Abstract
Introduction:
Recent evidence suggests that female severely burned children have higher endogenous anabolic hormone levels and a shorter ICU stay compared with males. The purpose of this study was to analyze the influence of age and gender on resting energy expenditure (REE) in severely burned children from acute hospitalization through 12 months postburn.
Methods:
A total of 100 pediatric patients with >40% total body surface area (TBSA) burn were enrolled in a prospective study and followed by indirect calorimetry measurements. The REE was expressed as actual REE kcal/d, percent of predicted REE, and REE/ body mass index (BMI). Statistical analysis was performed by Student t test and one-way ANOVA for repeated measures. Significance was accepted at P < 0.05.
Results:
The measured REE was significantly higher in males versus females at all time points (P < 0.05). The percent of predicted REE was significantly higher in males versus females during the acute hospitalization, at discharge, 6 and 9 months postburn (P < 0.05). The REE/BMI showed a significant difference between males and females at the acute and discharge time period (P < 0.05). In children 3 to 9.9 years of age, the measured REE and the percent of predicted REE were significantly higher in males versus females during the acute study, at discharge and 6 months postburn (P < 0.05). The measured REE at discharge, 9 and 12 months postburn for children >10 years of age was significantly higher in males compared with females (P < 0.05).
Conclusion:
Data show that female children exert a decreased hypermetabolic response compared with male children, which may improve burn outcomes in females.
Female severely burned children have higher endogenous anabolic hormone levels and a shorter ICU stay compared with males. The purpose of this study was to analyze the influence of age and gender on resting energy expenditure in severely burned children acutely and long-term. Data show that female children exert a decreased hypermetabolic response compared with male children, which may improve burn outcomes in females.
Characteristic for critically ill patients suffering from sepsis, major operations, and severe trauma, including a burn injury is a debilitating hypermetabolism, which is proportional to the degree of the insult.1–3 The hypermetabolic response to a severe burn injury is associated with increased systemic energy expenditure,4,5 severe muscle catabolism and wasting,6–8 and linear growth delays.9 Primary mediators of the hypermetabolic response are pro-inflammatory cytokines and endogenous plasma catecholamines levels,10,11 which increase as much as 10- to 15-fold after the insult.12,13 Hypermetabolism after the burn injury was thought to recede with closure of the burn wound.14,15 However, we have previously shown that pediatric patients with large burns (>40% total body surface area [TBSA] burn) exert muscle protein catabolism for 9 months and growth delays for at least 2 years after injury indicating perseverance of hypermetabolism over the same time period.7,9 The clinical results of this persistent pathophysiologic process is a decrease in lean body mass and severe muscle wasting.
There is evidence that female burned pediatric patients have higher endogenous anabolic hormone levels and had a shorter ICU stay compared with males.16 This study analyzes whether there is an attenuated hypermetabolic response in female patients compared with males. In nonburn healthy children, the resting energy expenditure (REE) is greater in boys when compared with girls,17–21 consistent with gender differences found in adults.22,23 Goran et al have shown that prepubertal boys are more hypermetabolic than girls.18 Whether this age and gender difference is similar in severely burned children has yet to be determined. The aim of this study was to analyze the effect of age and gender on REE in a large group of severely burned children from acute hospitalization through 12 months postburn.
METHODS
Patients and Clinical Care
One hundred pediatric burn patients who underwent acute treatment at the Shriners Hospital for Children, Galveston Burn Hospital from 1998 to 2002 were included into the present study. Only patients with greater than 40% TBSA burn were eligible for enrollment in this study. Burn size was assessed clinically by mapping on Lund and Browder charts24 during excisional surgery in the acute phase of the injury.
Inclusion criteria were age <18 years, TBSA burn of over 40%, acute burn treatment at Shriners Burn Hospital, and consent to return at 6, 9, and 12 months postburn. Patients were excluded if they had one or more of the following: anoxic brain injury, severe psychologic disorders, quadriplegia, or severe behavioral or cognitive disorders.
This study was performed under a University of Texas Medical Branch Institutional Review Board-approved protocol. Informed written consent was obtained from each patient's guardian with assent of the patient before enrollment into the study.
All subjects admitted to the Shriners Burns Hospital for Children were treated in an identical manner by the same team of burn surgeons. Standard treatment included early excision of the burn wound, systemic antibiotic therapy, and continuous enteral feeding. Within 48 hours of admission, each patient underwent total burn wound excision and grafting with autograft skin and allograft. Patients returned to the operating room when autograft donor sites healed and became available for reharvest (usually 6–10 days). Sequential staged surgical procedures for repeat excision and grafting were undertaken until the wounds were healed.
