Abstract
Objective:
To investigate the role of increased intra-abdominal pressure (IAP) on the intratumoral accumulation and the antitumor effect of intraperitoneal cisplatin in rats with advanced peritoneal carcinomatosis. To evaluate the tolerance of IAP in pigs, as it is a large animal with a body size equivalent to humans.
Summary Background Data:
To investigate if an active convection, driven by a positive IAP, increases cisplatin penetration and antitumor effectiveness in a model of advanced peritoneal carcinomatosis in rats.
Experimental Design:
BDIX rats with macroscopic peritoneal tumors received cisplatin administered as intravenous injection (IV), conventional intraperitoneal injection (IP), or sustained intraperitoneal injection of cisplatin given in a large volume of solvent for maintaining IAP for 1 hour. Platinum tissue concentration was measured by atomic absorption spectroscopy (AAS), and platinum distribution into the tumor nodules was assessed by the particular-induced x-ray emission (PIXE) method. The antitumor effect was assessed in a survival experiment. The hemodynamic, local, and systemic tolerance of IAP, with or without cisplatin, was evaluated in Large White pigs.
Results:
The maximum tolerated IAP was 22 mm Hg for 1 hour in nonventilated rats. IAP, in comparison with IV or conventional IP injections, resulted in the increased concentration and depth of diffusion of platinum into diaphragm and peritoneal tumor nodules. Consequently, IAP treatment induced an extended survival of rats treated at an advanced stage of carcinomatosis. In 7 50- to 70-kg ventilated pigs, a 40-mm Hg IAP was well tolerated when maintained stable for 2 hours. Renal failure occurred in pigs receiving a total dose of 200 and 400 mg of cisplatin with IAP, but a dose of 100 mg was well tolerated.
Conclusions:
Intraperitoneal chemotherapy with increased IAP, in comparison with conventional IP or IV chemotherapy, improved the tumor accumulation and the antitumor effect of cisplatin in rats bearing advanced peritoneal carcinomatosis. In preclinical conditions, the tolerance of sustained IAP was manageable in ventilated pigs.
The authors reported that a positive intra-abdominal pressure leads to increased penetration and antitumor effect of intraperitoneal cisplatin in an experimental model of rat peritoneal carcinomatosis. Hemodynamic tolerance of intra-abdominal pressure was demonstrated in pigs.
Ovarian and digestive cancers often spread throughout the entire peritoneal cavity through cell exfoliation and dissemination from the initial tumor site, resulting in peritoneal carcinomatosis. The prognosis of patients with extensive stage III ovarian carcinoma remains poor with only approximately 20% 5-year survivors, despite improvements in debulking surgery and systemic chemotherapy based on platinum derivatives.1 Peritoneal carcinomatosis from colorectal cancer has long been considered to be incurable. However, a new treatment strategy combining cytoreductive surgery and intraperitoneal chemotherapy with moderate hyperthermia (41°C–43°C) in the operating room now supports a curative approach to carcinomatosis from colorectal cancer.2–6 In both ovarian and digestive tumors, the main condition for a possible cure is the macroscopic completeness of surgical cytoreduction preceding intraperitoneal (IP) chemotherapy. Experimental models revealed that the antitumor effect of IP chemotherapy is strongly limited by the poor penetration (<1 mm) of anticancer drugs into peritoneal nodules.7–9 Thus, the therapeutic effect of IP chemotherapy in clinical studies is restricted to microscopic or millimeter residual tumors.10,11 The limited diffusion of intraperitoneally injected anticancer drugs into large neoplastic peritoneal nodules is attributed to the peritoneum-tumor barrier.12 This barrier is anatomically defined by the peritoneal mesothelium, the extracellular matrix surrounding the tumor, and the successive layers of tightly attached tumor cells. Thus, drugs have to diffuse through the tumor mass by the paracellular or transcellular pathway to reach all tumor cells, as every cell layer and the extracellular matrix slow drug penetration. Moreover, the anatomic peritoneum-tumor barrier is strengthened by a functional component, due to high interstitial tumor pressure13 and the effect of the capillary network, which drains drugs out of the tumor tissue. In previous papers, we showed that epinephrine, a potent vasoconstrictor, enhanced the local accumulation and antitumor activity of intraperitoneal cisplatin by limiting the amount of drug draining through the tumor vessels.14–16 However, the epinephrine-potentiating effect is restricted to the functional, but not the anatomic, component of the peritoneum-tumor barrier, and only millimeter-size peritoneal carcinomatosis nodules were cured by the epinephrine-cisplatin combination in rats.14 Simple passive diffusion is then inadequate for homogeneously bringing drugs inside the entire depth of supra-millimeter tumors. Jacquet et al have proposed to increase the intra-abdominal pressure (IAP) for improving the tissue penetration of doxorubicin after intraperitoneal administration.17
In the present study, we investigated if an active convection, driven by a positive IAP, could increase cisplatin penetration and antitumor effectiveness in rats bearing an advanced peritoneal carcinomatosis. As a preclinical investigation, the tolerance of increased IAP alone and the local and systemic tolerance of IP cisplatin with IAP were evaluated in pigs.
