Abstract
Objective:
To determine the role of chemokine receptor (CR) expression in patients with melanoma and colorectal cancer (CRC) liver metastases.
Summary Background Data:
Murine and in vitro models have identified CR as potential factors in organ-specific metastasis of multiple cancers. Chemokines via their respective receptors have been shown to promote cell migration to distant organs.
Methods:
Patients who underwent hepatic surgery for melanoma or CRC liver metastases were assessed. Screening cDNA microarrays of melanoma/CRC cell lines and tumor specimens were analyzed to identify CR. Microarray data were validated by quantitative real-time RT-PCR (qRT) in paraffin-embedded liver metastases. Migration assays and immunohistochemistry were performed to verify CR function and confirm CR expression, respectively.
Results:
Microarray analysis identified CXCR4 as the most common CR expressed by both cancers. qRT demonstrated CXCR4 expression in 24 of 27 (89%) melanoma and 28 of 29 (97%) CRC liver metastases. In vitro treatment of melanoma or CRC cells with CXCL12, the ligand for CXCR4, significantly increased cell migration (P < 0.001). Low versus high CXCR4 expression in CRC liver metastases correlated with a significant difference in overall survival (median 27 months vs. 10 months, respectively; P = 0.036). In melanoma, low versus high CXCR4 expression in liver metastases demonstrated no difference in overall survival (median 11 months vs. 8 months, respectively; P = not significant).
Conclusions:
CXCR4 is expressed and functional on melanoma and CRC cells. The ligand for CXCR4 is highly expressed in liver and may specifically attract melanoma and CRC CXCR4 (+) cells. Quantitative analysis of CXCR4 gene expression in patients with liver metastases has prognostic significance for disease outcome.
Activation of chemokine receptors results in cell migratory events and has been linked to organ-directed metastasis of numerous cancers. Melanoma and colorectal cancer share a predilection to metastasize to the liver. Here, we demonstrate that chemokine receptor CXCR4 is expressed and correlates with disease outcome in both types of liver metastases.
The mechanisms of cancer metastasis are still debated more than 100 years after the “seed and soil” theory was hypothesized by Paget.1 Through his experience with breast cancer patients, Paget theorized that cancers metastasize preferentially to organs or sites that support and/or nurture the growth of cancer cells. Ewing later countered that patterns of blood flow from the primary tumor could entirely account for the patterns of metastasis.2 His “mechanical” hypothesis predicted that the circulation would deliver the greatest metastatic tumor burden to the first organ encountered.3 Neither model is absolute, as clinical examples of exceptions to both exist.4
In light of these deficiencies, new mechanisms have been sought and a recent addition to these theories is the signaling or “homing” hypothesis. This mechanism is based upon the complex signaling patterns that occur in organogenesis, development, hematopoeisis, and immune responses. As such, it incorporates principles from both seed-and-soil and mechanical hypotheses. In the signaling or homing mechanism for cancer metastasis, cancer cells are drawn to specific metastatic sites as a result of a complex interchange of signals.5 Key mediators in this mechanism are chemokines, which are chemoattractive signaling molecules that function in a myriad of cell trafficking events.
Chemokines attained, perhaps, their greatest notoriety when the chemokine receptor CXCR4 was identified as a coreceptor for T-tropic HIV-1 and HIV-2.6–8 Since then, intense research with chemokines has led to the discovery that these chemoattractive molecules may play a significant role in cancer metastasis. Currently, chemokines and their respective receptors have been identified as contributing metastatic factors in numerous cancers.9–20 Müller et al9 and Zeelenberg et al10 have provided compelling in vivo data in murine models to implicate chemokine receptors in the metastatic progression of cancers to the liver. Such chemokines and receptors are typically quiescent in many normal tissues, with the notable exception of immune cells, and appear to be activated or up-regulated in cancer. Activation of chemokine receptors promotes the growth, adhesion, and most importantly directional migration of immune cells during antigen-specific inflammatory responses.21–23 Evidence now indicates that cancer cells exploit these innate signaling mechanisms to yield specific targeted metastasis.
Recently, our group identified the significance of chemokine receptor CXCR4 expression in patients with colorectal cancer (CRC).24 We demonstrated a significant association between high CXCR4 gene expression of primary CRC tumors and poor clinical outcomes. This finding was concordant with reports in other cancers identifying a correlation of CXCR4 expression with disease outcome.19,20,25–27 Our report also noted an up-regulation of CXCR4 gene expression in liver metastases compared with primary tumors and identified a potential chemoattractive relation between CRC CXCR4 receptors and the liver, which is a rich source of CXCL12, the specific ligand for CXCR4.9
In the present study, we hypothesized that cancers that have metastasized to the liver may express the same or similar chemokine receptors (CR). We selected CRC based on our previous report24; we selected melanoma because of its propensity for hepatic metastasis demonstrated by postmortem examination.28,29 Our objective was to determine the role of CR in patients with liver metastases of these 2 different cancers and to evaluate the clinical relevance of CR expression in both groups of patients.
