Abstract
Introduction:
Laparoscopic colon resection (LCR) is a safe and effective treatment of benign and malignant colonic lesions. There is little question that a steep learning curve exists for surgeons to become skilled and proficient at LCR. Because of this steep learning curve, debate exists regarding the appropriate hospital setting for LCR. We hypothesize that outcomes of LCR performed early in the learning curve at a regional medical center (New Hanover Regional Medical Center; NHRMC) and a university medical center (Baylor College of Medicine; BCM) would not be significantly different.
Methods:
The first 50 consecutive LCRs performed at each institution between August 2001 and December 2003 were reviewed. Age, mean body mass index (BMI), gender, history of previous abdominal surgery (PAS), operative approach [laparoscopic (LAP) versus hand/laparoscopic assisted (HAL)], conversions (Conv), operative time (OR time), pathology (benign vs. malignant), lymph nodes (LN) harvested in malignant cases, length of stay (LOS), morbidity and mortality were obtained. Continuous data were expressed as mean ± SD. Data were analyzed by χ2, Fisher exact test, or t test.
Results:
NHRMC patients were on average older females with a higher incidence of PAS. A LAP approach was more frequently performed at BCM (86%), whereas HAL was used more frequently at NHRMC (24%). Conversions to open were similar at both institutions (12%). Benign disease accounted for the majority of operations at both institutions. In cases of malignancy, more LN were harvested at BCM. OR time and LOS were shorter at NHRMC. Complication rates were similar between institutions. There were no anastomotic leaks or deaths.
Conclusions:
LCR can be performed safely and with acceptable outcomes early in the learning curve at regional medical centers and university medical centers. Outcomes depend more on surgeons possessing advanced laparoscopic skills and adhering to accepted oncologic surgical principles in cases of malignancy, than on the size or location of the healthcare institution.
Laparoscopic colectomy is a safe and effective treatment for benign and malignant colorectal diseases. This study demonstrates that laparoscopic colectomy can be performed safely with acceptable outcomes early in the learning curve at community and university medical centers.
Laparoscopic colon resection (LCR) was first described in 1991.1 Initially, LCR was slow to gain acceptance. In 2002, only 8% of all colon resections were performed laparoscopically and 7% to 9% were performed using a hand-assisted laparoscopic approach.2 In the current era of evidenced-based medicine, enthusiasm for laparoscopic colorectal surgery is rapidly gaining momentum. The trend toward minimally invasive colorectal surgery is being driven by an ever-increasing body of literature demonstrating the significant advantages of LCR, including less postoperative analgesia use, shorter time to resuming diet, shorter length of stay, and lower rates of postoperative morbidity.3–7 The reluctance of some surgeons to perform LCR is in part related to the rather steep learning curve.8–10 As more physicians and patients become educated on the safety and advantages of LCR, it is likely that many surgeons will be facing increasing pressure to perform LCR in the future.
Widely accepted indications for LCR include: diverticulitis, colorectal polyps, and inflammatory bowel disease.4,7,11–16 Malignancy was once considered by many to be a contraindication to performing LCR. Concern existed for issues such as port site recurrence, inadequate margins of resection, and inadequate lymphadenectomy leading to understaging and decreased long-term survival.17,18 Lujan et al reported a retrospective 5-year follow up on 102 consecutive patients who underwent LCR for malignancy and demonstrated a similar long-term survival to open resection.19 These investigators concluded that LCR was safe and feasible to perform for malignancy in the private practice setting. Recently, several prospective, randomized studies have reported that these initial concerns were unfounded,20–22 provided that the surgeon uses proper oncologic technique when performing LCR.23 Notably, 3 clinical trials; the COST, COLOR, and CLASICC, are providing level I data supporting LCR for colon cancer.3,24,25 These landmark trials are paving the way for properly trained surgeons to proceed with LCR for the treatment of colon cancer.
Because of the steep learning curve associated with LCR, debate exists regarding the appropriate hospital setting for LCR. This study was designed to compare the outcomes for LCR performed in a regional medical center to LCR performed in a large university medical center by laparoscopic surgeons during their initial learning curve. We hypothesized that outcomes of LCR performed early in the learning curve at a regional medical center and a university medical center will not be significantly different. The goal was to establish adequacy of resection for laparoscopic procedures and to determine if a significant difference existed between the short-term outcomes of the 2 separate institutions.