Indirect Calorimetry
As part of our routine clinical practice, all patients underwent REE measurements within 1 week following hospital admission and weekly thereafter during their acute hospitalization. To adjust for the ebb and flow phase of the hypermetabolic response, the first and second metabolic study was averaged and the results were defined as the acute study. This and subsequent measurements of REE were performed between midnight and 5 AM while the patients were asleep and receiving continuous feeding. REE was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA). Subjects were tested in a supine position while under a large, clear, ventilated hood. The REE was calculated from the oxygen consumption and carbon dioxide production by equations described by Weir.25 All REE measurements were made at ambient temperatures of 30°C, which is the standard environmental setting for all patient rooms in our acute burn intensive care unit. The REE measurements were used to guide nutritional management and to assess the level of metabolism. The discharge REE measurement was used to determine the level of hypermetabolism when the burn wounds were 95% healed and were included as part oft his study. Measured values were compared with predicted norms based upon the Harris-Benedict equation26 and corrected by body mass index (BMI). REE studies were repeated at 6, 9, and 12 months postburn when the patients returned for outpatient surgery. Assessments of REE at these time points were completed utilizing the previously described methodology and environmental settings as described above.
The REE was expressed in 3 different ways: actual REE in kcal per day, percent of predicted REE, and actual REE divided by the BMI.
Outcome Measurements and Group Selection
The main outcome measurement was REE as measured by indirect calorimetry. Secondary outcome measurements were age and gender. Patients were initially evaluated as a heterogenous group to determine the level of hypermetabolism over time. Patients were then randomized into groups of females and males to determine if gender had any effect of the level of hypermetabolism. All patients were then stratified for age. The first group included children <3 years of age, the second group included ages 3 to 9.9 years, and the last group included all children from 10 to 18 years of age. Finally, patients were stratified by age and gender to determine differences between males and females at different age groups.
Statistical analyses was performed using paired Student t test to determine differences within groups and unpaired Student t test were done to analyze differences between groups at similar time points. One-way ANOVA for repeated measures with post hoc Bonferroni was used to determine differences over time. Demographic data are expressed as mean ± SD. Metabolic data are expressed as mean ± SEM. Significance was accepted at P < 0.05.
RESULTS
Demographics
A total of 100 severely burned children that returned for clinic visits and had indirect calorimetry measurements were evaluated. Demographics are shown in Table 1. Overall, male patients were significantly older when compared with female patients; however, the severity of the burn was similar in both groups.
TABLE 1. Demographics
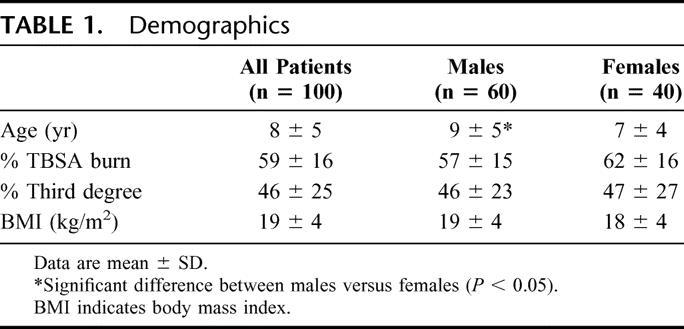
Resting Energy Expenditure Over Time
All Patients
The pattern of the REE over time is depicted in Figure 1. Acute REE and REE at discharge were significantly increased. REE decreased throughout the study period, however, remained elevated 12 months after burn. The percent of predicted REE also showed a significant decrease from the acute study to 6, 9, and 12 months postburn. The normalization of the REE to BMI showed a significant decrease from the acute study at 9 and 12 months postburn. All patients were hypermetabolic at the initial study period relative to the basal metabolic rate predicted by the Harris-Benedict equation, then decreased by 15% to 20%, and remained hypermetabolic throughout the 12-month time frame.
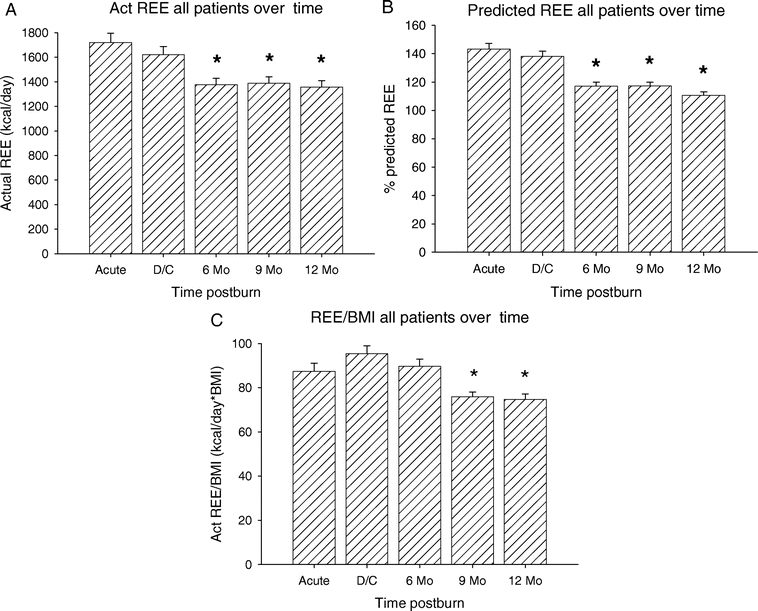
FIGURE 1. Pattern of the REE over time. A, Acute REE and REE at discharge were significantly increased compared with 6, 9, and 12 months. B, Percent of predicted REE showed a significant decrease from the acute study to 6, 9, and 12 months postburn. C, The normalization of the REE to body mass index (BMI) showed a significant decrease from the acute study at 9 and 12 months postburn. *Significant difference compared with acute and discharge (P < 0.05).
