and 11%, respectively. In multivariable analysis, use of a multivisceral (with or without liver) transplant (P = 0.002), induction with daclizumab (P = 0.005), patient at home prior to transplant (P = 0.007), and age at transplant ≥1 year (P = 0.02) favorably influenced patient survival. Multivisceral transplant was protective with respect to the mortality rate due to rejection, while an older age at transplant was associated with both a lower incidence rate of developing respiratory infection and lower risk of mortality following the respiratory infection. Survivors are off parenteral nutrition and have demonstrated significant growth catch-up.
Conclusions:
Itx in children still is a high-risk procedure but has now become a viable option for children who otherwise have no hope for survival. Control of respiratory infection is of particular importance in the younger children.
We performed141 intestinal transplants in 123 children. Survival has significantly improved in recent years with fewer severe rejection episodes mainly due to improved immunosuppression and greater use of multivisceral transplantation. Problems in achieving long-term successful outcomes remain, particularly the increased mortality due to infection in infant recipients.
The outcomes of children who suffer from neonatal (congenital) diseases such as gastroschisis, necrotizing enterocolitis, and intestinal atresia have improved dramatically with recent advances in both medical and surgical neonatal care.1–6 Some survivors, however, develop irreversible intestinal failure and are not able to absorb nutrition or fluid without parenteral administration of calorie and/or hydration. Some children are able to survive long-term with home parenteral nutrition and maintain a relatively good quality of life, but others will develop serious side effects that can eventually lead to death. Intestinal transplantation (Itx) was developed as a life-saving procedure for those suffering from life-threatening complications of parenteral nutrition.
Early outcomes of Itx in children were not satisfactory.7 Various reasons for these poor outcomes included the immunosuppressive therapy and lack of a center's experience, along with possibly poor patient selection. When the Intestinal Transplant Registry began in 1994, only about 25 cases of pediatric Itx were being collected per year worldwide. In 2004, the annual number performed in children increased to 98 (cumulative cases have reached 746) with substantially improved outcomes (Smith R and Grant D, personal communication). Since 1994, we have performed 141 cases of Itx in 123 children at the University of Miami/Jackson Memorial Holtz Children's Hospital. Here, we report our center's complete experience of using this procedure in children.
METHODS
Patients
A total of 141 transplants in 123 children under the age of 18 were performed, with 123 primary grafts and 18 retransplants. The median age at transplant was 16 months (range, 4 months to 17 years) (Fig. 1), and the median body weight at transplant was 9.5 kg (range, 4.4–67 kg).
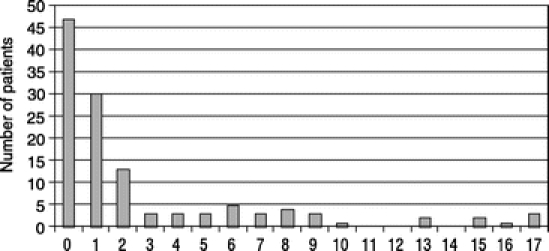
FIGURE 1. Frequency distribution of 123 primary Itx cases by age at transplant.
We analyzed our results in 3 separate periods based on our postoperative protocol: period 1 (August 1994 to December 1997; 25 patients), period 2 (January 1998 to March 2001; 29 patients), and period 3 (April 2001 to August 2005; 69 patients). Most patients in period 1 received no induction regimen, and induction with daclizumab began at the start of period 2. In period 3, indications for multivisceral transplantation were expanded, and patients received one of two different induction regimens (nonrandomized), daclizumab or alemtuzumab (Campath-1H). Therefore, we further divided patients in period 3 into 2 groups based on the induction regimen and formed 4 groups for comparison: group 1 (Itx in period 1), group 2 (Itx in period 2), group 3a (Itx in period 3 with daclizumab induction), and group 3b (Itx in period 3 with alemtuzumab induction). Patient follow-up was available through November 15, 2005.
Diagnosis
All patients had irreversible intestinal failure and complications of total parenteral nutrition (TPN). Patients were not placed on the waiting list unless they developed or were at risk for developing a life-threatening complication of TPN. Causes of intestinal failure are listed in Table 1. Most common diagnoses of short gut syndrome due to massive bowel resection were gastroschisis (n = 33), necrotizing enterocolitis (n = 25), and intestinal atresia (n = 16), while other diagnoses included functional abnormalities of the intestine such as Hirschsprung's disease (n = 11) and chronic pseudoobstruction/megacystis microcolon syndrome (n = 14).
TABLE 1. Diagnoses
Indications for primary transplantation were: liver failure in 86 patients (70%), cholestatic liver disease (reversible) in 10 (8%), and loss of central venous access or recurrent line sepsis in 27 (22%). Younger children were more likely to have liver failure as the indication: 86% (57 of 66) among patients age <1.5 years versus 51% (29 of 57) among patients age ≥1.5 years (P = 0.00002).
Pretransplant Evaluation
All organ systems were thoroughly assessed in each patient. Serious irreversible damage to a nonabdominal organ precluded patients from candidacy (eg, irreversible chronic lung disease). When kidney failure was imminent, transplantation of the kidney was included in the surgical procedure.
Surgical Technique
Principles of the surgical techniques for organ harvesting and transplantation have been described elsewhere.8–10 Types of grafts used in primary transplants were as follows: isolated intestinal transplant (n = 28, 23%), composite liver-intestine transplant (n = 7, 6%), noncomposite liver-intestinal transplant (n = 5, 4%), composite liver-intestine-pancreas transplant (n = 15, 12%), multivisceral transplant (n = 61, 49%), and modified multivisceral transplant (multivisceral transplant without the liver) (n = 7, 6%) (Fig. 2).
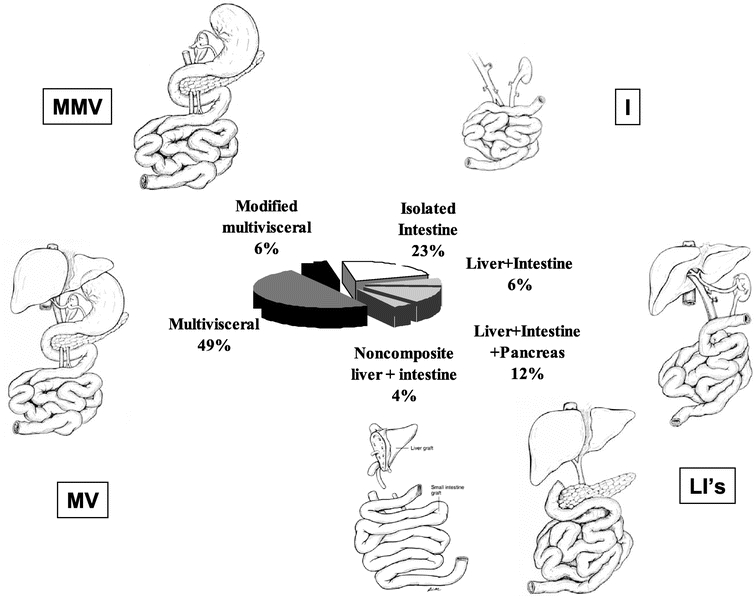
FIGURE 2. Types of grafts used in 123 primary Itx performed at this center since 1994.