MATERIALS AND METHODS
Animals
Male inbred BD IX strain rats 4 to 6 months old, weighing 300 to 350 g, were bred in constant conditions of temperature, hygrometry, and exposure to artificial light. Five-month-old Large White female pigs, weighing 50 to 70 kg, were operated and kept until death under licensed animal facilities. Experimental protocols were consistent with the “Guidelines on the protection of experimental animals” published by the Council of the European Community (1986). The Burgundy University Animal Care and Use committee approved all of the procedures.
Chemicals and Drugs
Cisplatin was obtained from Sigma-Aldrich (L'Isle d’ Abeau, France) and was diluted in sterile isotonic saline solution (9 g/L NaCl) before intravenous (IV) or IP injection.
Cancer Cells and Tumor Model
The DHD/K12 cell line originated from a dimethylhydrazine-induced colon tumor in BD IX rats. Its PROb clone was chosen for its regular tumorigenicity when injected into syngenic rats.18 PROb cells were maintained in culture in Ham's F10 medium supplemented with 10% fetal bovine serum. Cells were detached with trypsin and EDTA and centrifuged in the presence of complete culture medium with fetal bovine serum to inhibit trypsin. Tumor cells were suspended in 5 mL of serum-free Ham's F10 medium and then intraperitoneally injected (2 × 106/rat) in anesthetized rats. As previously reported,14 the size of the peritoneal tumor nodules depended upon time: no tumor nodules were visible at day 2 and multiple nodules of 0.5 to 3 mm were seen throughout the mesenteric and parietal peritoneum at day 14 after cell injection. At day 21 (advanced carcinomatosis), tumor nodules were confluent in the epiploic area and extended to the entire peritoneum wall, including the diaphragmatic areas. Untreated rats regularly died an average of 40 days after cell injection from extensive peritoneal carcinomatosis with hemorrhagic ascites, but without macroscopically evident extraperitoneal metastases, notably in the liver or the lungs.
Treatment of Animals
Male BD IX rats were treated 14 days or 21 days after the IP injection of PROb cancer cells. Control animals received no treatment. Intravenously (IV) treated rats received a single bolus of cisplatin, 3 mg/kg body weight, in 1 mL of isotonic saline through the penis vein. This dose, which represented approximately 1 mg cisplatin per rat, was previously determined as the maximum tolerated dose in the BD IX rat strain.19 Rats under conventional IP treatment received a single IP bolus of cisplatin in 20 mL of isotonic saline. For the sustained IAP group, cisplatin was diluted in 150 mL of isotonic saline and infused by gravity through a transcutaneous IP drip catheter (Terumo-Surfo*, 14G × 21/2). The drip was connected through a 3-way connector to a water column to monitor the IAP. Before IP infusion, the IAP was zero. An accurate measurement of pressure was checked by the synchronized oscillations of the water column with breathing. The required level of IAP was obtained in approximately 5 minutes by a rapid infusion and then the output was reduced and regulated to maintain a constant pressure. Preliminary studies determined that the maximum IAP tolerated for 1 hour in anesthetized, but not ventilated, male BD IX rats was an average of 22 mm Hg. Rats often died when the IAP was in excess than 22 mm Hg or when time exceeded 1 hour for a 22 mm Hg IAP. Intravenous infusion of isotonic saline into the right jugular vein, to prevent insufficient blood return from the inferior vena cava into the heart, did not permit an increase in the maximum tolerated IAP. Rats were killed 1 hour after starting treatment. For IP-treated animals (conventional IP or IAP), the peritoneal cavity was emptied, then washed with 40 mL of drug-free isotonic saline through a short laparotomy before tumor and organ sampling. Blood was collected by cardiac puncture. The volume of recovered peritoneal liquid varied, but was always less than 3 mL after a conventional IP injection, whereas a varying volume, always greater than 130 mL, was recovered after the IAP procedure. In the survival study, the peritoneal cavity was also emptied and washed with 40 mL of isotonic saline 1 hour after the beginning of treatment in all treatment groups, including IV. Animals were kept until they spontaneously died or were killed 120 days after treatment.
