Abstract
Background:
Liver tumors with inferior vena cava (IVC) involvement may require combined resection of the liver and IVC. This approach, with its high surgical risks and poor long-term prognosis, was precluded until the development of neoadjuvant chemotherapy, portal vein embolization, reinforced vascular prostheses, and technical advances in liver transplantation.
Methods:
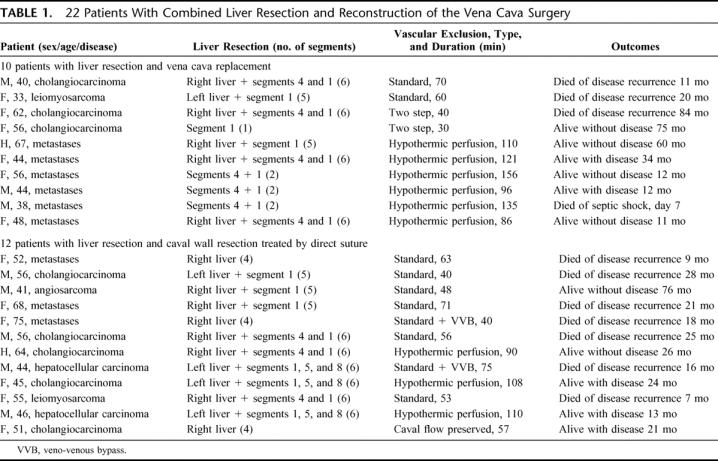
We reviewed 22 cases of hepatectomy with retrohepatic IVC resection and reconstruction. The patients had a median age of 51.5 years (range, 32.8–75.3 years). Indications for resection were: liver metastases (n = 9), cholangiocarcinoma (n = 8), hepatocellular carcinoma (n = 2), other cancers (n = 3). The liver resections carried out included 18 first, 3 second, and one third hepatectomy. Segment 1 (caudate lobe) was included in the specimen in 19 cases (86%). Resection concerned 1 to 6 liver segments (median = 5.0). Vascular control was achieved by vascular exclusion of the liver preserving the caval flow (n = 1), standard vascular exclusion of the liver (n = 12), in situ cold perfusion of the liver (n = 9). Ex situ surgery was not necessary in any case. Venovenous bypass was used in 12 cases. The IVC was reconstructed with a ringed Gore-Tex tube graft (n = 10), primarily (n = 8), or by caval plasty (n = 4). A main hepatic vein was reimplanted in 6 cases: into the native IVC (n = 4) or into a Gore-Tex tube graft (n = 2).
Results:
One patient died (4.5%) due to catheter infection, 7 days after in situ cold perfusion with replacement of the vena cava. Eight patients (36%) had no complications and 14 patients (64%) had 23 complications. In all but 1 case, the complications were transient and successfully controlled. The patients stayed in intensive care for 3.3 ± 2.0 days and in the hospital for 17.7 ± 7.8 days. All vascular reconstructions were patent at last follow-up. With median follow-up of 19 months, 10 patients died of tumor recurrence and eleven were alive with (n = 5) or without (n = 6) disease. Actuarial 1-, 3-, and 5-year survival rates were 81.8%, 38.3%, and 38.3%, respectively.
Conclusions:
IVC resection and reconstruction combined with liver resection can be safely performed in selected patients. The lack of alternative treatments and the spontaneous poor prognosis justify this approach, provided that surgery is carried out at a center specialized in both liver surgery and liver transplantation. The development of adjuvant chemotherapy regimens is required to improve the long-term results of this salvage surgery.
Combined resection of the liver and inferior vena cava is safe, and the long-term results are better than those for patients not undergoing surgery.
Liver resection is the only potentially curative treatment of most primary and metastatic tumors of the liver. Untreated liver cancers are fatal, with survival usually measured in months.1,2 Even with the best regimens, tumor response to chemotherapy is limited, rarely complete and median survival is reported to be generally poor for the most frequent tumors (ie, <24 months for metastases from colorectal cancer, <12 months for hepatocellular carcinoma and intrahepatic cholangiocarcinoma). Efforts have therefore been directed at achieving the resection of liver tumors. Multidisciplinary approaches have been developed, including neoadjuvant chemotherapy to decrease tumor burden,3–9 two-step hepatectomies,10 rehepatectomies,11 and portal vein embolization to increase the volume of the future remnant liver,12,13 together with in situ destruction of tumors.14–16 Involvement of the hepatocaval confluence or IVC was long considered a contraindication for liver resection, due to the risks of gas embolism and massive hemorrhage. Liver resection has become more common with the adoption of hepatic vascular exclusion17–19 venovenous bypass, hypothermic perfusion of the liver (in situ, ante situm, or ex situ,20). The resected IVC may be replaced by various materials, and most of the available reports are case reports and/or include limited data.21–35 We report here the experience of our unit with concomitant hepatic and IVC resection in 22 patients with various liver tumors. The surgical strategy was adapted to the specific topography of the tumor and the surgical techniques described above were used to achieve resectability.