Patients having intestinal failure without terminal liver disease received an isolated intestinal transplant (I), except for those whose stomach and/or pancreas had to be replaced. In these cases of patients having a severe gastric dysmotility, a modified multivisceral (MMV) transplant was performed (en bloc transplant of the stomach, pancreas, and intestine without the liver).
In periods 1 and 2, patients with liver and intestinal failure received composite liver-intestinal (LI) transplant, composite liver-intestine-pancreas (LIP) transplant, or noncomposite LI transplant. Composite LIP transplant was developed for small children to avoid backtable injury to the hepatic hilar structure while removing the pancreas from the composite graft.11–13 Noncomposite LI transplant was developed to accommodate any major size discrepancies between donor and recipient.14 Multivisceral (MV) transplants (en bloc transplant of the stomach, pancreas, intestine and liver) were used only in patients with liver and intestinal failure whose stomach and/or pancreas had to be replaced.
In period 3, we elected to use MV transplantation as an alternative to LIP transplant for the following reasons. First, in the use of a LIP transplant, the organ replacement is not orthotopic (the donor pancreaticoduodenal complex is placed on top of the native one), requiring more space in the abdominal cavity to perform this operation. Use of an MV transplant requires less space and thus may have a significant advantage in small children. Second, the native stomach and pancreas are not necessarily normal; they are often affected by the underlying pathology of portal hypertension, dense abdominal adhesion, and prior surgical manipulations. By retaining them, drainage of the native portal system is required. Third, an MV transplant preserves the entire vascular network of abdominal viscera, which may offer greater perfusion to the transplanted organs. Lastly, MV transplant may offer immunologic advantages as was demonstrated in an animal model.15 In keeping with this strategy, patients with liver and intestinal failure in this period received MV transplant exclusively (n = 47), except for 2 patients who received a noncomposite LI graft.
Donor colon together with the ileocecal valve was included in the primary graft of 34 cases, and 32 patients in period 3 received donor spleen with MV grafts. A total of 9 patients received a kidney transplant, 1 as part of a LIP graft, 1 as part of a MMV graft, and 7 as part of a MV graft. Upper GI anastomoses were: esophagogastrostomy (n = 35), gastrogastrostomy (n = 31), gastrojejunostomy (n = 2), duodenojejunostomy (n = 17), and jejunojejunostomy (n = 38). Lower anastomoses were: ileocolostomy (n = 46), ileoproctostomy (n = 5), ileoileostomy (n = 7), colocolostomy (n = 29), Mikulicz ileocolostomy (n = 26), Mikulicz colocolostomy (n = 4), and no anastomosis (n = 6). Types of distal stoma were: terminal ileostomy (or colostomy) with side-to-end ileocolostomy or colocolostomy (n = 55), Mikulicz-type double-barrel stoma (n = 29), loop ileostomy (n = 26), terminal ileostomy or colostomy with no anastomosis (n = 4), and others (n = 9).
Immunosuppression
Tacrolimus (Prograf, Astellas, Deerfiled, IL) and corticosteroids were used as maintenance immunosuppression in all periods except for the recipients of alemtuzumab (Campath, Ilex Oncology, Berlex, Montville, NJ) induction, whose maintenance regimen was tacrolimus monotherapy. During period 1, most patients received no induction therapy, except for a few patients who received OKT3 (n = 4) or cyclophosphamide (n = 3). For patients who received no induction therapy, mycofenolate mofetil (Cell Cept, Roche, Nutley, NJ) was added to the maintenance regimen. During period 2, daclizumab (Zenapax, Roche) was introduced as induction immunosuppression. Daclizumab was administered at a dose of 2 mg/kg every 2 weeks for the first 3 months and then 1 mg/kg every 2 weeks for the next 3 months. Sirolimus was introduced as a rescue agent in cases of refractory rejections and tacrolimus toxicity. During period 3, alemtuzumab was introduced as a tolerance induction protocol. However, it was discovered that the results of alemtuzumab induction were not satisfactory, particularly in small children. Therefore, after July 2002, its use was limited to patients at least 4 years of age.
Postoperative Monitoring of Rejection
Protocol surveillance endoscopy and biopsy were started in 1998. The surveillance schedule included: endoscopies with biopsies performed twice a week during the first 2 weeks posttransplant, then weekly for 3 to 8 weeks, then once a month until the stoma closure. Transplant ileostomies were created at the time of surgery to facilitate the procedure. Most endoscopies did not require patient sedation. Zoom endoscopy was available for use in older children.16 Immediate endoscopies were performed for symptoms of rejection such as fever, diarrhea, and increased or bloody stoma discharge. Biopsies were read by dedicated transplant pathology staff and scored according to current protocols.17
Once the diagnosis of rejection was determined by endoscopic appearance, histologic features, and clinical symptoms, treatment of rejection was given. Mild acute rejections were usually treated with corticosteroid bolus. Rapidly evolving mild, moderate, and severe rejections were treated with OKT3. Graft removal or retransplantation was considered for treatment resistant rejections.
Statistical Analysis
Cox stepwise regression analyses were performed to determine a most important set of prognostic factors for the hazard rates of the following outcome variables: development of a first rejection episode (any grade), development of severe acute rejection, development of posttransplant lymphoproliferative disease (PTLD), development of a respiratory infection, and patient survival. A cause-specific hazard rate analysis of death was also performed using 3 distinct outcomes: death due to rejection, death due to infection not triggered by rejection, and death due to other causes. In the analysis of freedom-from-failure due to a particular cause, patients who failed first of a competing cause were treated as censored observations. For each of these analyses, the score χ2 test criterion was used. To avoid the possibility of obtaining spurious results with relatively small sample sizes, only variables with univariable P values ≤0.05 were considered for entry into the cause-specific hazard Cox models. Kaplan-Meier curves were performed for visual display of the prognostic factors’ effects on the cause-specific hazards along with logrank tests of their differences. Tests of association among the important prognosticators were performed using Pearson (uncorrected) χ2 and t tests, along with inclusion in the text of median (with the range) and mean (±standard error) values for selected patient characteristics.
