Abstract
Aim:
To assess the effect of portal vein embolization (PVE) on intrahepatic recurrence rate after right hepatectomy for unilobar colorectal liver metastases (CLM).
Summary and Background:
Recent research suggests that CLM could spread retrogradely through the portal vein. PVE may reduce tumor shedding by the occlusion of distal portal branches. However, no study reported the clinical effect of PVE on intrahepatic recurrence after CLM resection.
Patients and Methods:
Between 1995 and 2003, 44 patients requiring a right hepatectomy for unilobar CLM were operated in our institution. Right hepatectomy was performed after PVE in 23 patients (group A) and without PVE in 21 (group B). Surgical outcome and site of recurrence were analyzed.
Results:
The postoperative mortality was nil. Overall morbidity and transitory liver failure rates were similar in groups A and B (43.4% and 17.3% vs. 33.3% and 14.2%, respectively). The 3- and 5-year overall survival rates did not differ in group A and B patients (61.2% and 43.7% vs. 49.7% and 35.5%, respectively; P = 0.862). The disease-free survival rate was similar in both groups. Thirty patients (68.2%) developed recurrences. Recurrences were intrahepatic in 22 patients (50%) and extrahepatic in 27 (61.3%). Intrahepatic recurrence rate was significantly lower in group A compared with group B (26.0% vs. 76.1% respectively; P < 0.001). PVE, number of CLM, and administration of neoadjuvant chemotherapy were independent prognostic factors for intrahepatic recurrences.
Conclusion:
This study showed that PVE reduces intrahepatic recurrence rate after right hepatectomy for unilobar CLM.
Right portal vein embolization before right hepatectomy for unilobar colorectal liver metastases might contribute to reduce intrahepatic recurrence rate.
Liver resection has been recognized as the only potentially curative treatment of patients with colorectal liver metastases (CLM) offering long-term survival and the only hope for cure.1–3 However, recurrence after CLM resection occurs in more than 60% of the patients.4,5
Several prognostic factors associated with cancer recurrence after CLM resection have been reported. They include primary tumor stage and tumor differentiation, disease-free interval between diagnosis of primary tumor and emergence of CLM, carcinoembryonic antigen (CEA) blood level before liver resection, number, size, and distribution of CLM, extent of liver resection, and resection margin. Finally, several markers of biologic activity were described (Ki-67, microscopic growth pattern, doubling tumor time, tumor ploidy, and intrahepatic lymphatic invasion).1,6–19
Currently, in patients requiring major hepatectomy with too small future remnant liver (FRL) < 30% of liver volume, portal vein embolization (PVE) has been accepted as an efficient preoperative therapy to optimize the volume of the FRL. However, it has been advocated that PVE increases the tumoral growth of CLM.20–22 To the best of our knowledge, no report has yet analyzed the effect of preoperative PVE on recurrence after CLM resection.
The present study aimed to investigate whether preoperative PVE has any clinical influence on recurrence after CLM resection in an homogeneous group of patients presenting unilobar CLM and who underwent a right hepatectomy with or without preoperative PVE.
PATIENTS AND METHODS
Study Patients
The outcome of 44 consecutive patients presenting resectable right unilobar CLM who underwent from January 1995 to December 2003, a right hepatectomy were retrospectively reviewed (Fig. 1). Among these patients, those with a FRL of less than 30% underwent a preoperative PVE. Therefore, a right hepatectomy was carried out after preoperative PVE in 23 patients and without preoperative PVE in 21 patients. During the same period, 5 other patients with unilobar right CLM underwent PVE, but they were not resected owing to disease progression.
FIGURE 1. Selection of the study population. *Including 4 patients (with PVE in 3) considered preoperatively as unilobar disease; in all of them, a curative resection has been performed. †Includes mono-, bi-, and trisegmentectomies and wedge resections. Values are number of patients.
Patient Selection for Chemotherapy
Patients who received neoadjuvant chemotherapy were referred to our department after administration of chemotherapy. At the opposite, postoperative adjuvant chemotherapy was decided in our institution by a multidisciplinary committee including oncologists, gastroenterologists, radiologists, and surgeons.