Large White female pigs were used to evaluate the hemodynamic, local, and systemic tolerance of increased IAP with (3 animals) or without (4 animals) IP cisplatin. Anesthesia was induced by intramuscular injection of 2 g ketamine, 20 mg acepromazine acetate, and 1 mg atropine. Anesthesia was completed by IV ketamine and sufentanil until the trachea was cannulated in spontaneous ventilation. Mechanical ventilation was performed with a controlled volume ventilator. Animals were maintained under anesthesia by isoflurane (1.5% in a mixture of air/O2) and IV sufentanil and cisatracurium. Bladder catheterization was performed to measure urine output. The IAP was continuously monitored through a gas insufflation needle for laparoscopy (Surgineedle, Autosuture, Tyco, Norwalk, CT), which was percutaneously inserted away from the infusion site. Systemic arterial blood pressure (SAP) was monitored through a catheter inserted into the femoral artery (Sedicath, Plastimed, Saint Leu La Forêt, France). Central venous pressure (CVP), pulmonary arterial pressure (PAP), and pulmonary arterial occlusion pressure (PAOP) were monitored through a pulmonary artery catheter surgically introduced in the right jugular vein (Swan Ganz, Edwards Lifesciences, Irvine, CA). All pressure values (SAP, CVP, PAP, PAOP, and IAP) were measured with a conventional Hewlett Packard monitor. Heart rate, ECG, and nasal temperature were also monitored. Only the airway pressure was measured with the ventilator itself. Oxygen saturation (pulse oxymetry), cardiac output (L/min), and cardiac index (L/min/m2) were measured using the NICO system (Novametrix Medical Systems Inc., Wallingford, CT). Peranesthetic fluid resuscitation was achieved with isotonic saline, Ringer lactate and gelatin (Plasmion, Fresenius Kabi France, Sèvres, France), with a mean volume of 6 L per pig.
Animals, which received increasing IP cisplatin with a sustained 40 mm Hg IAP for 2 hours, were kept for 4 weeks or until spontaneous death. Biologic parameters (blood count, sodium, potassium, and creatinine serum level) were monitored weekly. Autopsy was performed at time of sacrifice or spontaneous death.
Atomic Absorption Spectrometry
The total concentration of platinum in organs or tumors was measured by atomic absorption spectrometry (AAS). After treatment, blood was collected by cardiac puncture and the abdominal cavity was washed with 40 mL isotonic saline. Peritoneal tumor nodules, diaphragm, and organs were dried on absorbent paper and kept at −80°C until AAS assay. The diaphragm, which is thin in rats, was considered as a valuable substitute for the parietal peritoneum. After weighing, frozen tissues were digested in a microwave digester (MLS-1200 Mega, Milestone, Sorisole, Italy). Platinum concentration was measured after dilution in distilled water, using a Zeeman atomic absorption spectrometer (Spectra-A; Varian, Les Ulis, France).
PIXE Microanalysis
Tumor nodules were cryofixed in dimethylbutane chilled with liquid nitrogen and stored in Nunc tubes at −80°C until analysis. Samples were sectioned in a cryostat at −25°C with a thickness of 20 ìm. Sections were mounted on fresh, thin (about 0.4 ìm) Formvar film stretched over aluminum holders and freeze-dried overnight in the cryostat. Analysis was performed using a nuclear microprobe with a 3 MeV proton microbeam focused down to approximately 10 ìm. Micro-PIXE analysis was carried out by scanning the beam over contiguous 1 mm2 areas to obtain a complete transversal distribution of platinum through the diameter of the peritoneal tumor nodule. Platinum was evaluated using the L x-ray lines induced by the interaction of protons with the sample.20 Since the local dry weight of sections was measured using Rutherford Backscattering Spectrometry, the platinum content of tissues could be expressed in terms of concentration (in ìg/g tissue dry weight).
Statistical Analysis
The nonparametric Kruskal-Wallis test was used to detect global statistically significant differences in the extent of platinum accumulation in organs and tumors among groups and if different, the Mann-Whitney's test was used for 2 × 2 comparisons between groups. The Kaplan-Meier method was used to assess the animal survival time, and the log-rank test was used to test differences between groups. A P value <0.05 was considered to be statistically significant. The correlation between IAP and the infused volume was analyzed using the calculation of the correlation coefficient r.