RESULTS
Waiting List Statistics, Donor and Operative Variables
Since 1994, 182 patients were placed on our waiting list, and 123 patients (68%) were transplanted here, with waiting times of 1 to 1604 days (median time, 53 days). Six waitlisted patients were transplanted at other centers (double listing), 7 are still on the waiting list, 25 died while on the waiting list (median time, 51 days) and 21 were removed from the list for deteriorating condition (n = 3), improved condition (n = 13), or other (n = 5). Observed waiting list mortality, including the 3 patients who were removed due to deterioration, was 11% (3 of 28), 15% (5 of 34), and 22% (20 of 89) in periods 1, 2, and 3, respectively. Twenty-four of 25 patients (96%) who died on the waiting list had liver failure. Only one isolated intestine candidate died on the waiting list due to line sepsis.
Donor age ranged from 5 days-59 years (median, 1.67 years). Median body weight of donors was 11 kg (range, 2.7–67 kg). Donor/recipient body weight ratio ranged from 0.25 to 11.1 (median, 1.04). Human leukocyte antigen (HLA) was randomly selected (mean HLA ABDR match, 0.89; range, 0–3; mean HLA DR match, 0.39; range, 0–1). Donor cytomegalovirus (CMV) serology negative was preferred in period 1, but no preference was given in periods 2 and 3. CMV serology (donor/recipient) was −/− in 41 (33%), −/+ in 17 (14%), +/− in 39 (32%), and +/+ in 26 (21%). Mean cold ischemia time was 425 ± 8 minutes, and mean warm ischemia time was 41 ± 2 minutes.
Acute Rejection
A total of 191 distinct episodes of rejection were observed in this cohort, and the overall incidence rate of rejection was 6.2 ± 0.5 rejections per 100 patient-months of follow-up. The observed highest degree of rejection was mild in 27 patients (22%), moderate in 31 (25%), and severe in 24 (20%) patients. Forty-one patients (33%) experienced no rejection. Among the 82 patients who experienced rejection, 1) the mean number of rejection episodes per patient was 2.32 ± 0.19, and 2) the time-to-first episode of rejection ranged from 3 to 1072 days (median, 16 days). Patients transplanted during periods 1, 2, and 3 who experienced no rejection were 20% (5 of 25), 24% (7 of 29), and 42% (29 of 69), respectively (P = 0.02, period 3 vs. periods 1 + 2); conversely, patients transplanted during these 3 periods who experienced a severe rejection episode were 32% (8 of 25), 24% (7 of 29), and 13% (9 of 69), respectively (P = 0.04, period 3 vs. period 1 + 2). However, among patients who experienced a rejection, the mean number of rejection episodes per patient did not differ by period. Finally, a freedom-from-rejection analysis found that patients transplanted during period 3 had a significantly lower rejection rate (P < 0.00001); no difference between patients transplanted in the first 2 periods was observed. The more favorable outcome for patients transplanted during period 3 was seen in both a higher overall percentage of patients who remained rejection-free (stated above) as well as in a longer time-to-first rejection distribution among those who experienced a rejection (P = 0.00004). Indeed, among those transplanted during periods 1 and 2 who experienced a rejection episode, the first rejection occurred during the first month posttransplant in 38 of 42 patients; this compares with only 18 of 40 among those transplanted during period 3 who experienced a rejection episode.
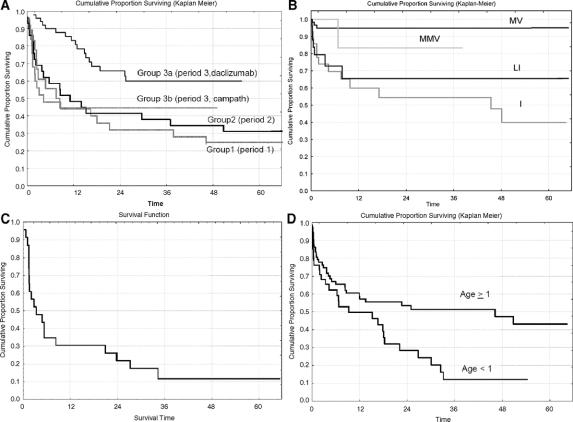
A Cox regression analysis looking at the impact of rejection (using time-dependent covariates) on patient survival was performed. The incidence of a mild or moderate rejection had no impact on patient survival, whether overall or cause-specific mortality was considered. However, the development of a severe rejection episode had a highly significant association with the subsequent hazard rate of death (P < 0.00001), particularly the hazard rate of death due to rejection (P < 0.00001). Indeed, 20 of 24 patients who experienced a severe rejection episode ultimately had graft failure due to rejection, and post-severe rejection survival was estimated to be only 35% ± 10% at 6 months (Fig. 3c).
FIGURE 3. A, Kaplan-Meier patient survival by group: group 1 (n = 25, 20 deaths), group 2 (n = 29, 20 deaths), group 3a (n = 51, 17 deaths), and group 3b (n = 18, 10 deaths) (P = 0.002 comparing the 4 groups, and P = 0.0003 comparing group 3a vs. the other 3 groups combined). Note: The x-axis label “Time” refers to “time (in months) following transplantation” (same for B and D). B, Kaplan-Meier freedom-from-the development of a severe rejection episode by type of transplant: MV (n = 61, 3 failures), MMV (n = 7, 1 failure), c-LI (n = 27, 7 failures), and I (n = 28, 13 failures) (P = 0.0001 comparing the 4 groups, and P = 0.00001 comparing I and c-LI combined vs. MV and MMV combined). C, Kaplan-Meier patient survival following the development of a severe rejection (n = 24, 20 deaths). Note: The x-axis label “Time” refers to “time (in months) following severe rejection.” D, Kaplan-Meier freedom from the development of a respiratory infection by age at transplant: age ≥1 year (n = 75, 35 failures), and age <1 year (n = 48, 31 failures) (P = 0.02).
