Abstract
Objective:
The aim of this study was designed to determine whether the period of drain insertion influences the incidence of postoperative complications.
Background Data:
The significance of prophylactic drains after pancreatic head resection is still controversial. No report discusses the association of the period of drain insertion and postoperative complications.
Methods:
A total of 104 consecutive patients who underwent pancreatic head resection were enrolled in this study. To assess the value of prophylactic drains, we prospectively assigned the patients into 2 groups: group I underwent resection from January 2000 to January 2002 (n = 52, drain to be removed on postoperative day 8); group II underwent resection from February 2002 to December 2004 (n = 52, drain to be removed on postoperative day 4). Postoperative complications in the 2 groups were compared.
Results:
The rate of pancreatic fistula was significantly lower in group II (3.6%) than in group I (23%) (P = 0.0038). The rate of intra-abdominal infections, including intra-abdominal abscess and infected intra-abdominal collections, was significantly reduced in group II (7.7%) compared with group I (38%) (P = 0.0003). Eighteen of 52 (34.6%) patients in group I had an inserted drain beyond 8 days, whereas only 2 of 52 (3.7%) patients in group II had an inserted drain beyond 4 days (P = 0.0002). Cultures of drainage fluid were positive in 16 of 52 (30.8%) patients in group I, and in 2 of 52 (3.7%) patients in group II (P = 0.0002). Intraoperative bleeding (>1500 mL), operative time (>420 minutes, and the period of drain insertion were significant risk factors for intra-abdominal infections (P = 0.043, 0.025, 0.0003, respectively). The period of drain insertion was the only independent risk factor for intra-abdominal infections by multivariate analysis (odds ratio, 6.7).
Conclusion:
Drain removal on postoperative day 4 was shown to be an independent factor in reducing the incidence of complications with pancreatic head resection, including intra-abdominal infections.
This study was prospectively designed to determine whether the period of drain insertion influences the incidence of postoperative complications in patients with pancreatic head resection. Drain removal on postoperative day 4 was shown to be an independent factor in reducing the incidence of complications with pancreatic head resection, including intra-abdominal infections.
Randomized clinical trials have demonstrated that prophylactic drains have not decreased the incidence of postoperative complications in elective hepatectomy, colectomy, and cholecystectomy.1–5 Yet, the significance of prophylactic drains after pancreatic head resection is still unclear. Only 1 randomized controlled trial in pancreatic surgery has suggested that routine drain insertion is unnecessary as drain insertion, compared with no drains, failed to reduce postoperative complications.6 However, the morbidity rate after pancreatic head resections still remains high in the range of 30% to 65%, although the mortality rate has decreased to less than 5% by recent advances in surgical techniques and perioperative management.7–15
Prophylactic drains after pancreatic head resection allow monitoring of the occurrence of intra-abdominal bleeding, as well as the detection and drainage of the pancreatic fistula.16,17 Therefore, prophylactic drains usually are inserted after pancreatic head resection, even in high-volume centers of pancreatic surgery, and usually are removed around postoperative day (POD) 7.9,13,18 It has been considered that surgically placed drains provide a risk of intra-abdominal infections by providing a route for ascending infections. To our knowledge, no reports in the literature have evaluated the association between the period of drain insertion and intra-abdominal infections after pancreatic head resection. We conducted this prospective study to determine whether the period of drain insertion influences the incidence of postoperative complications.
METHODS
Patients
From January 2000 to December 2004, 104 consecutive patients underwent pancreatic head resection at Wakayama Medical University Hospital (WMUH) by 2 pancreatic surgeons (H.Y. with 25 years experience, M.T. with 20 years experience). We prospectively assigned the patients into 2 groups (by periods of time) to assess the value of prophylactic drains. In the period January 2000 to January 2002, the drains were to be removed on POD 8. Then, from February 2002 to December 2004, the drains were to be removed on POD 4 to determine whether the incidence of intra-abdominal infections could be reduced by early removal of drains, thereby inhibiting ascending infections. The protocol and study design of the present trial were approved and conducted according to the guidelines of the Ethical Committee of WMUH.