PATIENTS AND METHODS
From March 1987 to March 2004, 2109 liver resections were performed at our hepatobiliary surgery and liver transplantation center. The population studied consisted of 22 patients (2%) who underwent liver resection combined with resection of part (12 cases) or all (10 cases) of the vena cava. The characteristics of the patients are summarized in Table 1. We studied 11 men and 11 women, with a mean age of 51.9 ± 10.9 years. The primary tumor was metastatic cancers in 9 cases (41%), intrahepatic cholangiocarcinoma in 8 cases (36%), primary leiomyosarcoma of the vena cava invading the liver in 2 cases (9%), hepatocellular carcinoma in 2 cases (9%), and angiosarcoma in one case (5%). The underlying liver parenchyma was histologically healthy in 20 cases, cirrhotic in 1 case, and fibrotic in 1 case. Written informed consent was obtained from all patients.
TABLE 1. 22 Patients With Combined Liver Resection and Reconstruction of the Vena Cava Surgery
Preoperative Management
All patients were screened as indicated to exclude extrahepatic malignant disease. Portal vein embolization was performed in 2 cases, as described elsewhere.12,13 All patients were evaluated by CT and Doppler ultrasound scans. Cavograms were not obtained. The mean number of liver nodules was 2.0 ± 2.1, and the mean diameter of the largest lesion was 12.2 ± 6.7 cm. Preoperative liver and kidney function tests results were as follows: prothrombin time = 87% ± 13% of normal level, bilirubin = 12 ± 6 ìmol/L, AST = 55 ± 99 IU/L, ALT = 32 ± 20 IU/L, indocyanine green retention rate at 15 minutes = 8.9% ± 6.4% of normal (normal <10%), creatinine = 68 ± 14 ìmol/L. Sixteen patients (73%) received a mean of 10.5 ± 7.1 courses of systemic chemotherapy before surgery. Three of the 6 patients who did not receive chemotherapy underwent one to three courses of transarterial chemoembolization before surgery.
Anesthetic Management
Patients were monitored during surgery by standard non invasive techniques. In addition, a Swann-Ganz catheter and an arterial line were systematically used. Warming therapy was applied to minimize intraoperative hypothermia.
Liver Resection
The surgical techniques used for liver resection in our unit have been described elsewhere.36 A bilateral subcostal incision was used in all cases. In 5 cases, radial phrenotomy or resection of the right diaphragm was also performed to mobilize the liver (3 cases) and/or for tumoral invasion of the muscle. During surgery, we carefully searched the abdominal cavity for recurrent local disease, extrahepatic metastases, and peritoneal seeding. Any suspicious nodule and systematically at least one lymph node from the hepatic pedicle were examined by frozen sections. A complete examination of the liver by palpation and ultrasonography was carried out during surgery, to confirm the number and size of the lesions, to define their relationship to intrahepatic vascular structures, and to look for occult liver metastases. parenchyma was dissected with an ultrasonic dissector (Cavitron Ultrasonic Aspirator, Valley Laboratory Inc., Boulder, CO).
The liver resections studied here were the first liver resection in 18 cases (82%), the second in 3 cases (14%), and the third in one case (4%). The 22 liver procedures included the resection of 4.7 ± 1.7 segments.36,37 As detailed in Table 1, the resection included 6 segments in 10 cases, 5 segments in 3 cases, 4 segments in 5 cases, 2 segments in 3 cases, and 1 segment in 1 case. Overall, segment 1 (caudate lobe) was included in the specimen in 19 cases (86%). Additional nonanatomic resection was performed on the remnant liver in 3 cases (14%).
Surgery of the Vena Cava and Hepatic Veins
The type of treatment varied according to the location of the tumor and the extent of caval involvement, which was further evaluated during surgery. If less than 30% of the circumference of the IVC wall was involved, it was sutured longitudinally (n = 8 cases). If wall involvement was between 30% and 50%, the IVC was sutured transversely (as for pyloroplasty) to prevent stenosis of the vein (n = 4 cases). If ≥50% of the circumference of the wall was involved, the IVC was resected and replaced by a 20-mm-diameter external ring-reinforced PTFE (n = 10 cases).
Only one main hepatic vein remained in 16 cases (73%). The segment of this hepatic vein was resected and its stump reimplanted into the native vena cava in 4 cases, and into the replaced vena cava in 2 cases. In the other 10 cases, the root of the remaining hepatic vein in the native vena cava remained untouched.
Vascular Control
This was planned following preoperative morphologic analysis (Table 1). Vascular control was then adapted during surgery, with the aim of 1) minimizing the need for transfusion, 2) shortening ischemia time as much as possible, 3) maintaining stable systemic hemodynamics, and 4) improving the tolerance of the remnant liver to ischemia-reperfusion injury. The vena cava was controlled in the pericardium in 5 cases for a safer control of the suprahepatic vena cava and/or to ensure that a sufficiently long stump of suprahepatic vena cava was available for vascular reconstruction. The pericardium was opened via an abdominal approach in 4 cases and by sternotomy in 1 case.
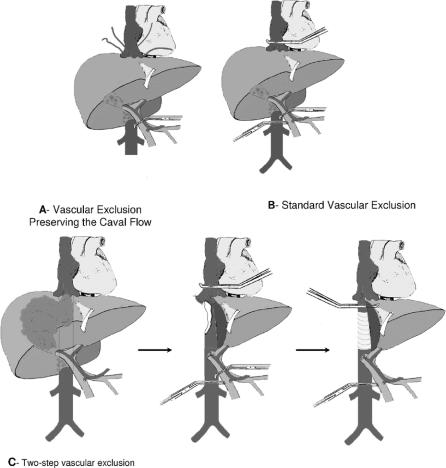
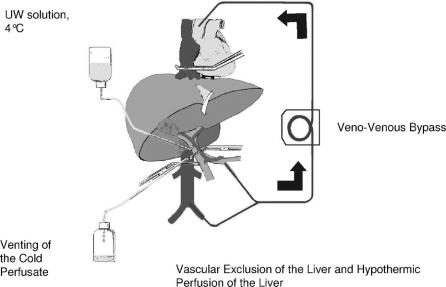
Four different vascular control techniques were used in this series (Figs. 1, 2).