RESULTS
Platinum Tissue Concentration and Distribution
Platinum concentration in organs of rats with advanced carcinomatosis was measured by AAS 1 hour after the IV or conventional IP injection, or at the end of a 1-hour infusion of cisplatin with an increased 22 mm Hg abdominal pressure (IAP). Cisplatin dose for the IV treatment (1 mg/rat; 3 mg/kg) was previously determined as the maximum tolerated in survival experiments. As the injected volume was different between conventional IP (20 mL) and IAP administration (150 mL), the problem was to separately evaluate the effect of concentration and drug total dose. As drug excess was flushed from the peritoneal cavity at the end of experiment, the total amount of cisplatin absorbed by a rat could not be precisely estimated. Cisplatin concentration was 50 mg/L in 150 mL isotonic saline (7.5 mg/rat; 22.5 mg/kg) for the IAP group. So AAS experiments were performed for conventional IP (20 mL) both at a constant 50 mg/L concentration (1 mg/rat; 3 mg/kg), then at a constant 22.5 mg/kg total dose of cisplatin (7.5 mg/rat; 375 mg/L). Under both experimental conditions, IAP led to a significant increase in platinum accumulation in the peritoneal tumor nodules and in the diaphragm (Fig. 1). In contrast, no significant difference was found among treatment groups for blood and extraperitoneal organs (Fig. 2). As AAS gave only information on the global amount of platinum in tissue, it was completed by the PIXE method, which allowed for the study of platinum distribution throughout the entire depth of the tumor after various IP treatments (Fig. 3). Cisplatin was used at a higher dose in PIXE experiment than for AAS to allow for the detection of platinum in tissue. IAP was compared with conventional IP treatment, either at the same concentration or at the same total dose of cisplatin in rats. For the same 250 mg/L cisplatin concentration, IAP resulted in a 3-fold increase in platinum concentration in the tumor compared with conventional IP. For an equivalent total dose (37.5 mg/rat, and then a 1875 mg/L concentration for conventional IP and 250 mg/L for IAP), IAP again resulted in an increased local concentration of platinum, but mainly in the tumor center.
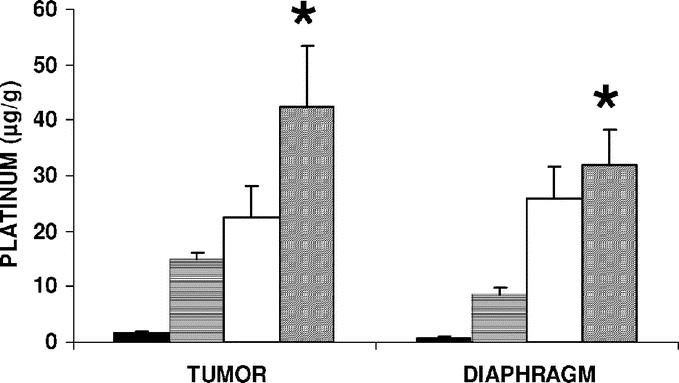
FIGURE 1. Platinum concentration in peritoneal tumors and diaphragm after IV, IP, or IAP cisplatin treatment. Rats with 21-day-old carcinomatosis (5 per group) were treated with cisplatin by intravenous (IV) or conventional intraperitoneal (IP) injection, or by a 1-hour intraperitoneal infusion with a sustained 22 mm Hg intra-abdominal pressure (IAP). The cisplatin dose was 1 mg/rat (3 mg/kg) for the IV administration (dark bars) and 7.5 mg/rat (22.5 mg/kg; 50 mg/L in 150 mL of isotonic saline) for the IAP treatment (diagonal bars). For the conventional IP treatments, cisplatin was given either to obtain the same concentration (50 mg/L in 20 mL; 1 mg/rat; 3 mg/kg; horizontal bars) or the same total dose (7.5 mg/rat; 22.5 mg/kg; 375 mg/L in 20 mL; clear bars) as for the IAP treatment. Each point is the average of 5 determinations and presented as a mean value; bars = SD. *Statistically significant difference between the IAP group and both the conventionally treated IP groups, either at the same concentration or at the same total dose of cisplatin (P < 0.05, Kruskal-Wallis test).
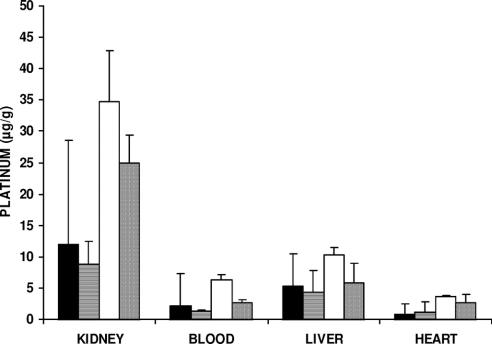
FIGURE 2. Platinum concentration in blood and extraperitoneal organs after IV, IP, or IAP cisplatin treatment. The experimental conditions are the same as those in Figure 1. No significant difference was seen in each organ with the various treatments (Kruskal-Wallis test).
