Abstract
Objective:
We sought to test whether postconditioning, a series of brief mechanical interruptions of reperfusion applied during the onset of reperfusion, can prevent neurologic injury of the spinal cord after transient ischemia.
Summary Background Data:
Ischemia-reperfusion injury of the spinal cord is the principal mechanism leading to the paraplegia after surgery for descending and thoracoabdominal aortic aneurysms. Postconditioning has recently been demonstrated to confer cardioprotection by attenuating reperfusion injury.
Methods:
Spinal cord ischemia was induced in rabbits by infrarenal aorta occlusion for 25 minutes. Control animals underwent no additional intervention. Two groups of animals underwent postconditioning consisting of 4 or 6 cycles of 1-minute occlusion/1-minute reperfusion, respectively, which were applied 1 minute after the start of reperfusion. In 2 additional groups, 6 cycles of postconditioning started 5 or 10 minutes after the onset of reperfusion, respectively. Hind-limb motor function was assessed during a 10-day recovery period using the modified Tarlov criteria. Histologic examination of the spinal cord was performed, and the number of intact motor neurons was counted.
Results:
Compared with controls, 4 cycles of postconditioning significantly increased the Tarlov score and the number of intact motor neurons. Six cycles of postconditioning did not further improve the neuroprotection. Postconditioning starting 5 minutes after reperfusion still resulted in powerful neuroprotection, but the neuroprotection disappeared completely when postconditioning was delayed for 10 minutes.
Conclusions:
Postconditioning prevents neurologic injury of the spinal cord after ischemia, and the first few minutes of reperfusion are crucial to neuroprotection by postconditioning.
Postconditioning, a series of brief mechanical interruptions of early reperfusion, prevents neurologic injury of the spinal cord after transient ischemia in rabbits. The first few minutes of reperfusion during which postconditioning is applied are crucial to its neuroprotection.
Paraplegia remains a major devastating and unpredictable complication after surgical repair of descending and thoracoabdominal aortic aneurysms.1 Although this complication has been attributed to multiple factors, the principal root is thought to be related primarily to ischemia and reperfusion injury of the spinal cord.2,3 Numerous surgical techniques and pharmacologic interventions have been used to protect the spinal cord, but the complication still cannot be prevented completely.4
In 1986, the concept of ischemic preconditioning was first introduced as a potent endogenous form of cardioprotection against ischemia-reperfusion injury.5 Ischemic preconditioning has also been shown to have powerful protective effects against ischemia-reperfusion injury in neural system, including the spinal cord.6–9 Another endogenous form of cardioprotection, termed postconditioning, has been recently reported, in which a short series of repetitive cycles of brief reperfusion and reocclusion of the coronary artery were applied immediately at the onset of reperfusion, and this procedure has been shown to reduce myocardial injury to an extent comparable to that of ischemic preconditioning.10 The cardioprotective effect of postconditioning has been further confirmed in several models and species, and the underlying mechanisms have been suggested to include a reduction in neutrophil accumulation and decreased endothelial dysfunction,10 attenuation of oxidative stress,11 inhibition of the mitochondrial permeability transition,12 and activation of the phosphatidylinositol 3-kinase-Akt pathway.13 These data show that postconditioning can engage endogenous mechanisms to attenuate reperfusion injury specifically, which strongly suggests that it may also induce protective effects against reperfusion injury in tissues other than the myocardium. However, it is unknown whether postconditioning can confer neuroprotective effects on spinal cord ischemia. Therefore, the current study was designed to test the hypothesis that postconditioning would provide neuroprotection in a rabbit model of spinal cord ischemia after a relatively long recovery period (10 days).
METHODS
Animal Care and Surgical Procedure
Japanese white rabbits weighing 1.8 to 2.6 kg were used in the study. The animal protocol was approved by the Ethics Review Committee for Animal Experimentation of Hamamatsu University School of Medicine and was in accordance with the NIH guide for the use and care of laboratory animals.