MATERIALS AND METHODS
Patients, Tissues, and Cell Lines
Patients who underwent surgical resection for melanoma or CRC liver metastases were selected from institutional melanoma and GI databases. Matched benign colon and liver specimens were also identified from the databases and evaluated for this study. These specimens were procured from patients with primary CRC or isolated liver metastasis, respectively. All patients were treated at John Wayne Cancer Institute (JWCI)/Saint John's Health Center (SJHC), Santa Monica, CA, between 1996 and 2003, and underwent operative exploration with curative intent. All melanoma patients were followed up at JWCI for their treatment course. CRC patients were evaluated at JWCI within the early postoperative period, and then were returned to the care of their primary physician when appropriate. Standard clinicopathologic factors were obtained from database and chart review. Patients provided informed consent for tissue procurement, and Institutional Review Board (IRB) approval was obtained prior to study initiation.
Operative specimens were collected immediately following resection, snap frozen, and stored at −80°C for later use. Paraffin-embedded archival tissue (PEAT) blocks were stored at and retrieved from the Department of Surgical Pathology of SJHC. Established CRC cell lines (DLD1, LoVo, HT-29, SW480, SW620, and WiDr) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in ATCC-recommended media. The CRC cell line, CX-1, was provided by Dr. Peter Thomas (Boston University School of Medicine, Boston, MA). Melanoma cell lines (MA, MB, MC, MD, ME, MF, MG, MH, and MI) were developed from melanoma metastases at JWCI. These cell lines, including CX-1, were maintained in RPMI 1640 medium (Gibco, Carlsbad, CA) with 10% (vol/vol) heat-inactivated fetal calf serum and 1% penicillin/streptomycin at 37°C with 5% CO2.
RNA Isolation and Microarray Analysis
RNA from frozen tissues and cell lines was extracted, isolated, and purified using Tri-Reagent (Molecular Research Center, Cincinnati, OH) as previously described.30,31 PEAT total RNA was extracted using modifications of the RNAWiz Isolation Kit (Ambion, Austin, TX).32 RNA was quantified and assessed for purity by ultraviolet spectrophotometry and RIBOGreen detection assay (Molecular Probes, Eugene, OR) as previously described.30
Total RNA (5 μg) from CRC specimens (cell line, primary CRC, and liver metastasis) and from melanoma specimens (cell line, primary melanoma, and liver metastasis) was screened for CR expression by microarray analysis (Human Chemokine & Receptor Q Series GEArray kit, SuperArray Bioscience Corp., Bethesda, MD). cDNA probes were constructed according to the manufacturer's instructions and hybridized onto GEArray nylon membranes. After incubation with a chemifluorescent substrate (ECF, Amersham Biosciences, San Francisco, CA), signal intensities were measured on a Storm 840 PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and image analysis was performed as previously described.24
Primers and Probes and Quantitative RT-PCR
Primer and probe sequences were designed and verified as previously described.24 Specific primers were designed to sequence at least one exon-exon region and to optimally amplify cDNA with amplicons less than 150 bp to account for fragmented RNA in PEAT samples. The primers and FRET probe sequences used were: CXCR4 (145 bp): 5′-GGAGGGGATCAGTATATACA-3′ (forward); 5′-GAAGATGATGGAGTAGATGG-3′ (reverse); and 5′-FAM-CGAGGAAATGGGCTCAGGGG-BHQ-1-3′ (FRET probe). CXCL12 (112 bp):5′-CCCGAAGCTAAAGTGGATTC-3′ (forward); 5′-TTCAGAGCTGGGCTCCTACT-3′ (reverse); and 5′-FAM-TGAGAGGGTCAGACGCCTGAGG-BHQ-1-3′ (FRET probe). Glyceraldehyde-3-phoshate dehydrogenase (GAPDH; 136 bp): 5′-GGGTGTGAACCATGAGAAGT-3′ (forward); 5′-GACTGTGGTCATGAGTCCT-3′ (reverse); and 5′-FAM-CAGCAATGCCTCCTGCACCACCAA-BHQ-1-3′ (FRET probe).