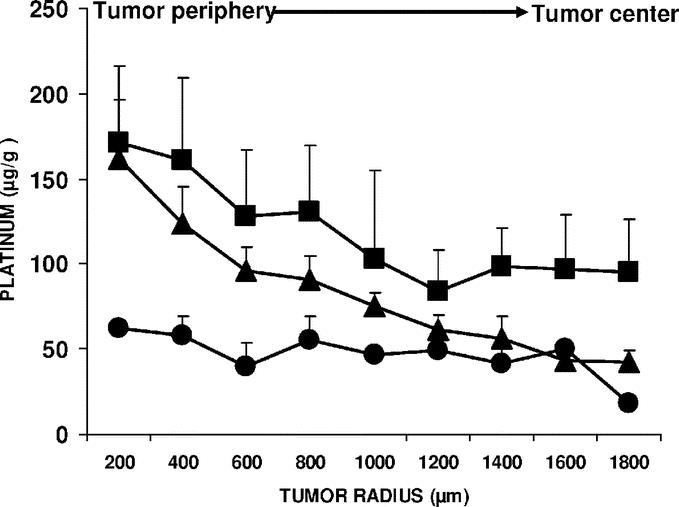
FIGURE 3. Distribution of platinum into peritoneal tumor nodules after conventional IP or IAP cisplatin treatment. Rats with 21-day-old carcinomatosis (4 per group) were treated with cisplatin through a conventional intraperitoneal injection (IP) or an intraperitoneal infusion with increased intra-abdominal pressure (22 mm Hg for 1 hour; IAP). Local platinum concentration was measured along the radii of peritoneal tumor nodules by the PIXE method. The platinum distribution in 400 × 800 ìm2 analyzed areas was plotted from the periphery to the tumor center. In conventionally treated IP groups, the cisplatin concentration in the peritoneal liquid was either 250 mg/L in 20 mL isotonic saline (5 mg/rat; 15 mg/kg; •), or 1875 mg/L in 20 mL isotonic saline (37.5 mg/rat; 112.5 mg/kg; ▴) to compare groups exposed to the same concentration or the same total dose of cisplatin. Cisplatin concentration was 250 mg/L in 150 mL isotonic saline (37.5 mg/rat; 112.5 mg/kg) for the IAP-treated group (▪). Each point is the mean of 4 determinations ± SD. A significant difference among the 3 IP treatments was detected (P = 0.0125, Kruskal-Wallis test). The Mann-Whitney test indicated that the difference between both of the upper curves was only significant between a depth of 1400 and 1800 ìm (P = 0.0421).
Antitumor Effect
Intravenous or intraperitoneal chemotherapy, with or without increased abdominal pressure, was evaluated in a survival assay (Fig. 4). Rats received an IP injection of 2 × 106 PROb cells and then various treatments were performed 14 days later. All of the rats in group A (no treatment, control group), group B (IV cisplatin at 3 mg/kg; 1 mg/rat), and group C (treated for 1 hour with a conventional 20 mL IP injection of a 50 mg/L cisplatin solution; 1 mg/rat; 3 mg/kg) died of a progressive peritoneal carcinomatosis, with median survival times of 39, 57, and 58 days, respectively. Survival times of the rats in groups B and C were significantly longer (log-rank test, P < 0.001) than in the control group A. Animals in group D (treated with 50 mg/L cisplatin in 150 mL isotonic saline for 1 hour with a sustained 22 mm Hg IAP; 7.5 mg/rat; 22.5 mg/kg) survived longer than the animals which received the conventional IP treatment (log-rank test, P = 0.0157), with 6 of 8 rats surviving until they were killed (120 days), with no tumors found at autopsy. The early death (day 12 after treatment) of 2 animals in group D was attributed to the cisplatin toxicity, confirming that IAP treatment was done at nearly the maximum tolerated dose.
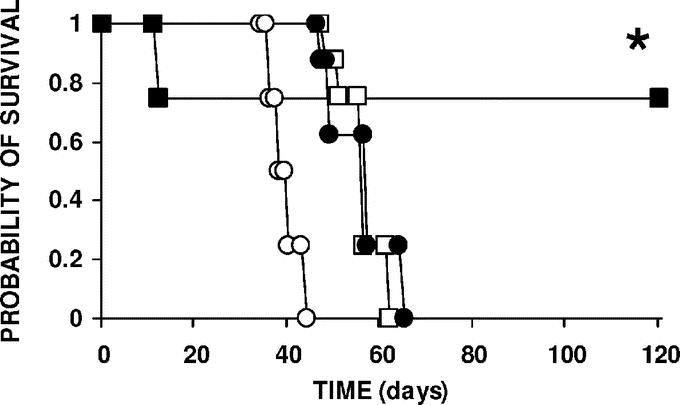
FIGURE 4. Animal survival according to treatment. Rats with 14-day-old carcinomatosis (8 per group) were treated with cisplatin by the intravenous (•) (IV) or the intraperitoneal (IP) route with (IAP, ▪) or without increased intra-abdominal pressure (IP conventional; □). Control animals (○) were left untreated. Cisplatin was given at the maximum tolerated dose for IV administration (1 mg/rat; 3 mg/kg). For conventional IP chemotherapy, rats received 20 mL of the 50 mg/L cisplatin solution (1 mg/rat; 3 mg/kg) as a bolus into the peritoneal cavity. For IP chemotherapy with IAP, cisplatin was administered in 150 mL of isotonic saline solution, 50 mg/L (7.5 mg/rat; 22.5 mg/kg), and continuously infused for 1 hour to maintain a constant 22 mm Hg intra-abdominal pressure. In both IP-treated groups, the peritoneal cavity was emptied and washed with drug-free saline 1 hour after starting the treatment. Animals were kept until spontaneous death or sacrifice at 120 days. *Significantly prolonged survival in the group treated with IP cisplatin with IAP compared with the conventionally treated IP group (log-rank test, P = 0.0157).