METHODS
Patients
Following Institutional Review Board approval from both institutions, a single physician reviewer (D.J.R.) conducted a retrospective chart review of the initial 50 laparoscopic colon resections performed at New Hanover Regional Medical Center (NHRMC) in Wilmington, North Carolina and at Baylor College of Medicine (BCM) in Houston, Texas. NHRMC actively supports an independent surgical residency program affiliated with the University of North Carolina-Chapel Hill. BCM supports a large surgical residency as well as an advanced minimally invasive fellowship. The operations reviewed were conducted between August 2001 and December 2003. Data were gathered from operative reports, inpatient records, pathology reports, and physician records of outpatient postoperative visits. Patient demographic information obtained included age, gender, race, ASA classification, body mass index (BMI), indication for colectomy, and a history of previous abdominal surgery (PAS). Operative data recorded included skin-to-skin operative time, operative procedure, operative technique, and the frequency for conversion to an open procedure. Postoperative data obtained included length of hospital stay, morbidity and mortality, final pathologic diagnosis (benign vs. malignant), and in cases of malignancy, the proximal and distal margins and number of lymph nodes harvested.
Operative Technique
The procedures were stratified with regard to location of resection and by the technique used to perform the resection. A “totally laparoscopic” resection indicates that the dissection, devascularization, and division of the bowel were all performed intracorporeally. In the case of right hemicolectomies and transverse hemicolectomies, the upper midline trochar incision was enlarged to approximately 5 cm to extract the specimen and externalize the ends of the bowel. A GIA stapler was then used to create a side-to-side, functional end-to-end anastomosis. The common enterotomy was either closed with a GIA or with a two-layered hand-sewn anastomosis. The mesenteric defect was closed using interrupted Vicryl sutures. The bowel was then placed back into the abdomen, the wound closed, and pneumoperitoneum restored. The scope was reinserted to evaluate the anastomosis in situ. In the case of a sigmoid colectomy or low-anterior resection, the bowel was removed and the intracorporeal colorectal anastomoses created by enlarging a trochar incision to approximately 3 to 4 cm to extract the specimen and externalize the proximal end of transected colon. A 29- to 31-mm EEA anvil was inserted in the proximal colon and secured with a purse-string suture. The colon was then placed back into the abdomen and the port site closed. Pneumoperitoneum was restored and an EEA circular stapling device was placed transanally to create an end-to-end anastomosis under laparoscopic visualization. The anastomosis was tested intraoperatively with air instilled via a rigid sigmoidoscope. Drains were not routinely placed. A procedure was deemed a “laparoscopic-assisted” resection, if the majority of the dissection was performed laparoscopically, including mobilization of the splenic flexure, followed by a minimal incision to complete the vascular ligation, resect the bowel, and create the anastomosis. A procedure was deemed a “hand-assisted” resection when a GelPort (Applied Medical) was placed in a lower midline incision to perform a hand-assisted dissection and anastomosis.
All procedures performed at NHRMC used a small Protractor (MedSurg Innovations) wound protector placed prior to removal of specimen and externalization of bowel for extracorporeal anastomosis. All procedures performed at BCM used a large laparoscopic polyurethane retrieval bag to secure the specimen prior to extraction. Preoperative localization and tattoo placement at colonoscopy for colonic polyps was preferred; however, not all lesions were tattooed preoperatively. All specimens were opened and examined in the operating room to ensure adequate margins of resection.
Statistical Analysis
Continuous data were expressed as mean ± SD and compared by t test. Categorical data were analyzed by χ2 or Fisher exact test when sample size dictated appropriateness. All conversions to an open procedure were included in the database, and unless otherwise stated, the data were included in final results. A P value less than or equal to 0.05 was used to define significance in all analyses.
RESULTS
Patient Demographics
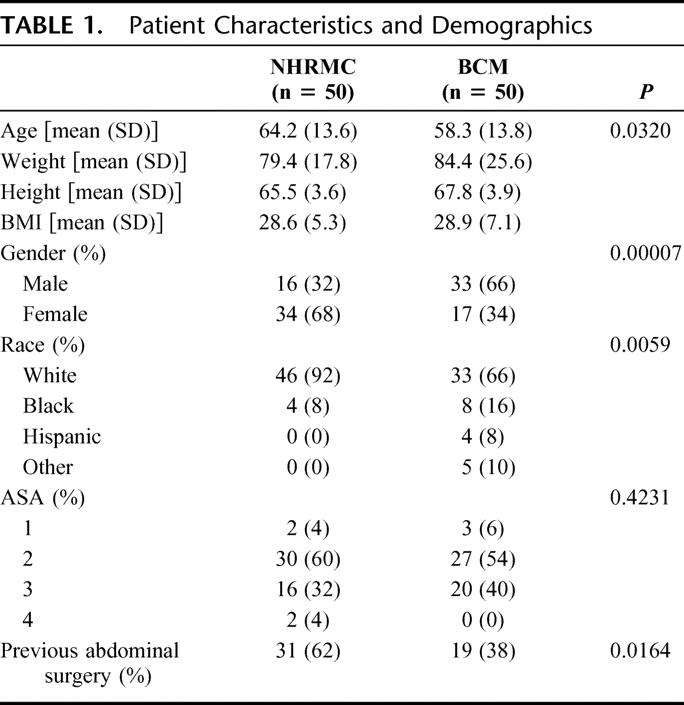
The first 50 consecutive laparoscopic colon resections performed at NHRMC and BCM were evaluated. The patient characteristics and demographics are listed in Table 1. Patients at NHRMC were significantly older, more commonly female, and predominately white, with a higher incidence of previous abdominal surgery when compared with patients from BCM. There were no significant differences in patient weight, height, and BMI or ASA classification between the 2 institutions.
