Abstract
Objective:
We have previously shown that cardiac uncoupling (reduced heart rate variability) in the first 24 hours of trauma ICU stay is a robust predictor of mortality. We hypothesize that cardiac uncoupling over the entire ICU stay independently predicts mortality, reveals patterns of injury, and heralds complications.
Methods:
A total of 2088 trauma ICU patients satisfied the inclusion criteria for this study. Cardiac uncoupling by outcome was compared using the Wilcoxon rank sum test. Risk of death from cardiac uncoupling and covariates (age, ISS, AIS Head Score, total transfusion requirements) was assessed using multivariate logistic regression models at each ICU day. Univariate logistic regression was used to assess risk of death from uncoupling irrespective of covariates at each ICU day.
Results:
A total of 1325 (63.5%) patients displayed some degree of uncoupling over their ICU stay. The difference in uncoupling between survivors and nonsurvivors is both dramatic and consistent across the entire ICU stay, indicating that the presence of uncoupling is unrelated to the cause of death. However, the magnitude of uncoupling varies by day when data is stratified by cause of death.
Conclusions:
Cardiac uncoupling: 1) is an independent predictor of death throughout the ICU stay, 2) has a predictive window of 2 to 4 days, and 3) appears to increase in response to inflammation, infection, and multiple organ failure.
Cardiac uncoupling, reduced heart rate variability, is a robust predictor of death throughout ICU stay. Uncoupling reflects risk of mortality better than age, injury severity, degree of traumatic brain injury, and units of total blood transfused, has a median predictive window of 2–4 days, and potentially reflects complications (inflammation, infection, and multiple organ failure) superimposed on anatomic injury.
Our previous work in trauma patients suggests that cardiac uncoupling (percent low heart rate variability) is a biomarker for increased mortality,1–3 diminished physiologic reserve,4 and is potentially associated with alterations in the autonomic nervous system.5 We believe cardiac uncoupling reflects a deterioration of multiple command and control mechanisms linking systems, organs, cells, proteins, and genes. The best documented of these mechanisms include the autonomic nervous system and other neuroendocrine mechanisms.6,7
This manuscript is organized to highlight 4 new characteristics of cardiac uncoupling in trauma patients, illustrated by this study. These characteristics demonstrate:
The predictive power of cardiac uncoupling over the entire ICU stay.
Uncoupling outperforms other covariates associated with mortality (age, injury severity, transfusion requirements8 and the presence of severe traumatic brain injury).
Uncoupling is associated with mortality from multiple causes.
Uncoupling appears to increase in patients who die of inflammation, infection and multiple organ failure.
These 4 characteristics illuminate a small portion of our overall program for the identification of new biomarkers. This work is part of a multiyear, multi-institutional, and multidisciplinary program designed to bring new information management tools to the bedside. These tools will define new biomarkers. These new biomarkers will predict outcome, define organ dysfunction, identify early patient deterioration (trajectory), and stratify patients in real time for therapy and research.
We hypothesize: 1) Cardiac uncoupling stratifies ICU patients by mortality risk over the entire ICU stay well in advance of death and independent of the cause of death. 2) Cardiac uncoupling measured over ICU stay is a more robust predictor of outcome than previously reported measures during the first 24 hours. 3) Continuous measures of cardiac uncoupling may illustrate unique patterns of injury and herald the onset of complications.
METHODS
Setting
Vanderbilt University Medical Center (VUMC) is the only level I trauma center serving an 80,000 square-mile catchment area; 3500 trauma patients are admitted annually, 1900 of which are admitted to a 31-bed dedicated trauma unit. Fourteen of the 31 beds are ICU beds equipped with continuous physiologic monitoring capability, SIMON (Signal Interpretation and Monitoring), and accommodate 700 to 800 admissions per year.
Data Sources
VUMC's clinical information infrastructure provided the linked patient physiologic, demographic, and outcome required for this study. Key components of the infrastructure relevant to this analysis include:
SIMON
The SIMON (Signal Interpretation and Monitoring) project is an ongoing collaborative effort between the VUMC Division of Trauma and Vanderbilt University School of Engineering.3 Since December 2000, physiologic data from bedside medical devices have been continuously captured and stored from trauma ICU beds. Physiologic parameters captured include heart rate, invasive and noninvasive blood pressures, intracranial and cerebral perfusion pressures, arterial and venous oxygen saturations, core temperature, pulmonary and central venous pressures, cardiac index, and end diastolic volume index. As of July 2005, data have been collected on 3760 patients for their entire length of ICU stay in a SIMON-monitored bed. This represents more than 310,000 total hours of continuous monitoring and over 3 billion data points.
TRACS
The VUMC Division of Trauma has maintained a trauma registry since 1986 and has participated in the Trauma Registry of the American College of Surgeons (TRACS) since 1996. All patients admitted to VUMC with trauma or burns are entered into this database, which includes all patients with SIMON data. Data are maintained locally and shared quarterly with the national repository. Currently, more than 300 parameters are captured via retrospective chart review, including patient demographics, injuries, diseases, operative procedures, hospital dispositions, complications, costs, resource utilization, and length of stay at various levels of care.
De-identified Repository
Both SIMON and TRACS meet regulatory requirements for data repository status and are approved as such by the Vanderbilt University Institutional Review Board. SIMON data are prospectively captured during the course of clinical care, while TRACS data are captured retrospectively. For this and other studies, data requests are processed in accordance with institution and HIPAA regulations, including de-identification prior to analysis.