Finally, a Cox stepwise regression analysis of freedom-from-severe rejection found 2 significantly unfavorable predictors of this outcome: receiving an I or c-LI transplant (c-LI represents the combined group of LI, noncomposite LI, and LIP) (P = 0.00002, vs. MV or MMV, Fig. 3b) and receiving no induction agent (P = 0.05). The observed percentage of patients who experienced a severe rejection was 20 of 55 among those who received I or c-LI in comparison with only 4 of 68 among those who received MV or MMV. Although freedom-from-severe rejection was significantly more favorable among patients transplanted during period 3 (P = 0.01), these patients were more likely to have received a MV or MMV transplant (P < 0.00001, 77% vs. only 28% in the first 2 periods). Thus, once the effect of transplant type was selected into the Cox model, the comparison of periods 1 and 2 versus period 3 was no longer significant (P = 0.54). It should be noted that the protective effect of MV transplantation does not appear to be associated with the inclusion of the liver. Indeed, among the patients who received a liver, freedom-from-severe rejection was significantly more favorable in the MV group in comparison with the c-LI group (P = 0.001). In addition, there was no difference in freedom-from-severe rejection between the I and c-LI transplanted groups (P = 0.46).
Infectious Complications
Most of the patients, 91% (112 of 123), developed at least one type of infection during the posttransplant follow-up period. The median number of infections observed per patient was 5 (range, 0–32). Among the total of 619 distinct infections that were observed among the 123 patients, 39% (242 of 619) occurred during the first 3 months posttransplant, 26% (163 of 619) occurred during months 4 to 12 posttransplant, and 35% (214 of 619) occurred after 12 months posttransplant. The great majority of the infections, 90% (559 of 619), had a bacterial component: 526 were strictly bacterial, 27 were also fungal, and 6 were also viral. Sixty-six of the infections (11%) included a fungal component, 40 being solely fungal. Twenty-three of the infections (4%) included a viral component, 17 being solely viral.
Analysis by location of infection shows that 277 (45%) were found in blood, 113 (18%) respiratory, 56 (9%) wound, 49 (8%) at the catheter site, 47 (7%) in the urine, 41 (7%) intraabdominal, 26 (4%) intestinal, and 10 (2%) other. There was no association of infection with date of transplant, other organs transplanted (liver, spleen, etc.), induction agent, or pretransplant status. However, there were associations of recipient age with infection. Specifically, stepwise linear regression found that the number of infections seen per patient was significantly higher in younger patients (P = 0.03). This association remained consistent over time posttransplant as well as across location of infection, ie, higher rates of blood, respiratory, and in-urine infections were seen in younger patients. In addition, an analysis of freedom-from-development of a respiratory infection found a significantly higher incidence rate in the younger patients (P = 0.02 for age, both as a continuous variable and in comparing age < vs. ≥1 year) (Fig. 3d).
There appeared to be no association of the development of a urine, abdominal, wound, or intestinal infection with the subsequent rate of death. However, patients who developed a respiratory infection or bacteremia (blood infection) had significantly higher death rates following the infection in comparison with the mortality rate while patients remained free of developing these infections (P < 0.00001 for the impact of respiratory infection; P = 0.004 for the impact of bacteremia after controlling for the impact of respiratory infection). While the development of a respiratory infection was highly associated with the subsequent mortality rate due to infection (P < 0.00001), it was also significantly associated, but to a slightly lesser degree, with the mortality rates due to rejection (P = 0.0006) and other causes (P = 0.00003). While the development of a bacteremia did not influence the subsequent mortality rate due to infection (P = 0.24) and only marginally affected the subsequent mortality rate due to rejection (P = 0.07), it was significantly associated with the subsequent mortality rate due to other causes (P = 0.002). Thus, while the development of a respiratory infection is clearly associated with the patient's subsequent risk of dying from infection, it also appears that the development of a respiratory infection and to a lesser degree a blood infection may play some role in exacerbating any other ongoing clinical problems in the patient, which, in fact, may lead to death.
Finally, 9 of the 22 patients who died of an infection had respiratory infection (ie, pneumonia) as the triggering cause of death. The risk of death due to a respiratory infection was clearly associated with age, with the observed percentage who died of a respiratory infection in patients <1.5 and ≥1.5 years of age being 13.6% (9 of 66) and 0.0% (0 of 57), respectively (P = 0.003, logrank test). Within the group of 66 patients who had developed a respiratory infection, the observed percentage who subsequently died of a respiratory infection in patients <1.5 and ≥1.5 years of age was 22.5% (9 of 40), and 0.0% (0 of 26), respectively (P = 0.009).
PTLD
Posttransplant lymphoproliferative disease (PTLD) was seen in 14 children (11%, or 6% per year based on an overall incidence rate 0.50 ± 0.13 per 100 patient-months of follow-up). Median onset of PTLD, among those who developed it, was 21 months, with a range of 1.6 to 65.6 months. All 14 had pathologic diagnosis of PTLD, with detailed data for type of PTLD being available in 11; monoclonal B cell type, EBV positive (n = 4), polymorphic B cell type, EBV positive (n = 6), polymorphic T cell type, EBV negative (n = 1). Incidence of PTLD in periods 1, 2, and 3 were 8.0% (3.3% per year), 20.7% (12.7% per year), and 8.7% (4.9% per year), respectively. Two patients died of PTLD. The first patient developed PTLD in 1997 and subsequently died despite receiving chemotherapy and surgical resection. In the second patient, PTLD was only discovered at an autopsy. The remaining 12 patients received antiviral and rituximab therapy with reduction of immunosuppression. All 12 of these patients responded to the therapy with complete resolution; 3 subsequently died of causes unrelated to PTLD. Therefore, with an overall mortality due to PTLD being only 1.6% (2 of 123), the occurrence of PTLD had no significant impact on patient survival. Finally, a preliminary analysis of predictors of the incidence rate of developing PTLD found one significant factor. The use of OKT3 as treatment of a rejection episode (as a time-dependent covariate) appeared to be associated with a significantly higher incidence rate (P = 0.0004), with the observed percentages of OKT3 and non–OKT3-treated patients who developed PTLD being 23.7% (9 of 38) and 5.9% (5 of 85), respectively.
GVHD
Six patients developed confirmed graft versus host disease (GVHD). The observed incidence was somewhat higher (although nonsignificant) among MV and MMV recipients, with 7.4% (5 of 68) of these patients developing GVHD in comparison with only 3.6% (1 of 28) of the I recipients and 0.0% (0 of 27) of the c-LI recipients. However, mortality following GVHD was poor, with 4 of these 6 patients dying of GVHD (66.7%), 3 from acute GVHD and 1 from chronic GVHD.