Resting Energy Expenditure Over Time
Females Versus Males
The patient population was then stratified for gender (Figure 2). The measured REE was significantly higher in males versus females at all time points (P < 0.05) (Fig. 2A). Similarly to the measured REE, the percent predicted REE showed a significant difference between male and females during the acute study, at discharge, 6 and 9 months postburn, with the males being more hypermetabolic than females (P < 0.05) (Fig. 2B). Normalization of the REE to BMI showed a significant difference between males and females from acute to 9 months postburn with males having a significantly higher REE/BMI index compared with females (P < 0.05) (Fig. 2C).
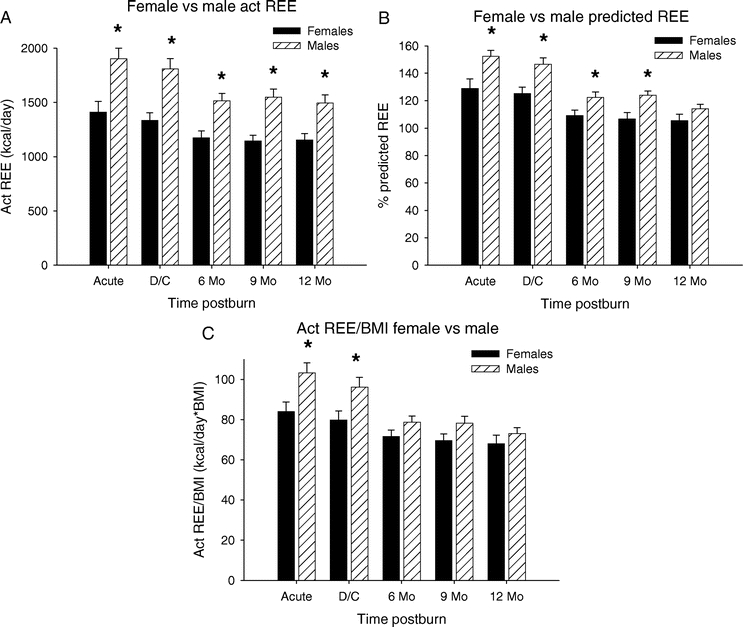
FIGURE 2. Resting energy expenditure over time: females versus males. A, The measured REE was significantly higher in males versus females at all time points. B, Percent predicted REE showed a significant difference between males and females during the acute study, at discharge, 6 and 9 months postburn. C, Normalization of the REE to BMI showed a significant difference between males and females from acute to 9 months postburn. *Significant difference compared with acute and discharge (P < 0.05).
Resting Energy Expenditure
All Patients Stratified for Age
All patients were divided into 3 age groups: 0 to 2.9, 3 to 9.9, and 10 to 18 years. The measured REE for patients <3 years of age were significantly lower at all time points when compared with the patients from 10 to 18 years and at acute, discharge, and at 12 months postburn compared with 3 to 9.9 years (P < 0.05) (Fig. 3A). Additionally, there was a significant difference between patients in the 3- to 9.9-year age group compared with the 10- to 18-year age group at all time points with the 3- to 9.9-year-old being less hypermetabolic compared with the 10- to 18-year-old patients (P < 0.05) (Fig. 3A). The percent predicted REE for patients in the <3-year age group was significant lower when compared with the 10- to 18-year group during the acute study and upon discharge (P < 0.05) (Fig. 3B). Normalization of the REE to the BMI showed that children <3 years of age had the lowest REE/BMI index at all time points compared with 10 to 18 years and at acute, discharge, and at 12 months compared with the 3 to 9.9 years (P < 0.05) (Fig. 3C). Children 3 to 9.9 years of age had lower REE/BMI index acutely, at discharge and 6 months postburn compared with 10- to 18-years of age (P < 0.05) (Fig. 3C).