Preoperative Evaluation
The diagnosis of CLM was made on the basis of a raised level of tumors markers and/or after detection of liver tumors by ultrasonography, CT scan and/or MRI. Before liver resection all the patients underwent a preoperative evaluation including abdominal and thoracic CT scan.
PVE and Liver Surgery
Right PVE was decided preoperatively when the FRL was less than 30% of the total functional liver volume. The technique of PVE has been previously described.23 Right hepatectomy was performed using a standardized technique. In brief, after adequate exposure of the operative field through an abdominal approach, the right liver was mobilized by sectioning the falciform and right triangular ligaments. The inferior vena cava was controlled and encircled with a tape above and below the cavo-hepatic confluence. The hepatic pedicle was then dissected and lymph nodes in the hepatoduodenal ligament were analyzed. The common bile duct was isolated to avoid bile duct clamping during Pringle maneuver. Intraoperative ultrasound was routinely performed. Liver parenchyma transection was performed by clamp-crush technique and/or ultrasound aspiration dissector under right selective or total pedicle clamping, or without clamping. Liver resection was achieved with a macroscopic tumor free margin of ≥1 cm whenever possible.
Extrahepatic Disease
Patients in whom resectable extrahepatic disease was intraoperatively detected underwent liver resection provided that a complete macroscopic resection of the extrahepatic disease could be achieved at the time of hepatectomy.
Postoperative Care
After operation, intravenous albumin was administered to maintain blood levels over 30 g/L. Prophylactic antibiotics were given during 24 hours postoperatively. A cholangiogram was performed at day 6 and was followed by removal of abdominal drain in the absence of biliary leak.
Follow-up
After surgical resection, all patients were followed up by a physical examination, CEA and CA 19–9 serum levels control, abdominal ultrasonography, and CT scan every 3 to 6 months. No patient was lost during the follow-up period.
Statistics
Results are expressed as mean ± standard error of mean. Fisher exact, χ2, and Mann-Whitney U tests were used. Kaplan-Meier survival was calculated from the date of liver surgery, and significant differences were examined with log-rank test. Independent variables influencing postoperative recurrences were determined by using a stepwise logistic regression analysis. A difference was considered significant when the P value was less than 0.05. All statistical analysis were performed with the SPSS 10.0 for Windows computer software (SPSS, Inc., Chicago, IL).
RESULTS
Patient Characteristics
The mean age of the 44 patients at hepatectomy was 64.1 ± 9.9 years (range, 44–80 years). Sixteen patients were older than 70 years. The sex M/F ratio was 25:19. At the time of hepatectomy, 14 patients were ASA score 1, 23 ASA score 2, and 7 ASA score 3. The primary tumor was located in the colon in 27 patients and in the rectum in 17 patients. Primary tumor was well, moderately differentiated, and poorly differentiated in 9, 29, and 6 patients, respectively. According to pTNM classification (M excluded), the primary tumor was staged I, II, and III in 7, 10, and 27 patients, respectively. Three patients underwent a simultaneous resection of the primary tumor and of the liver metastases. The mean interval between primary tumor resection and hepatectomy was 32 ± 29 months (range, 3–150 months) in the remaining 41 patients.
Metastases Characteristics
Seventeen patients had a solitary liver metastases, 8 had 2 metastases, 13 had 3 metastases and 6 had more than 3 metastases. The mean size of largest metastases was 86 ± 43 mm (range, 15–200 mm). Twenty-two patients had synchronous CLM. Six patients underwent associated procedures at the time of hepatectomy. They included resection of solitary peritoneal carcinomatosis (n = 4), resection of loco-regional primary tumor recurrence (n = 1), and partial resection of the right diaphragm (n = 1). During laparotomy for liver resection, no patient presented macroscopic evidence of hepatic pedicle lymph node involvement. Thirty-eight of 44 patients underwent hepatic pedicle and retroduodenopancreatic lymph node dissection. Among them, 6 patients (15.8%) presented hepatic pedicle lymph node metastases at pathologic examination.