TABLE 1. Patient Characteristics and Demographics
Preoperative Indications, Operative Procedure, and Technique
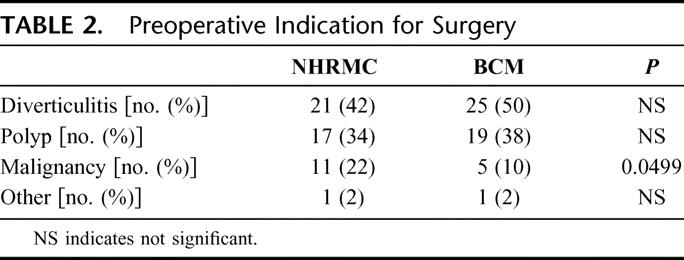
LCR was primarily performed for benign disease at both institutions (Table 2). The most common indication for surgery was diverticulitis followed by a colonic polyp not amenable to colonoscopic resection. Eleven NHRMC patients had a known diagnosis of cancer preoperatively compared with 5 patients at BCM (P = 0.0499).
TABLE 2. Preoperative Indication for Surgery
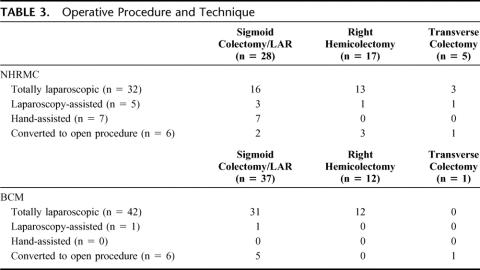
The operative procedures performed and techniques used at each institution are listed in Table 3. Laparoscopic sigmoid colectomy/LAR was the most frequent procedure performed at each institution, followed by laparoscopic right hemicolectomy and laparoscopic transverse colectomy. A significantly higher number of laparoscopic-assisted and hand-assisted resections were performed at NHRMC, with the majority of these performed for sigmoid colectomy/low-anterior resection. There were a total of 6 conversions at each institution. At NHRMC, conversion was due to extensive adhesions in 5 patients and an inability to identify the lesion in 1 patient. At BCM, conversion was due to adhesions in 3 patients, and a large tumor (>7 cm), bleeding, and inability to identify the lesion in 1 patient each.
TABLE 3. Operative Procedure and Technique
Operative Times, Complications, Diet, Length of Stay
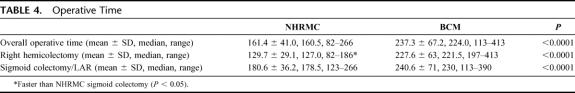
Operative times are listed in Table 4. The overall mean operative times for laparoscopic colectomy were significantly shorter at NHRMC when compared with BCM, as were operative times for laparoscopic right hemicolectomy and laparoscopic sigmoid colectomy/low anterior resection. In addition, operative times for laparoscopic right hemicolectomy performed at NHRMC were significantly shorter than operative times for laparoscopic sigmoid colectomy/low anterior resection performed at NHRMC. Figure 1 demonstrates that a progressive decline in operative time was seen at NHRMC over the course of the study period, while operative times at BCM remained relatively level. Two attending surgeons were scrubbed on the majority of laparoscopic colectomies undertaken at NHRMC (31 of 50, 62%), while all laparoscopic colectomies undertaken at BCM were completed by an attending surgeon and a chief resident or minimally invasive surgery fellow.