Reverse transcription of total RNA was performed using Moloney Murine Leukemia Virus RT (Promega, Madison, WI). Oligo dT (Gene Link, Hawthorne, NY) and random hexamers (Roche, Indianapolis, IN) were added to the PEAT reaction mixtures for more robust cDNA production as previously described.32 The quantitative real-time RT-PCR (qRT) assay was performed with the iCycler iQ RealTime PCR Detection System (Bio-Rad Laboratories, Hercules, CA) using 250 ng of total RNA for each reaction. Each PCR reaction was subjected to 45 cycles at 95°C for 60 seconds, 60°C for 60 seconds, and 72°C for 60 seconds for CXCR4 and CXCL12; and 45 cycles at 95°C for 60 seconds, 55°C for 60 seconds, and 72°C for 60 seconds for GAPDH. Each sample was assayed in triplicate with appropriate positive and negative tissue controls and reagent controls as previously described.24 The expression of the housekeeping gene GAPDH was assessed in each sample to verify mRNA integrity.
Immunohistochemistry
Immunohistochemistry (IHC) was performed to confirm the translation of CXCR4 mRNA to protein in PEAT melanoma and CRC liver metastases. Paraffin-embedded tumor blocks were sectioned (5 μm), dried overnight at 37°C, and then deparaffinized with xylene. The sections were treated with an antigen retrieval solution (Target Retrieval, DakoCytomation, Carpinteria, CA) at 95°C for 15 minutes, cooled to room temperature, and then treated with dilute hydrogen peroxide to block endogenous peroxidase. Nonspecific antibody binding was diminished with 1% albumin. The sections were incubated overnight at 4°C with a monoclonal mouse antihuman CXCR4 (12G5) antibody (Santa Cruz Biotechnology; Santa Cruz, CA) at a dilution of 1:200. Isotype control slides were incubated with an antimouse IgG2a antibody (Santa Cruz Biotechnology). The next day, sections were labeled with a secondary Link-Streptavidin HRP solution (Dako Corp.), developed with diaminobenzidine (DAB), counterstained with hematoxylin and examined at 400× magnification.
Chemotaxis Assay
Cell lines were assessed for migration utilizing a modified Boyden chamber chemotaxis assay to assess response to CXCL12, the specific ligand of CXCR4.33 For this assay, 6.5-mm-diameter chambers with 8-μm pore filters (HTS Transwell-24 System; Corning, Acton, MA) were used. Laminin (20 μg/mL) was coated on the lower surfaces of the filter inserts.34 CXCL12 (Peprotech, Rocky Hill, NJ), the specific ligand to CXCR4, was added to the lower wells of the Boyden chamber with serum-free medium and 0.1% albumin. Selective cells were treated with an anti-CXCR4 neutralizing antibody (10 ng/mL) (R&D Systems, Minneapolis, MN) 2 hours prior to assay performance. Melanoma or CRC cells (104) were placed into the insert and incubated overnight at 37°C. The next day, the cells within the inserts were removed using cotton-tipped applicators, fixed in 100% ethanol, washed with phosphate buffer solution, and then stained with crystal violet. Cells were assessed and evaluated as previously described.32
Statistical Analysis
Disease-free and overall survival curves for patients with high or low CXCR4 expression levels were constructed using Kaplan-Meier's method. The log-rank test was used to compare the equality of the 2 curves. Univariate analysis of prognostic factors was assessed by the log-rank test. The prognostic significance of high versus low CXCR4 expression was assessed by multivariate analysis of established clinical prognostic factors using the Cox proportional hazard regression model. A stepwise method was chosen for covariate selection. Analyses were performed using SAS (SAS /STAT User's Guide, version 8; SAS Institute Inc, Cary, NC). All tests were 2-sided, and P values were considered significant when <0.05.
RESULTS
Patients
All patients had undergone operative resection for melanoma or CRC liver metastases at JWCI. Twenty-seven melanoma patients with available PEAT operative hepatic specimens were identified and accrued for analysis. None of the patients had received a melanoma cancer vaccine as part of their initial treatment regimen for primary melanoma. All eligible patients received adjuvant therapy following hepatic resection. Adjuvant therapy was not administered to one patient that died in the perioperative period (within 30 postoperative days).
A comparison group of 29 consecutive CRC patients was selected from a JWCI GI database. Patients who had stage IV disease at time of initial diagnosis of the colorectal tumor underwent operative resection prior to initiation of chemotherapy. All eligible patients received adjuvant chemotherapy after hepatic resection. Chemotherapy was not administered by hepatic arterial infusion pump for any patient with CRC liver metastasis. One patient died in the immediate perioperative period secondary to fulminant hepatic failure. Additionally, histologically benign liver (n = 15) and colon (n = 8) specimens were accrued to compare CXCL12 gene expression levels. The demographic data of both CRC and melanoma patients with liver metastasis are presented in Table 1.