Chemotherapy Administration
Neoadjuvant Chemotherapy for CLM
Nineteen patients did not receive chemotherapy. Among them, 11 had metachronous and 8 synchronous CLM, including 2 patients in whom a simultaneous resection of the primary tumor and of liver metastases was performed. The remaining 25 patients received different regimens of systemic chemotherapy before right hepatectomy. The chemotherapy regimens included 5-fluorouracil (5-FU) + acid folinic (n = 8), 5-FU + acid folinic + oxaliplatin (n = 14), and 5-FU + acid folinic + irinotecan (n = 3). The median number of cycles was 6 (range, 2–12). Only 1 patient developed severe neuropathy after 3 cycles of oxaliplatin and received a second line of chemotherapy with a shift from 5-FU + acid folinic + oxaliplatin to 5-FU + acid folinic (17 cycles). Among the 25 patients receiving neoadjuvant chemotherapy, 21 had stabilization and 4 had partial response of CLM.
Adjuvant Chemotherapy for CLM
Five patients presenting synchronous and 9 metachronous liver metastases did not receive adjuvant chemotherapy. The remaining 30 patients received different regimens of systemic chemotherapy after CLM resection. The chemotherapy regimens included 5-FU + acid folinic (n = 12), 5-FU + acid folinic + oxaliplatin (n = 12) and 5-FU + acid folinic + irinotecan (n = 6). The median number of cycles was 6 (range, 3–12).
Comparability of Patients Undergoing Hepatectomy With or Without Preoperative PVE
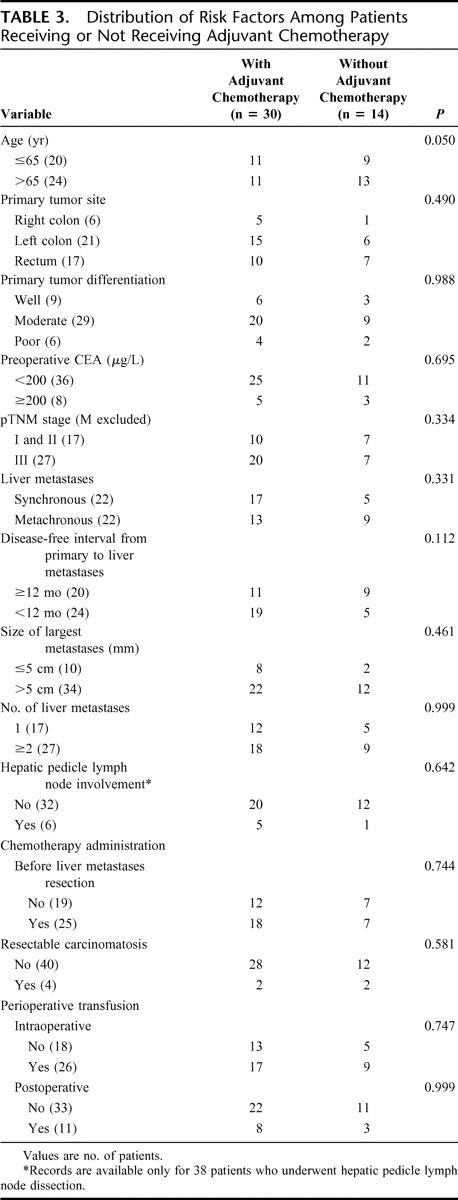
Age, gender, ASA score, primary tumor site, primary tumor differentiation, pTNM stage, preoperative CEA blood level, type of liver metastases, disease-free interval from primary to liver metastases, size of largest liver metastases, resection of solitary peritoneal carcinomatosis nodule, hepatic pedicle lymph node involvement, perioperative blood transfusion, and chemotherapy administration (neoadjuvant or adjuvant for CLM) were similar in patients with or without preoperative PVE (Table 1). However, patients undergoing hepatectomy with preoperative PVE had significantly higher rate of single liver metastases (56.5%) compared with patients undergoing hepatectomy without preoperative PVE (19.0%, P = 0.025). Oncological risk factors were homogenously distributed among the patients receiving or not receiving respectively neoadjuvant or adjuvant chemotherapy as shown in Tables 2 and 3.