Tolerance of Increased Intra-abdominal Pressure in Pig
Intra-abdominal pressure increased as a function of the infused volume (Fig. 5; r = +0.99). A stable 40 to 50 mm Hg IAP was achieved by the rapid injection, in approximately 30 minutes, of an average volume of 10 L of isotonic saline. IAP was maintained at a constant level for 2 hours, without life-threatening side effects, using a slow infusion at an average output of 500 mL/h. Intra-abdominal pressure in excess of 50 mm Hg was poorly tolerated with increased heart rate and a decrease in oxygen blood saturation and cardiac index. If necessary, lowering pressure to or below 50 mm Hg through limited draining of the abdominal fluid restored acceptable hemodynamic parameters. Four pigs were submitted to a stable 40 mm Hg abdominal pressure for 2 hours using drug-free isotonic saline and were kept alive for 4 weeks before being killed. No change in blood count, ions, and creatinine blood level was registered and no macroscopic alteration of the peritoneum and the intra-abdominal organs were seen at autopsy. Three animals were treated for 2 hours with a 100, 200, or 400 mg cisplatin dose to achieve a 10, 20, or 40 mg/L drug concentration in isotonic saline, respectively, while maintaining a 40 mm Hg IAP. Both animals receiving 200 and 400 mg cisplatin developed severe renal failure (maximum creatinine level 569 and 791 μM/L, respectively, for a normal level <100 μM/L). No significant neutropenia, thrombocytopenia, or sodium or potassium change occurred. The pig treated with 400 mg cisplatin spontaneously died at day 16 after treatment, while the 200 mg cisplatin-treated animal survived with persistent biologic renal failure until sacrifice at day 28 after treatment. In contrast, the blood creatinine level and the other blood parameters did not change in the 100 mg cisplatin-treated pig. At autopsy, easily cleavable peritoneal adherences, mostly between the liver and the facing parietal peritoneum, were most marked in the cisplatin-treated animals than in those that received only the isotonic saline and IAP. All other intra-abdominal or extra-abdominal organs were macroscopically normal. A severe tubular necrosis was microscopically observed in the kidneys of both animals, which developed renal failure after receiving the highest doses of cisplatin.
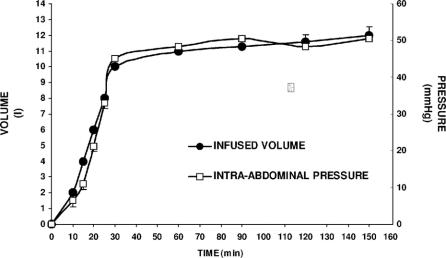
FIGURE 5. Relationship between the intra-abdominal pressure and the infused volume. Intra-abdominal pressure (approximately 40 mm Hg) was rapidly generated through the intraperitoneal infusion of isotonic saline and then maintained constant for 120 minutes in anesthetized and ventilated animals. Each point is the average of measurements from 7 animals and presented as a mean value; bars = SD.
DISCUSSION
In this study, we demonstrated that an IAP of 22 mm Hg for 1 hour elicited the penetration and antitumor effect of intraperitoneal cisplatin on advanced carcinomatosis in rats. Jacquet et al first proposed an increase in IAP to improve the effectiveness of intraperitoneal chemotherapy.17 They studied the impact of a 10 to 30 mm Hg positive IAP for 10 to 60 minutes on pharmacokinetics and tissue distribution of IP doxorubicin in tumor-free rats. Pressure was generated through the armband of an air sphygmomanometer wrapped around the rat abdomen. No significant increase in doxorubicin concentration was found in the abdominal wall, bladder, or diaphragm after 60 minutes of IP chemotherapy at the highest pressure. Moreover, prolonged IAP was associated with a high incidence of intestinal ischemia. We did not observe such immediate ischemia or late toxicity in the peritoneal wall or intestine of rats. Discrepancies between both studies could be attributed to either the technique or the drug. Generating an IAP through the infusion of a large amount of liquid (150 mL for rats weighing an average of 300 g) is probably less traumatic than a mechanical device. Moreover, depth of anthracycline penetration into the peritoneal tumor nodule is only limited to the outer cells,21 and in addition, this class of anticancer agents is only able to cure microscopic implants of peritoneal carcinomatosis.22 The high molecular weight of anthracyclines (579.9 for doxorubicin, 627.6 for pirarubicin) probably contributes to this poor tissue diffusion. Because of its relatively low molecular weight (330) cisplatin could penetrate deeper than anthracyclines into tumor nodules. Increased IAP is thought to generate a convective flux that drives cisplatin from the peritoneal cavity deeply into the tumor core. The concept of convection-enhanced diffusion through a mild constant pressure has been developed for the intratumoral chemotherapy of brain tumors.23 We indicate here that convection-enhanced diffusion by IAP is valuable for intraperitoneal chemotherapy. Extrinsic pressure could counterbalance the interstitial pressure, which is high in solid tumors (15 mm Hg in breast cancer, 22 mm Hg in cervical cancer, 19 to 33 mm Hg for head and neck cancer, 38 mm Hg in renal cancer, and 6 to 30 mm Hg in melanoma xenografts).24,25 Moreover, increased IAP could counteract the hydraulic capillary pressure, which drives water- and blood-borne molecules out of the vascular compartment.26 Capillary pressure averages 22 to 24 mm Hg in the intestinal muscle, 30 to 33 mm Hg in the mesentery, and 13 to 15 mm Hg in the mucosal villi,27 but is unknown in peritoneal tumors. The PIXE method showed that IAP lead to a platinum penetration into peritoneal tumor nodules, which is far beyond the classic 1 mm limit advocated by Los et al with conventional intraperitoneal chemotherapy.8,9 This directly translates to a greater antitumor effect of chemotherapy with IAP on advanced carcinomatosis, since the inner tumor cells are reached by a sufficiently high anticancer drug concentration to kill them.