Preoperative biliary drainage was performed by percutaneous transhepatic catheter drainage (PTCD) or endoscopic nasal biliary drainage (ENBD), when the serum levels of total bilirubin were greater than 5 mg/mL, or dilatation of the intrahepatic bile duct and hepatic dysfunction (transaminase, >100 IU/mL) were detected. Patient characteristics and perioperative and postoperative parameters in the 2 groups were reviewed for the following 21 clinical variables: patient age, gender, preoperative serum level of albumin, total bilirubin and amylase, history of jaundice, history of diabetes mellitus, preoperative biliary drainage, type of resection; pancreaticoduodenectomy (PD) or pylorus-preserving pancreaticoduodenectomy (PpPD), operative time, intraoperative bleeding, red blood cell transfusion, pancreatic texture (soft or hard), presence or absence of dilatation of the main pancreatic duct, histologic diagnosis (malignant or benign), degree of lymph node dissection (D1 or D2), intended period of drain insertion (POD 4 or 8), serum amylase level on POD 1 and 4, and amylase level of drainage fluid on POD 1 and 4.
Perioperative Management
Twenty patients received PD with Child reconstruction, and 84 patients received PpPD with Traverso reconstruction. Pancreatic anastomosis after PD and PpPD was performed by duct-to-mucosal, end-to-side pancreaticojejunostomy in all enrolled patients. External suture rows were performed as a single suture between the remnant pancreatic capsule, parenchyma, and jejunal seromuscular by using an interrupted sutured of 4-0 Novafil (polybutester, Tyco Healthcare Co.). Internal suture rows, duct-to-mucosa, were performed between the pancreatic ductal and jejunal mucosa by using 8 interrupted 5-0 PDS-II (polydioxanone, Johnson and Johnson Co.). A 5-French polyethylene pancreatic duct drainage tube (Sumitomo Bakelite Co.) was used in all patients. A stent placed in the pancreatic duct removed on POD 21, and the timing of stent removal was the same for both groups. No stent was used for the biliary anastomosis. Two intra-abdominal drains routinely were placed near the pancreatic and biliary anastomosis. One 10-mm Penrose drain, a silicon, multitubular flat drain, routinely was placed near the pancreaticojejunostomy, and a 10-mm silicon tube drain (Kaneka Medics Co.) was routinely placed near the cholangiojejunostomy. These drains were connected to a closed drainage system. The collection system was changed to new one daily to prevent contamination of drain system, and it was identical over the 4 years of this study. The drains were to be removed on POD 8 in group I and on POD 4 in group II, when the drainage fluid was clear, and pancreatic fistula and bacterial contamination were absent. Dilatation of the pancreatic duct was defined as a diameter greater than 3 mm and was judged intraoperatively. The amylase of serum and drainage fluid was measured on POD 1 and 4. Drainage fluid on POD 7 in group I and on POD 4 in group II was cultured in all patients.
No patient received radiotherapy preoperatively and postoperatively. All patients received prophylactic antibiotics intraoperatively and for 3 days postoperatively. Prophylactic octreotide was not administered to prevent pancreatic fistula.
Postoperative Complications
Pancreatic fistula was defined as more than 50 mL drainage fluid per day with 3-fold serum amylase level on POD 4. An international study group of pancreatic surgeons (ISGPF) has proposed a consensus definition and clinical grading about postoperative pancreatic fistula, and defined as follows: grade A, called “transient fistula,” it has no clinical impact; grade B, required a change in management or adjustment in the clinical pathway; grade C, a major change in clinical management or deviation from the normal clinical pathway.19 Biliary fistula was defined as the presence of bile in drainage fluid that persisted by POD 4. Intra-abdominal abscess was defined as intra-abdominal fluid collection with positive cultures identified by ultrasonography (US) or computed tomography (CT) associated with persistent fever and elevations of white blood cells. Infected intra-abdominal fluid was defined as drainage fluid having a positive culture with clinical signs, but without detected intra-abdominal abscess. Delayed gastric emptying was defined as output from a nasogastric tube of greater than 500 mL per day that persisted beyond POD 10, or the failure to maintain oral intake by POD 14.