TABLE 1. Comparison of Clinicopathologic Features in Patients Undergoing a Right Hepatectomy With or Without Preoperative Portal Vein Embolization for Unilobar Colorectal Liver Metastases

TABLE 2. Distribution of Risk Factors Among Patients Receiving or Not Receiving Neoadjuvant Chemotherapy

TABLE 3. Distribution of Risk Factors Among Patients Receiving or Not Receiving Adjuvant Chemotherapy
Operative Outcome
The average time period between the time of PVE and hepatectomy was 2 ± 1 months. There was no operative mortality. After hepatectomy, 27 patients (61.4%) had uneventful postoperative course. Seventeen patients (38.6%) developed 26 postoperative complications. Among them, 11 patients presented specific complications related to liver surgery and 8 patients developed general complications. Records of these postoperative complications are summarized in Table 4.
TABLE 4. Postoperative Outcome

Survival and Recurrence
The mean follow-up after hepatectomy was 35 ± 28 months, respectively (range, 6–115 months). The 1-, 3-, and 5-year overall survival rates were 92.5%, 54.7%, and 38.7%, respectively. The 3- and 5-year overall survival rates were 61.2% and 43.7% in patients undergoing hepatectomy after PVE and did not differ from that observed in patients operated on without PVE (49.7% and 35.5%; P = 0.862). The 1-, 3-, and 5-year disease-free survival rates were 52.4%, 29.0%, and 18.8%, respectively. The disease-free survival rate was similar in both groups.
Thirty patients (68.2%) developed recurrences. Among them, 19 patients died of recurrences after a mean follow-up of 29 ± 17 months (range, 7–71 months) and 11 were alive with recurrent disease after a mean follow-up of 41 ± 36 months (range, 8–115 months). One patient died without detectable recurrence 24 months after hepatectomy. The remaining 13 patients did not develop any recurrence after a mean follow-up of 40 ± 34 months (range, 6–87 months).
Intrahepatic and Extrahepatic Recurrences
Recurrences were intrahepatic in 22 patients (50%) and extrahepatic in 27 (61.3%). Nineteen patients (43.1%) developed both intrahepatic and extrahepatic recurrences. Intrahepatic and extrahepatic recurrences were simultaneously diagnosed in 9 patients. Extrahepatic recurrences were diagnosed 12 ± 10 months (range, 2–30 months) after intrahepatic recurrences in 8 patients. In the remaining 2 patients, intrahepatic recurrences were detected 8 and 11 months after extrahepatic recurrences. Three patients developed isolated intrahepatic recurrences 5, 23, and 33 months, respectively, after hepatectomy. Isolated extrahepatic recurrences occurred in 8 patients 20 ± 16 months (range, 3–46 months) after hepatectomy. Extrahepatic recurrences were located as follows: lungs in 21 patients, bones in 5, peritoneum in 4, primary tumor site in 3, brain in 2. Rarer sites of metastases were the adrenal gland, the kidney, and the gluteal region, each accounting for one occurrence. In 9 patients, extrahepatic recurrences occurred in 2 (n = 7) or 3 (n = 2) sites. First intrahepatic or extrahepatic recurrence occurred within 18 months after liver resection in 18 (81.8%) and 20 patients (74.0%), respectively, as shown in Figure 2.
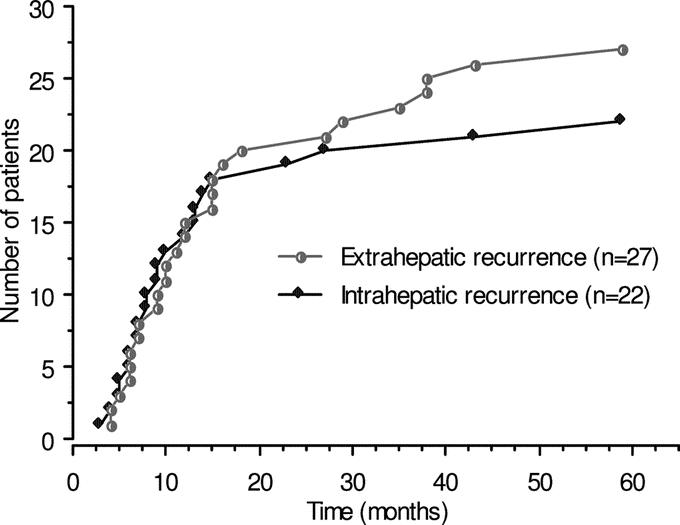
FIGURE 2. Kaplan-Meier cumulative risk of intrahepatic and extrahepatic disease recurrence after right hepatectomy for unilobar colorectal liver metastases. Note that 81.8% and 74.0%, respectively, of these recurrences occurred within 18 months after hepatectomy.