TABLE 4. Operative Time
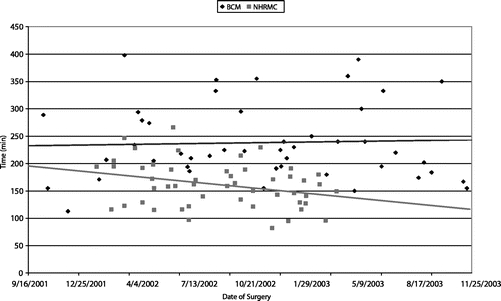
FIGURE 1. Operative times at NHRMC and BCM.
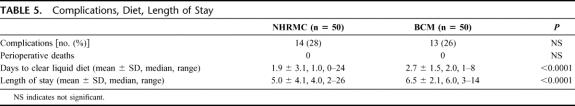
The incidence of complications at both institutions was similar, with the majority of these complications being minor (eg, wound infection, urinary tract infection) (Table 5). There was, however, 1 wound dehiscence at NHRMC and 3 wound dehiscences at BCM. There were no deaths at either institution. Days to clear liquid diet and overall length of hospital stay were significantly shorter at NHRMC when compared with BCM.
TABLE 5. Complications, Diet, Length of Stay
Final Pathology, Lymph Nodes Harvested, Margins
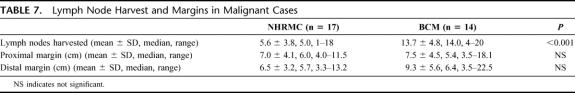
The final pathology yielded additional malignancies at both institutions (Table 6). The mean number of lymph nodes harvested in these malignant cases was on average more than 2-fold greater at BCM when compared with NHRMC. There were no significant differences in the mean proximal or distal margins between the 2 institutions (Table 7).
TABLE 6. Postoperative Pathology
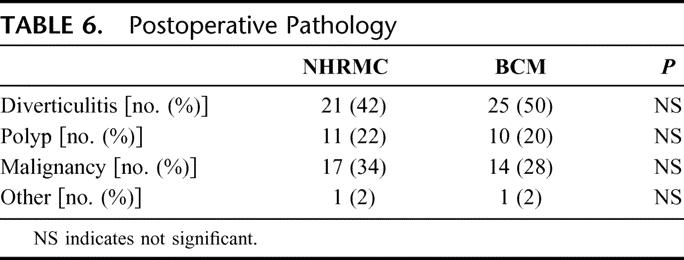
TABLE 7. Lymph Node Harvest and Margins in Malignant Cases
DISCUSSION
The first reported cases of laparoscopic cholecystectomy in the United States were initially greeted with skepticism regarding the advantage of laparoscopy, as well as concern for the safety of the procedure. Since that time, laparoscopic cholecystectomy has proven its superiority and become the gold standard for treatment of symptomatic gallbladder disease. Using the advent of laparoscopic cholecystectomy as a model, we submit that LCR will in time become the gold standard for treatment of benign and malignant colorectal diseases.
This begs 2 simple questions. 1) Who should be performing LCR? 2) In what hospital setting should LCR be performed? The learning curve for this advanced procedure is known to be steep.8–10 Therefore, it is paramount that surgeons offering this procedure are adequately trained and qualified to perform advanced laparoscopic procedures. Many well-trained general and colorectal surgeons are finding it necessary to obtain additional laparoscopic training to keep up with the pressure by patients and referring physicians to perform LCR. The current study was designed in an attempt to answer the second question. In an attempt to answer this question, the first 50 LCRs performed by laparoscopic surgeons in a regional medical center were compared with first 50 LCRs performed in a large university medical center.
The 2 patient populations from NHRMC and BCM differed demographically. The reasons for age and gender differences between the 2 institutions are hard to explain, although the geographic locations of each institution may have played a small role in these differences. NHRMC is located on the Southeastern Atlantic coast of North Carolina, while BCM is located in Houston, TX. Patients from NHRMC were greater than 90% white, while the race distribution of the BCM patients was more heterogeneous, which is reflective of a major urban center. The higher incidence of previous abdominal surgery in the NHRMC patients is in part related to the number of female patients having a past history of total abdominal hysterectomy. The most common indication for LCR at both institutions was diverticulitis, although there were more patients with a preoperative diagnosis of malignancy at NHRMC.
Operative times for LCR were significantly shorter at NHRMC when compared with BCM, despite the higher incidence of previous abdominal surgery in NHRMC patients. The greater use of hand-assisted and laparoscopic-assisted approaches to LCR and the fact that the majority of cases performed at NHRMC were completed by 2 attending surgeons (31 of 50) are certainly factors here. At BCM, all cases were completed by a single attending surgeon and either a senior general surgery resident or minimally invasive surgery fellow. Although operative times were longer, there was no compromise to patient care. Conversion rates were similar and well within the reported range.20–22 There was a conversion at each institution secondary to inability to identify the lesion during surgery. This reinforces the importance of preoperative tattooing of lesions with India ink or the availability of intraoperative colonoscopy to make sure that the lesion is removed with appropriate margins. Complications were similar at both institutions, and neither institution had any deaths or anastomotic leaks.