Effect of Donor Spleen
Among the 68 patients who received a MV or MMV graft, the number who received/did not receive a donor spleen were 32 and 36 patients, respectively. Although the spleen group showed a greater observed proportion of patients experiencing no rejection (56% vs. 28% in the no spleen group), this difference was not significant. In addition, none in the spleen group have developed PTLD so far, whereas 5 of 36 (14%) developed PTLD in no spleen group. Finally, GVHD was observed in 3 of 32 (9.4%) in the spleen group in comparison with 2 of 36 (5.6%) in no spleen group.
Other Complications
Among recipients that had follow-up greater than 1 year with complete serum creatinine and anthropometric data (n = 31), estimated glomerular filtration rate (GFR) was calculated using Schwartz's formula.18 The average serum creatinine level at pretransplant, 1 year, and 2 years posttransplant was 0.36 ± 0.03, 0.60 ± 0.06, and 0.56 ± 0.05 mg/dL, respectively. Average estimated GFR at pretransplant, 1 year and 2 years posttransplant was 117.8 ± 6.3, 105.5 ± 7.8, and 113.3 ± 7.1 mL/min, respectively. Incidence of renal insufficiency defined as estimated GFR <90 mL/min increased from 13% at pretransplant to 48% at 1 year and decreased slightly to 35% at 2 years. Although the sample size was small, there was sufficient evidence to suggest a linear correlation between a higher patient average prograf (12-hour trough) level during the first 3 months posttransplant and 1) a lower estimated GFR at both 12 and 24 months posttransplant (P = 0.07 and 0.01, respectively), and 2) a greater decrease in estimated GFR at both 12 and 24 months posttransplant from the pretransplant level (P = 0.05 and 0.06, respectively). Indeed, an average prograf (12-hour trough) level during the first 3 months posttransplant ≥18 ng/mL appeared to indicate poorer GFR outcomes (data not shown).
A stepwise linear regression analysis was performed of the patient's average prograf (12-hour trough) level during the first 3 months posttransplant (data were available on 88 patients). Two variables were selected containing a significant association with this level: patients transplanted during the first 2 periods had higher levels (P < 0.00001), and patients who received Campath-1H induction had lower levels (P < 0.00001). No differences in the average prograf (12-hour trough) levels beyond 3 months posttransplant were seen, although individual patients’ prograf levels appeared to decrease over time. The mean ± SE of the average prograf level during the first 3 months posttransplant for patients in groups 1 and 2 combined, group 3a, and group 3b was 20.1 ± 0.7 (n = 30), 15.6 ± 0.3 (n = 48), and 11.1 ± 1.1 (n = 10), respectively. Most of the patients transplanted during the most recent period had an average prograf level during the first 3 months that was <18 ng/mL.
Seventy-four patients required 107 re-explorations for complications (excluding staged abdominal closure); thus, the average rate of bring-back surgeries per patient was 0.87. Major reasons for re-explorations were: intraperitoneal bleeding (n = 15), wound dehiscence (n = 10), intraabdominal sepsis (“wash out” procedure for intraabdominal infection) (n = 9), intestinal obstruction (n = 7), intestinal anastomotic leak (n = 5), reflux of esophagogastrostomy (n = 3), pancreatitis (n = 3), leak at esophagogastrostomy (n = 1), bolioenteric anastomotic leak (n = 2), and bowel perforation (n = 2). The average number of bring-back cases in periods 1 to 3 were 1.00, 1.07, and 0.74, respectively.
Growth/Nutrition
Height and weight for age Z-scores were calculated with Epi Info software (Center for Disease Control, Atlanta, GA) on 45 patients whose anthropometric data were available for more than 1 year of follow-up. Average pretransplant Z scores were: −3.27 ± 0.53 (height for age Z), and −1.80 ± 0.36 (weight for age Z-score) (a Z score value of 1 represents one standard deviation difference from the standard growth chart). In those whose growth was severely delayed pretransplant (Z score <−2.0), the average Z scores increased from −4.71 to −3.59 (height, P = 0.06) and −4.20 to −1.65 (weight, P = 0.0003) at 1 year, and to −2.43 (height, P = 0.02) and −0.74 (weight, P = 0.00004) at 2 years.
All survivors are off TPN except for 2 patients who require intermittent TPN to maintain weight.
Long-term Outcomes
As of November 15, 2005, 41 patients have survived more than 3 years (36 are currently alive), 16 more than 5 years (15 alive), and 2 more than 10 years (both are still alive). Among those who lived more than 3 years, most children (24 of 27) who reached school age were attending school regularly, and the remaining number are receiving home schooling.
Survival and Cause-Specific Mortality Analysis
Of the 123 patients, 67 deaths have been observed. Actuarial patient survival by group is displayed in Figure 3a. One- and 3-year patient survivals were 44%/32%, 52%/38%, 83%/60%, and 44%/44% in group 1 (n = 25), group 2 (n = 29), group 3a (n = 51), and group 3b (n = 18), respectively (P = 0.0003 favoring group 3a over the other 3 groups). Results for graft survival (73 graft losses/deaths) were similar and are not shown. The Cox stepwise regression analysis of patient survival found 4 significantly unfavorable predictors of this outcome: receiving an I or c-LI transplant (P = 0.002, versus MV or MMV transplant), not receiving daclizumab (ie, being transplanted prior to 1998 or receiving Campath-1H, P = 0.005), being in the hospital (vs. at home) pretransplant (P = 0.007), and age-at-transplant <1 year (P = 0.02). The major difference in survival between patients in periods 2 and 3 who received induction with daclizumab (group 2 vs. group 3a) appears to be explained by the type of transplant effect: the percentage of daclizumab-receiving patients who received a MV or MMV transplant was only 21% (6 of 29) during period 2 versus 76% (39 of 51) during period 3 (P < 0.00001).
Finally, among the 67 patient deaths, 20 were due to rejection (16 due to severe, 3 due to refractory/vascular, and 1 due to chronic), 22 were due to infection (not triggered by rejection), and 25 were due to other causes. Separate stepwise Cox regression analyses of these 3 cause-specific hazard rates were performed, yielding the following results. Two factors contained a significantly unfavorable prognosis for the mortality rate due to rejection: receiving an I or c-LI transplant (P = 0.0004) and not receiving an induction agent (P = 0.001). Conversely, age-at-transplant <1 year was the single factor associated with a significantly higher mortality rate due to infection (P = 0.001). The observed percentage of patients who died of an infection was 29.2% (14 of 48) versus 10.7% (8 of 75) among those <1 versus ≥1 year of age, respectively. Lastly, 2 factors contained significantly unfavorable prognosis for the mortality rate due to other causes: being in the hospital pretransplant (P = 0.01) and not receiving daclizumab (P = 0.02).