Treatment of Recurrences
Intrahepatic recurrences occurred as a single metastases in 9 patients or multiple metastases in 13. Surgical treatment of intrahepatic recurrences was achieved in 8 patients and consisted in a repeat hepatectomy in 6 patients and in percutaneous radiofrequency ablation in 2. In 3 cases (2 with PVE), intrahepatic recurrences were located near the liver resection margin. Among them, 2 patients with PVE had resection margin of less than 1 cm.
Analysis of Factors Influencing Intrahepatic Recurrences
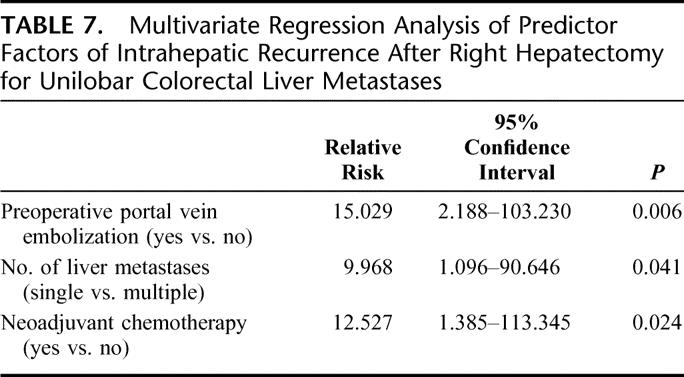
The results of univariate analysis showed that preoperative PVE (P < 0.001) and single liver metastases (P = 0.030) were significantly associated with a decrease rate of intrahepatic recurrences (Table 5). Remarkably, intrahepatic recurrence has been observed in 76.1% of the patients who underwent a right hepatectomy for CLM without PVE and in 26.0% of the patients who underwent PVE (Table 5; P < 0.001). The rate of intrahepatic recurrences remained unchanged in patients without PVE despite the number of liver metastases. On the contrary, intrahepatic recurrence rate in patients with PVE was influenced by the number of liver metastases. A 2 contingency table summarizes the intrahepatic recurrence rates according to preoperative PVE and number of liver metastases (Table 6). A multivariate analysis showed that preoperative PVE, single liver metastases, and administration of neoadjuvant chemotherapy were independent factors for intrahepatic recurrences (Table 7).
TABLE 5. Univariate Analysis of Predictors for Intrahepatic Recurrence After Curative Right Hepatectomy for Unilobar Colorectal Liver Metastases

TABLE 5. (Continued)

TABLE 6. Two Contingency Analyses for Intrahepatic Recurrences

TABLE 7. Multivariate Regression Analysis of Predictor Factors of Intrahepatic Recurrence After Right Hepatectomy for Unilobar Colorectal Liver Metastases
DISCUSSION
The present series showed that preoperative PVE was significantly associated with a reduced rate of intrahepatic recurrence in patients undergoing right hepatectomy for unilobar CLM. This study suggests that preoperative PVE could contribute to reduce perioperative tumor shedding and intraoperative intrahepatic dissemination.
After CLM resection, most of the recurrences occur during the first 2 years (Fig. 2).19 Currently, the reported recurrence rates in Europe, United States, and Japan range from 40% to 79%.1–5,7,14,19,23 Similarly, in the present series, the overall intrahepatic recurrence rate was 50%. The number of liver metastases, administration of neoadjuvant chemotherapy, and particularly PVE were significantly associated with a decreased rate of intrahepatic recurrence. The present single institutional retrospective study is the first series showing a significant reduction of intrahepatic recurrence rate after right hepatectomy for unilobar CLM provided that a preoperative PVE has been used.