Surgical preparation was conducted according to the method described previously.14 The rabbits were anesthetized with intravenous sodium pentobarbital (25 mg/kg) and allowed to breathe spontaneously; 0.5% lidocaine was administered at the site of the skin incision as local anesthesia. The left common carotid artery was cannulated with a 24-gauge catheter for monitoring the arterial pressure. Core body temperature was continuously monitored with a rectal probe and was maintained at about 38.5°C with the aid of a heating lamp. The infrarenal abdominal aorta was exposed through a transperitoneal approach. After systemic heparinization (200 U/kg), spinal cord ischemia was induced by crossclamping the abdominal aorta just distal to the renal arteries and just above the aortic bifurcation. At the end of the procedure, the clamps were released and the flank was closed in 2 layers.
Experimental Protocol
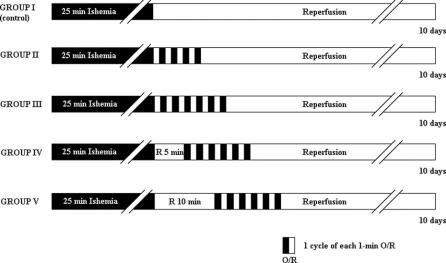
Rabbits were assigned to 5 groups, as shown in Figure 1. All groups were subjected to 25 minutes of spinal cord ischemia, and then all except the control group underwent a defined number of postconditioning cycles (one cycle comprised 1-minute occlusion and 1-minute reperfusion) at a defined time after the start of full reperfusion. Group I (control, n = 6): rabbits subjected to spinal cord ischemia only, without any further intervention. Group II (n = 7): 4 cycles of postconditioning were performed 1 minute after the start of full reperfusion. Group III (n = 7): 6 cycles of postconditioning were performed 1 minute after the start of full reperfusion. Group IV (n = 6): 6 cycles of postconditioning were performed 5 minutes after the start of full reperfusion. Group V (n = 6): 6 cycles of postconditioning were performed 10 minutes after the start of full reperfusion.
FIGURE 1. Experimental groups and protocol. O, occlusion; R, reperfusion
Neurologic Assessment
On days 1, 2, 5, and 10 after reperfusion, hind-limb motor function was assessed by blinded observers, using the modified Tarlov scale:15 0, no movement; 1, slight movement; 2, sit with assistance; 3, sit alone; 4, weak hop; 5, normal hop.
Histologic Study
All animals were killed by a lethal injection of pentobarbital (200 mg/kg) 10 days after the operation. Lumbar spinal cords (L4–L6) were quickly removed, fixed in 10% formalin, and embedded in paraffin. Transverse sections (4 μm) were cut and stained with hematoxylin and eosin. In cases in which the cytoplasm was diffusely eosinophilic, the large motor neuron cells were considered to be “necrotic or dead.” When the cells demonstrated basophilic stripling (containing Nissl substance), the motor-neuron cells were considered to be “viable or alive.”16 An investigator who was unaware of the animal groups and neurologic outcomes examined each slide. The intact motor neurons were counted in the ventral gray matter (anterior to a line drawn through the central canal perpendicular to the vertical axis) in 3 sections for each rabbit, and the numbers were then averaged.
Statistical Analysis
Statistical analyses of the neurologic score and the number of intact large motor neurons were performed with Mann-Whitney U tests. Physiological data are presented as means ± SD and were processed by analysis of variance for repeated measures. Statistical significance was defined as a P value less than 0.05.
RESULTS
Physiologic Parameters
All the animals survived the entire observation period. Body weight, rectal temperature and mean arterial blood pressure (MAP) at baseline, 15 minutes after ischemia and 15 minutes after reperfusion are shown in Table 1. There were no significant differences in the weight of the animals, and rectal temperature and MAP were not significantly different among the 5 groups at each time point.