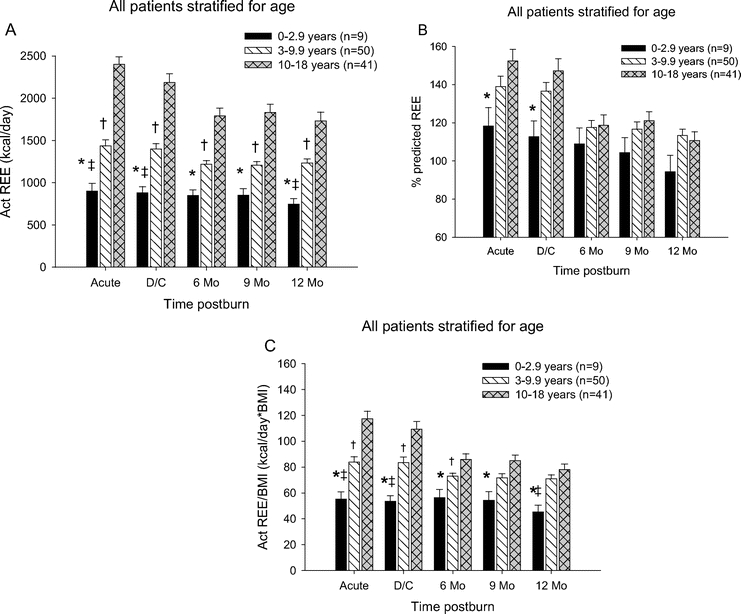
FIGURE 3. Resting energy expenditure patients stratified for age. A, The measured REE for patients <3 years of age were significantly lower at all time points when compared with the patients from 10 to 18 years and at acute, discharge and at 12 months postburn compared with 3 to 9.9 years. There was a significant difference between patients in the 3- to 9-year age group compared with the 10- to 18-year age group at all time points. B, Percent predicted REE for patients in the <3-year age group was significantly lower compared with the 10- to 18-year group during the acute study and upon discharge. C, Children <3 years of age had the lowest REE/BMI index at all time points compared with 10 to 18 years and at acute, discharge and at 12 months compared with the 3 to 9.9 years. Children 3 to 9.9 years had lower REE/BMI index acutely, at discharge and 6 months postburn compared with 10 to 18 years. *Significant difference between 0 to 2.9 years and 10 to 18 years (P < 0.05). †Significant difference between 3 to 9.9 compared with 10 to 18 years (P < 0.05). ‡Significant difference between 0 to 2.9 compared with 3 to 9.9 years (P < 0.05).
Females Stratified by Age
We determined the REE in the female children and stratified these patients into the 3 different age groups. The measured REE was significantly lower in the <3-year age group compared with the to the 10- to 18-year age group at all time periods (P < 0.05). There was no difference between <3 compared with 3- to 9.9-year-old ones. The measured REE for patients in the 3- to 9.9-year age group versus the 10- to 18-year-olds showed a significant difference in REE acutely, at discharge and at 6 months postburn (P < 0.05) (Fig. 4A). The percent predicted REE for patients in the <3-year age group compared with the 3- to 9.9-year age group showed no significant differences. The percent of predicted REE in the <3-year age group compared with the 10- to 18-year age group showed a significant difference at acute and at discharge (P < 0.05). For patients in the 3- to 9.9-year age group compared with the 10- to 18-year age group, the percent of predicted REE was significantly different only during the acute study (P < 0.05). Normalization of the REE to BMI showed no significant difference in the <3-year age group compared with the 3- to 9.9-year age group (Fig. 4C). When the <3-year age group was compared with the 10- to 18-year age group, the REE/BMI index was significantly different during the acute study and at discharge (P < 0.05). The age group 3 to 9.9 years of age had a significantly lower REE/BMI index acutely when compared with females 10 to 18 years of age (P < 0.05) (Fig. 4C).