Measurements
Cardiac Uncoupling
Our measurement of interest, cardiac uncoupling (percent low heart rate variability), reflects the percent of time in a 24 hour period that a patient's short-term heart rate variability falls within a critically low range. It is computed as follows: Each patient's daily heart rate data is split into 5-minute intervals. The standard deviation of integer heart rate for each interval is computed, providing a measure of short-term heart rate variability reflecting duration (5 minutes) and intensity (standard deviation) of variability. The percentage of these intervals that fall within a critically low range (0.3–0.6 bpm)4 provides our measurement of cardiac uncoupling.
The 5-minute time interval follows established practices for data collection of heart rate variability, and our analysis resembles time-series techniques for assessing heart rate variability (ie, SDANN).9 Our data, however, differ from that used in traditional heart rate variability analysis because precise instantaneous heart rate is not acquired at every beat. SIMON samples heart rate from a standard monitor (Philips Viridia) at an average rate of once every 1 to 4 seconds. Thus, a typical 5-minute interval will contain between 75 and 300 heart rate data samples for a single patient. The standard deviation of these points is our basic parameter of short-term heart rate variability, and the units of this measure are beats per minute (bpm). Intervals containing less than 60 data samples are discarded (<5.4% of available intervals).
Demographics and Outcome
Patient age in years, gender, ethnicity, Injury Severity Score (ISS), hospital disposition, length of stay, cause of death, and units packed red blood cells transfused (uPRBC) were obtained from TRACS. Survivors were defined as those patients discharged from the hospital alive. Causes of death, as recorded in TRACS, were defined as: sepsis (n = 21), multiple organ failure (n = 31), hemorrhage (n = 5), preexisting condition (n = 10), severe traumatic brain injury (n = 139), and other, including those patients for whom cause of death could not be reliably determined (n = 36).
Predictive Window
For each ICU day, we defined the predictive window for death (date of death minus date of observation). Both the mean and median days are reported.
Study Group
This IRB-approved study includes all trauma admissions to VUMC who:
Were admitted in the 48-month period, December 1, 2000 through November 30, 2004, recorded in TRACS as initial hospital admissions, rather than in-hospital transfers or readmissions (n = 13,972), and
Were admitted to a trauma ICU bed (n = 3227), and
Arrived in the trauma ICU within 24 hours of ED admission, and
Had 12 hours or more of heart rate data within the first 5 days of ICU stay (n = 2088).
Statistics
Comparisons of Cardiac Uncoupling by Outcome
Cardiac uncoupling was compared between outcome groups in 2 ways: First, distributions of short-term heart rate variability were graphed by outcome group for all data in the ICU stay. Percent cardiac uncoupling is shown for each group at each ICU day as follows: T-bars denote 10th (lower) and 90th (upper) percentiles, boxes denote 25th (lower) and 75th (upper) percentiles, and dots show the median percent uncoupling. Second, at each ICU day the Wilcoxon rank sum test was used to assess differences in cardiac uncoupling between survivors and nonsurvivors, in both the entire population and in those with severe traumatic brain injury (Abbreviated Injury Scale [AIS] Head Score ≥5). A P value of 0.05 defined statistical significance in all tests.
Univariate Models
Logistic regression was used to assess the risk of cardiac uncoupling for death independent of covariates at each ICU day. Odds ratios were used to determine the relative increase in risk of death for a one unit increase in the input variable. Area (AUC) under the receiver operator characteristic (ROC) curve and its standard error (SE) were used to assess the performance of these measures.
Multivariate Models
At each of the first 11 ICU days, logistic regression was used to assess the risk of cardiac uncoupling and covariates (age, ISS, AIS Head Score, and total blood requirements) for death. Additionally, at ICU days 1 to 5, the population was randomly divided into 2 equally sized groups for model validation. Models were developed on the first group and evaluated on the second group. Odds ratios and AUC were used as above.
RESULTS
The Dataset
This is a population-based study comprised of 2088 patients whose demographics are outlined in Table 1. This population is typical for admission to the Trauma ICU in our catchment area. All patients had SIMON data recorded. There were approximately 260 million heart rate measurements representing 182,676 continuous hours of monitoring. Of these, 763 patients (36.5%) showed no evidence of cardiac uncoupling (no time spent with heart rate variability in critically low range) and 1325 (63.5%) showed some degree of uncoupling during their ICU stay.
TABLE 1. Population Demographics
Cardiac Uncoupling and Mortality
Our first aim is to demonstrate the predictive power of uncoupling. Our current measures of cardiac uncoupling reflect the duration that a patient's heart rate shows low variability during a 24-hour period. Figure 1 demonstrates how cardiac uncoupling during ICU stay stratifies patients by outcome. Patients who die clearly spend more time with low heart rate variability in the critical range (0.3–0.6 bpm). Figure 2 demonstrates the same data in a different fashion. At each individual ICU day, cardiac uncoupling continues to stratify patients by mortality. Furthermore, the risk of death (odds ratio) and the predictive window (2–4 days) associated with cardiac uncoupling remains consistent through ICU stay (Table 2).