TABLE 1. Demographics of Patients With Liver Metastases
Microarray Analysis
Melanoma and CRC RNA derived from heterogeneous sources (cell line, primary tumor, and hepatic metastasis) was analyzed using the Human Chemokine & Receptor Q Series GEArray Kit (SuperArray). Of the 96 spots on the GEArray microarray membrane, 16 were assessed for CR. The 2 sets of samples demonstrated expression of 6 different CR. Only CXCR4 was detected in greater than half the samples (n = 4 of 6). Therefore, it was selected for further investigation in this study.
CXCR4 Expression and Clinical Outcomes
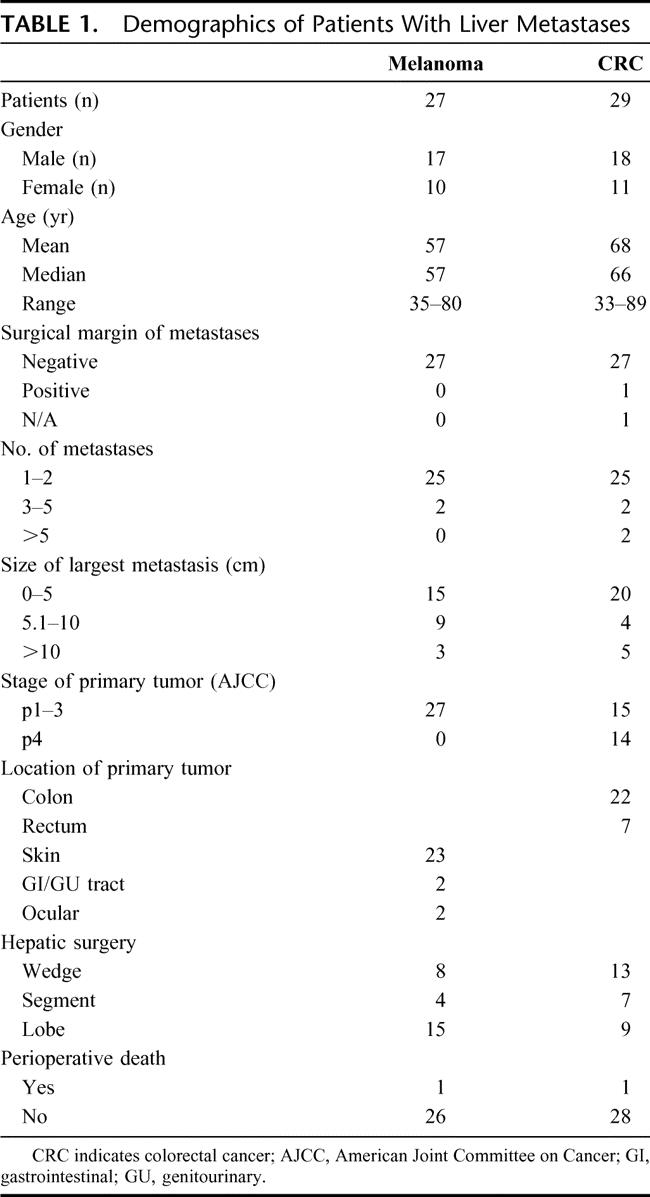
CXCR4 gene expression was initially assessed by qRT in melanoma and CRC cell lines. Six of the 7 CRC lines and all 9 of the melanoma cell lines were positive for CXCR4 gene expression (Fig. 1). PEAT melanoma and CRC metastatic liver specimens were then assessed by qRT. Eighty-nine percent (n = 24 of 27) and 97% (n = 28 of 29) of melanoma and CRC liver metastases, respectively, had CXCR4 gene expression (Fig. 2).