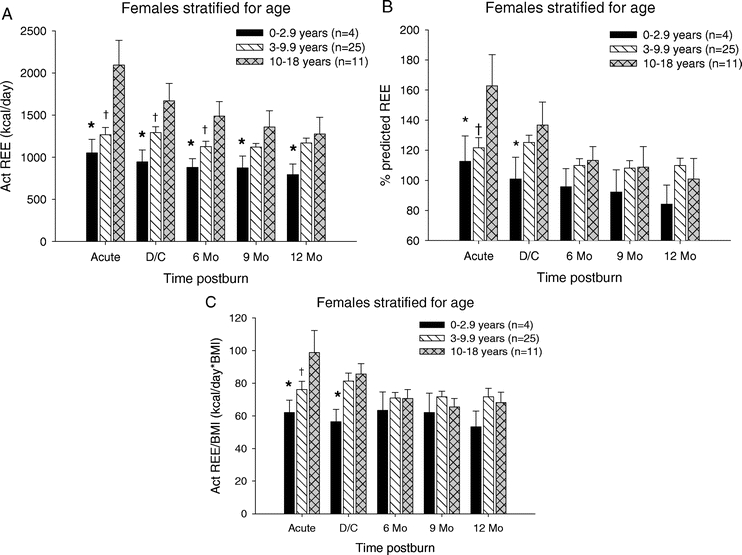
FIGURE 4. Resting energy expenditure females stratified by age. A, REE was significantly lower in the <3-year age group compared with the 10- to 18-year age group at all time periods. REE for patients in the 3- to 9.9-year age group versus the 10- to 18-year age group showed a significant difference in REE acutely, at discharge and at 6 months postburn. B, Percent predicted REE in the <3-year age group compared with the 10- to 18-year age group showed a significant difference at acute and at discharge. In the 3- to 9.9-year age group compared with the 10- to 18-year age group, percent predicted REE was significantly different only during the acute study. C, In the <3-year age group, REE/BMI was significantly lower during the acute study and at discharge compared with the 10- to 18-year age group. The age group 3 to 9.9 years had a significantly lower REE/BMI index acutely when compared with females 10 to 18 years. *Significant difference between 0 to 2.9 years and 10 to 18 years (P < 0.05). †Significant difference between 3 to 9.9 compared with 10 to 18 years (P < 0.05). ‡Significant difference between 0 to 2.9 compared with 3 to 9.9 years (P < 0.05).
Males Stratified by Age
We determined the REE in the male children and stratified these patients into the 3 different age groups (Figure 5). The measured REE for male children <3 years of age was significantly lower at all study periods when compared with the other 2 age groups (P < 0.05) (Fig. 5A). Additionally, there was a significant difference between the 3- to 9-year age group and the 10- to 18-year age group at all time periods (P < 0.05) (Fig. 5A). The percent predicted REE was significantly lower in the <3-year age group compared with the 10- to 18-year age group at discharge (P < 0.05) (Fig. 5B). Similar to the measured REE, the REE/BMI index showed that male children <3 years of age had significantly lower REE/BMI at all study periods when compared with the other 2 groups (P < 0.05) (Fig. 5C). Additionally, the REE/BMI index was significantly different between the 3- to 9-year age group and the 10- to 18-year age group from the acute study through 9 months postburn (P < 0.05) (Fig. 5C).
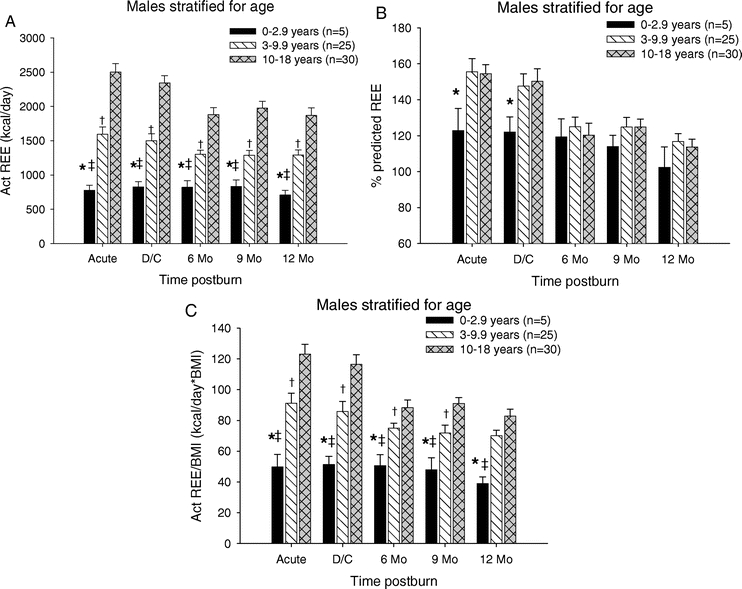
FIGURE 5. Resting energy expenditure males stratified by age. A, REE for male children <3 years of age was significantly lower at all study periods when compared with the other 2 age groups. In the 3- to 9-year age group, the REEE was significantly decreased compared with 10- to 18-year age group at all time periods. B, Percent predicted REE was significantly lower in the <3-year age group compared with the 10- to 18-year age group at discharge. C, REE/BMI index showed that male children <3 years of age had significantly lower REE/BMI at all study periods when compared with the other 2 groups. Index was also lower in the 3- to 9-year age group compared with the 10- to 18-year age group from the acute study through 9 months postburn. *Significant difference between 0 to 2.9 years and 10 to 18 years (P < 0.05). †Significant difference between 3 to 9.9 compared with 10 to 18 years (P < 0.05). ‡Significant difference between 0 to 2.9 compared with 3 to 9.9 years (P < 0.05).