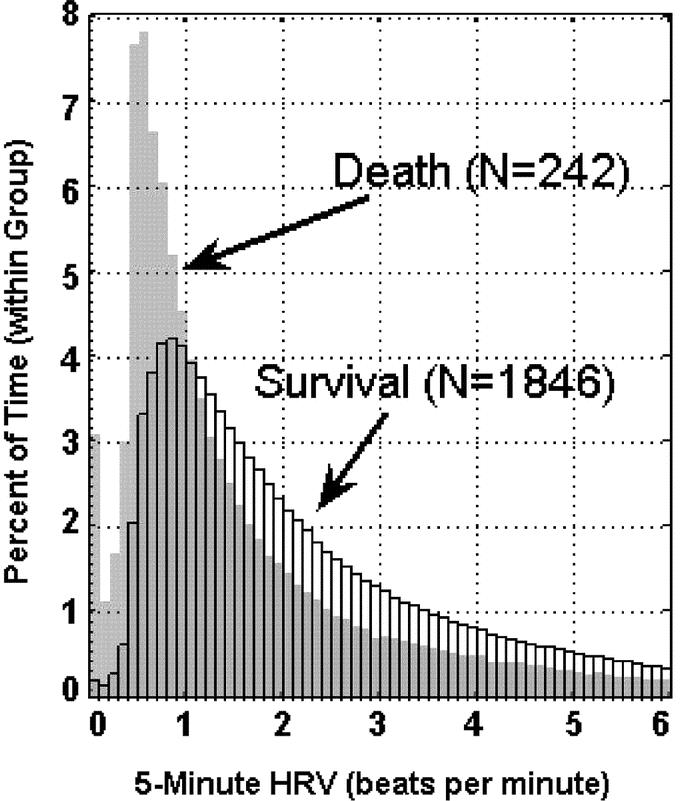
FIGURE 1. Reduced heart rate variability during ICU stay stratifies patients by mortality. Distribution of all heart rate variability measurements obtained during patients’ ICU stay is shown for 1846 survivors and 242 nonsurvivors.
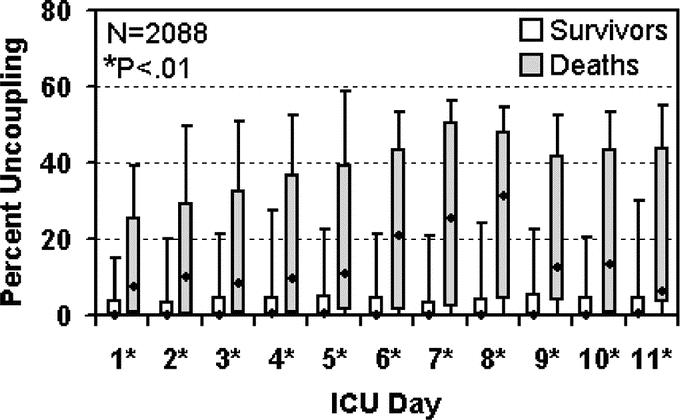
FIGURE 2. Cardiac uncoupling over time by mortality. Percent cardiac uncoupling is shown for survivors and nonsurvivors at each ICU day as follows: T-bars denote 10th (lower) and 90th (upper) percentiles, boxes denote 25th (lower), and 75th (upper) percentiles, and dots show the median percent uncoupling within each group. Differences in cardiac uncoupling between survivors and nonsurvivors were assessed using the Wilcoxon rank sum test. Statistically significant differences in daily percent cardiac uncoupling between survivors and nonsurvivors were evident through ICU day 11. While the total population comprises 2088 distinct cases, at each ICU day the number of cases with available data is less due to patients being transferred to/from the ICU (Table 2 for number of cases at each day).
TABLE 2. Univariate Regression Results
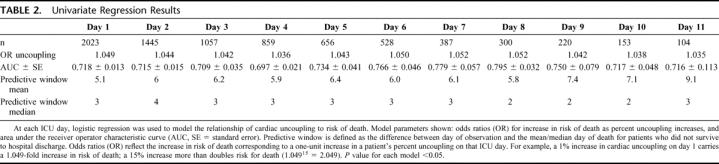
Contribution of Covariates to Mortality
Once we defined cardiac uncoupling as a robust predictor of mortality throughout the ICU stay, we determined its predictive efficacy in conjunction with currently accepted known risk factors for mortality (injury severity, transfusion requirements, presence of traumatic brain injury, and age). Table 3 illustrates that, contrary to our expectations, injury severity loses its predictive value early, at day 2. We hypothesize that the relatively high acuity levels seen in this patient population diminish the discriminatory power of the ISS. Transfusion requirement also loses power in the model relatively early, despite its association with infection.
TABLE 3. Multivariate Regression Results
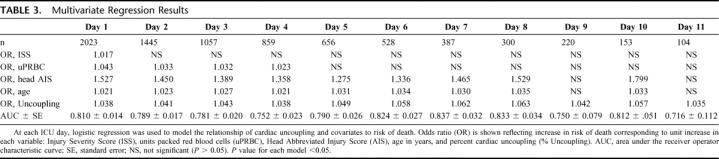
Age and grade of traumatic brain injury are more robust predictors. Increasing grade of traumatic brain injury increases the risk of death during the first week, and then the risk declines. It may be that if one survives traumatic brain injury longer than one week, chances of being discharged from the hospital, independent of functional outcome, are good. Increasing age is robustly associated with mortality until day 10. The impact of advancing age is demonstrated in Table 1.
Finally, cardiac uncoupling, unlike the covariates, is a dynamic parameter changing over time in concert with the patient's clinical course. It continues to be a robust predictor of outcome throughout the entire ICU stay and, consequently, outperforms the other covariates in the mortality model.