Statistical Analysis
The study design to predict the number of patients necessary for statistical validity (two-sided) was based on the premise of improving intra-abdominal infection rate from 35% to 10%, with the α set at 0.05 and the β set at 0.2, yielding a power of 80%.6,9,17 We calculated that 52 patients were required in each arm of this study, for a total study population of 104 patients. Data were expressed as means ± SD. Patient characteristics and perioperative and postoperative factors between 2 groups were compared by using χ2 statistics, Fisher exact test, and Mann-Whitney U test. Variables with P < 0.100 were entered into a logistic regression model to determine independent risk factors of postoperative complications. The independent risk factors of the variables were expressed as odds ratios with their 95% confidence intervals. Statistical significance was defined as P < 0.05.
RESULTS
Comparison of Histologic Diagnosis, Patient Characteristics, and Perioperative Status
A total of 104 patients who underwent pancreatic head resection were divided into 2 groups: 52 patients in group I (drain to be removed on POD 8) and 52 in group II (drain to be removed on POD 4).
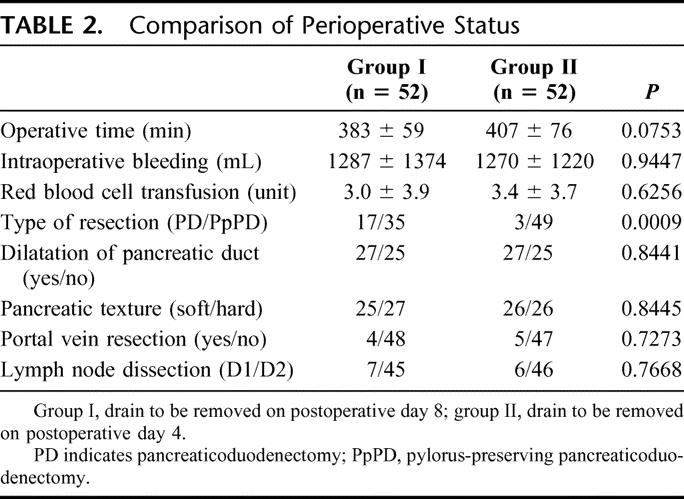
Table 1 shows the results of histologic analysis of the resected specimens, patient characteristics, and preoperative status. There was no significant difference concerning malignant (group I, n = 37; group II, n = 43) and benign tumors (group I, n = 15; group II, n = 9) between the 2 groups. There were no significant differences between the 2 groups concerning other background data.
TABLE 1. Characteristics of Enrolled Patients
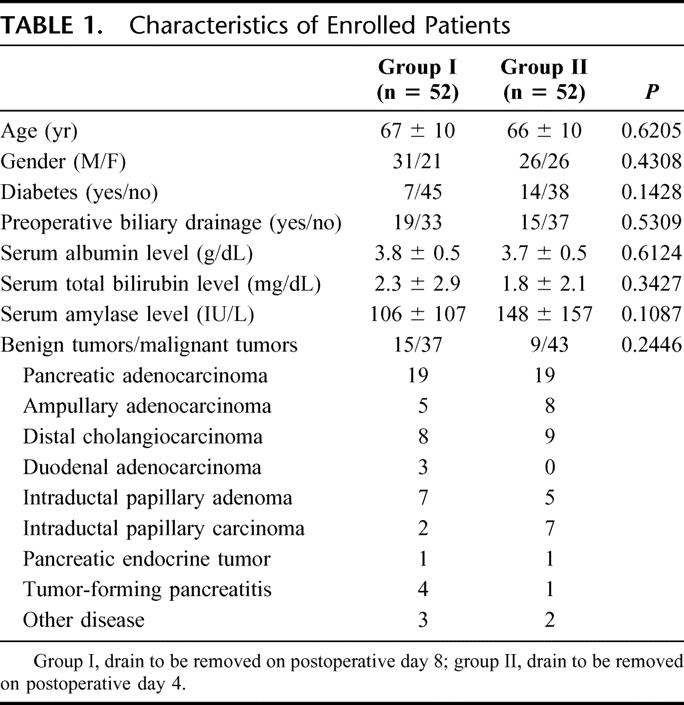
Table 2 shows the results of perioperative status. There were no significant differences between the 2 groups concerning perioperative status except for the type of resection.