Another potential explanation for the longer operative times at BCM may be the extent of the lymphadenectomy performed during the mesenteric dissection in suspected cases of malignancy. The key to obtaining the highest number of lymph nodes during a colon resection for cancer is to isolate and divide the main vascular pedicle as it arises off the superior mesenteric artery (right and transverse colectomies) or the aorta (sigmoid colectomy and low anterior resection). This can be tedious and time-consuming, but it always results in a high lymph node harvest during pathologic examination. There was a 2-fold greater difference in the number of lymph nodes examined pathologically at BCM when compared with NHRMC. The 2000 guidelines for colon and rectal cancer surgery published in the Journal of the National Cancer Institute attributes a >90% accuracy in staging if a minimum of 12 nodes are examined.23,26 The mean number of nodes examined at the NHRMC was only 6, suggesting that a wide lymphadenectomy was not performed. However, proximal and distal margins were more than adequate, suggesting that a wide dissection and resection were completed. An explanation for the difference in node count may be in part due to the lack of standardized pathology reporting within or between institutions, as well as variability in each pathologist's technique for identifying and tabulating mesenteric lymph nodes. In review of the pathology reports, the use of enzymatic mesenteric fat dissolution to improve identification of mesenteric lymph nodes was documented only once. Different techniques used to count nodes based on the preoperative diagnosis (neoplasm vs. diverticular disease), as well as the lack of use of fat-clearing techniques may in part explain the low nodal yield at NHRMC. It is vital to stress the importance of performing a wide lymphadenectomy for potentially malignant resections, as well as establishing a protocol for pathologic examination of mesenteric fat to maximize nodal yield. Early reports on sentinel lymph node mapping in colorectal surgery show promise.27–29 If the technique is refined and found to be accurate, it may find an application in LCR.
Differences related to length of stay, although statistically significant, are also difficult to explain, as there was no standardized postoperative pathway at either institution. Protocols emphasizing early feeding and ambulation should be used for both open and laparoscopic colorectal surgery. A more aggressive approach to early diet initiation and discharge has been initiated at BCM with a decrease in time to diet and length of stay approaching those reported for NHRMC in this study.
A few potential shortcomings of this study must be addressed. The sample size is small and makes broad application of the results questionable. A prospective study with larger numbers of patients and clearly standardized patient care protocols would be more optimal. Longer follow-up of patients with a diagnosis of malignancy on final pathology is also warranted to be certain that there was no compromise of oncologic technique during LCR at either institution. Nonetheless, we feel this study demonstrates that successful implementation of LCR does not require large tertiary care university hospitals with access to profoundly expensive and elaborate equipment. Rather, the keys to success lie in the adequate training and skills of the surgeon performing advanced minimally invasive procedures and the support of the healthcare institution in which this care is delivered. There is little question that surgeons in the community will begin to feel pressure to perform routine colon resections laparoscopically. It is paramount that surgeons in the community are given access to proper training in advanced laparoscopic techniques and that today's general surgery residency programs incorporate this training into their curriculum. Lastly, this study clearly shows that properly trained surgeons can achieve acceptable short-term outcomes during the initial learning curve for LCR.
Discussions
Dr. B. Todd Heniford (Charlotte, North Carolina): There are many of us in surgery and many of us in this room who have known the anatomy in the steps of a traditional operation and learned a new method of technique and has joined the 2 of them in an effort to advance our ability to care for our patients.
Despite how common this phenomenon is, there are few papers that demonstrate the great learning curve of this marriage as the authors have this morning. The additional proponent of comparing two different surgical centers, the university based as well as the regional medical center, adds to the importance of the presentation and lends itself to many questions.
First, Dr. Sweeney, you stated that the conversion rate from the laparoscopic to open in both centers was 12%. Did you note any change in the need to convert from the beginning of the trial to the end of the trial as the surgeons became more comfortable with the procedure? Additionally, was there any difference in outcomes between those patients that required conversions as compared to the laparoscopic group? You noted several hand-assisted cases. Was this hand-assist added in an attempt to prevent conversion to a true laparotomy in an attempt to maintain some minimally invasive approach?
The most distressing factor of the study is that one of your centers only harvested an average of 5.6 lymph nodes per cancer case. In the paper you supplied me early ānd I appreciate that w̄here were several reasons you listed for this.