DISCUSSION
Achieving successful outcomes in children who receive Itx has been very challenging, because children with intestinal failure undergo transplants at a very young age due to the congenital nature of the disease. The median age-at-transplant of our patients was only 16 months, and these patients were more likely to have liver failure in addition to intestinal failure. As we described previously, there seems to be a subpopulation of children who do not tolerate TPN and develop liver failure early.19
Management of young-age children is particularly difficult due to their respiratory system's immaturity. Our results indicate that younger children have a significantly increased rate of developing respiratory infections and that this increased rate is consequently associated with a significantly increased risk of mortality. In addition, even among patients who develop a respiratory infection, a younger age portends a significantly worse prognosis with respect to the risk of subsequent death due to infection. Further refinements of immunosuppressive strategy and prophylactic therapy may improve the outcome of these patients.
Our early outcomes were significantly affected by the high frequency of severe rejection. We learned that successful Itx is most likely not possible without the use of an induction agent (use of no induction therapy in period 1 was significantly associated with a higher hazard rate of death due to rejection). The use of daclizumab induction appeared to immediately affect patient outcome starting in period 2. In an attempt to promote tolerance in period 3, we tested alemtuzumab induction therapy. The results of alemtuzumab induction were not satisfactory, particularly in small children.20 Its poor tolerance in the smaller children may possibly be explained by difficulty in choosing an appropriate dosing of this antibody in such children.
We decided to expand indications for MV transplantation in 2001. Since then, small children with liver and intestinal failure have received a MV instead of a LIP transplant. Although the main reasons for this protocol modification were not based on the immunologic aspect, the use of MV transplantation was associated with a significantly reduced risk of death due to rejection.10,19 In our current analysis, we further clarified these results by analyzing tacrolimus levels and HLA data, and showed that type of transplant has the strongest association with the hazard rate of death due to rejection (even more important than the use of no induction therapy). The reason for the protective effect of a MV transplant on intestinal rejection is still not clear. Other investigators have suggested that this effect is due to the liver's inclusion;21 however, our data do not support this hypothesis. Indeed, in our analyses of the rates of developing severe rejection and death due to rejection, among patients who received a liver, the prognosis was significantly poorer among those receiving a c-LI in comparison with an MV graft.
One possible explanation for the protective effect of a MV transplant is the removal of native lymphoid tissue such as spleen, mesenteric lymph nodes, and intestinal mucosal lymphoid tissue. During MV (and MMV) transplantation, these lymphoid tissues are extensively removed. The degree of removal of native lymphatic tissue is significantly more than in c-LI transplantation. Secondary lymphoid tissue provides an environment for antigen presenting cells to initiate allorecognition and activation of lymphocytes. An animal model suggests that the elimination of secondary lymphoid organs can lead to indefinite acceptance of a cardiac allograft.22 More precise explanations of the protective effect of a MV transplant (ie, whether this effect is due to tolerance or some other mechanism), however, will require further investigation.
PTLD and GVHD were both observed in our children. Incidence of PTLD remained high even in recent periods. However, outcome of treatment of PTLD markedly improved and related mortality was reduced. As reported earlier, long-term use of Rituximab appears to be the reason for such improvement.23 Outcomes following treatment of GVHD, on the other hand, are still dismal. The incidence rate of GVHD occurrence is not high, but it remains a difficult complication to treat in Itx patients.
Most extrarenal organ transplants have been associated with the development of posttransplant renal insufficiency; indeed, Itx was reported to have the highest incidence of renal failure from Scientific Registry of Transplant Recipients data.24 It seems that early reduction of the tacrolimus levels may protect against the development of renal insufficiency. Further analysis with prospective data collection will be necessary to develop kidney protective regimens of immunosuppression.
The results of growth in children after Itx are very encouraging. Among patients whose anthropometric data were available, patients whose growth was significantly delayed prior to transplant showed significant catch-up in growth following their Itx. Long-term survivors seem to have a relatively good quality of life with oral intake of calories alone. Some children took a very long time to start oral intake, and these patients showed a significant developmental delay. Early intervention for these children after Itx may further improve their outcomes.
CONCLUSION
Our data indicated that short-term outcomes of Itx in children have improved significantly over time. Acute rejection and infection are the 2 major complications that lead to mortality. More liberal use of MV transplantation appears to have improved the outcomes. However, the achievement of favorable long-term survival in this patient population is an elusive goal that remains to be achieved. Continuing efforts to further refine the postoperative monitoring and management of these patients should be maintained until the results of Itx reach long-term success, thus further establishing its solid role in the management of irreversible intestinal failure in children.
Discussions
Dr. Ronald W. Busuttil (Los Angeles, California): This is truly an important presentation because it is one of the largest single-centered experiences with intestinal transplantation in children. In fact, it actually accounts for over 10% of the world experience.
As you have heard, over a 10-year period, 141 intestinal, composite, and multivisceral grafts have been placed in 123 pediatric patients. This comprehensive experience covers not only the important issues of technique in patient and graft survival but also adds important information on wait list statistics, infectious complications, lymphoproliferative orders, and nutritional parameters. Additionally, they describe an evolution of immunosuppressive management, which allows a substantial improvement in reducing allograft rejection.
Dr. Kato, I have several questions for you.
First, multivisceral grafts are your preferred option because technically they do allow easier implantation and they may confer an immunologic advantage. But does this actually justify removing the pancreaticoduodenal splenic complex, which has in and of itself several disadvantages? Both Pittsburgh and our own data from UCLA show a higher patient mortality for recipients who undergo recipient splenectomy. I wonder if you would comment on that.
Secondly, the inclusion of the donor colon is quite controversial, and you included it in 34 of your cases. Pittsburgh reported worst outcomes in their recipients of intestinal transplantation in which the colon was retained, and most of the deaths were due to sepsis. A series from France has also described outcomes that are not as good when the colon is retained. So I was wondering if would you provide us with your algorithm and rationale for why you retained the donor colon.