FIGURE 1.
FIGURE 2.
Vascular Exclusion Without Caval Clamping
The anterior face of the vena cava was freed as much as possible, leaving any tumoral adhesion in place (Fig. 1A). The hepatic veins were controlled.38–40 Vascular exclusion was performed by clamping successively the portal triad and the hepatic veins. Resection was then performed via an anterior approach.41 When the adhesion of the tumor to the cava was reached, the cava was clamped laterally and the specimen and a patch of the cava were removed en bloc. This technique was used in 1 case. Reperfusion was performed after 57 minutes of vascular exclusion of the liver following longitudinal suture of the vena cava.
Standard Total Vascular Exclusion of the Liver
This method involved mobilization of the liver, and isolation of the suprahepatic and infrahepatic vena cava and the hilum (Fig. 1B). The infrahepatic vena cava, hilum, and suprahepatic vena cava were serially clamped following systematic ligation and division of the adrenal vein.18,19 A venovenous bypass (see below) was installed in 2 cases of hemodynamic intolerance, defined as a decrease in mean arterial pressure >30% and/or a decrease in cardiac index >50% upon vascular exclusion, despite adequate fluid loading.42 After parenchymal division, circulation was restored by unclamping successively the suprahepatic vena cava, the infrahepatic vena cava, and the portal triad. This technique was used in 10 cases. Vascular exclusion lasted from 40 to 75 minutes (median, 58 minutes).
Two-Step Vascular Exclusion of the Liver
In 2 patients, standard vascular exclusion of the liver was performed, leaving a sufficiently long IVC stump below the confluence of the hepatic veins for replacement of the suprahepatic caval clamp by another clamp on the retrohepatic vena cava, below the confluence of the hepatic veins (Fig. 1C). En bloc resection of the specimen and of a segment of the vena cava could then be completed, with revascularization of the remnant liver. The retrohepatic vena cava was replaced by a prosthesis in these 2 cases and vascular exclusion lasted 30 and 40 minutes.
In Situ Hypothermic Perfusion of the Liver
In situ hypothermic perfusion of the liver was applied when the planned procedure included vascular reconstruction in addition to vena cava surgery, ie, hepatic vein and/or portal vein surgery, potentially prolonging beyond 1 hour the total vascular exclusion (TVE) indicated for the resection per se (Fig. 2).
In situ hypothermic perfusion of the liver was performed as described by Fortner et al,43 with some modifications.20 The liver was first mobilized as for TVE. A venovenous bypass was systematically installed from the inferior mesenteric vein, and the right femoral vein to the left internal jugular vein. The femoral and internal jugular veins were punctured under ultrasonic control and percutaneous 15-Fr cannulas were installed (Medtronic laboratory, Paris, France.44,45 Following TVE and venovenous bypass, the portal vein was catheterized above the portal triad clamp with a Silastic (silicone elastomer) catheter (internal diameter, 2.5 mm; external diameter, 4.5 mm). In situ hypothermic perfusion of the liver was carried out with 2 to 4 L of UW solution, chilled to 4°C, from a height of 0.5 m, during resection and vascular reconstruction. A cavotomy was performed immediately above the inferior caval clamp for the insertion of a 30-Fr catheter to drain the cold perfusate and to prevent a decrease in central temperature. When liver resection and vascular reconstruction were completed, the liver was flushed with serum albumin via the portal vein. The portal catheter for perfusion was removed. The portotomy and the cavotomy were closed. Circulation was restored as for TVE. The venovenous bypass was removed as a last step, after hemodynamic stabilization. The inferior mesenteric vein was ligated and cutaneous sutures were applied to the femoral and jugular puncture sites. This technique was used in 9 cases and vascular exclusion lasted from 86 to 156 minutes (median, 110 minutes). Some of these cases were recently reported elsewhere.20
Associated Procedures
Ten patients (45.5%) had 16 associated procedures: atypical resection in the remnant liver (3 cases), right nephrectomy (1 case), right adrenal gland resection (1 case), right inferior lung lobectomy via phrenotomy (1 case), biliary reconstruction with a Roux-en-Y bypass for resection of the biliary confluence (4 cases), porto-portal anastomosis for segmental resection of the portal vein (2 cases, including 1 direct anastomosis and 1 with interposed 8-mm-diameter external ring reinforced PTFE). Resection of the right diaphragm was performed in 4 cases. The defect was directly sutured in 1 case and replaced by a nonabsorbable synthetic mesh in 3 cases.