TABLE 1. Physiologic Parameters
Neurologic Assessment
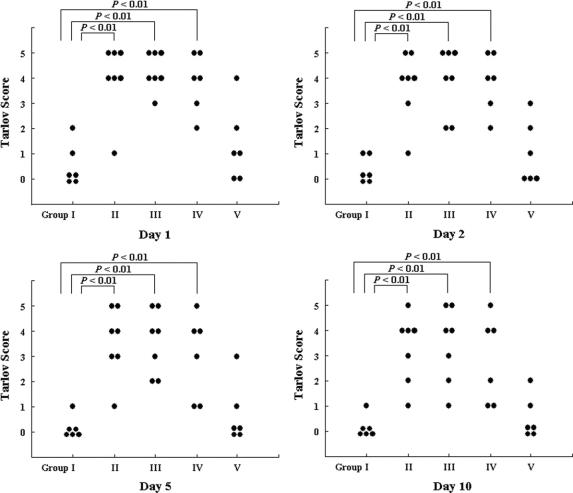
The individual neurologic scores of the 5 groups 1, 2, 5, and 10 days after reperfusion are shown in Figure 2. A 25-minute period of infrarenal aortic occlusion resulted in severe lower-extremity neurologic deficits in group I (the control group), in which 4 of the 6 rabbits showed complete paralysis (Tarlov score = 0) 1 day after reperfusion, with still worse scores found 10 days after reperfusion (five rabbits scored 0 points and one scored 1 point). Postconditioning (groups II and III) remarkably enhanced the recovery of motor function in the hind limb after the operation (P < 0.01 for group II and group III vs. the control group, at each of the 4 time points). Furthermore, the Tarlov scores for the 2 postconditioning groups did not decrease significantly, even 10 days after reperfusion (P > 0.05 vs. Tarlov scores assessed 1 day after reperfusion, for both group II and group III). Although the outcomes in group III appeared to be better, compared with group II, the difference did not reach statistical significance. Markedly higher Tarlov scores were still observed when the 6 cycles of postconditioning were performed 5 minutes after reperfusion (group IV, P < 0.01 vs. the control group, at each of the 4 time points), but there were no significant differences in neurologic outcome between the control animals and the rabbits that received 6 cycles of postconditioning 10 minutes after reperfusion (group V, P > 0.05 vs. the control group, at each of the 4 time points).
FIGURE 2. Neurologic function assessed 1, 2, 5, and 10 days after reperfusion. Solid cycles represent individual rabbits.
Histologic Assessment
Four rabbits that underwent a sham operation were also enrolled in the histologic study. Representative photographs of sections stained with hematoxylin and eosin are shown in Figure 3, and the results of counting viable motor neurons are summarized in Figure 4. In the sham-operated animals, the spinal cord was intact and many large motor neurons were present in the anterior horn. Twenty-five minutes of ischemia induced severe neuronal damage in control animals (group I) 10 days after reperfusion, as evidenced by vacuolization and frank necrosis, and almost 80% of the motor neurons were lost. In contrast, only slight changes in the spinal cord were found in groups II and III, and most motor neurons remained intact (P < 0.01 vs. the control group for both group II and group III). Many motor neurons were also preserved after 10-day reperfusion in group IV, and the number of motor neurons was much higher than that in the control group (P < 0.01). Apparent neuronal damage was observed in the spinal cords of animals in group V, and there was no significant difference in the motor neuron count between group V and the control group (P > 0.05).
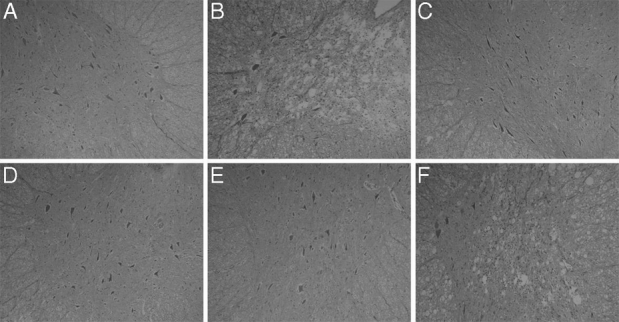
FIGURE 3. Representative sections of lumbar spinal cords stained with hematoxylin and eosin (original magnification, ×100). A, sham-operated group. B, Group I (control). C, Group II (4 cycles of postconditioning). D, Group III (6 cycles of postconditioning). E, Group IV (6 cycles of postconditioning with a 5-minute delay). F, Group V (6 cycles of postconditioning with a 10-minute delay). Severe histologic damage was found in groups I and V, whereas viable large motor neurons were preserved to a much greater extent in groups II, III, and IV.