We all recognize that lymphadenectomy by itself in colon cancer can save lives. It also significantly factors in the appropriate staging of the patient and possible addition of chemotherapy. At the center in question, was any effort made to compare the number of lymph nodes harvested from open resections at the same time of the study or just prior to the initiation of the study?
Additionally, you mentioned the change in technique of lymph node retrieval by the pathologist in the paper in the institution where they harvested only 5.6 lymph nodes per case. Following the study, this was changed and subsequently an increase in number of lymph nodes was resected by those surgeons performing the procedure laparoscopically. You stated you felt it was possibly due to the pathologist and the change of technique. I would counter and ask you if you think indeed it was because of the surgeon's increasing experience after 50 cases?
Of the patients undergoing surgery for polyps, in the study 39% had cancer in their final path. This study again demonstrates that laparoscopic surgery for a polyp is not a “free shot.” Were the polyp colectomies approached like a cancer? If not, was this a reason for the decrease in lymph nodes noted at that one center?
Lastly, please describe how your patients were considered for surgery, especially when you had performed no previous cancer operations. Did you tell them that and list the number of cases you had done?
Dr. John F. Sweeney (Houston, Texas): I will address your last question first. I can't speak to how the regional medical center handled their initial experience with the laparoscopic colon resections. At Baylor, we started initially performing laparoscopic colon resection for benign disease. We then started doing resections for cancer under an IRB-approved prospective protocol. Any patient who was having a laparoscopic colectomy for cancer was entered into this protocol and followed at established time points. It was not our intent to reproduce the COST trial; rather, it was more to make sure that the patients were followed in a standard manner.
In regards to lymph nodes harvested, looking back through the data, I don't see any increase or any improvement in the lymph node harvest at either institution associated with the progression through the learning curve. Also, I have no data to compare to the number of lymph nodes harvested during an open colectomy prior to initiation of a laparoscopic colectomy cancer at either institution. My statement in the manuscript I supplied is purely anecdotal at best in that I don't have any strong data, they haven't repeated the study, to show that initiation of a standardized protocol for harvesting lymph nodes by the pathologist has truly increased the numbers. And that is something I think that needs to be addressed.
The use of hand-assisted laparoscopy at New Hanover was a planned procedure. Early on, a hand was placed into the abdomen to facilitate the procedure. And patients who had a hand-assisted or laparoscopic-assisted procedure did not really have that much of a difference in their perioperative outcomes.
You asked about the 12% conversion rate at each institution. I did not see any difference along the learning curve specifically, but I would have to go back and really review the data to be able to tell you that for sure.
Dr. C. Daniel Smith (Atlanta, Georgia): First, semantics. A steep learning curve is one where you gain proficiency over a short number of trials. That means the curve is steep. I think semantically we are really talking about a prolonged or long learning curve. I know it is a subtle distinction, but I can't miss the opportunity to make that point.
Now, along the lines of the learning curve, and in particular your conclusion. You talked about how it doesn't matter where you deploy, as long as the skills of the surgeon are good. But you didn't tell us anything about the skills of the surgeons and the experience of the surgeons that were involved in this series.
So one question is: what kind of experience did surgeons at both sites have with open colon resection for cancer and benign disease? Also, what type of experience did surgeons have in other advanced procedures? This will in part tell us where they started on this so-called learning curve. Or were these just the first 50 procedures at that particular site and had the surgeons come to those sites with extra skill and experience? I think it is going to be important for us in understanding what you are talking about in learning curve.
Next, can you tell us if you stratified your outcomes based on the cancer diagnosis? Some of these cancers that might be undertaken laparoscopically may be more challenging than the benign disease or the small polyps. So did you stratify any of these outcomes strictly to the cancer diagnosis?
Finally, I want to make a comment about the operative time question. In your manuscript, you commented that the prolonged operative time didn't have any consequence to patient outcome. But yet your length of stay at Baylor was longer. You also had longer operative time there. Could you make a comment about whether you thought that longer operative time might have impacted on length of stay and time to return of GI function?
Dr. John F. Sweeney (Houston, Texas): This is a difficult learning curve, with relation to the semantics. I think that difficult may be more appropriate to describe the learning curve.
In regards to the skills of the surgeons at each institution, what I can say is that these are Board-certified general surgeons with extensive open surgical experience that have either significant advanced laparoscopic experience and/or have undergone laparoscopic or minimally invasive surgery fellowship training. So these aren't surgeons who are doing an occasional lap chole and then trying to do a laparoscopic colectomy. These are surgeons who have advanced laparoscopic skills and then embark on the field of laparoscopic colectomy.