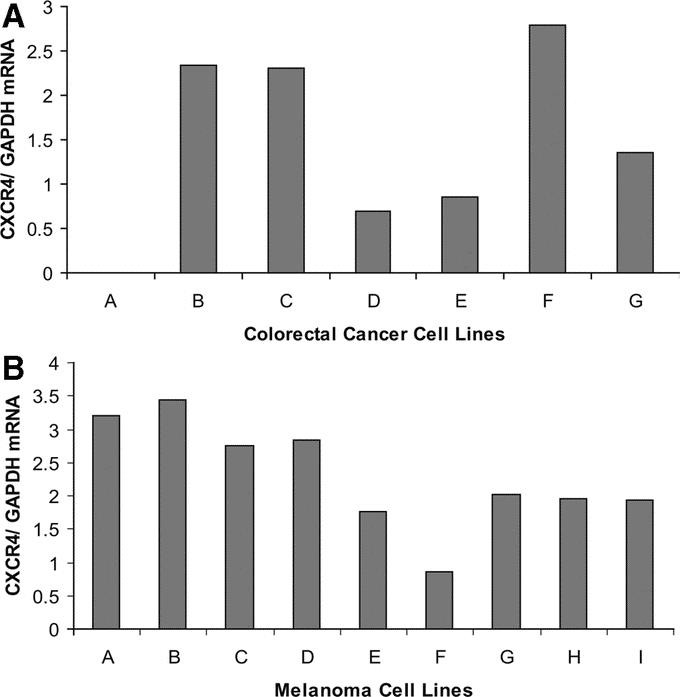
FIGURE 1. A, CXCR4 screening of CRC cell lines. B, CXCR4 screening of melanoma cell lines. Cell lines A and B (MA and MB), which were established from melanoma liver metastases, had the highest levels of CXCR4 gene expression.
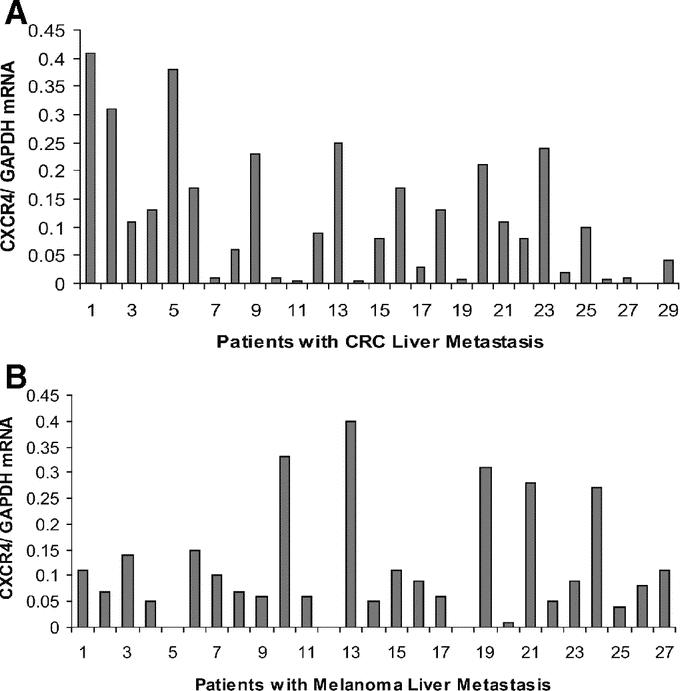
FIGURE 2. A, CXCR4 gene expression in patients with CRC metastasis to the liver. B, CXCR4 expression in patients with melanoma metastasis to the liver.
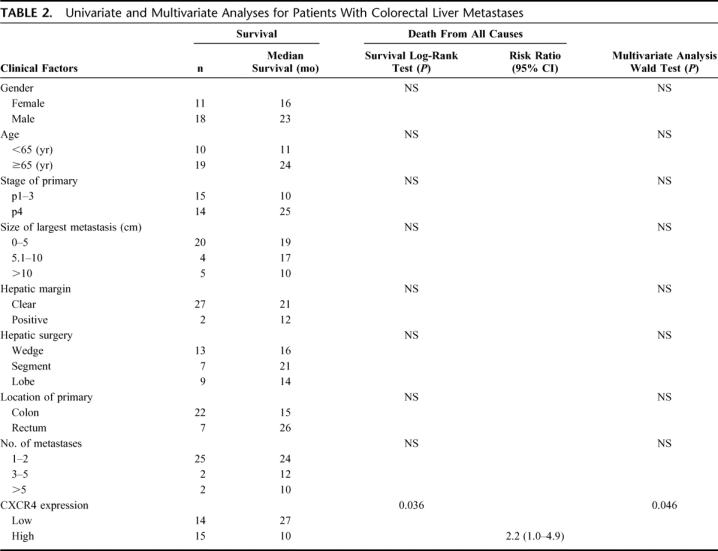
Since a standard range of CXCR4 gene expression values has not yet been established, we used the median CXCR4 gene expression value for each cancer to dichotomize liver metastases into high and low CXCR4-expressing tumors. Clinical outcomes were assessed according to this dichotomization. In patients with CRC, comparison by the Kaplan-Meier method for low versus high CXCR4 expression showed a significant difference in overall survival (median survival 27 months vs. 10 months, respectively; P = 0.036) (Fig. 3); the hazard ratio was also significant (HR 2.2; 95% CI, 1.0–4.9, P = 0.046). Univariate and multivariate analyses identified CXCR4 expression as an independent factor for overall survival of patients with CRC liver metastases. No other factor examined was statistically significant based on univariate testing (Table 2).