TABLE 2. Comparison of Perioperative Status
Postoperative Changes in Amylase Level of Serum and Drainage Fluid
Table 3 shows the fluctuation of amylase levels in serum and drainage fluid. The serum amylase level on POD 4 in group I and group II was restored to the normal range. The mean amylase level of drainage fluid on POD 1 was higher than on POD 4 in group I and group II (1982 ± 3374 IU/L vs. 603 ± 1604 IU/L; 2025 ± 4448 IU/L vs. 700 ± 2047 IU/L) (P = 0.0267 and .0342, respectively).
TABLE 3. Amylase Levels in Serum and Drainage Fluid
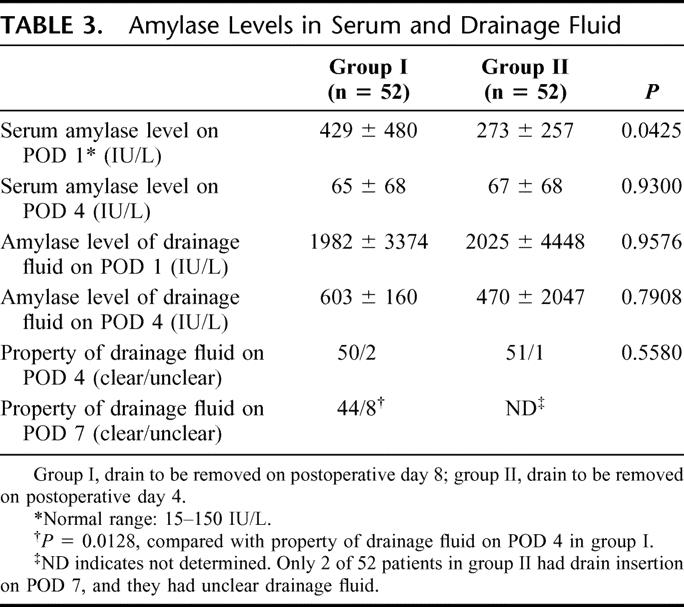
The drainage fluid on POD 4 was clear in 50 of 52 (96%) of group I and 51 of 52 (98%) of group II patients (P = 0.5580). However, the drainage fluid on POD 7 had become unclear, compared with drainage fluid on POD 4 in group I (15% vs. 4%, P = 0.0128).
Postoperative Changes in Leukocyte Counts and C-Reactive Protein Level
Postoperative changes in leukocyte counts and C-reactive protein (CRP) level were analyzed in both groups. Leukocyte counts on POD 7 in group I were significantly higher than those in group II (P = 0.011), whereas there was no difference in either group on POD 1 and 4. Leukocyte counts in group I were lower on POD 4 than on POD 1 (7944 ± 2729/mm3 vs. 10,596 ± 3024/mm3, P = 0.0001); however, the counts in group I increased again on POD 7 (9325 ± 3419/ mm3) (P = 0.013), whereas in group II leukocyte counts on POD 7 were similar to those on POD 4. On the other hand, there was no significant difference in CRP levels on POD 1, 4, and 7 between the 2 groups.