Outcomes were not stratified at all by tumor size. I would say for the initiation of this particular series we tried to avoid patients with large tumors, and we only had one patient with a very large tumor.
With regards to longer operative times, I think that clearly the participation of a senior resident or a fellow who is learning how to do this procedure significantly impacts the operative time. There is no doubt about that. But the other issue is: I don't think that the operative times, the length of the operative times, affected the length of stay. I think that is more that a defined patient protocol was not in place. And by now being much more aggressive with early initiation of diet and discharge, we are able to shorten these numbers along the lines of what we see at the regional medical center.
Dr. J. Gary Maxwell (Wilmington, North Carolina): As you heard, this study compares the first 50 cases of laparoscopic colon resection done for both benign and malignant conditions in two hospital settings, and the study concludes that early outcomes in both settings are acceptable and not greatly different from one another.
Although Dr. Sweeney in his discussion compared the two settings somewhat, the manuscript does not provide sufficient detail about New Hanover Regional Medical Center as a representative of the universe of community hospitals nor does it provide enough detail about Baylor as a representative of the universe of university hospitals for us to understand the distinction. As Dr. Sweeney pointed out, New Hanover Regional Medical Center is a 628-bed hospital, it is a general medical, surgical, trauma, and oncologic hospital serving more than 600,000 people, and has independent residency programs, and is a major teaching hospital for Chapel Hill and other medical schools in North Carolina. So my first question, Dr. Sweeney, is: are there not more similarities than differences between the two hospitals chosen for the comparison of outcomes?
The second conclusion made by the authors in their manuscript is that their study “clearly shows that properly trained surgeons,” can achieve acceptable early outcomes. Again, no data are presented, and this was pointed out by Dr. Smith, in the manuscript to define what constitutes a properly trained surgeon or to compare the surgeons of the community medical center and those of the university medical center. The two named authors from New Hanover Regional Medical Center are both fellowship trained, one in laparoscopic surgery. Both have served on the faculty of medical schools, both have authored surgical papers, both are involved in prospective clinical research and are committed to the education of residents and medical students. So my second question is: are there not more similarities than differences between the surgeons chosen for comparison?
Somewhere out there, there is a department chair in a university medical center who has said to a young faculty, “I want you to go take a course and get involved and get our laparoscopic colon surgery program going here at Prestigious University Medical Center.” Similarly, there is a surgeon in the community hospital saying, “I am going to go out there and take a course and be the first surgeon in our hospital or in our city to do laparoscopic colon resections.” I don't want either of these surgeons, either the university one or the community hospital surgeon, to cite your study as a justification of what in both cases is an ill-devised plan. So please clarify for us how we are to recognize the quality medical center in the community and the quality surgeon so that there won't be a misapplication of your study.
Dr. John F. Sweeney (Houston, Texas): With regard to the similarities both between the surgeons and the institutions, I think on face value you can make that statement. But the major metropolitan area of Houston includes a population of between 4 and 6 million people. The Texas Medical Center itself is the largest medical center in the world. The institutions are inherently different.
But I think the point that we are trying to make with this particular study is that, with appropriate laparoscopic training, this operation can be initiated or implemented in an institution, again with appropriate institutional support, whether it is in a place like Baylor, which is in a huge medical center, or in a regional medical center, or a community medical center like New Hanover.
I think the key issue is appropriate training. My intent is not to stand here and discuss credentialing for advanced laparoscopic procedures, which is a whole different issue. However, I firmly believe that only surgeons who can document appropriate training and outcomes for laparoscopic colectomy should be credentialed to perform this procedure.
Footnotes
Supported in part by an educational grant from United States Surgical Corporation.
Reprints: John F. Sweeney, MD, Division of General Surgery, Minimally Invasive Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, 1709 Dryden, Suite 1500, Houston, TX 77030. E-mail: jsweeney@bcm.edu.