Postoperative Treatment and Follow-up
Patients in whom the vena cava was replaced received continuous intravenous anticoagulant treatment with heparin. Treatment was initiated in the operating theater, at 1 mg/kg body weight/24 hours, and was adapted to maintain coagulation time at 1.5 to 2 times the normal level. Intravenous heparin treatment was continued until day 8 after surgery and then replaced by a daily injection of low-molecular weight heparin for 1 month. All other patients received one daily injection of low-molecular weight heparin from day 1 to discharge from hospital. No patient was lost to follow-up (27.0 ± 24.0 months; median, 19.0 months). At the time of last follow-up, 17 of the 21 patients surviving the perioperative period were receiving adjuvant systemic chemotherapy.
Definition of Postoperative Complications
Patients were defined as having postoperative liver insufficiency when total bilirubin concentration was >90 ìmol/L or prothrombin time <30% of normal levels within 5 days of surgery. Asterixis and alteration of consciousness after excluding drug effects were considered signs of liver failure, even if they occurred in isolation. We also checked for other complications such as intra-abdominal hemorrhage requiring reoperation, biliary fistula, clinically significant ascites (abdominal drain output >500 mL per day over more than 3 days), renal insufficiency (with serum creatinine concentration >150 ìmol/L), pleuropulmonary complications requiring pleural drainage, infection (defined as temperature ≥38.5°C, a white blood cell count ≥10 × 1010/L, and either positive blood cultures or a documented septic focus). All other complications were also recorded.
Data Analysis
Results are expressed as means ± standard deviations unless otherwise stated. A P value <0.05 was considered significant. All statistical analyses were performed using SAS software (SAS institute, Inc., Cary, NC). We compared the group of patients who underwent surgery under warm ischemia (without in situ hypothermic perfusion) with the group of patients who underwent surgery under cold ischemia (with in situ hypothermic perfusion) for the main intraoperative and postoperative events.
RESULTS
Intraoperative Events
Overall, vascular exclusion lasted 78 ± 34 minutes. The duration of vascular exclusion was significantly longer for patients undergoing surgery under hypothermic perfusion of the liver than in those not receiving such perfusion (112 ± 22 vs. 54 ± 14 minutes, respectively, P < 10−4). The number of segments resected was similar in the group operated under hypothermic perfusion (4.7 ± 2.1 segments) compared with the group operated under warm ischemia (4.7 ± 1.4 segments, P > 0.9). Patients required transfusion with a mean of 7.7 ± 5.8 blood units (8.4 ± 5.9 vs. 7.2 ± 5.9 blood units for patients with vs. without hypothermic perfusion of the liver, respectively, P = 0.6). The mean duration of operation was 455 ± 121 minutes (range, 290–795 minutes; median, 439 minutes), and was significantly longer under cold ischemia (506 ± 124 minutes) than under warm ischemia (402 ± 108 minutes, P = 0.05).
In-Hospital Mortality
The in-hospital mortality rate was 4.5% (1 case). Following 26 courses of chemotherapy for synchronous metastases from a colon cancer, this 38-year-old patient underwent a third hepatectomy (segmentectomy 4 + 1 on the remnant left liver) en bloc with resection of the portal vein bifurcation (and reconstruction with an interposed reinforced Goretex 8 mm in diameter), the biliary confluence (reconstructed with a bilio-digestive anastomosis with a Roux-en-Y bypass) and the retrohepatic vena cava (replaced by a PTFE prosthesis). The patient suffered sudden septic shock 7 days after hepatectomy, after transfer to the ward, with normal liver (prothrombin time = 70% of normal, bilirubin = 24 ìmol/L) and kidney (creatinine = 87 ìmol/L) function. Emergency laparotomy identified no cause of sepsis and vessels were found to be patent on intraoperative Doppler ultrasound scan. He died 12 hours after surgery, from sepsis and multiple organ failure. Two days later, blood cultures from arterial and venous central catheters were found to be positive for Klebsiella and Staphylococcus.
Morbidity
Eight patients (36%) had no complication and 14 patients (morbidity rate = 64%) had 23 complications. All but one of these complications were transient and successfully controlled. Only one complication was fatal, as described above. The following complications were recorded: transient liver insufficiency (7 cases), ascites (6 cases), biliary fistula (2 cases), subphrenic abscess treated percutaneously (1 case), septic shock (1 case, described above), reoperation for hemostasis (1 case), pleuropulmonary complication (1 case), renal failure (3 cases, including 1 case of drug toxicity), and transient radial nerve palsy (1 case).
Postoperative Liver and Kidney Function Tests
All preoperative liver and kidney function tests were similar in the 2 groups with and without hypothermic perfusion of the liver (data not shown). When comparing the 2 groups with and without hypothermic perfusion, the peak concentrations within 10 days of surgery of AST (521 ± 245 vs. 828 ± 515 IU/L, respectively, P = 0.1), ALT (366 ± 168 vs. 600 ± 359 IU/L, P = 0.1), and bilirubin (79 ± 27 vs. 76 ± 60 ìmol/L, P = 0.9), and the minimum value of prothrombin time (28.9% ± 9.1% vs. 31.4% ± 12.6% of normal, P = 0.6) were similar. Likewise the peak creatinine concentration was similar in both groups (96 ± 81 vs. 86 ± 35 ìmol/L, P = 0.7).