Patterns of Cardiac Uncoupling
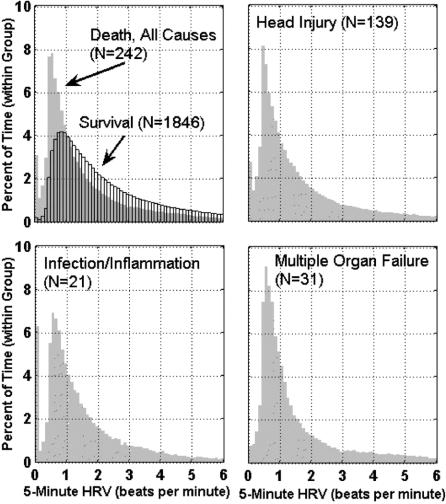
Our third aim is to demonstrate the constancy of cardiac uncoupling's predictive power, independent of the cause of death. Figure 3 shows the inordinate amount of time spent with cardiac uncoupling in 3 subpopulations of patients who die due to: severe traumatic brain injury, infection and inflammation, and multiple organ failure. Figure 4 illustrates the time course of uncoupling in these groups. In all groups, uncoupling appears at day one and appears to increase at day 6, the anticipated time frame for the onset of infection. This pattern of increased uncoupling at day 6 occurs independent of the cause of death (traumatic brain injury, infection/inflammation, multiple organ failure), suggesting that cardiac uncoupling reflects a superimposed complication.
FIGURE 3. Distribution of heart rate variability by cause of death. Distribution of all heart rate variability measurements obtained during patients’ ICU stay is shown for: all patients (1846 survivors and 242 nonsurvivors), 139 patients who died due to traumatic brain injury, 21 patients who died due to infection and/or inflammation, and 31 patients who died due to multiple organ failure.
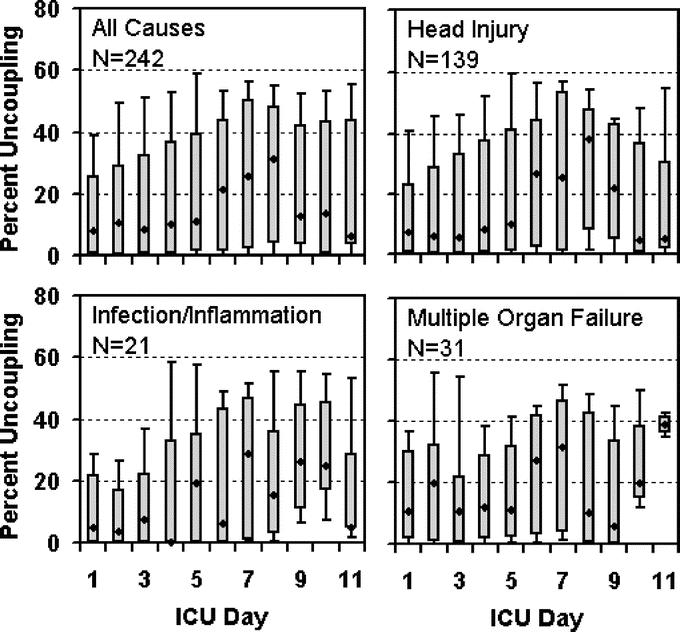
FIGURE 4. Cardiac uncoupling over time by cause of death. Percent cardiac uncoupling is displayed at each ICU day for patients who died of all causes, traumatic brain injury, infection and/or inflammation, and multiple organ failure. The 10th, 25th, 50th (median), 75th, and 90th percentiles of uncoupling are shown at each day (see Fig. 2 legend).
Patients who die of multiple organ failure (Fig. 4D) experience higher uncoupling early in their stay (days 1–2) compared with those who die of other causes. We have previously shown early uncoupling to be associated with the failure of resuscitation. Therefore, the pattern demonstrated here is compatible with the “second hit” theory of multiple organ failure, ie, the first hit occurs early with the failure of resuscitation, and the second hit occurs at day 6 with the onset of infection.
Patients with severe traumatic brain injury (AIS ≥ 5) are stratified into survivors and nonsurvivors early in their hospital course (Fig. 5). Of greater interest is the increase in the median cardiac uncoupling of the nonsurvivors at day 6, suggesting an inflammatory component associated with traumatic brain injury and poor outcome.
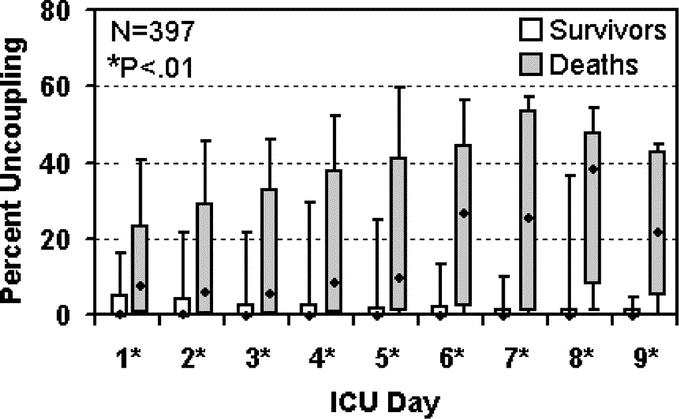
FIGURE 5. Cardiac uncoupling over time by mortality in patients with severe traumatic brain injury. Percent uncoupling for patients with severe traumatic brain injury (AIS Head ≥ 5) who survived versus those who died in the hospital is displayed at each ICU day, illustrating the 10th, 25th, 50th (median), 75th, and 90th percentiles of cardiac uncoupling within each group (see Fig. 2 legend). Differences between survivors and nonsurvivors were assessed at each day using the Wilcoxon rank sum test. Of 397 patients with severe traumatic brain injury, 139 died and 258 survived to hospital discharge.
DISCUSSION
In this manuscript, we examine cardiac uncoupling and reduced heart rate variability in 2088 ICU patients. We found cardiac uncoupling to be robustly associated with mortality. Furthermore, risk of mortality increases as uncoupling increases. This relationship remains consistent throughout the ICU stay. Additionally, the median window of prediction remains remarkably consistent at 3 to 4 days through the ICU stay, suggesting that cardiac uncoupling is more than a premorbid phenomenon.