The higher the IAP, the better the antineoplastic drug diffusion is into the tumor or the peritoneum, which could be contaminated with microscopic tumor cell foci. However, the level of the IAP is limited by respiratory and hemodynamic tolerance. Respiratory failure with sudden death precluded a sustained IAP over 22 mm Hg for 1 hour in nonventilated rats. Pulmonary dysfunction due to an acute elevation of the IAP is well characterized both experimentally and clinically. Reduction of the diaphragm course and compression of lung parenchyma by IAP results in the reduction of pulmonary compliance, leading to hypoventilation, hypoxia, and hypercapnia.28–30 In ventilated pigs, higher IAP was tolerated and a pressure of 40 to 50 mm Hg was maintained for 2 hours with acceptable tolerance. A detailed report on respiratory and hemodynamic change with IAP will be published later. In contrast to other authors,17,31–33 we did not observe intestinal or peritoneum ischemia in rats or pigs submitted to IAP. In 3 animals that received IP cisplatin with IAP, tolerance was limited by the known nephrotoxicity at the highest dose, but not by unexpected local or systemic toxicity related to IAP. Because of the systemic drug passage, which is probably increased by IAP, higher cisplatin doses than those usually given by the IV route are not recommended for the first step of a future clinical trial.
CONCLUSION
By counterbalancing tumor interstitial and capillary pressure, intra-abdominal pressure increases the depth of cisplatin penetration in millimeter peritoneal tumor nodules and, as a consequence, its antitumor effect on rats with peritoneal carcinomatosis. IAP levels higher than those tolerated in rats were tolerated in ventilated pigs, a large animal with a body size equivalent to humans. However, caution should be paid to the increased drug systemic passage. The concept that IAP can improve the therapeutic efficiency of intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from digestive or ovarian origin needs to be validated in clinical trials.
ACKNOWLEDGMENTS
The authors thank François Martin and Eric Solary for their helpful criticism, Franck Bonnetain and Fabien Pillard for advice on statistical methods, and Jonathan Ewing for his help in revising the manuscript.
Footnotes
Supported by a grant from the French National League against Cancer (Committees of Saône et Loire and Nièvre).
Reprints: Philippe Esquis, MD, Service de Chirurgie Digestive, Hôpital Général, 3 rue du Faubourg Raines 21000 Dijon. France. E-mail: esquisp@yahoo.fr.