FIGURE 3. Kaplan-Meier survival curves demonstrate prolonged overall survival in colorectal cancer patients whose liver metastases had lower CXCR4 expression (log-rank test, P = 0.036).
TABLE 2. Univariate and Multivariate Analyses for Patients With Colorectal Liver Metastases
In melanoma, significant differences in overall survival (median 11 months vs. 8 months, P = 0.19) and increased survival at 3 years and 5 years (23% vs. 0% and 8% versus 0%, respectively; P = 0.19) with low versus high CXCR4 expression were not identified (Fig. 4). Because the overall survival differences were not statistically significant, additional univariate and multivariate analyses were not performed.
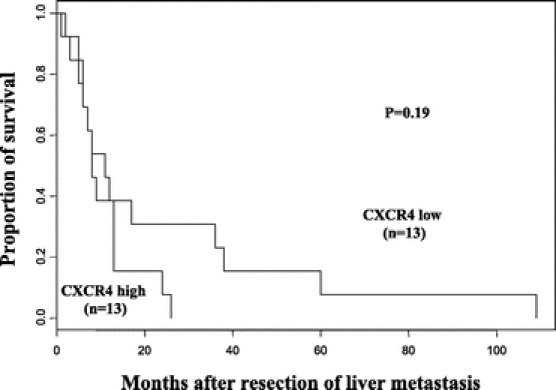
FIGURE 4. Kaplan-Meier curves demonstrate a nonsignificant increase in overall survival of melanoma patients whose liver metastases had low CXCR4 expression (log-rank test, P = 0.19).
Analysis of CXCR4 Expression by IHC
When IHC was performed on PEAT sections of liver metastases, membrane and cytoplasmic patterns of immunostaining for CXCR4 protein were observed in both CRC and melanoma cells (Fig. 5a). IHC of PEAT sections of normal liver demonstrated generally weak, and in some cases no detectable staining in benign hepatocytes (Fig. 5b).
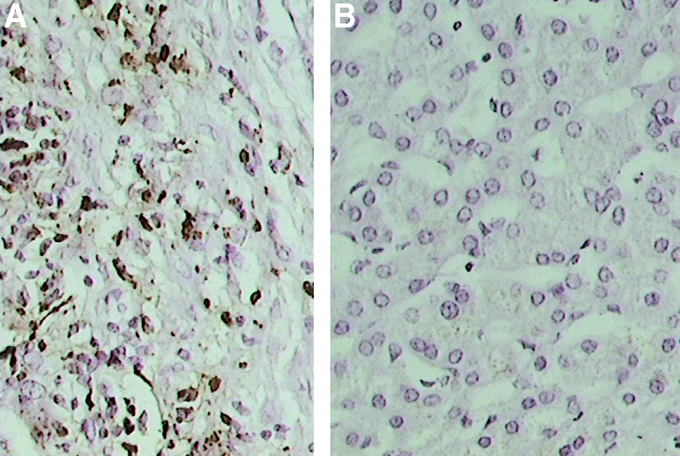
FIGURE 5. A, Representative liver metastasis specimen demonstrating strong immunoreactivity of the CXCR4 protein in melanoma cells. B, Normal liver specimen demonstrating minimal to absent cytoplasmic immunostaining of the CXCR4 protein in hepatocytes (hematoxylin stain, original magnification ×400).
CXL12 Expression and Chemotaxis Assay
Müller et al9 found that CXCL12, the specific ligand to CXCR4, is produced constitutively by many cells but has the highest levels in the lung, liver, lymphoid tissue, and bone marrow. We also found by qRT that CXCL12 expression is 10-fold higher in histologically benign liver tissues compared with benign colonic tissues (P = 0.015).
To demonstrate the functional role of CXCR4 receptors in relation to CXCL12, migrational assays were performed. Studies demonstrated responses of melanoma and CRC cells to CXCL12. The number of migrating melanoma or CRC cells after stimulation with CXCL12 (50 ng/mL) was significantly greater than untreated controls (P < 0.001 and P < 0.001, respectively). The incubation of cells with a monoclonal anti-CXCR4 antibody blocked both melanoma and CRC cell migration by 38% (P < 0.001). These studies demonstrated the functional status of the CXCR4 receptor on melanoma and CRC and its responsiveness to CXCL12.