The third involves the donor spleen. And you included that in 32 recipients. This clearly carries immunological potential for complications. In our recipients, when the donor spleen is removed after reperfusion, we have seen our lowest incidence of rejection. What is your opinion regarding the risk benefits of retaining the spleen vis-à-vis post-splenectomy sepsis and the development of graft-versus-host-disease and allograft rejection?
Fourth, the donor data are very interesting. In your report, you used neonatal donors, some as young as 5 days of age. Have you used premature infants? As you well know, in isolated liver transplantation, donor grafts from the 1- to 2-month-old child do not do as well. Is there a protective effect on these young donors by using multivisceral grafts?
Based on your results, is there ever an indication for Campath? There doesn't seem to be.
Finally, because the cause of death in your long-term survivors was not delineated, what are these patients dying of 3 and 4 and 5 years after transplantation?
Dr. Tomoaki Kato (Miami, Florida): The first question was in reference to the removal of the native pancreas when performing the multivisceral transplant. There are two main reasons why we remove this portion of the native foregut. First, although present, the native stomach duodenum and pancreas are not necessarily normal organs in these patients. Many have long-standing portal hypertension. The stomach especially is often congested. The stomach and duodenum are often dilated due to chronic distal obstruction. Gastric emptying is often grossly abnormal. A second reason for removing the native stomach duodenum and pancreas is for providing sufficient room in the abdominal cavity for the multivisceral graft and to assure that the three-dimensional alignment and position of the graft is acceptable. Placing the multivisceral graft on top of the native stomach and pancreatic duodenal complex in a sort of heterotopic position may compromise the three-dimensional orientation of the graft.
The second question was in reference to the inclusion or the exclusion of the colon in the multivisceral graft. An early study from the University of Pittsburg concluded that inclusion of the colon had a detrimental effect on survival. However, this preliminary observation has not been validated in other series. When we analyze our results, inclusion of the colon did not seem to have an adverse effect on survival. Also, inclusion of ileocecal valve and a portion of the colon is functionally advantageous, reducing stool output and thus decreasing the chances of problems related to dehydration.
The third question was in reference to the inclusion of the spleen. We have two reasons for including the spleen in the multivisceral graft. The inclusion of the spleen may mitigate the risk of infection seen in asplenic patients, especially infections due to encapsulated organisms. The second reason is that inclusion of the spleen has a potential immunological advantage. Although I did not show that data in my presentation, data presented in the manuscript show that the spleen was included in 32 of the multivisceral graft recipients and was excluded in 36 multivisceral recipients. In the group that received the spleen as part of the composite graft, more than half of the patients did not experience a single rejection episode, whereas in the group that did not receive a spleen only 28% of these patients escaped at least a single episode of rejection. PTLD has been observed in 14% of the recipients that did not receive a spleen. No patient in the group that received the spleen experienced PTLD so far. Graft-versus-host-disease, on the other hand, was more common in the spleen group. Patients receiving a spleen had a 9% incidence of graft versus host disease compared with 6% incidence in the group that did not receive the spleen.
The fourth question was regarding the use of neonatal donors. Specifically, regarding our 5-day-old donor. We have not used any organs from pre-term infant donors. In our early experience, we had technical difficulties with very young donors, not so much in terms of graft function but more in terms of the handling of the tissue. In these donors, the intestine is paper thin and is quite fragile, making it very susceptible to perforation due to the ischemia reperfusion injury or a mechanical injury. The dilemma is that more recently the younger potential recipients are being referred to us for evaluation probably due to the realization of the success of the procedure. This change in the referral pattern has required us to reconsider our use of neonatal donors. While we are now using them, we really have to be very careful, especially with regard to our handling of the graft during the transplant and post-transplant.
The fifth question was regarding the use of Campath in this patient population. In our experience, Campath was associated with significant problems in very small and young children. Three children developed an ARDS-like respiratory problem which did not reverse. It is not clear whether this was attributable to the Campath or not. But there is no question that the group of children that received Campath did not do as well as the non-Campath cohort. Although we stopped using Campath in small children, we are still using it in children age 4 and older. These children don't seem to experience the same problems, especially the respiratory problems. And the results with Campath in this group of older children have been quite reasonable. We hope to be able to use it again in the future in the very small children. This will require very careful monitoring.
The sixth question was regarding late graft loss. Whereas with other solid organ transplants, where the graft loss rate levels off after the first year, we still see significant graft loss after the first year. One reason for this might be associated with the fact that a number of children seem to do poorly after their stoma is closed and GI continuity is restored. Many of these patients, especially those transplanted at a very young age, have never had a normal bowel movement in their life. Once GI continuity is restored and the native colonic segment and rectum are being used for the first time, we have seen significant enteritis involving both the native distal bowel and the transplanted bowel. This may be due to the overgrowth of bacteria in a previously unused segment. Also, once the stoma is closed, monitoring for rejection becomes more difficult because of the relative inaccessibility of the graft mucosa for endoscopic evaluation. In response to this potential problem, we have decided not to close the stoma for approximately 2 years. And this seems to have contributed to the improved survival in the most recent cases.
Dr. J. Alex Haller, Jr. (Baltimore, Maryland): One of the things we have been interested in is whether a heavy load of donor antigen would induce in these younger children true tolerance. You gave some implications that you believe this might be happening. Do you have any actual data using transfer skin grafts or any other way of checking to see whether this is true induction of tolerance that could account for the better results?
The second question I wanted to ask you is: in this group of children, you mentioned that the best results were with the multivisceral transplants but then suggested that there was a concern of graft versus host in them because of that big load. Yet you did not have any deaths associated in this group with graft versus host, so obviously you were able to control it in your suppression. But would you address that question of whether there is any good evidence that the young child with a young transplant has an opportunity to develop true tolerance?
Dr. Tomoaki Kato (Miami, Florida): The massive donor antigen load these young children receive may help to create a state of tolerance or state that promotes the possibility of donor specific tolerance. But there may be another explanation for the observed protective effect of the multivisceral graft with respect to intestinal rejection. It may be the removal of the native lymphoid tissue when doing the multivisceral transplant procedure. The evisceration of the recipient prior to the implantation of the multivisceral graft results in removal of all the native intestinal mucosal tissue, including the spleen, and the bulk of the native mesenteric lymph nodes. The secondary lymphoid tissues play a role in allorecognition, and their absence in the multivisceral patients may in part explain why there is less rejection. Whether this is in fact tolerance or not still needs to be investigated. We have been freezing donor tissue, specifically donor spleen cells when available and/or donor lymph nodes when the spleen is transplanted. We are beginning, but these are not ready to be presented at this time. At this time, we still don't know if donor-specific tolerance can be induced by the multivisceral transplant procedure. The second question was in reference to graft-versus-host-disease. In our experience, graft-versus-host-disease was a fatal complication. Although the incidence of this complication is low in our experience, this is a disadvantage in doing multivisceral transplantation. The incidence of this complication has been lower than expected.