REFERENCES
- 1.McGuire WP 3rd, Markman M. Primary ovarian cancer chemotherapy: current conventionals of care. Br J Cancer. 2003;89:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Peritoneal carcinomatosis: is cure an option? J Clin Oncol. 2003;21:762–764. [DOI] [PubMed] [Google Scholar]
- 3.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatoses treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Successful management of microscopic residual disease in a large bowel cancer. Cancer Chemother Pharmacol. 1999;43:15–25. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatoses from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. [DOI] [PubMed] [Google Scholar]
- 7.Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 8.Los G, Mutsaers PH, van der Vijgh WJ, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res. 1989;49:3380–3384. [PubMed] [Google Scholar]
- 9.Los G, Mutsaers PH, Lenglet WJ, et al. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25:389–394. [DOI] [PubMed] [Google Scholar]
- 10.Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277–283. [DOI] [PubMed] [Google Scholar]
- 11.Piccart MJ, Floquet A, Scarfone G, et al. Intraperitoneal cisplatin versus no further treatment: 8-year results of EORTC 55875, a randomized phase III study in ovarian cancer patients with a pathologically complete remission after platinum-based intravenous chemotherapy. Int J Gynecol Cancer. 2003;13:196–203. [DOI] [PubMed] [Google Scholar]
- 12.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. [DOI] [PubMed] [Google Scholar]
- 14.Duvillard C, Benoit L, Moretto P, et al. Epinephrine enhances penetration and anti-cancer activity of local cisplatin on rat sub-cutaneous and peritoneal tumors. Int J Cancer. 1999;81:779–784. [DOI] [PubMed] [Google Scholar]
- 15.Favoulet P, Magnin G, Guilland JC, et al. Pre-clinical study of the epinephrine-cisplatin association for the treatment of intraperitoneal carcinomatosis. Eur J Surg Oncol. 2001;27:59–64. [DOI] [PubMed] [Google Scholar]
- 16.Chauffert B, Favoulet P, Polycarpe E, et al. Rationale supporting the use of vasoconstrictors for intraperitoneal chemotherapy with platinum derivatives. Surg Oncol Clin North Am. 2003;12:835–848. [DOI] [PubMed] [Google Scholar]
- 17.Jacquet P, Stuart OA, Chang D, et al. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs. 1996;7:596–603. [DOI] [PubMed] [Google Scholar]
- 18.Caignard A, Martin MS, Michel MF, et al. Interaction between two cellular subpopulations of a rat colonic carcinoma when inoculated to the syngeneic host. Int J Cancer. 1985;36:273–279. [DOI] [PubMed] [Google Scholar]
- 19.Chauffert B, Dimanche-Boitrel MT, Genne P, et al. Experimental chemotherapy of peritoneal carcinomatosis of colonic origin in rats. Gastroenterol Clin Biol. 1992;16:215–219. [PubMed] [Google Scholar]
- 20.Ortega R, Moretto P, Fajac A, et al. Quantitative mapping of platinum and essential trace metals in cisplatin resistant and sensitive human ovarian adenocarcinoma cells. Cell Mol Biol. 1996;42:77–88. [PubMed] [Google Scholar]
- 21.Ozols RF, Locker GY, Doroshow JH, et al. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res. 1979;39:3209–3214. [PubMed] [Google Scholar]
- 22.Favoulet P, Benoit L, Osmak L, et al. Prevention of peritoneal carcinomatosis from colon cancer cell seeding using a pirarubicin solution in rats and nude mice. World J Surg. 2004;28:451–456. [DOI] [PubMed] [Google Scholar]
- 23.Vogelbaum MA. Convection enhanced delivery for the treatment of malignant gliomas: symposium review. J Neurooncol. 2005;73:57–69. [DOI] [PubMed] [Google Scholar]
- 24.Gutmann R, Leunig M, Feyh J, et al. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res. 1992;52:1993–1995. [PubMed] [Google Scholar]
- 25.Tufto I, Rofstad EK. Interstitial fluid pressure in human melanoma xenografts: relationship to fractional tumor water content, tumor size, and tumor volume-doubling time. Acta Oncol. 1995;34:361–365. [DOI] [PubMed] [Google Scholar]
- 26.Heldin CH, Rubin K, Pietras K, et al. High interstitial fluid pressure: an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. [DOI] [PubMed] [Google Scholar]
- 27.Gore RW, Bohlen HG. Pressure regulation in the microcirculation. Fed Proc. 1975;34:2031–2037. [PubMed] [Google Scholar]
- 28.Mutoh T, Lamm WJ, Embree LJ, et al. Abdominal distension alters regional pleural pressures and chest wall mechanics in pigs in vivo. J Appl Physiol. 1991;70:2611–2618. [DOI] [PubMed] [Google Scholar]
- 29.Obeid F, Saba A, Fath J, et al. Increases in intra-abdominal pressure affect pulmonary compliance. Arch Surg. 1995;130:544–547. [DOI] [PubMed] [Google Scholar]
- 30.Cullen DJ, Coyle JP, Teplick R, et al. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med. 1989;17:118–121. [DOI] [PubMed] [Google Scholar]
- 31.Samel ST, Neufang T, Mueller A, et al. A new abdominal cavity chamber to study the impact of increased intra-abdominal pressure on microcirculation of gut mucosa by using video microscopy in rats. Crit Care Med. 2002;30:1854–1858. [DOI] [PubMed] [Google Scholar]
- 32.Diebel LN, Dulchavsky SA, Brown WJ. Splanchnic ischemia and bacterial translocation in the abdominal compartment syndrome. J Trauma. 1997;43:852–855. [DOI] [PubMed] [Google Scholar]
- 33.Bongard F, Pianim N, Dubecz S, et al. Adverse consequences of increased intra-abdominal pressure on bowel tissue oxygen. J Trauma. 1995;39:519–524. [DOI] [PubMed] [Google Scholar]