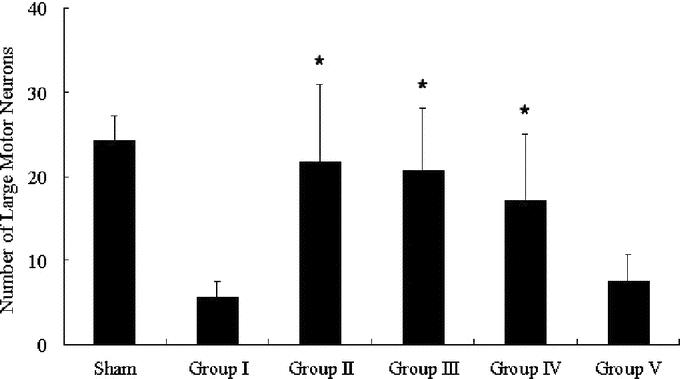
FIGURE 4. Number of large motor neurons in the ventral gray matter. *P < 0.01 versus group I (control).
DISCUSSION
The salient findings of the current study can be summarized as follows: 1) repetitive cycles of briefly interrupted reperfusion performed at the onset of full reperfusion, which is termed postconditioning, significantly reduces neurologic injury due to spinal cord ischemia in a rabbit model; and 2) neuroprotection by postconditioning is lost if the intervention is delayed for 10 minutes, which suggests that the timing of the application of postconditioning is critical.
Postconditioning was initially described and named by Zhao et al.10 In their study, 3 cycles of 30-second reperfusion and 30-second reocclusion preceding full reperfusion was found to confer potential cardioprotection against ischemia-reperfusion injury in a canine model, including a reduction in infarct size and preservation of endothelial function. In subsequent studies, the cardioprotective effects of postconditioning were further confirmed both in vivo and in vitro using rabbit and rat models.11–13,17,18 In the present study, we investigated the possible neuroprotective effects of postconditioning on spinal cord ischemia. In a reproducible rabbit model, we confirmed that 4 cycles of 1-minute occlusion/1-minute reperfusion applied 1 minute after release of 25-minute infrarenal occlusion significantly increased hind-limb motor function and the number of intact large motor neurons in the anterior horn of the lumbar spinal cord. The neuroprotective effects were not significantly improved with extension of the postconditioning algorithm to 6 cycles, which is in agreement with the findings of Yang's group, who showed that increasing the number of cycles of postconditioning did not increase the amount of salvaged myocardium.17 After ischemia, the motor neurons of the spinal cord undergo necrosis, as well as delayed neuron death. Despite recovery of blood flow, motor neurons that initially appear to have survived the ischemic insult go on to die a few days later.19,20 This phenomenon is compatible with delayed deterioration of neurologic function after spinal cord ischemia. In a similar rabbit model, ischemic preconditioning was found to protect the spinal cord against neuronal damage at 24 hours but not at 7 days after reperfusion, indicating that the irreversible neural injury was simply delayed, rather than reduced.21 In contrast, the current results show that the neuroprotective effects induced by postconditioning did not decrease markedly and were still significant after a 10-day recovery period, suggesting that postconditioning actually prevented but not only delayed the neural injury including delayed neuron death after spinal cord ischemia.