Outcome
Patients remained in the intensive care unit (ICU) for 3.3 ± 2.0 days and in the hospital for 17.7 ± 7.8 days. The durations of ICU (P = 0.6) and hospital (P = 0.9) stays were similar in patients undergoing surgery under warm or cold ischemia of the liver. All 21 survivors of the perioperative period were discharged from hospital and able to function independently. Resection margins were more than 1 cm from the tumor in 6 patients (27%). In 16 cases (73%), the resection margin was less than 1 cm from the tumor. The wall of the vena cava showed true invasion of the tumor in 5 cases (22.7%), and no true invasion in 17 cases (77.3%). In 2 of these 17 patients, the vena cava was obstructed by a tumoral thrombus, in both cases from a hepatocellular carcinoma. At last follow-up, all but 2 of the surviving patients were receiving adjuvant systemic chemotherapy.
Long-term Results
Recurrence occurred in 15 patients (68%), within a mean of 12.0 ± 15.6 months following liver resection. Recurrence initially occurred in the liver (8 cases), liver and lung (1 case), lungs (4 cases), bones (1 case), or was diffuse (1 case). One patient underwent surgery again for recurrence in segment 4, 23 months after right hepatectomy and replacement of the vena cava under hypothermic perfusion of the liver for colorectal metastases. This patient is alive and disease-free 60 months and 37 months after the first and second hepatectomy, respectively. With a median follow-up of 19 months, 1 patient died in the postoperative period, 10 patients died of disease, 5 patients were alive with disease, and 6 patients were alive without disease. Overall actuarial survival was 81.8%, 38.3%, and 38.3% at 1, 3, and 5 years, respectively.
DISCUSSION
We report here our experience with 22 cases of liver resection combined with resection of the vena cava, including 10 cases of caval replacement by a prosthetic material. Perioperative mortality (4.5%, 1 of 22) and morbidity (64%) are acceptable given the nature of the procedures performed.
Our results compare favorably with those of the few other available series of combined liver and vena cava resection. Lessons learned from liver transplantation have made it possible to increase the safety of resecting liver tumors involving the vena cava and/or the hepatocaval confluence. It was only to be expected that the first attempt at liver resection combined with caval replacement21 and the first successful surgery of this type22 would be performed by a team pioneering liver transplantation. The liberal use of venovenous bypass46 during vascular exclusion of the liver with caval clamping has solved the problems of hemodynamic intolerance47 and splanchnic congestion.48 The percutaneous venovenous bypass technique has several advantages over the cut-down technique. It is less time-consuming, insertion is simple (by the Seldinger technique), without the need for open dissection, making it possible to avoid nerve injuries, seromas, and lymphoceles. Puncturing the femoral and internal jugular veins under ultrasonic control also decreases the risk of arterial and pleural injuries. These advantages are obtained for flow rates similar to those obtained with the open technique.49 The use of in situ hypothermic perfusion43 has solved the problem of prolonged ischemia and reduced the risk of liver failure.20 In the present series, postoperative liver and kidney function tests were similar in patients with hypothermic perfusion of the liver compared with those without. Indeed, we observed a trend toward a better tolerance to ischemia-reperfusion injury in the group with hypothermic perfusion as assessed by a lower peak AST concentration (521 ± 245 IU/L) compared with the group operated under warm ischemia (828 ± 516 IU/L, P = 0.1) despite a significantly longer duration of vascular exclusion in the former group (P < 10−4). In addition to their intrinsic advantages, the use of venovenous bypass and hypothermic perfusion of the liver increase the time for which vascular exclusion may be safely maintained, making it easier to teach this type of surgery.
Given the higher mortality rate (28%) following ex situ liver resection under hypothermic perfusion than for in situ resection (8%) (for review see Azoulay et al20), we did not use this technique in this series. Even those who initially promoted the ex situ technique now seem to have abandoned it (Oldhafer KJ, personal communication). As a means of minimizing the duration of vascular exclusion and the subsequent ischemia-reperfusion of the liver, some authors have proposed initiating parenchymal transection without vascular exclusion or with intermittent portal triad clamping until the hepatocaval intersection is reached.50,51 The optimal approach between ours, favoring hypothermic perfusion of the liver, as compared with performing the majority of the liver resection without vascular exclusion and then performing the vascular resection/reconstruction during a shorter ischemic period with or without cold perfusion remains to be found.
Preoperative morphologic explorations currently available are inaccurate to differentiate malignant infiltration of the caval wall from simple tumoral adhesion to the vein. It is therefore difficult to identify indications for vena cava resection before surgery. As in other reports, the vena wall was actually invaded by the tumor in only 5 of the 22 cases of the present series.51 In many cases, the final decision to resect the vena cava is taken during surgery. The use of intravascular ultrasound scans might make it easier to diagnose accurately tumoral invasion of the caval wall.52,53 Indeed, this might not always prevent the resection of the vena in certain cases to prevent to crack into the tumor plane.