Females Versus Males 3 to 9 Years of Age
The relationship between REE and gender for children in the 3- to 9-year age group is summarized in Figure 6. The measured REE was significantly higher in males during the acute study period, at discharge and 6 months postburn (P < 0.05) (Fig. 6A). Similar to the measured REE, the percent predicted REE was significantly higher in males during the acute study, at discharge and 6 months postburn (P < 0.05) (Fig. 6B). The REE/BMI index showed no significant difference between males and females in this age group (Fig. 6C).
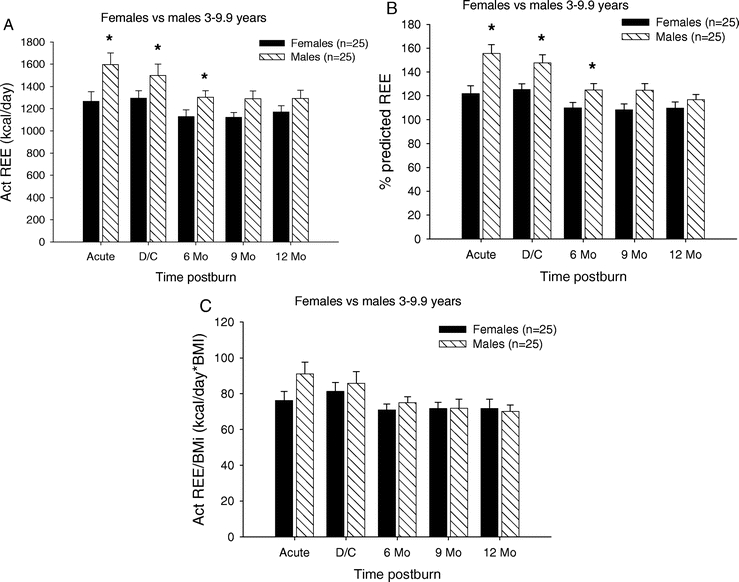
FIGURE 6. Resting energy expenditure (females vs. males 3–9 years). A, REE was significantly higher in males during the acute study period, at discharge and 6 months postburn. B, Percent predicted REE was significantly higher in males during the acute study, at discharge and 6 months postburn. C, The REE/BMI index showed no significant difference between males and females in this age group. *Significant difference between females versus males (P < 0.05).
Females Versus Males 10 to 18 Years of Age
The relationship between REE and gender for children in the 10- to 18-year age group are summarized in Figure 7. The measured REE was significantly higher in males at discharge and at 9 and 12 months postburn when compared with females (P < 0.05) (Fig. 7A). The percent predicted REE showed no significant difference between males and females at any study period in this age group. The REE/BMI index showed a significant difference between males and females at the 9- and 12-month time period, with males having a higher index compared with females (P < 0.05) (Fig. 7C).
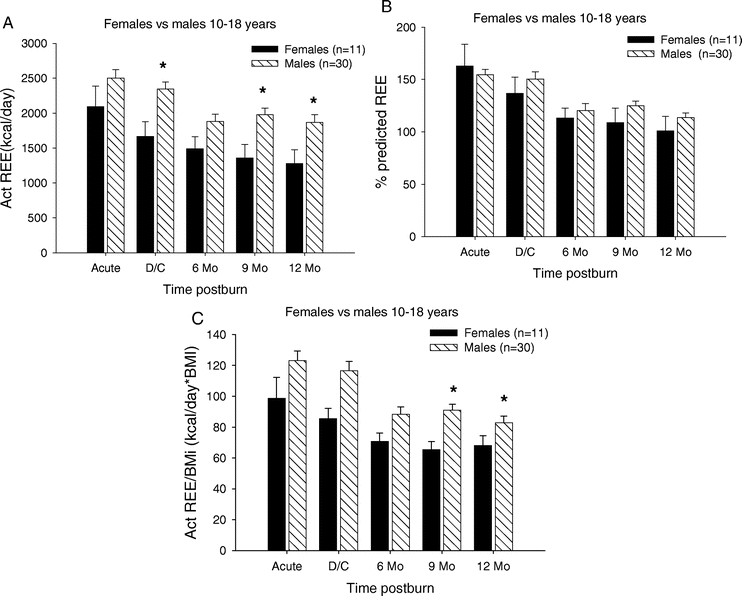
FIGURE 7. Resting energy expenditure females versus males 10 to 18 years. A, REE was significantly higher in males at discharge and at 9 and 12 months postburn when compared with females. B, Percent predicted REE showed no significant difference between males and females at any study period in this age group. C, The REE/BMI index showed a significant difference between males and females at the 9- and 12-month time period. *Significant difference between females versus males (P< 0.05).
DISCUSSION
Hypermetabolism after a major operation or injury during acute hospitalization leads to protein degradation followed by increased incidence of infection or even multiorgan failure.27 In addition, long-term, hypermetabolism leads to growth arrest and failure for bone synthesis.28 Hypermetabolism was thought to persist only over a short period. In the present study, we found that hypermetabolism persists at least over a period of 12 months after burn injury. The increased REE persistence needs to be investigated in a more long-term study that will address the REE over 24 months.