Another important message conveyed by the present study is that immediate application of postconditioning during the first few minutes of reperfusion is critical to neuroprotection. Although neuroprotection with postconditioning started 5 minutes after reperfusion was of comparable strength to that induced by postconditioning at the very onset of reperfusion, neuroprotection was lost with delay of the application of postconditioning until 10 minutes after reperfusion. A previous study has reported that cardioprotective effects conferred by postconditioning applied at the very onset of reperfusion disappear completely when the procedure is delayed for only 1 minute.11 Therefore, the powerful neuroprotection observed with postconditioning started 5 minutes after reperfusion was somewhat unexpected. Hence, it seems that the timing of the application of postconditioning for neuroprotection against spinal cord ischemia is not as critical as that for cardioprotection, a discrepancy that may be attributable to the differences in the organs with respect to ischemia-reperfusion injury.
Timely reperfusion is thought to be an effective strategy to salvage neural tissue after prolonged ischemia. However, there is convincing evidence that sudden and full restoration of blood flow to ischemic cerebral tissue may paradoxically exaggerate injury that is not present at the end of ischemia. In clinical trials, patients treated with thrombolytic therapy have shown a 6% rate of intracerebral hemorrhage, which was balanced against a 30% improvement in functional outcome over controls. Destruction of the microvasculature and extension of the infarct area occur after cerebral reperfusion.22 The manifestations of reperfusion injury are initiated during the early period of reperfusion, which is characterized by a burst of inflammatory-like reactions and may be exacerbated as the reperfusion continues. It has been suggested that reperfusion injury is a complex process involving several cellular and molecular signals, such as an inflammatory response occurring after the reestablishment of circulation, the excess formation of free radicals, mitochondrial calcium overload, and metabolic dyshomeostasis.22,23 To attenuate reperfusion injury, staged or controlled reperfusion with retarded restoration of full coronary blood flow and perfusion pressure has been proposed for many years.24,25 Recently, postconditioning, which can also be thought of as staged or controlled reperfusion, has been shown to provide powerful cardioprotection. These studies suggest that endogenous mechanisms are put into action within the first few minutes of reperfusion and specifically attenuate reperfusion injury. We have previously shown that pharmacological blockade of Na+/Ca2+ channels after reperfusion attenuates neural damage caused by spinal cord ischemia, indicating that neuroprotection can also be achieved by targeted reduction of reperfusion injury.14 In the present study, we confirmed that postconditioning conveys powerful neuroprotection against spinal cord ischemia. Postconditioning may reduce reperfusion injury by modifying the biochemical and metabolic perturbations that occur in the transition from ischemia to reperfusion and render the neurons more resistant to the insult after spinal cord ischemia. However, the mechanisms through which postconditioning induces neuroprotection against spinal cord ischemia remain to be defined in detail.
CONCLUSION
Our study demonstrates for the first time that postconditioning induces robust neuroprotective effects against spinal cord ischemia, and the early period of reperfusion is critical to the neuroprotection resulting from postconditioning. The observations of the current study provide compelling evidence of a novel method of spinal cord protection from reperfusion injury specifically. As a very simple procedure, postconditioning possesses a potential clinical value in the prevention of neurologic injury after thoracic aneurysm surgery.
Footnotes
Supported by Department of Anesthesiology, Hamamatsu University School of Medicine. Dr. Shi is supported by Japan Society for the Promotion of Science. Xiaojing Jiang and Enyi Shi are currently affiliated with China Medical University.
Reprints: Shigehito Sato, MD, Department of Anesthesiology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, #431-3192, Japan. E-mail: jiangxj@hama-med.ac.jp.