Recently, Tanaka et al reported that the number of liver metastasis affects intrahepatic recurrences after hepatectomy for CLM.14 The present series confirmed that even in case of unilobar disease the presence of multiple CLM was associated with a higher rate of intrahepatic recurrence compared with single CLM. Moreover, in contrast to the results of Tanaka et al the number of CLM was an independent prognostic factor.14
Several studies analyzed the value of neoadjuvant chemotherapy for the treatment of CLM, particularly in case of nonresectable CLM.4,24–32 Adam et al reported a significant reduction of intrahepatic recurrence rate in patients who responded to neoadjuvant chemotherapy.4 In the present series, administration of neoadjuvant chemotherapy was significantly associated with a reduction of intrahepatic recurrence rate. Indeed, all the patients who underwent hepatectomy after neoadjuvant treatment had at least stabilization of the disease. However, different chemotherapy regimens as well as different number of preoperative cycles have been used and no conclusion can be made. Furthermore, the indications for neoadjuvant chemotherapy in case of resectable CLM need to be established.
Weitz et al have demonstrated that patients undergoing major liver resection for CLM are at high risk for intraoperative tumor cells dissemination.33 The authors concluded that the extensive manipulation of the tumor during liver mobilization result in a high incidence of tumor cell shedding. Moreover, the same group have recently demonstrated that colorectal tumor cells dissemination are related to the number of liver metastases and to chemotherapy administration.34,35 Experimental models36 and histologic investigations of the vascularization of human CLM37,38 have shown that portal vein contribute to blood supply of CLM particularly at the tumor periphery. Moreover, Miles et al have recently showed that portal vessels around CLM have more a draining rather than a blood supplying function.39 According to these findings, it seems possible to hypothesize that tumor shedding may occur retrogradely through the portal vessels as previously described for dissemination of hepatocellular carcinomas.40–42 This “retrograde portal dissemination” theory suggests that preoperative PVE might contribute to reduce intrahepatic tumoral spreading by intraluminal occlusion of the peripheral portal branches. Indeed, in the present series, patients who underwent preoperative PVE, suffered from significantly lower intrahepatic recurrence rate than patients who did not underwent preoperative PVE. However, further studies should be conducted to confirm the existence of tumoral shedding through the portal vessels and to demonstrate its clinical relevance. An alternative explanation for the observed reduction of intrahepatic recurrence rate in the group of patients who underwent preoperative PVE can be proposed. Preoperative PVE allows a better selection of surgical patients by providing a period of time of about 1 to 3 months between PVE and liver resection. Indeed, in our experience, 22% (n = 5) of the patients requiring preoperative PVE developed disease progression while waiting liver hypertrophy and these patients were excluded from the study as they never underwent liver resection.23 However, if the reduction of intrahepatic recurrence after PVE would be explained only by the selection of patients with a favorable tumor biology, we would expect to observe an improved overall survival. At the opposite, in the present series PVE did not affect survival but was associated with a reduction of intrahepatic recurrence rate. Indeed, PVE may have a locoregional effect reducing intrahepatic recurrences without any impact on patient outcome. These results support the recent reports concerning the retrograde cells dissemination through the portal vessels.39–42 However, further investigations are needed to confirm this hypothesis.