Comparison of Postoperative Status and Complications
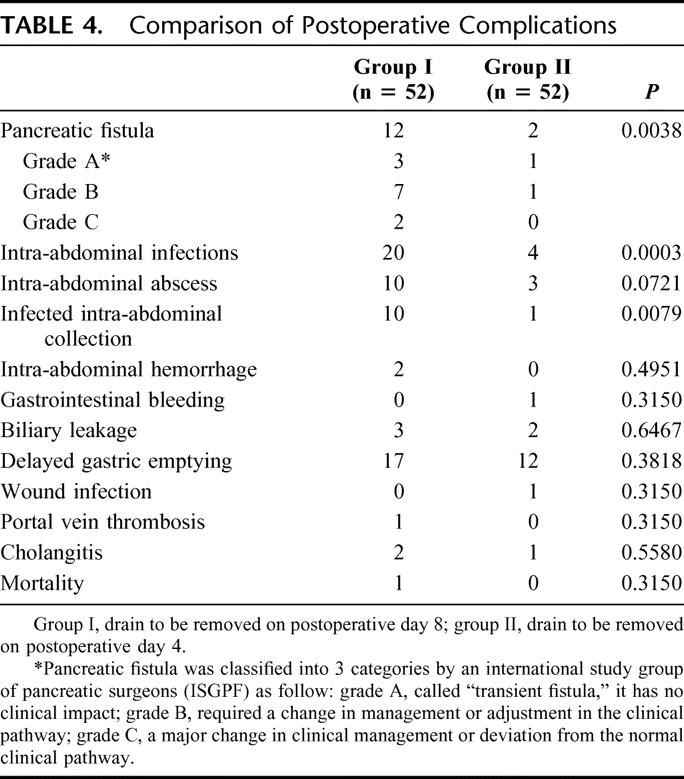
Postoperative complications were compared with clarify whether the period of drain insertion influenced postoperative complication rates (Table 4). The overall rate of pancreatic fistula after pancreatic head resection was 13% (14 of 104 patients); the rate of pancreatic fistula was significantly lower in group II (3.6%) than group I (23%) (P = 0.0038). There were no patients in either group with major leakage of pancreaticojejunostomy, which was defined as leakage of pancreatic juice with contamination of bile or intestinal contents. Pancreatic fistula was classified into 3 categories by ISGPF, and 12 patients of pancreatic fistula in group I were classified into grade A (n = 3), grade B (n = 7), grade C (n = 2), and 2 patients in group II into grade A (n = 1), grade B (n = 1) (Table 4). There were no significant differences between the 2 groups with regard to hemorrhage and DGE. Most importantly, the rate of intra-abdominal infections, including infected intra-abdominal collections and intra-abdominal abscess, was significantly lower in group II than group I (7.7% vs. 38%, P = 0.0003). The rate of infected intra-abdominal collections was significantly reduced in group II, compared with group I (1.9% vs. 19%, P = 0.0079).
TABLE 4. Comparison of Postoperative Complications
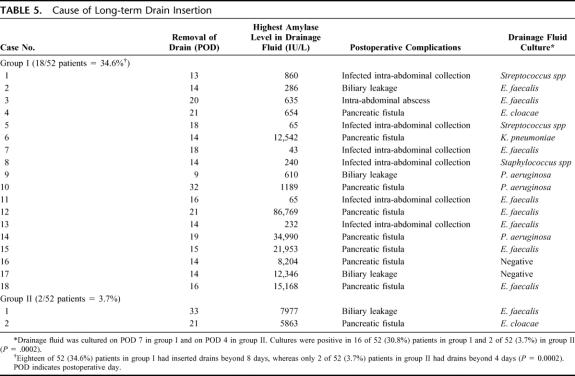
Cause of Long-term Drain Insertion
Eighteen of 52 (34.6%) patients in group I had inserted drains beyond 8 days, whereas only 2 of 52 (3.7%) patients in group II had inserted drains beyond 4 days (P = 0.0002). Table 5 shows the details of all patients with inserted drains beyond 8 days in group I and 4 days in group II. The period of drain insertion in 18 patients was 17 ± 5 days in group I (range, 12–32 days) because of 8 pancreatic fistula, 3 biliary leakage, and 7 intra-abdominal infections (6 infected intra-abdominal collections, 1 intra-abdominal abscess). Positive culture of drainage fluid was 16 of 18 (94%) cases. The period of drain insertion in 2 patients was 15 ± 1 day in group II (range, 14–16 days) because of 1 pancreatic fistula and 1 biliary leakage. Cultures of drainage fluid were positive in both patients (100%). Cultures of drainage fluid were positive in 16 of 52 patients (30.8%) in group I, and in 2 of 52 patients (3.7%) in group II (P = 0.0002).
TABLE 5. Cause of Long-term Drain Insertion
Risk Factors of Intra-abdominal Infections
Univariate and multivariate analysis were used to reveal risk factors for intra-abdominal infections. Table 6 shows the results of 20 parameters univariately examined as potential risk factors for the 24 patients with intra-abdominal infections versus the 80 patients without intra-abdominal infections. Two intraoperative factors (intraoperative bleeding, >1500 mL, P = 0.043; operative time, >420 minutes, P = 0.025) and 1 postoperative factor (intended period of drain insertion: 4 days vs. 8 days, P = 0.0003) differed significantly between these 2 groups. No significant preoperative factors, including preoperative biliary drainage, were associated with the incidences of postoperative intra-abdominal infections.