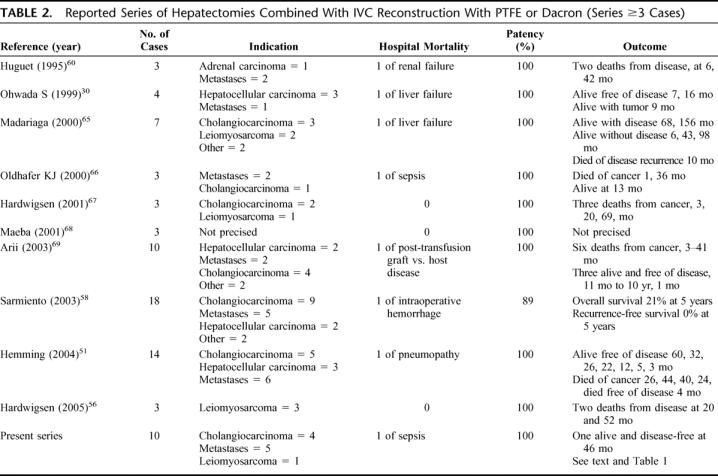
Various materials have been used to replace segments of the vena cava, including xenografts35 allografts,21 autologous grafts,25 and Dacron.22 There is currently a consensual preference for reinforced PTFE, based on experimental54,55 and clinical data (Table 2). This rules out the debate concerning arterialization of the graft to enhance its patency proposed for other materials. However, some authors56 still use arteriovenous fistula with PTFE grafts.
TABLE 2. Reported Series of Hepatectomies Combined With IVC Reconstruction With PTFE or Dacron (Series ≥3 Cases)
The incidence of infection of synthetic caval grafts in the abdomen seems to be low. Indeed, only 1 such case has been reported.57 In this case, an occluded graft became infected 18 days after resection of abdominal liposarcoma without hepatectomy and with intraoperative radiation. The infection of the graft led to duodenal leakage, multiple organ failure, and death 4 months after surgery. In our case of death due to sepsis, no septic focus was identified by laparotomy. It was not possible to perform autopsy and so the prosthesis could not be cultured. An omental wrap has been used to protect prosthetic caval grafts from infection when performing combined liver resection and caval reconstruction.51,58 This precaution was taken in the last few cases of our series.
The long-term patency of the graft was not a problem in the present series, as in most other reported series (Table 2). The anticoagulation protocol used in this series was simply a pragmatic application of that used for prosthetic H-graft portocaval shunt in our unit. However, it remains to be demonstrated that anticoagulation is required.
Some authors have suggested that the suprarenal IVC may be safely resected and not replaced.59 It has been suggested that long-term venous sequelae rarely occur because vena cava occlusion occurs gradually with tumor enlargement. However, it is impossible to predict late venous sequelae, such as subsequent renal insufficiency and lower extremity edema, on the basis of preoperative venous signs, symptoms, or imaging.60,61 We, like others, favor caval replacement in patients with unobstructed IVC requiring resection for tumor clearance, in those without well-developed venous collaterals, in those whose collateral veins had to be ligated or resected for tumor removal.
The 5-year overall actuarial survival rate of 38.3% obtained cannot in itself be considered a justification for surgery because of the heterogeneity of the diseases treated. However, given the poor results of nonsurgical treatment, we consider an aggressive surgical attitude to be justified for a selected group of patients able to undergo such major surgery with reasonable morbidity and mortality rates: those with no significant renal, liver, or cardiopulmonary dysfunction and those responding to or at least stable under neoadjuvant chemotherapy.62 The formidable challenge presented by these procedures and the importation of techniques and technologies from the field of liver transplantation into liver surgery require a center specialized in both hepatobiliary surgery63 and liver transplantation.64 Given the rarity of patients amenable to surgery, a prospective trial comparing surgery with nonsurgical management would be very difficult, if not impossible. In addition, given the poor results obtained with nonsurgical treatments, such a study would be ethically questionable.
CONCLUSION
Combined liver resection and reconstruction of the vena cava is feasible in a certain subset of patients with acceptable morbidity and mortality and fair long-term results. This procedure may be carried out provided that the selection of patients is stringent and based on both oncologically sound indications and risk assessment. Such surgery should be performed only in centers with experience in both liver surgery and liver transplantation. This expertise allows a technical and technological escalation adapted to the planned and the actual procedure performed. Adjuvant treatments are required to improve the long-term results of this surgery.
Footnotes
Reprints: Daniel Azoulay, MD, PhD, Centre Hépato-Biliaire, Hôpital Paul Brousse, 94800, Villejuif, France. E-mail: daniel.azoulay@pbr.ap-hop-paris.fr.