As we hypothesized, cardiac uncoupling appears to be a generic predictor of death, ie, the association with death appears to be independent of the cause of death. To our surprise, the pattern of uncoupling appears to provide additional discriminatory power.
First, there appears to be a relationship between the temporal pattern of uncoupling and the cause of death. Cardiac uncoupling increases at day 6 to 10 in patients who ultimately die of infection and inflammation. Others have described a relationship between inflammation and waveform heart rate variability.10–12 While this study was not designed to show a relationship between cardiac uncoupling and inflammation, if such a relationship exists, it should occur in the timeframe of day 6 and would increase the value of cardiac uncoupling as a biomarker. Our current database on this population of patients contains sufficient information to directly address the relationship between uncoupling and inflammation in the future.
Second, the presence of uncoupling provided remarkable discriminatory power between survivors and nonsurvivors in patients with the most severe grade of traumatic brain injury. While we and others13–15 have observed the association between traumatic brain injury and uncoupling, we did not expect uncoupling to robustly stratify mortality in the AIS ≥ 5 group. Furthermore, the temporal pattern of uncoupling suggests that traumatic brain injury survivors and nonsurvivors can be stratified very early in their ICU course. In the future, this information may have therapeutic and mechanistic implications. For instance, if inflammation occurs early in traumatic brain injury, and if inflammation is associated with intracranial hypertension, and if cardiac uncoupling serves as a noninvasive biomarker for this process, then uncoupling would mandate early ICP monitoring. We are prospectively collecting inflammatory markers in cerebral spinal fluid.
Perspective
Failure of physiologic regulatory mechanisms is common in critically ill surgical patients. These failures provide opportunities to observe and quantify communication pathways linking organs, cells, proteins, and genes. Defining these relationships requires novel methods of clinical information management. Specifically, we must capture, store, analyze, and display new types of information.16 We have begun this process and appear to have uncovered new biomarkers that identify patients at increased risk of dying in the ICU.
Measurement of heart rate variability is not new.17–19 A large body of work has examined heart rate variability measured using spectral analysis of the EKG waveform. These techniques have demonstrated that high-frequency waveform spectra are associated with abnormalities of the parasympathetic nervous system, whereas low-frequency variation is associated with abnormalities of the sympathetic nervous system. Waveform measurements have been done, infrequently, in critically ill patients and have shown an association with outcome in multiple disease states, including infection, multiple organ failure,20–22 and myocardial infarction.23 Unfortunately, waveform measurements of heart rate variability are difficult to execute routinely in a working ICU. They are measured episodically, usually once in a 24-hour period, and are difficult to automate, often requiring manual review prior to analysis.
Strengths
The strengths of this large population-based study are clear: The patient population is homogeneous and the data set is robust and unique. Only trauma patients with sufficient acuity to warrant ICU admission are included in the study population. All patients are managed by a small number of faculty practicing under a single set of evidence-based protocols.
Our evolving clinical information management system allows linkage of 300 fields of outcome and demographic data, tens of thousands of laboratory values, and billions of physiologic data points. This results in a uniquely powerful phenotypic portrait of our patient population.
Additionally, we provide data on a new physiologic biomarker, cardiac uncoupling, and we define its utility in a single setting, the trauma ICU. It is possible that this noninvasive tool will be used in other settings as well. In the prehospital environment, it may predict patient deterioration and impending physiologic exhaustion.4,24 On the battlefield, cardiac uncoupling may provide new, real-time information for combat casualty triage and management. In the step-down setting, it may define a patient's clinical trajectory and serve as the foundation for a new generation of alarm systems.
Limitations
This work is nascent and not yet ready for routine deployment in the clinical setting. The concepts are not only new, but the tools required to advance this work are untested. We have yet to refine our measurements, optimize our analysis, define interactions, and explore populations other than trauma patients.
The number of deaths is small. While the data set contains millions of heart rate data points, our outcome variable, mortality, is a product of the number of deaths (n = 242). The proportionately small number of deaths limits our ability to analyze the relationship of uncoupling to mortality in patient subgroups. While we have data on patients with SIMON stay greater than 11 days, and while uncoupling appears common in this population, there are insufficient deaths to determine if a statistically significant relationship between cardiac uncoupling and mortality exists beyond ICU day 10.
Standard deviation may not be the optimal measurement of cardiac uncoupling. We hypothesize that the heart is acting autonomous of central control. We have defined one method of continuously measuring uncoupling: the standard deviation of integer heart rate over a 5-minute interval. While this method demonstrates the association of uncoupling with death, it may not be the optimal tool to demonstrate dynamic changes in heart rate variability. The use of other analytical measures of variation such as percentile analysis holds great promise for improving our ability to detect real time changes in patient trajectory. Finally, advances in technology may allow the efficient capture, storage, and real-time analysis of wave form data, which may potentially provide useful mechanistic and therapeutic information.
Temporal relationships between physiologic and clinical events are complex. To date, we have explored only a few techniques for defining these relationships. Time series analysis requires sophisticated computational power and algorithms. In this study, 260 million data points were captured, stored, organized, and analyzed. Our methods for executing these tasks remain rudimentary, but as we refine our informatics infrastructure we will engage new disciplines (mathematics, statistics, and computer science) and new analyses (machine learning, wavelets, chaos theory). These results may strengthen or attenuate our current conclusions.