REFERENCES
- 1.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic resection). Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 2.IHS Health Group (Medical Data International). U.S. Surgical Procedure Volumes: 2001. Englewood, CO: IHS Health Group (Medical Data International), 2001. [Google Scholar]
- 3.Nelson H, Sargent D, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 4.Guller U, Jain N, Hervey S, et al. Laparoscopic vs open colectomy. Arch Surg. 2003;138:1179–1186. [DOI] [PubMed] [Google Scholar]
- 5.Gibson M, Byrd C, Pierce C, et al. Laparoscopic colon resections: a five-year retrospective review. Am Surg. 2000;66:245–248; discussion 248–249. [PubMed]
- 6.Weeks JC, Nelson H, Gelber S, et al. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321–328. [DOI] [PubMed] [Google Scholar]
- 7.Hong D, Lewis M, Tabet J, et al. Prospective comparison of laparoscopic vs open resection for benign colorectal disease. Surg Laparosc Endosc Percutan Tech. 2002;12:238–242. [DOI] [PubMed] [Google Scholar]
- 8.Schlachta CM, Mamazza J, Seshadri PA, et al. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44:217–222. [DOI] [PubMed] [Google Scholar]
- 9.Tekkis P, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kincler S, Koller MT, Steurer J, et al. Multidimensional analysis of learning curves in laparoscopic sigmoid resection: eight-year results. Dis Colon Rectum. 2003;46:1371–1378. [DOI] [PubMed] [Google Scholar]
- 11.Chung CC, Tsang WWC, Kwok SY, et al. Laparoscopy and its current role in the management of colorectal disease. Colorectal Dis. 2003;5:528–543. [DOI] [PubMed] [Google Scholar]
- 12.Joo JS, Amarnath L, Wexner SD. Is laparoscopic resection of colorectal polyps beneficial? Surg Endosc. 1998;12:1341–1344. [DOI] [PubMed] [Google Scholar]
- 13.Eijsbouts QAJ, Heuff G, Sietses C, et al. Laparoscopic surgery in the treatment of colonic polyps. Br J Surg. 1999;86:505–508. [DOI] [PubMed] [Google Scholar]
- 14.Wu JS, Birnbaum EH, Kodner IJ, et al. Laparoscopic-assisted ileocolic resections in patients with Crohn's disease: are abscess, phlegmons, or recurrent disease contraindications? Surgery. 1997;122:682–689. [DOI] [PubMed] [Google Scholar]
- 15.Reissman P, Salky BA, Edye M, et al. Laparoscopic surgery in Crohn's disease: indications and results. Surg Endosc. 1996;10:1201–1203. [DOI] [PubMed] [Google Scholar]
- 16.Bauer JJ, Harris MT, Grumbach NM, et al. Laparoscopic-assisted intestinal resection for Crohn's disease: which patients are good candidates? J Clin Gastroenterol. 1996;23:44–46. [DOI] [PubMed] [Google Scholar]
- 17.Stocchi L, Nelson H. Laparoscopic colectomy for colon cancer: trial update. J Surg Oncol. 1998;68:255–267. [DOI] [PubMed] [Google Scholar]
- 18.Alexander RJ, Jaques BC, Mitchell KG. Laparoscopic assisted colectomy and wound recurrence. Lancet. 1993;341:249–250. [DOI] [PubMed] [Google Scholar]
- 19.Lujan H, Plasencia G, Jacobs M, et al. Long-term survival after laparoscopic colon resection for cancer. Dis Colon Rectum. 2002;45:491–501. [DOI] [PubMed] [Google Scholar]
- 20.Braga M, Frasson M, Vignali A, et al. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum. 2005 Oct 3; Epub ahead of print. [DOI] [PubMed]
- 21.Kitano S, Intomata M, Sato A, et al. Randomized controlled trial to evaluate laparoscopic surgery for colorectal cancer: Japan Clinical Oncology Group Study JCOG 0404. Jpn J Clin Oncol. 2005;35:475–477. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser AM, Kang JC, Chan LS, et al. Laparoscopic-assisted vs. open colectomy for colon cancer: a prospective randomized trial. J Laparoendosc Adv Surg Tech A. 2004;14:329–334. [DOI] [PubMed] [Google Scholar]
- 23.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. JNCI. 2001;93:583–596. [DOI] [PubMed] [Google Scholar]
- 24.Bonjer HJ, Haglind E, Jeekel J, et al. Laparoscopic surgery vs. open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. [DOI] [PubMed] [Google Scholar]
- 25.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional vs laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 26.Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg. 1989;76:1165–1167. [DOI] [PubMed] [Google Scholar]
- 27.Codignola C, Zorzi F, Zaniboni A, et al. Is there any role for sentinel node mapping in colorectal cancer staging? Personal experience and review of the literature. Jpn J Clin Oncol. 2005 Nov 7; Epub ahead of print. [DOI] [PubMed]
- 28.Broderick-Villa G, Anr D, Haigh PI, et al. Ex vivo lymphatic mapping: a technique to improve pathologic staging in colorectal cancer. Am Surg. 2004;70:937–941. [PubMed] [Google Scholar]
- 29.Bertogilio S, Sandrucci S, Percivale P, et al. Prognostic value of sentinel lymph node biopsy in the pathologic staging of colorectal cancer patients. J Surg Oncol. 2004;85:166–170. [DOI] [PubMed] [Google Scholar]