DISCUSSION
Numerous reports now demonstrate a role for chemokines and their respective receptors in cancer metastasis. Although these studies appear to support homing or signaling, the strength of these reports is in the incorporation, rather than disregard, of principles from both seed and soil and mechanical theories. It is clear that the bloodstream or vascular channels are requisite for transport of cancer cells to their target; therefore, the specific routes may impart some bias to a particular target organ. Here, we have examined CRC, which has direct vascular drainage to the liver and may appear to overwhelmingly favor a mechanical route for metastasis. However, a recent report demonstrates that all 3 aforementioned theories likely have contributing roles.10 Zeelenberg et al conducted studies in a murine model and discovered that CXCR4 was essential for preferential metastasis of CRC to the liver and lung.10 However, they noted that only cancer cells that had implanted in the liver were able to flourish and grow. Specific homing or signaling in organ-specific metastasis may have a more central role in melanoma with its various metastatic targets. Our group has demonstrated that expression of chemokine receptor CCR7 is specifically associated with metastasis to lymph nodes, where the specific ligand CCL21 is abundant.32
The liver may be a specific target for CXCR4-expressing cells because of its relative abundance of CXCL12. CXCL12, previously known as stromal derived factor-1 (SDF-1), was characterized shortly after CXCR4 was discovered.35,36 Current studies demonstrate that ligand receptor binding of CXCL12 to CXCR4 results in cell proliferation, adhesion, and directional migration.21–23 The CXCR4/CXCL12 axis has been implicated in the metastasis of over 20 different cancers.37 Thus, we were not surprised to identify frequent expression of CXCR4 in hepatic metastases of both CRC and melanoma. However, chemokines and receptors are typically quiescent and not expressed in many normal tissues. In hepatic parenchymal tissues, CXCR4 gene expression levels were minimal; qRT assay of 18 benign liver specimens demonstrated no CXCR4 expression in 15 specimens and minor CXCR4 expression in 3 specimens.
Most, if not all, studies on chemokine receptors have been conducted in murine models or as in vitro studies. Studies that evaluate clinical specimens and provide correlative outcome data are lacking. It was our aim to show the potential translational relevance of CXCR4 expression after the establishment of liver metastases in patients with 2 very different cancers. Activation of CXCR4 can result in specific interaction with other metastasis-related genes.13,38,39 Previously, we have demonstrated the significance of CXCR4 gene expression in primary CRC.22 Here, we show that gene expression of CXCR4 correlates with clinical outcomes in patients with CRC or melanoma liver metastases. In CRC, overall survival was significantly longer in patients that had lower gene expression of CXCR4. Even when factors that have been associated with outcomes were evaluated by multivariate analysis, CXCR4 expression remained the only significant factor that correlated with overall survival.40–42 The prognostic significance of CXCR4 may be explained by the rich hepatic production of CXCL12, the specific ligand to CXCR4. We hypothesize that activation of this receptor continues in the liver. Therefore, cells that have higher levels of CXCR4 may have greater potential for local growth and invasion within the liver.
In melanoma, overall survival was prolonged in patients with lower values of CXCR4 gene expression, but the results were not significant. We provide an explanation to account for the lack of statistical significance in melanoma as opposed to CRC. Melanoma metastasis to the liver has among the worst outcomes in metastatic melanoma and effective adjuvant treatment strategies are lacking beyond surgery.26,43 Larger cohort studies may better account for these shortcomings in melanoma treatment to delineate the potential correlation of CXCR4 expression with disease outcomes in metastatic melanoma. Nevertheless, our study highlights the need to therapeutically target this receptor to prevent or treat liver metastases.
The data presented in this study build on the foundation of published in vitro studies. As a translational study, it emphasizes the significance of CR and identifies similar expression and function of the same CR in 2 very different types of liver metastases. These significant findings were observed not only in vitro, but also in patients with metastatic CRC or melanoma. Recently, specific inhibitors to CXCR4 have been effective in arresting the downstream actions of the CXCR4 receptor.44–48 These CXCR4 antagonists have reached phase II trials and represent a new dimension of developing therapies to attack molecular targets of malignancies metastatic to the liver.
Footnotes
Presented in part at the 2004 Surgical Forum, American College of Surgeons Clinical Congress, October 10–14, 2004, New Orleans, LA.
Supported in part by P01 CA12582 and CA29605, NCI, NIH; Rod Fasone Memorial Cancer Fund, Indianapolis, IN; and Martin H. Weil and Roy E. Coats Research Laboratories, John Wayne Cancer Institute at Saint John's Health Center.
Reprints: Dave S. B. Hoon, PhD, Department of Molecular Oncology, John Wayne Cancer Institute, 2200 Santa Monica Blvd, Santa Monica, CA 90404. E-mail: hoon@jwci.org.