Finally, disruption of communication pathways between systems, organs, and cells appears to be signaled by uncoupling of numerous organ systems from physiologic command and control. Cardiac uncoupling is the prototype, illuminated by merging dense physiologic data capture and a unique clinical information management system. Other factors, alone or in combination with cardiac uncoupling, may demonstrate a more robust relationship between dense physiologic data and patient trajectory.
The Future
Effective information management will be to 21st century health care what the introduction of anesthesia was to the 19th, and the discovery of antibiotics was to the 20th century. Informatics will be the tool by which we redesign health care. Future healthcare processes will increasingly focus on disease management, not patient management. Data will be captured, linked, and aggregated across populations of patients. Tomorrow's electronic data capture and display tools will automatically leverage aggregate population data to assist clinicians in making individual patient care decisions.
This transformation requires new methods of capturing, linking, and delivering information. Data must be captured electronically, integrated into the electronic medical record using a standardized vocabulary, aggregated across multiple sources and patients into secure, linked repositories, and shared among institutions to support patient care, quality, and benchmarking. Novel displays will deliver this information to the point of medical decision making, whether at the physical or “virtual” bedside.
This work barely scratches the surface of this opportunity. Our next task is to migrate dense data capture techniques to different settings in the hospital (step-down, floor beds) and diverse arenas (prehospital, battlefield). We must link physiologic data with pharmacy, laboratory, and genetic information to show how these factors affect the predictive power of biomarkers.
SIMON contains 12 other physiologic parameters. While this study analyzes more than 260 million heart rate data points, it represents less than 20% of the physiologic data; we have captured and stored in this population of 2088 patients. Other parameters, alone or in concert, may enhance our predictive ability.
Additionally, we must expand this technology to investigators at other institutions. Only in this way will we rapidly obtain statistical power needed to investigate subgroups of patients and ultimately realize the benefits of disease management.
CONCLUSION
The effective management of vast amounts of clinical information will be required to care for patients in the future. This work begins the process of illustrating the association between heart rate variability, cardiac uncoupling, and the risk of death in trauma patients. Specifically, the presence of cardiac uncoupling: 1) is an independent predictor of death throughout the ICU stay, 2) has a predictive window of 2 to 4 days, and 3) appears to increase in response to inflammation, infection, and multiple organ failure.
Discussions
Dr. Edward E. Cornwell, III (Baltimore, Maryland): I would like to publicly thank Dr. David Chang, a research associate in our department at Hopkins, who has expertise in outcomes research as well as in database management for reviewing the manuscript as well.
The authors have amplified on their earlier work suggesting that cardiac uncoupling, low heart right variability, in the early post-injury period was associated with an increased mortality. This study, covering 2088 patients admitted to a trauma ICU over a 4-year period of time, suggests on multivariate analysis that this low heart rate variability remains a robust predictor of mortality across a host of mechanisms of injury and post-injury complications as well as for the entirety of the ICU stay.
I have an observation and three questions. The positive impact of heart rate control perioperatively in ICU patients on their outcome is one of the more compelling developments in critical care medicine in the last decade. On the other hand, it appears that the calculation of heart rate variability is mathematically not greatly impacted by the actual magnitude of the heart rate. Could it be that low heart rate variability is a surrogate for persistent tachycardia that is seen in critically ill and injured patients? In other words ānd you may not have enough mortality to stratify this w̄ould you expect to see a change in the predictive ability of cardiac uncoupling when you compare it with a patient whose heart rates are consistently in the 120 to 130 range versus the 70 to 75 range?
Question Number 2 relates to clinical significance. It appears in the manuscript of Table 3 that the relative risk for death on multivariate analysis was higher for increasing severity of head injury, increasing head AIS, but that the relative risk for mortality among patients with low heart rate variability was similar for increasing injury severity score overall for transfusions and for age. Given this, would you assign independent clinical significance to the low heart rate variability over and above those other risk factors?
Finally, Number 3, could you speculate on therapeutic intervention if, in fact, these observations are duplicated across a host of ICUs and across a host of increased numbers of deaths?
In summary, the authors have accurately asserted in their manuscript that information management will be to 21st century health care what anesthesia was to the 19th century and antibiotics were to the 20th century. I congratulate the authors for bringing us into that 21st century, perhaps kicking and screaming, as we search for the right biomarker that identifies patients at risk for poor outcomes at a point where interventions may offer hope for effecting that outcome.
Dr. John A. Morris, Jr. (Nashville, Tennessee): Your first question about heart rate variability and tachycardia. We don't think that heart rate variability is reflecting just tachycardia. We have work that shows that if you compare time intervals between heart rate variability and cardiac index, which is a function of heart rate, that heart rate variability is providing very different information than cardiac index.
We know that heart rate variability and cardiac index in the hypothermic patient are low. In the hyperthermic patient, that relationship reverses. So you are getting different information from heart rate variability than you are from invasive continuous cardiac index measurements. So we are confident that heart rate variability is telling us something different than just myocardial performance.
Second, the clinical significance. We are getting more comfortable that we are able to stratify groups of patients that will allow us to manage people differently. We feel very strongly that one of the things that we have to do early on is look at beta blockade in this population of patients and determine if beta blockade influences heart rate variability and outcome. We are merging multiple databases to answer that question, but it is probably going to be this time next year before we get that information.
We do think, however, that we can stratify patients, for instance, having high risk of certain abnormalities, such as adrenal insufficiency, so that we can take a large group of patients, stratify them into a subgroup of patients, and then apply specific testing to those patients to see whether adrenal insufficiency, for instance, is present.