REFERENCES
- 1.Fong Y. Surgical therapy of hepatic colorectal metastases. CA Cancer J Clin. 1999;49:231–255. [DOI] [PubMed] [Google Scholar]
- 2.Song T-J, Wai Kit Ip E, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127(suppl):248–260. [DOI] [PubMed] [Google Scholar]
- 3.Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lévi F, Zidani R, Brianza S, et al. A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma: International Organization for Cancer Chronotherapy. Cancer. 1999;85:2532–2540. [DOI] [PubMed] [Google Scholar]
- 5.De Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 6.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. [DOI] [PubMed] [Google Scholar]
- 7.Rougier P, Van Custem E, Bajetta E, et al. Randomized trial of irinotecan vs fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;35:1407–1412. [DOI] [PubMed] [Google Scholar]
- 8.Douillard J-Y, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387–394. [DOI] [PubMed] [Google Scholar]
- 10.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883 , discussion: 883–884. [DOI] [PMC free article] [PubMed]
- 12.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339. [DOI] [PubMed] [Google Scholar]
- 15.Elias D, Gohanin A, El Otmany A, et al. Usefulness of intraoperative radiofrequency thermoablation of liver tumors associated or not with hepatectomy. Eur J Surg Oncol. 2000;26:763–769. [DOI] [PubMed] [Google Scholar]
- 16.Parikh AA, Curley SA, Fornage BD, et al. Radiofrequency ablation of hepatic metastases. Semin Oncol. 2002;29:168–182. [DOI] [PubMed] [Google Scholar]
- 17.Heaney JP, Stanton WK, Halbert DS, et al. An improved technique for vascular isolation of the liver: experimental study and case reports. Ann Surg. 1966;163:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huguet C, Nordlinger B, Galopin J, et al. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689–693. [PubMed] [Google Scholar]
- 19.Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azoulay D, Eskenazy R, Andreani P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. 2005;241:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Koep LJ, Weil R III, et al. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet. 1980;150:208–214. [PMC free article] [PubMed] [Google Scholar]
- 22.Iwatsuki S, Todo S, Starzl TE. Right trisegmentectomy with a synthetic vena cava graft. Arch Surg. 1988;123:1021–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumada K, Shimahara Y, Fukui K, et al. Extended right hepatic lobectomy: combined resection of inferior vena cava and its reconstruction by PTFE graft (goretex). Case report. Acta Chir Scand. 1988;254:481–483. [PubMed] [Google Scholar]
- 24.Risher WH, Arensmn RM, Ochsner JL, et al. Retrohepatic vena cava reconstruction with polyfluoroethylene graft. J Vasc Surg. 1990;12:367–370. [DOI] [PubMed] [Google Scholar]
- 25.Miller CM, Schwartz ME, Nishizaki T. Combined hepatic and vena caval resection with autogenous caval graft replacement. Arch Surg. 1991;126:106–108. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Hishiki S, Nakamura S, et al. Extension incision for renal carcinoma including invaded vena cava and right lobe of the liver. Urology. 1992;39:285–288. [DOI] [PubMed] [Google Scholar]
- 27.O'Malley KJ, Stuart RC, McEntee GP. Combined resection of the inferior vena cava and extended right hepatectomy for leiomyosarcoma of the retrohepatic cava. Br J Surg. 1994;81:845–846. [DOI] [PubMed] [Google Scholar]
- 28.Yagyu T, Shimizu R, Nishida M, et al. Reconstruction of the hepatic vein to the prosthetic inferior vena cava in right extended hemihepatectomy with ex situ procedure. Surgery. 1994;115:740–744. [PubMed] [Google Scholar]
- 29.Grazi GL, Mazziotti A, Jovine E, et al. Total vascular exclusion of the liver during hepatic surgery: selective use, extensive use, or abuse? Arch Surg. 1997;132:1104–1109. [DOI] [PubMed] [Google Scholar]
- 30.Ohwada S, Kawashima Y, Ogawa T. Extended hepatectomy with ePTFE graft vena caval replacement and hepatic vein reconstruction: a case report. Hepatogastroenterology. 1999;46:1151–1155. [PubMed] [Google Scholar]
- 31.Enoki T, Hayashi D, Inokuchi T, et al. Combined right hepatic and retrohepatic caval resection with reconstruction using a polyfluoroethylene graft for primary leiomyosarcoma of the liver: report of a case. Surg Today. 1999;29:67–70. [DOI] [PubMed] [Google Scholar]
- 32.Zografos GN, Palmer S, Papastratis G, et al. Aggressive surgical management of fibrolamellar hepatocellular carcinoma in puberty. Eur J Surg Oncol. 1997;23:570–572. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto Y, Terajima H, Ishikawa Y, et al. In situ pedicle resection in left trisegmentectomy of the liver combined with reconstruction of the right hepatic vein to an inferior vena caval segment transpositioned from the infrahepatic portion. J Am Coll Surg. 2001;192:137–141. [DOI] [PubMed] [Google Scholar]
- 34.Lechaux D, Magavand JM, Raoul JL, et al. Ex vivo right trisegmentectomy with reconstruction of inferior vena cava and ‘flop’ reimplantation. J Am Coll Surg. 2002;194:842–845. [DOI] [PubMed] [Google Scholar]
- 35.Del Campo C, Konok GP. Use of a pericardial xenograft patch in repair of resected retrohepatic vena cava. Can J Surg. 1994;37:59–61. [PubMed] [Google Scholar]
- 36.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 37.Couinaud C. Le foie Etudes anatomiques et chirugicales. Paris: Masson et Cie, 1957. [Google Scholar]
- 38.Nagasue N, Yukaya H, Ogawa Y, et al. Segmental and subsegmental resections of the cirrhotic liver under inflow and outflow occlusion. Br J Surg. 1985;72:565–568. [DOI] [PubMed] [Google Scholar]