The 2 groups were similar regarding the clinical and the pathologic characteristics except the number of CLM. Indeed, the repartition of patients with single liver metastases was significantly different between patients with and without preoperative PVE. This difference could have influenced the interpretation of the presented data regarding the effect of PVE on postoperative intrahepatic recurrence rate. However, our results showed that preoperative PVE as the number of liver metastases were independent factors significantly associated with a reduced rate of intrahepatic recurrences. Moreover, even in patients with multiple liver metastases, the intrahepatic recurrence rate was higher in patients without preoperative PVE compared with patients with preoperative PVE (76.5% vs. 40%). Recently, Kokudo et al suggested that PVE can be associated with an enhanced recurrence rate after CLM resection.22 However, the authors compared patients with major liver resection to those with minor liver resection, and they included patients with unilobar and bilobar CLM. Therefore, the extent of their inclusion criteria resulting in a heterogeneous population could have affected their final conclusion. Elias et al reported that PVE can increase the growth rate of CLM.20 Similarly, in our experience, PVE could have stimulated the growth of previously undetected contralateral micrometastases at least in 3 patients. However, PVE is still indicated before right hepatectomy in case of a small FRL (≤30%).23,43,44
CONCLUSION
This study suggests, for the first time, that preoperative PVE may reduce intrahepatic recurrence rate after liver resection for unilobar CLM. Further studies are warranted to confirm this interesting message delivered by these preliminary results. Whether or not preoperative PVE should be routinely performed before right hepatectomy to reduce intrahepatic recurrences is an important question, which could be answered by a prospective randomized trial.
Footnotes
Reprints: Daniel Jaeck, MD, PhD, FRCS, Centre de Chirurgie Viscérale et de Transplantation, Hôpital de Hautepierre, Hôpitaux Universitaires de Strasbourg–Université Louis Pasteur, Avenue Molière, 67098 Strasbourg Cedex, France. E-mail: Daniel.Jaeck@chru-strasbourg.fr.
REFERENCES
- 1.Nordlinger B, Jaeck D, Guiguet M, et al. Surgical resection of hepatic metastases: multicentric retrospective study by the French Association of Surgery. In: Nordlinger B, Jaeck D, eds. Treatment of Hepatic Metastases of Colorectal Cancer. Paris: Springer-Verlag, 1992:129–146. [Google Scholar]
- 2.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 3.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Br J Surg. 1997;84:977–980. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukunaga K, Takada Y, Otsuka M, et al. Resection of localized recurrences after hepatectomy of colorectal cancer metastases. Hepatogastroenterology. 2003;50:1894–1897. [PubMed] [Google Scholar]
- 6.Weber SM, Jarnagin WR, DeMatteo RP, et al. Survival after resection of multiple hepatic colorectal metastases. Ann Surg Oncol. 2000;7:643–650. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JC, Bachellier P, Oussoultzoglou E, et al. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br J Surg. 2003;90:956–962. [DOI] [PubMed] [Google Scholar]
- 9.Jaeck D, Nakano H, Bachellier P, et al. Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: a prospective study. Ann Surg Oncol. 2002;9:430–438. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi J, Komuta K, Matsuzaki S, et al. Mode of infiltrative growth of colorectal liver metastases is a useful predictor of recurrence after hepatic resection. World J Surg. 2002;26:1122–1125. [DOI] [PubMed] [Google Scholar]
- 11.Cady B, Jenkins RL, Steele GD Jr, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagakura S, Shirai Y, Yokoyama N, et al. Major hepatic resection reduces the probability of intrahepatic recurrences following resection of colorectal carcinoma liver metastases. Hepatogastroenterology. 2003;50:779–783. [PubMed] [Google Scholar]
- 13.Taylor M, Forster J, Langer B, et al. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173:467–471. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Shimada H, Miura M, et al. Metastatic tumor doubling time: most important prehepatectomy predictor of survival and nonrecurrence of hepatic colorectal cancer metastasis. World J Surg. 2004;28:263–270. [DOI] [PubMed] [Google Scholar]
- 15.Lise M, Bacchetti S, Da Pian P, et al. Patterns of recurrence after resection of colorectal liver metastases: prediction by models of outcome analysis. World J Surg. 2001;25:638–644. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi A, Kurosaka Y, Kanno M, et al. Analysis of hepatic recurrence of colorectal cancer after resection of hepatic metastases. Int Surg. 1993;78:16–19. [PubMed] [Google Scholar]