We also believe that we may be able to predict patients using heart rate variability who would be at high likelihood of needing intercranial pressure monitoring. And we would then have a noninvasive test which potentially could tell us when to move to an invasive environment.
Dr. Timothy C. Fabian (Memphis, Tennessee): Although the concept is fairly simple (relatively fixed heart rate is associated with poor outcomes), the statistical analyses overwhelm me. Asking me to discuss this elaborate methodology was truly like “throwing pearls at the feet of swine!” Then, when I got to Figure 4 in the manuscript, the surface contour plot, I was further humiliated because it took me back to John Siegel's contour plots, which were always way over my head.
With those apologies, let me gather a few mundane questions. In the abstract, you refer to the “LHRVI,” low heart rate variability index, while in the manuscript instead you refer to “cardiac uncoupling.” Why did you change nomenclature? I have always noted that elderly patents do not mount a tachycardic response to all phases of injury as compared to the persistent tachycardia of youth at essentially all stages of injury. I generally assumed this is due to coronary artery disease and its effect on the conduction system. Have you compared cardiac uncoupling by increments of age to outcomes? Similarly, do the young patients who die display similar uncoupling patterns to the older nonsurvivors?
Next question. Have you considered comparing in those with substantial uncoupling, survivors to nonsurvivors to interrogate differences in those cohorts? As predictors of poor outcomes, base deficit and pH on admission have proven to be valuable, why did you not use those as covariates in your analyses of outcome predictors? Finally, in order to address associations and causations, do you have plans to do correlative cytokine analyses?
In conclusion, an observation and a warning to Nashville's neighbors: In the Methods Section of the November 2005 publication in the Journal of Surgical Research, these authors noted a 60,000 square mile catchment area for these patients, and in the current manuscript it has metastasized to 80,000 square miles! That translates into a 160-mile radius. Louisville is 176 from Nashville. Birmingham is 192; Knoxville, 180; Chattanooga, 133; Memphis, 212. I have visions of Pac-Man, and I think we're all doomed.
Dr. John A. Morris, Jr. (Nashville, Tennessee): The question about the differences between the manuscript and the abstract is very germane. We have moved to this concept of cardiac uncoupling because it is a lot easier to explain. We kept saying “the reduction in low heart rate variability,” and the sentences just became untenable. Additionally, in the time between the abstract being submitted and the manuscript being written, we think that we are seeing other organ systems that are demonstrating this uncoupling phenomenon: the adrenal gland, as Dr. Britt and I have discussed. Certainly changes in glucose homeostasis, whether this is insulin related, pancreas related, or muscle bed related. Clearly, insulin and glucose are things that we need to look at within this coupling-uncoupling model. So that is why we have changed our terminology.
Age clearly is a factor to be taken into account. All of the early work that we did in this area, and have published, has shown that age increases mortality independent of injury severity. And we have to work through some of that in greater detail, but it clearly is a factor that affects.
Base deficit. We didn't include base deficit lactate and coagulopathy in this manuscript because we just finished submitting one to the Journal of Trauma looking at the effects of uncoupling and time to lactate clearance and so on and so forth. And it is from that work that we made the conclusion that we think uncoupling does reflect failure of resuscitation and reconstitution of reserve.
Finally, your comment about cytokines is right on. We think that this is especially important in head injury. We are looking at cytokines in subgroups of these patients. And specifically we are looking at cytokines and inflammatory markers in CSF in patients who get pressure intercranial pressure monitoring.
Dr. Lewis M. Flint, Jr. (Tampa, Florida): When you talk about trajectory, we know that if you graduate from the ICU that doesn't mean you're out of the woods. Have you got any long-term data that relate your heart rate variability findings to late death in your patients?
Dr. John A. Morris, Jr. (Nashville, Tennessee): No. But we have applied for funds to be able to deliver this kind of monitoring device to our step-down and non-ICU beds. That is the critical issue. If this works to be able to identify people who are deteriorating, then we will have something that is truly significant.
Dr. Robert M. Mentzer, Jr. (Lexington, Kentucky): These are clearly intriguing findings with significant clinical implications. I am curious if you have taken some of these observations back to the laboratory to determine, first of all, whether this phenomenon exists in a pre-clinical situation, and second, whether you can use the pre-clinical environment to elucidate the underlying mechanism of action?
Dr. John A. Morris, Jr. (Nashville, Tennessee): We haven't taken it back to the laboratory. We have a fairly robust group at Vanderbilt who look at autonomic dysfunction. They are temperamentally a little bit different than the trauma surgeon group, and it has taken a while for us to be able to engage them in this kind of work. But we think that they have a tremendous value in being able to take some of their clinical testing not purely to the lab but to the bedside and to be able to determine some of the mechanistic things going on, especially in regards to what we believe is one of the real failures of command and control, the failure of the autonomic nervous system.
Dr. C. Gillion Ward (Miami, Florida): I commend you for introducing us into the arena of chaos theory and also beginning to tease open the concepts of what I call quantum medicine: in 100 patients you know the percentage that will have results, but you don't know which ones. And in that regard, my question is: of the group that you saw the uncoupling, could you determine which ones were going to have a death or more severe complication?
Dr. John A. Morris, Jr. (Nashville, Tennessee): Not yet. I don't think we are ready to take this to an individual patient. We are simply looking, as you pointed out, at population phenomena. And, yes, we are treading in domains such as chaos therapy and entropy. My second grade arithmetic teacher is spinning in her grave at the thought of me standing here using those concepts. I am sure glad there is a math department at Vanderbilt.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): There are other medications besides beta blockers that can affect heart rate, such as analgesics and anesthetic agents. Additionally, temperature affects metabolic rate and cooling can lower heart rate. We also need to know how variability relates to heart rate per se? If there is profound bradycardia, is variability greater or less? How do these other factors influence variability independent of the patient's condition? Thirdly, it sounds to me like the monitoring system you utilize might be expensive. Consequently, we need to know what it is better than. Is it better than John Morris standing at the foot of the bed saying, “This patient is very sick”?