- 39.Elias D, Lasser P, Debaene B, et al. Intermittent vascular exclusion of the liver (without vena cava clamping) during hepatectomy. Br J Surg. 1995;82:1535–1539. [DOI] [PubMed] [Google Scholar]
- 40.Cherqui D, Malassagne B, Colau P, et al. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg. 1999;230:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azoulay D, Hargreaves GM, Castaing D, et al. The anterior approach: the right way for right massive hepatectomy. J Am Coll Surg. 2001;192:412–417. [DOI] [PubMed] [Google Scholar]
- 42.Delva E, Barberousse JP, Nordlinger B, et al. Hemodynamic and biochemical monitoring during major liver resection with use of hepatic vascular exclusion. Surgery. 1984;95:309–318. [PubMed] [Google Scholar]
- 43.Fortner JG, Shiu MH, Kinne DW, et al. Major hepatic resection using vascular isolation and hypothermic perfusion. Ann Surg. 1974;180:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oken AC, Frank SM, Merritt WT, et al. A new percutaneous technique for establishing venous bypass access in orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 1994;8:58–60. [DOI] [PubMed] [Google Scholar]
- 45.Budd JM, Isaac JL, Bennett J, et al. Morbidity and mortality associated with large-bore percutaneous venovenous bypass cannulation for 312 orthotopic liver transplantations. Liver Transplantation. 2001;7:359–362. [DOI] [PubMed] [Google Scholar]
- 46.Shaw BW, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belghiti J, Noun R, Zante E, et al. Portal triad clamping or hepatic vascular exclusion for major liver resection: a controlled study. Ann Surg. 1996;224:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu DL, Jeppsson B, Hakansson CH, et al. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442–447. [DOI] [PubMed] [Google Scholar]
- 49.Johnson SR, Marterre WF, Alonso MH. A percutaneous technique for venovenous bypass in orthotopic cadaver liver transplantation and comparison with the open technique. Liver Transpl Surg. 1996;2:354–361. [DOI] [PubMed] [Google Scholar]
- 50.Berney T, Mentha G, Morel P. Total vascular exclusion of the liver for the resection of lesions in contact with the vena cava or the hepatic veins. Br J Surg. 1998;85:485–488. [DOI] [PubMed] [Google Scholar]
- 51.Hemming AW, Reed AI, Langham MR, et al. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaneko T, Nakao A, Nomoto S, et al. Intracaval endovascular ultrasonography for preoperative assessment of retrohepatic inferior vena cava infiltration by malignant tumors. Hepatology. 1996;24:1121–1127. [DOI] [PubMed] [Google Scholar]
- 53.Kikumori T, Imai T, Kaneko T, et al. Intracaval endovascular ultrasonography for large adrenal and retroperitoneal tumors. Surgery. 2003;134:989–993. [DOI] [PubMed] [Google Scholar]
- 54.Herring M, Gardner A, Peigh P, et al. Patency on canine vena cava grafting: effects of graft material, site, and endothelial seeding. J Vasc Surg. 1984;1:877–887. [DOI] [PubMed] [Google Scholar]
- 55.Smith D, Hammon J, Anane-Sefah R, et al. Segmental venous replacement: a comparison of biological and synthetic substitutes. J Thorac Cardiovasc Surg. 1975;69:589–598. [Google Scholar]
- 56.Hardwigsen J, Balandraud P, Ananian P, et al. Leiomyosarcoma of the retrohepatic portion of the inferior vena cava: clinical presentation and surgical management in five patients. J Am Coll Surg. 2005;200:57–63. [DOI] [PubMed] [Google Scholar]
- 57.Bower TC, Nagorney DM, Cherry KJ Jr, et al. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000;31:270–281. [DOI] [PubMed] [Google Scholar]
- 58.Sarmiento JM, Bower TC, Cherry KJ, et al. Is combined partial hepatectomy with segmental resection of inferior vena cava justified for malignancy? Arch Surg. 2003;138:624–631. [DOI] [PubMed] [Google Scholar]
- 59.Takayama T, Makuuchi M, Kosuge T, et al. A hepatoblastoma originating in the caudate lobe radically resected with the inferior vena cava. Surgery. 1991;109:208–213. [PubMed] [Google Scholar]
- 60.Huguet C, Ferri M, Gavelli A. Resection of the suprarenal inferior vena cava: the role of prosthetic replacement. Arch Surg. 1995;130:793–797. [DOI] [PubMed] [Google Scholar]
- 61.Dekett JW, Gittes RF. Resection of the inferior vena cava for adjacent malignant disease. Surg Gynecol Obstet. 1973;136:711–716. [PubMed] [Google Scholar]
- 62.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glasgow RE, Showstack JA, Katz PP, et al. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg. 1999;134:30–35. [DOI] [PubMed] [Google Scholar]
- 64.Edwards EB, Roberts JP, McBride MA, et al. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–2053. [DOI] [PubMed] [Google Scholar]
- 65.Madariaga JR, Fung J, Gutierrez J, et al. Liver resection combined with excision of vena cava. J Am Coll Surg. 2000;191:244–250. [DOI] [PubMed] [Google Scholar]
- 66.Oldhafer KJ, Lang H, Schlitt HJ, et al. Long-term experience after ex situ liver surgery. Surgery. 2000;127:520–527. [DOI] [PubMed] [Google Scholar]
- 67.Hardwigsen J, Baqué P, Crespy B, et al. Resection of the inferior vena cava for neoplasms with or without prosthetic replacement: a 14-patients series. Ann Surg. 2001;233:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maeba T, Okano K, Mori S, et al. Retrohepatic vena cava replacement of hepatic malignancies without using total hepatic vascular exclusion or extracorporeal bypass. Hepatogastroenterology. 2001;48:1455–1460. [PubMed] [Google Scholar]
- 69.Arii S, Teramoto K, Kawamura T, et al. Significance of hepatic resection combined with inferior vena cava resection and its reconstruction with expanded polytetrafluoroethylene for treatment of liver tumors. J Am Coll Surg. 2003;196:243–249. [DOI] [PubMed] [Google Scholar]