TABLE 6. Univariate Analysis of Risk Factors Influencing Intra-abdominal Infection
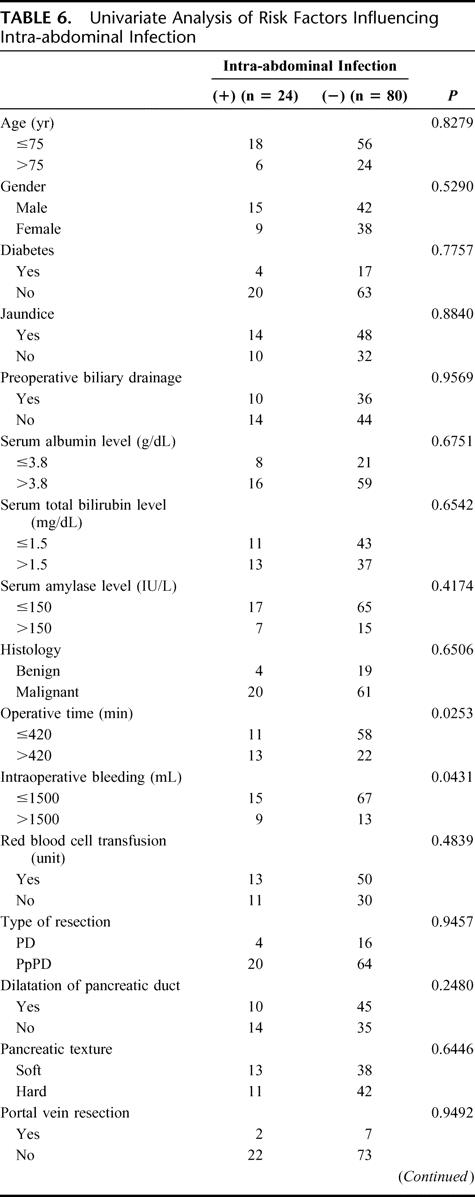
TABLE 6. (Continued)
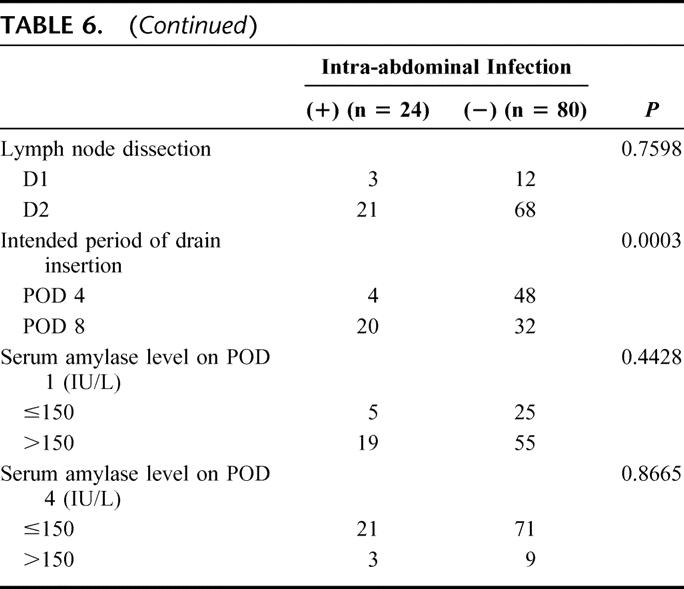
A multivariate logistic regression analysis in intra-abdominal infections revealed that the intended period of drain insertion was the only independent risk factor (P = 0.0024; Table 7).