REFERENCES
- 1.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 2.Ewing J. Neoplastic Diseases, ed 6. Philadelphia: Saunders, 1928. [Google Scholar]
- 3.Reynolds T. Why tumors travel to certain sites still puzzles researchers. J Natl Cancer Inst. 1998;90:491–493. [DOI] [PubMed] [Google Scholar]
- 4.Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis. 1992;10:191–199. [DOI] [PubMed] [Google Scholar]
- 5.Stetler-Stevenson WG, Kleiner DE Jr. Molecular biology of cancer: invasion and metastasis. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins, 2001:123–136. [Google Scholar]
- 6.Feng Y, Broder CC, Kennedy PE, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. [DOI] [PubMed] [Google Scholar]
- 7.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. [DOI] [PubMed] [Google Scholar]
- 8.Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. [DOI] [PubMed] [Google Scholar]
- 9.Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. [DOI] [PubMed] [Google Scholar]
- 10.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 11.Burger M, Glodek A, Hartmann T, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. [DOI] [PubMed] [Google Scholar]
- 12.Klein RS, Rubin JB, Gibson HD, et al. SDF-1α induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. [DOI] [PubMed] [Google Scholar]
- 13.Staller P, Sulitkova J, Lisztwan J, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. [DOI] [PubMed] [Google Scholar]
- 14.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 15.Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–45105. [DOI] [PubMed] [Google Scholar]
- 16.Taichman RS, Cooper C, Keller ET, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 17.Libura J, Drukala J, Tomescu O, et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597–2606. [DOI] [PubMed] [Google Scholar]
- 18.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. [DOI] [PubMed] [Google Scholar]
- 19.Longo-Imedio MI, Longo N, Trevino I, et al. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. In press. [DOI] [PubMed]
- 20.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. [DOI] [PubMed] [Google Scholar]
- 21.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- 22.Epstein FH. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. [DOI] [PubMed] [Google Scholar]
- 23.Ganju RK, Brubaker SA, Meyer J, et al. The α-chemokine, stromal cell-derived factor-1α, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Takeuchi H, Lam ST, et al. CXCR4 expression in patients with colorectal cancer promotes liver metastasis. J Clin Oncol. 2005;23:2744–2753. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Kitayama J, Kazama S, et al. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rombouts EJ, Pavic B, Lowenberg B, et al. Relation between CXCR-4 expression, Flt3 mutations and unfavourable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–557. [DOI] [PubMed] [Google Scholar]
- 27.Spano JP, Andre F, Morat L, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–617. [DOI] [PubMed] [Google Scholar]
- 28.Rose DM, Essner R, Hughes TMD, et al. Surgical resection for metastatic melanoma to the liver. Arch Surg. 2001;136:950–955. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh EC, Essner R, Foshag LJ, et al. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100:122–129. [DOI] [PubMed] [Google Scholar]
- 30.Sarantou T, Chi DDJ, Garrison DD, et al. Melanoma-associated antigens as messenger RNA detection markers for melanoma. Cancer Res. 1997;57:1371–1376. [PubMed] [Google Scholar]
- 31.Takeuchi H, Bilchik A, Saha S, et al. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res. 2003;9:1480–1488. [PubMed] [Google Scholar]
- 32.Takeuchi H, Fujimoto A, Tanaka M, et al. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004;10:2351–2358. [DOI] [PubMed] [Google Scholar]
- 33.Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 34.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bleul CC. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. [DOI] [PubMed] [Google Scholar]
- 36.Oberlin E. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. [DOI] [PubMed] [Google Scholar]
- 37.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. [DOI] [PubMed] [Google Scholar]
- 38.Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. [DOI] [PubMed] [Google Scholar]
- 39.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. [DOI] [PubMed] [Google Scholar]
- 40.Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231–255. [DOI] [PubMed] [Google Scholar]
- 41.DeMatteo RP, Shah A, Fong Y, et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood TF, DiFronzo LA, Rose DM, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658–62. [DOI] [PubMed] [Google Scholar]
- 44.Tamamura H, Fujisawa M, Hiramatsu K, et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569:99–104. [DOI] [PubMed] [Google Scholar]
- 45.Takenaga M, Tamamura H, Hiramatsu K, et al. A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem Biophys Res Commun. 2004;320:226–232. [DOI] [PubMed] [Google Scholar]
- 46.Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:1095–1102. [DOI] [PubMed] [Google Scholar]
- 47.Mori T, Doi R, Koizumi M, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 48.Sachpatzidis A, Benton BK, Manfredi JP, et al. Identification of allosteric peptide agonists of CXCR4. J Biol Chem. 2003;278:896–907. [DOI] [PubMed] [Google Scholar]