Dr. John A. Morris, Jr. (Nashville, Tennessee): I hate to say publicly that it is better than John Morris. But it certainly allows John Morris to make rounds in the intensive care unit and go to the resident and say, “Tell me about this patient, tell me about this patient, and tell me about this patient, because these patients don't look right to me based on the heart rate variability data.”
Again, I think what we want you to understand is that we are identifying populations of patients at risk. We are not trying to say that one single vital sign can do the job of the house staff and the faculty. It simply says here is another piece of information to put in your armamentarium and determine whether a patient needs intervention.
And, Dr. Pruitt, I think that your equating heart rate and heart rate variability highlights the need to transition from one way of thinking to another. Heart rate variability is completely different than the rate of the heart. I would love to get “rate” out of “heart rate variability.” It is really the variability component that is the key component.
Footnotes
Reprints: John A. Morris, Jr., MD, 1211 21st Avenue South, 404 MAB, Nashville, TN 37212. E-mail: john.morris@vanderbilt.edu.
REFERENCES
- 1.Grogan EL, Morris JA Jr, Norris PR, et al. Reduced heart rate volatility: an early predictor of death in trauma patients. Ann Surg. 2004;240:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grogan EL, Norris PR, Speroff T, et al. Volatility: a new vital sign identified using a novel bedside monitoring strategy. J Trauma. 2005;58:7. [DOI] [PubMed] [Google Scholar]
- 3.Norris PR, Morris JA Jr, Ozdas A, et al. Heart rate variability predicts trauma patient outcome as early as 12 h: implications for military and civilian triage. J Surg Res. 2005;129:122–128. [DOI] [PubMed] [Google Scholar]
- 4.Morris JA Jr, Norris PR, Ozdas A, et al. Reduced heart rate variability signals cardiac uncoupling and deterioration of physiologic reserve: a study of 1425 trauma patients. J Trauma. 2006. [DOI] [PubMed] [Google Scholar]
- 5.Winchell RJ, Hoyt DB. Spectral analyses of heart rate variability in the ICU: a measure of autonomic function. J Surg Res. 1996;63:11–19. [DOI] [PubMed] [Google Scholar]
- 6.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002;8:311–315. [DOI] [PubMed] [Google Scholar]
- 7.Buchman TG. Nonlinear dynamics, complex systems, and the pathobiology of critical illness. Curr Opin Crit Care. 2004;10:378–382. [DOI] [PubMed] [Google Scholar]
- 8.Wudel JH, Morris JA Jr, Yates K, et al. Massive transfusion: outcome in blunt trauma patients. J Trauma. 1991;31:1–7. [PubMed] [Google Scholar]
- 9.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 10.Korach M, Sharshar T, Jarrin I, et al. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med. 2001;29:1380–1385. [DOI] [PubMed] [Google Scholar]
- 11.Toweill D, Sonnenthal K, Kimberly B, et al. Linear and nonlinear analysis of hemodynamic signals during sepsis and septic shock. Crit Care Med. 2000;28:2051–2057. [DOI] [PubMed] [Google Scholar]
- 12.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response endotoxin. Nature. 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 13.Haji-Michael PG, Vincent JL, Degaute JP, et al. Power spectral analysis of cardiovascular variability in critically ill neurosurgical patients. Crit Care Med. 2000;28:2578–2583. [DOI] [PubMed] [Google Scholar]
- 14.King ML, Lichtman SW, Seliger G, et al. Heart-rate variability in chronic traumatic brain injury. Brain Inj. 1997;11:445–453. [DOI] [PubMed] [Google Scholar]
- 15.Winchell RJ, Hoyt DB. Analysis of heart-rate variability: a noninvasive predictor of death and poor outcome in patients with severe head injury. J Trauma. 1997;43:927–933. [DOI] [PubMed] [Google Scholar]
- 16.Morris JA Jr, Norris PR. Role of reduced heart rate volatility in predicting death in trauma patients. Adv Surg. 2005;39:77–96. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. [DOI] [PubMed] [Google Scholar]
- 18.Liao D, Sloan RP, Cascio WE, et al. Multiple metabolic syndrome is associated with lower heart rate variability: the Atherosclerosis Risk in Communities Study. Diabetes Care. 1998;21:2116–2122. [DOI] [PubMed] [Google Scholar]
- 19.Nolan J, Batin PD Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UKHEART). Circulation. 1998;98:1510–1516. [DOI] [PubMed] [Google Scholar]
- 20.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–1116. [DOI] [PubMed] [Google Scholar]
- 21.Sauaia A, Moore FA, Moore EE, et al. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45:291–301. [DOI] [PubMed] [Google Scholar]
- 22.Tibby SM, Frndova H, Durward A, et al. Novel method to quantify loss of heart rate variability in pediatric multiple organ failure. Crit Care Med. 2003;31:2059–2067. [DOI] [PubMed] [Google Scholar]
- 23.Lampert R, Ickovics JR, Viscoli CJ, et al. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91:137–142. [DOI] [PubMed] [Google Scholar]
- 24.Holcomb JB, Helling TS, Hirshberg A. Military, civilian, and rural application of the damage control philosophy. Mil Med. 2001;166:490–493. [PubMed] [Google Scholar]