TABLE 7. Multivariate Analysis of Risk Factors Influencing Intra-abdominal Infection
DISCUSSION
The prophylactic value of drains after pancreatic head resection is still controversial. Recent reports have suggested that drain insertion after pancreatic resections failed to reduce postoperative complications,6,20 whereas drains usually are inserted after pancreatic head resection in most institutes. Moreover, drains placed surgically after abdominal surgery have been reported to result in increased rates of intra-abdominal infection.6,20,21 However, drains after pancreatic resections are necessary for early detection of pancreatic fistula, which can lead to hemorrhage and abdominal abscess, and is associated with a high mortality rate.21–25 No previous report has discussed the relationship between the period of drain insertion and postoperative complications. The present prospective study has been designed to clarify whether the intended period of drain insertion influenced postoperative complication rates after pancreatic head resection. Our results showed that postoperative complication rates concerning the incidence of pancreatic fistula and intra-abdominal infections were significantly lower when the prophylactic drains were to be removed on POD 4. With regard to complications after pancreas head resection, it has been suggested that high-volume centers have lower rates of morbidity and mortality.26–28 In the period of the present study, from 2000 to 2004, more than 20 pancreatic head resections per year have been performed at WMUH, and we have performed more than 15 distal pancreatectomies per year. Thus, we did not need to take into account the hospital volume in this analysis. However, this study was not truly a randomized study, and we could not perfectly deny the possibility that these improved results were not due in part to subtle improvements in operative technique honed over the first 2 years of this study.
In this study, positive cultures of drainage fluid on POD 7 increased to 31% in group I (drains to be removed on POD 8), although there was no difference in the properties of drainage fluid on POD 4 between the 2 groups. Three of 16 bacteria (19%) detected by culture of drainage fluid in group I were indigenous bacteria of skin (2 Streptococcus spp and 1 Staphylococcus spp). It is suggested that long-term insertion of drains might be a major cause of postoperative infectious complications. Leukocyte counts on POD 7 were thought to be significantly higher than those on POD 4 in group I, which suggested the ascending infection occurred around POD 7. Indeed, despite the absence of intra-abdominal abscess, pancreatic fistula, and biliary leakage, 6 of 16 patients in group I with positive cultures of drainage fluid on POD 7 had clinical signs such as elevated leukocyte counts and fever of greater than 38.0°C. Therefore, one should consider that the onset of infection by drainage routes would appear around POD 7, and we strongly propose that drain removal at POD 4 could reduce the rates of intra-abdominal infections. However, drain removal around POD4 such as day 3 or 5 may have provided equally good results.
Pancreatic fistula, with an incidence varying between 5% to 20% in most series, has been reported as the most serious complication of morbidity after pancreatic head resection.7,8,29,30 In the previous reports, many preoperative and perioperative risk factors of pancreatic fistula were evaluated, including age older than 65 years, preoperative jaundice, soft pancreatic parenchyma, small pancreatic duct, exocrine pancreatic function, longer operative time, high-volume transfusion, and intraoperative bleeding.21,29–31 In this study, the incidence of pancreatic fistula was significantly higher in patients in whom drains were removed on POD 8, compared with patients whose drains were removed on POD 4. However, pancreatic texture and size of the pancreatic duct, which generally are reported to be risk factors of pancreatic fistula, had no impact on the occurrence of postoperative complications. Because pancreaticojejunostomy has been performed by duct-to-mucosal anastomosis since 1999 in WMUH, the occurrence of postoperative pancreatic fistula is similar among patients, even in patients with a soft pancreas and a normal sized pancreatic duct.7
Ascending infections by the drain route secondarily may increase pancreatic fistula in patients with long-term drain insertion. It has been suggested that pancreatic juice should be activated by bacterial phospholipase and lipopolysaccharide.32–34
CONCLUSION
Prophylactic drains placed after pancreatic head resection should be better removed on POD 4, compared with POD 8 to prevent ascending infections, and postoperative peripancreatic fluid or intra-abdominal abscess should be treated with CT- or US-guided percutaneous drainage. We are now considering to proceed the randomized controlled trial, which would have made these data much more convincing.
Footnotes
Reprints: Hiroki Yamaue, MD, Second Department of Surgery, Wakayama Medical University, School of Medicine, 811-1 Kimiidera, Wakayama 641-8510, Japan. E-mail: yamaue-h@wakayama-med.ac.jp.