Dr. James A. O'Neill, Jr. (Nashville, Tennessee): These are the sorts of results we have been awaiting for years for children with what has been called short bowel syndrome. Now the alternate form of therapy has been a variety of procedures, including intestinal lengthening procedures, Bianchi operations, intestinal reversals, colon interpositions, so forth and so on.
Your paper with the current results makes us ask the following questions: Are these alternate procedures and by the way, the results at 1 year may be as good but at 4 years not as good as you reported today are these alternate procedures appropriate anymore? And if they are performed, do they prejudice the ease of your operation?
I think this will be very important information as guidance to people who deal with children's surgery who are trying to improve the lot of these patients or to tide them over to a point where they are candidates for intestinal transplantation. This is an important paper.
Dr. Tomoaki Kato (Miami, Florida): There is definitely still a role for lengthening procedure. The key is patient selection. Patients that present to us with very advanced disease, with portal hypertension, and severe cholestatic liver disease experience a lot of problems. These patients just don't tolerate total parenteral nutrition for extended periods of time, and we believe these patients should be referred to transplant instead of performing any lengthening procedure. Children that are less severely ill with less advanced disease may do well with lengthening procedure or other attempts of bowel rehabilitation. The patients that are transplanted after age 3 do better than those patients less than 1 year, especially with regard to respiratory infections. So clearly, there is a benefit to delaying transplantation for those patients that are not so severely ill at the time of presentation. We cannot emphasize enough the importance of patient selection when deciding on a course of either bowel rehabilitation with a lengthening procedure or intestinal transplantation. And this decision is especially important in the very young patient.
Footnotes
Supported in part by NIH Grant No. R03 DK 061445-01.
Reprints: Tomoaki Kato, MD, 1801 NW 9th Avenue, 5th floor, Miami, FL 33136. E-mail: tkato@med.miami.edu.
REFERENCES
- 1.Weber TR, Tracy T Jr, Conners RH. Short-bowel syndrome in children: quality of life in an era of improved survival. Arch Surg. 1991;126:841–846. [DOI] [PubMed] [Google Scholar]
- 2.Galea MH, Holliday H, Carachi R, et al. Short-bowel syndrome: a collective review. J Pedaitr Surg. 1992;27:592–596. [DOI] [PubMed] [Google Scholar]
- 3.Della Vecchia LK, Grosfeld JL, West KW, et al. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133:490–497. [DOI] [PubMed] [Google Scholar]
- 4.Dusick AM. Medical outcomes in preterm infants. Semin Perinatol. 1997;21:164–177. [DOI] [PubMed] [Google Scholar]
- 5.Ricketts RR. Surgical treatment of necrotizing enterocolitis and the short bowel syndromes. Clin Perinatol. 1994;21:365–387. [PubMed] [Google Scholar]
- 6.Dorney SE, Ament ME, Berquist WE, et al. Improved survival in very short small bowel of infancy with use of long-term parenteral nutrition. J Pediatr. 1985;107:521–525. [DOI] [PubMed] [Google Scholar]
- 7.Grant D. 1997 Report of the Intestinal Transplant Registry. Transplantation. 1997;67:1061–1064. [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Tzakis AG. Transplantation of the liver with digestive organs. In: Busuttil, Klintmalm, eds. Transplantation of the Liver, 2nd ed, Philadelphia: Elsevier Saunders, 2005:717–813. [Google Scholar]
- 9.Kato T, Tzakis A, Selvaggi G, et al. Surgical techniques used in intestinal transplantation. Curr Opin Organ Transpl. 2004;9:207–213. [Google Scholar]
- 10.Tzakis AG, Kato T, Levi DM, et al. 100 multivisceral transplants at a single center. Ann Surg. 2005;242:480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno J, Abu-Elmagd K, Mazariegos G, et al. Composite liver–small bowel allografts with preservation of donor duodenum and hepatic biliary system in children. J Pediatr Surg. 2000;35:291–295; discussion 295–296. [DOI] [PubMed]
- 12.Kato T, Romero R, Verzaro R, et al. Inclusion of entire pancreas in the composite liver and intestinal graft in pediatric intestinal transplantation. Pediatr Transplant. 1999;3:210–214. [DOI] [PubMed] [Google Scholar]
- 13.Sudan D, Iyer KR, Deroover A, et al. A new technique for combined liver/small intestinal transplantation. Transplantation. 2001;72:1846–1848. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Tzakis AG. Noncomposite simultaneous liver and intestinal transplantation. Transplantation. 2004;78:485; author reply 485–486. [DOI] [PubMed]
- 15.Murase N, Demetris AJ, Matsuzaki T, et al. Long term survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 16.Kato T, Gaynor J, Nishida S, et al. Zoom endoscopic monitoring of small bowel allograft rejection. Surg Endosc. In press. [DOI] [PubMed]
- 17.Ruiz P, Bagni A, Brown R, et al. Histological criteria for the identification of acute cellular rejection in human small bowel allografts: results of the pathology workshop at the VIII International Small Bowel Transplant Symposium. Transplant Proc. 2004;36:335–337. [DOI] [PubMed] [Google Scholar]
- 18.Schwatrz GJ, Haycock GB, Edelmanncm, Jr, et al. A simple estimate of glomerular filtration rate in children drived from body length and plasma creatine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 19.Kato T, Mittal N, Nishida S, et al. The role of intestinal transplantation in the management of babies with extensive gut resections. J Pediatr Surg. 2003;38:145–149. [DOI] [PubMed] [Google Scholar]
- 20.Kato T, Gaynor JJ, Selvaggi G, et al. Intestinal transplantation in children: a summary of clinical outcomes and prognostic factors in 108 patients from a single center. J Gastrointest Surg. 2005;9:75–89. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadi G, Alexandr A, Bogumila T, et al. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686. [DOI] [PubMed] [Google Scholar]
- 23.Berney T, Delis S, Kato T, et al. Successful treatment of posttransplant lymphoproliferative disease with prolonged rituximab treatment in intestinal transplant recipients. Transplantation. 2002;74:1000–1006. [DOI] [PubMed] [Google Scholar]
- 24.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]