Clinically, it would be ideal to attenuate hypermetabolism and improve the acute and long-term outcome. One feasible approach is the application of anabolic agents, such as recombinant human growth hormone, insulin-like growth factor-1, or insulin. To avoid unnecessary intervention, one aim of the present study was to identify pediatric patients, age and gender related, who are hypermetabolic and hence profit from drug treatment. First, we divided our patients into male and females. We found that pediatric males exert a significantly higher REE up to 12 months postburn when compared with females. This is in concert with a study by Goran et al in which the authors found that girls have a lower REE compared with boys.18 The underlying mechanisms are not clear, and we asked whether this is an age-dependent or hormone-dependent phenomenon. By stratifying all patients into 3 age groups, we found that children >10 years of age had a significant higher REE compared with younger children. Additionally, children <3 years of age demonstrated only a slight increase in REE. The REE/BMI index stratified by age demonstrated significant differences between groups. Children <3 years of age had significantly lower REE/BMI indexes when compared with older children. Similarly, children 3 to 9.9 years of age had significantly lower REE/BMI indexes when compared with children >10 years of age up to 9 months postburn. Our results indicate the older children have higher REE/BMI indices than younger children. Stratification by age and gender revealed that males and females <3 years of age demonstrate no significant differences in REE, percent of predicted REE or REE/BMI index. Males 3 to 9.9 years of age demonstrated significantly higher REE and percent of predicted REE up to 6 months postburn compared with females. In children >10 years of age, males demonstrated significantly higher REEs at discharge, 9 and 12 months postburn. Additionally, males demonstrated higher REE/BMI indexes at 9 and 12 months postburn when compared with females.
One major finding of the present study is that infants and toddlers do not undergo hypermetabolism and that there is no difference between males and females. In the age group 10 to 18 years, both sexes are hypermetabolic but males demonstrated a significant increased REE at discharge, 9 and 12 month. These data indicate that estrogens do not play a crucial role in attenuating hypermetabolism. However, there are several studies showing gender differences in the REE. In nonburn healthy children the REE is greater in boys when compared with girls,17–21 consistent with gender differences found in adults.22,23 Goran et al have shown that prepubertal boys are more hypermetabolic than girls.18
One criticism of the present study could be the heterogenicity of our patient population. We did not differentiate between American, white, Hispanic, or black patients. In prepubertal and pubertal age children, ethnic differences of the components of energy expenditure have been identified.29–32 However, several investigators have not found an ethnic difference in REE.18,33,34 The previous studies focused on black and white children. Since most of our patients are of Hispanic origin, it was not the intent to stratify patients into ethnic categories. Therefore, the initial design of our study was to stratify for gender and age and not ethnicity.
Another criticism could be the way we determined the predicted REE. The Harris Benedict Formula predicts the REE and is a well-established method in critically ill patients, however, whether this formula is accurate in children in not clear. Therefore, we attempted to normalize the actual REE to the BMI, which is also not established, and it is also not evident whether the normalization of the REE to BMI is appropriate. However, as we observed the same pattern in the 3 methods used, we think that the data demonstrate differences between different ages and gender independent from the method used. Based on our results, we would recommend to present metabolic data using these 3 different methods because the validity is much stronger.
In the present study, our data show that female children exert a decreased hypermetabolic response compared with male children, which could explain the improved outcome and higher endogenous hormone levels observed in female children. The mechanisms by which females have a lower REE are not defined and cannot be extrapolated from the present study. However, our data identify male children as an important patient population for drug intervention to attenuate hypermetabolism. As female and male teenagers have a high REE these patients are also identified to possibly benefit from anti-inflammatory and anabolic treatment to improve their outcome.
Footnotes
Supported by a grant from the National Institute for Disabilities and Rehabilitation Research (No. H133A70019) and a grant from the National Institutes of Health (No. P50-GM60338).
Reprints: Ronald P. Mlcak, PhD, Shriners Hospital for Children, Galveston Burns Unit, 815 Market Street, Galveston, TX 77550. E-mail: rmlcak@utmb.edu.