REFERENCES
- 1.Liu CL, Fan ST, Lo CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158–162. [DOI] [PubMed] [Google Scholar]
- 3.Merad F, Yahchouchi E, Hay JM, et al. Prophylactic abdominal drainage after elective colonic resection and suprapromontory anastomosis: a multicenter study controlled by randomization. Arch Surg. 1998;133:309–314. [DOI] [PubMed] [Google Scholar]
- 4.Merad F, Hay JM, Fingerhut A, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery. 1999;125:529–535. [PubMed] [Google Scholar]
- 5.Monson JR, Guillou PJ, Keane FB, et al. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery. 1991;109:740–746. [PubMed] [Google Scholar]
- 6.Kelvin CC, Daniel L, Dennis L, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tani M, Onishi H, Kinosita H, et al. The evaluation of duct-to mucosal pancreaticojejunostomy in pancreaticoduodenostomy. World J Surg. 2005;29:76–79. [DOI] [PubMed] [Google Scholar]
- 8.Tani M, Kawai M, Terasawa H, et al. Complication with reconstruction procedure in pylorus-preserving pancreaticojejunostomy. World J Surg. 2005;29:881–884. [DOI] [PubMed] [Google Scholar]
- 9.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomy in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.B̋uchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314. [DOI] [PubMed] [Google Scholar]
- 11.Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. [DOI] [PubMed] [Google Scholar]
- 12.Adam U, Makowiec F, Riediger H, et al. Risk factors for complications after pancreatic head resection. Am J Surg. 2004;187:201–208. [DOI] [PubMed] [Google Scholar]
- 13.Bottger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg. 1999;23:164–172. [DOI] [PubMed] [Google Scholar]
- 14.Povoski SP, Karpeh MS, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: 2. Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Nakano H, Midorikawa T, et al. Prediction of pancreatic fistula by amylase levels of drainage fluid on the first day after pancreatectomy. Hepatogastroenterology. 2003;50:1155–1158. [PubMed] [Google Scholar]
- 17.Cullen JJ, Sarr MG, Ilstrup DM. Pancreatic anastomotic leak after pancreaticoduodenectomy; incidence, significance, and management. Am J Surg. 1994;168:295–298. [DOI] [PubMed] [Google Scholar]
- 18.Tran KTC, Smeenk HG, van Eijck CHJ, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 20.Heslin MF, Harrison LE, Brooks AD, et al. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg. 1998;2:373–378. [DOI] [PubMed] [Google Scholar]
- 21.Pessaux P, Msika S, Atalla D, et al. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg. 2003;138:314–324. [DOI] [PubMed] [Google Scholar]
- 22.Rumstadt B, Schwab M, Korth P, et al. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grobmyer SR, Rivadeneira DE, Goodman CA, et al. Pancreatic anastomotic failure after pancreaticoduodenectomy. Am J Surg. 2000;180:117–120. [DOI] [PubMed] [Google Scholar]
- 24.Choi SH, Moon HJ, Heo JS, et al. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186–191. [DOI] [PubMed] [Google Scholar]
- 25.Dixon E, Vollmer CM, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American center. Ann Surg. 2005;241:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neoptolemos JP, Russell RCG, Bramhall S, et al. Low mortality following resection for pancreatic and periampullary tumors in 1026 patients: UK survey of specialist pancreatic units: UK Pancreatic Cancer Group. Br J Surg. 1997;84:1370–1376. [PubMed] [Google Scholar]
- 27.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Siewers AE, Finlayson EF, et al. Hospital volume and surgical mortality in the united states. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 29.Büchler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883–889. [DOI] [PubMed] [Google Scholar]
- 30.Munoz-Bongrand N, Sauvanet A, Denys A, et al. Conservative management of pancreatic fistula after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2004;199:198–203. [DOI] [PubMed] [Google Scholar]
- 31.Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy: current management. Arch Surg. 1992;127:945–950. [DOI] [PubMed] [Google Scholar]
- 32.Vaccaro MI, Dagrosa MA, Mora MI, et al. The effect of chronic intraperitoneal infusion of bacterial endotoxin on exocrine pancreas function in rats. Int J Pancreatol. 1996;19:49–54. [DOI] [PubMed] [Google Scholar]
- 33.Nakano T, Yanagisawa J, Nakayama F. Phospholipase activity in human bile. Hepatology. 1988;8:1560–1564. [DOI] [PubMed] [Google Scholar]
- 34.Traverso LW, MacFarlane SK. Pancreatic juice in the peritoneal cavity: antibiotics or omental preservation prevent mortality. J Surg Res. 1987;43:220–225. [DOI] [PubMed] [Google Scholar]