REFERENCES
- 1.Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–370. [PubMed] [Google Scholar]
- 2.Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3:389–404. [DOI] [PubMed] [Google Scholar]
- 3.Coselli JS, LeMaire SA, Miller CC 3rd, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg. 2000;69:409–414. [DOI] [PubMed] [Google Scholar]
- 4.Gharagozloo F, Larson J, Dausmann MJ, et al. Spinal cord protection during surgical procedures on the descending thoracic and thoracoabdominal aorta: review of current techniques. Chest. 1996;109:799–809. [DOI] [PubMed] [Google Scholar]
- 5.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 6.Zvara DA, Colonna DM, Deal DD, et al. Ischemic preconditioning reduces neurologic injury in a rat model of spinal cord ischemia. Ann Thorac Surg. 1999;68:874–880. [DOI] [PubMed] [Google Scholar]
- 7.Fan T, Wang CC, Wang FM, et al. Experimental study of the protection of ischemic preconditioning to spinal cord ischemia. Surg Neurol. 1999;52:299–305. [DOI] [PubMed] [Google Scholar]
- 8.Abraham VS, Swain JA, Forgash AJ, et al. Ischemic preconditioning protects against paraplegia after transient aortic occlusion in the rat. Ann Thorac Surg. 2000;69:475–479. [DOI] [PubMed] [Google Scholar]
- 9.Toumpoulis IK, Papakostas JC, Matsagas MI, et al. Superiority of early relative to late ischemic preconditioning in spinal cord protection after descending thoracic aortic occlusion. J Thorac Cardiovasc Surg. 2004;128:724–730. [DOI] [PubMed] [Google Scholar]
- 10.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–588. [DOI] [PubMed] [Google Scholar]
- 11.Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. [DOI] [PubMed] [Google Scholar]
- 12.Argaud L, Gateau-Roesch O, Raisky O, et al. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. [DOI] [PubMed] [Google Scholar]
- 13.Tsang A, Hausenloy DJ, Mocanu MM, et al. Postconditioning: a form of ‘modified reperfusion’ protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. [DOI] [PubMed] [Google Scholar]
- 14.Shi E, Kazui T, Jiang X, et al. NS-7, a novel Na+/Ca2+ channel blocker, prevents neurologic injury after spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg. 2005;129:364–371. [DOI] [PubMed] [Google Scholar]
- 15.Tarlov IM. Acute spinal cord compression paralysis. J Neurosurg. 1972;36:10–20. [DOI] [PubMed] [Google Scholar]
- 16.Mutch WA, Graham MR, Halliday WC, et al. Use of neuroanesthesia adjuncts (hyperventilation and mannitol administration) improves neurological outcome after thoracic aortic cross-clamping in dogs. Stroke. 1993;24:1204–1210. [DOI] [PubMed] [Google Scholar]
- 17.Yang XM, Proctor JB, Cui L, et al. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. [DOI] [PubMed] [Google Scholar]
- 18.Halkos ME, Kerendi F, Corvera JS, et al. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Horinouchi T, Sakurai M, et al. Cyclosporin A reduces delayed motor neuron death after spinal cord ischemia in rabbits. Ann Thorac Surg. 2003;75:1294–1299. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai M, Hayashi T, Abe K, et al. Delayed and selective motor neuron death after transient spinal cord ischemia: a role of apoptosis? J Thorac Cardiovasc Surg. 1998;115:1310–1315. [DOI] [PubMed] [Google Scholar]
- 21.Kakimoto M, Kawaguchi M, Sakamoto T, et al. Evaluation of rapid ischemic preconditioning in a rabbit model of spinal cord ischemia. Anesthesiology. 2003;99:1112–1117. [DOI] [PubMed] [Google Scholar]
- 22.Jean WC, Spellman SR, Nussbaum ES, et al. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–1396. [DOI] [PubMed] [Google Scholar]
- 23.Hossmann KA. Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock. 1997;8:95–101. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto F, Allen BS, Buckberg GD, et al. Studies of controlled reperfusion after ischemia XIV: reperfusion conditions: importance of ensuring gentle versus sudden reperfusion during relief of coronary occlusion. J Thorac Cardiovasc Surg. 1986;92:613–620. [PubMed] [Google Scholar]
- 25.Sato H, Jordan JE, Zhao Z, et al. Gradual reperfusion reduces infarct size and endothelial injury but augments neutrophil accumulation. Ann Thorac Surg. 1997;64:1099–1107. [DOI] [PubMed] [Google Scholar]