- 17.Sasaki A, Aramaki M, Kawano K, et al. Prognostic significance of intrahepatic lymphatic invasion in patients with hepatic resection due to metastases from colorectal carcinoma. Cancer. 2002;95:105–111. [DOI] [PubMed] [Google Scholar]
- 18.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 19.Topal B, Kaufman L, Aerts R, et al. Patterns of failure following curative resection of colorectal liver metastases. Eur J Surg Oncol. 2003;29:248–253. [DOI] [PubMed] [Google Scholar]
- 20.Elias D, De Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–788. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Ouellet JF, De Baere T, et al. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294–299. [DOI] [PubMed] [Google Scholar]
- 22.Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. [DOI] [PubMed] [Google Scholar]
- 23.Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038–2048. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, Shimada H, Kubota K, et al. Effectiveness of prehepatectomy intra-arterial chemotherapy for multiple bilobar colorectal cancer metastases to the liver: a clinicopathologic study of peritumoral vasculobiliary invasion. Surgery. 2005;137:156–164. [DOI] [PubMed] [Google Scholar]
- 26.Bathe OF, Dowden S, Sutherland F, et al. Phase II study of neoadjuvant 5-FU + leucovorin + CPT-11 in patients with resectable liver metastases from colorectal adenocarcinoma. BMC Cancer. 2004;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozzo C, Basso M, Cassano A, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. [DOI] [PubMed] [Google Scholar]
- 28.Rahman S, Toogood GJ, Lodge PJ, et al. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:1453. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka K, Adam R, Shimada H, et al. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963–969. [DOI] [PubMed] [Google Scholar]
- 30.Wein A, Riedel C, Bruckl W, et al. Neoadjuvant treatment with weekly high-dose 5-fluorouracil as 24-hour infusion, folinic acid and oxaliplatin in patients with primary resectable liver metastases of colorectal cancer. Oncology. 2003;64:131–138. [DOI] [PubMed] [Google Scholar]
- 31.Allen PJ, Kemeny N, Jarnagin W, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–115. [DOI] [PubMed] [Google Scholar]
- 32.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal liver metastases. Ann Surg Oncol. 2001;8:347–353. [DOI] [PubMed] [Google Scholar]
- 33.Weitz J, Koch M, Kienle P, et al. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000;232:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch M, Kienle P, Hinz U, et al. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg. 2005;241:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kienle P, Koch M, Autschbach F, et al. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: a first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann Surg 2003;238:324–330; discussion 330–331. [DOI] [PMC free article] [PubMed]
- 36.Kuruppu D, Christophi C, O'Brien PE. Microvascular architecture of hepatic metastases in a mouse model. HPB Surg. 1997;10:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikfarjam M, Muralidharan V, Malcontenti-Wilson C, et al. Scanning electron microscopy study of the blood supply of human colorectal liver metastases. Eur J Surg Oncol. 2003;29:856–861. [DOI] [PubMed] [Google Scholar]
- 38.Haugeberg G, Strohmeyer T, Lierse W, et al. The vascularization of liver metastases: histological investigation of gelatine-injected liver specimens with special regard to the vascularization of micrometastases. J Cancer Res Clin Oncol. 1988;114:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles KA, Leggett DA, Kelley BB, et al. In vivo assessment of neovascularization of liver metastases using perfusion CT. Br J Radiol. 1998;71:276–281. [DOI] [PubMed] [Google Scholar]
- 40.Sakon M, Nagano H, Nakamori S, et al. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137:94–99. [DOI] [PubMed] [Google Scholar]
- 41.Matsumata T, Kanematsu T, Takenaka K, et al. Lack of intrahepatic recurrence of hepatocellular carcinoma by temporary portal venous embolization with starch microspheres. Surgery. 1989;105:188–191. [PubMed] [Google Scholar]
- 42.Yamanaka N, Okamoto E, Fujihara S, et al. Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer. 1992;70:2263–2267. [DOI] [PubMed] [Google Scholar]
- 43.Kokudo N, Imamura H, Sugawara Y, et al. Surgery for multiple hepatic colorectal metastases. J Hepatobiliary Pancreat Surg. 2004;11:84–91. [DOI] [PubMed] [Google Scholar]
- 44.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691; discussion 691–693. [DOI] [PMC free article] [PubMed]