REFERENCES
- 1.Seashore JH, Seashore MR. Protein requirements of infants receiving total parental nutrition. J Pediatr Surg. 1976;11:645. [DOI] [PubMed] [Google Scholar]
- 2.Rubecz I, Mestyan P, Varga P, et al. Energy metabolism, substrate utilization, and nitrogen balance in parentally fed postoperative neonates and infants: the effects of glucose, glucose + amino acids, lipid + amino acids infused in isocaloric amounts. J Pediatr. 1981;98:42. [DOI] [PubMed] [Google Scholar]
- 3.Mickell JJ. Urea nitrogen excretion in critically ill children. Pediatrics. 1981;70:949. [PubMed] [Google Scholar]
- 4.Milner EA, Cioffi WG, Mason AD, et al. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma. 1994;37:167–170. [DOI] [PubMed] [Google Scholar]
- 5.Ireton-Jones CS, Turner WW Jr, Baxter CR. The effect of burn wound excision on measured energy expenditure and urinary nitrogen excretion. J Trauma. 1987;27:217–220. [DOI] [PubMed] [Google Scholar]
- 6.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720; discussion 720–712. [DOI] [PMC free article] [PubMed]
- 7.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. [DOI] [PubMed] [Google Scholar]
- 8.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. [DOI] [PubMed] [Google Scholar]
- 10.Harrison TS, Seaton JF, Feller I. Relationship of increased oxygen consumption to catecholamine excretion in thermal burns. Ann Surg. 1967;165:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilmore DW, Long JM, Mason AD Jr, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodall MC, Stone C, Hays BW Jr. Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957;145:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1187. [DOI] [PubMed] [Google Scholar]
- 14.Moore FD. Response to starvation and stress. In: Moore FD, ed. Metabolic Care of the Surgical Patient. Philadelphia: Saunders, 1959:222–275. [Google Scholar]
- 15.Warden GD, Heimbach DC. Burns. In: Schwartz SI, ed. Principles of Surgery. New York: McGraw-Hill, 1999:232–254. [Google Scholar]
- 16.Jeschke MG, Barrow RE, Mlcak RP, et al. Endogenous anabolic hormones, effects of trauma and gender. Ann Surg. 2005;241:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontvieille AM, Dwyer J, Ravussin E. Resting metabolic rate and body composition of Pima Indian and caucasian children. Int J Obes. 1992;16:535–542. [PubMed] [Google Scholar]
- 18.Goran MI, Kaskoun M, Johnson R. Determinants of resting energy expenditure in young children. J Pediatr. 1994;125:362–367. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths M, Payne PR, Stunkard AJ, et al. Metabolic rate and physical development in children at risk of obesity. Lancet. 1990;336:76–78. [DOI] [PubMed] [Google Scholar]
- 20.Maffeis C, Schultz Y, Micciolo R, et al. Resting metabolic rate in six- to ten year old obese and non-obese children. J Pediatr. 1993;122:556–562. [DOI] [PubMed] [Google Scholar]
- 21.Molnar D, Schultz Y. The effects of obesity, age, puberty and gender on resting metabolic rate in children and adolescents. Eur J Pediatr. 1997;156:376–381. [DOI] [PubMed] [Google Scholar]
- 22.Arciero PJ, Goram MI, Poehlman ET. Resting metabolic rate is lower in women compared to men. J Appl Physiol. 1993;75:2514–2520. [DOI] [PubMed] [Google Scholar]
- 23.Ferraro RT, Lillioja S, Fontvieille AM, et al. Lower sedentary metabolic rate in women compare to men. J Clin Invest. 1992;90:780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lund C, Browder NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79. [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with specific reference to protein metabolism. J Physiol. 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Carnegie Institution. 1919. [Google Scholar]
- 27.Low JFA, Barrow RE, Mittendorfer B, et al. The effects of short-term growth hormone on growth and energy expenditure in burned children. Burns. 2001;27:447–452. [DOI] [PubMed] [Google Scholar]
- 28.Low JFA, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3 year follow-up. Lancet. 1999;354:1789. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan AS, Zemel BS, Stalling VA. Differences in resting energy expenditure in prepubertal black and white children. J Pediatr. 1996;129:643–647. [DOI] [PubMed] [Google Scholar]
- 30.Morrison JA, Alfaro MP, Khoury P, et al. Determinants of resting energy expenditure in young black girls and young white girls. J Pediatr. 1996;129:637–642. [DOI] [PubMed] [Google Scholar]
- 31.Yanovski SZ, Reynolds J, Boyle AJ, et al. Resting metabolic rate in African American and Caucasian children. Obes Res. 1997;5:321–325. [DOI] [PubMed] [Google Scholar]
- 32.Wong WW, Butte NF, Ellis KJ, et al. Pubertal African American girls expend less energy at rest and during physical activity than Caucasian girls. J Clin Endocrinol Metab. 1999;84:906–911. [DOI] [PubMed] [Google Scholar]
- 33.Davies PS, Day JME, Lucas A. Energy expenditure in early infancy and later body fatness. J Obes. 1991;15:727–731. [PubMed] [Google Scholar]
- 34.Sun M, Gower BA, Nagy TR, et al. Total, resting, and activity related energy expenditure's are similar in Caucasian and African-American children. Am. J Physiology. 19918;274:E232–E237. [DOI] [PubMed]


