Abstract
Objective:
To investigate whether immunohistochemically demonstrated lymph node micrometastasis has a survival impact in patients with advanced gallbladder carcinoma (pT2–4 tumors).
Summary Background Data:
The clinical significance of immunohistochemically detected lymph node micrometastasis recently has been evaluated in various tumors. However, few reports have addressed this issue with regard to gallbladder carcinoma.
Methods:
A total of 1476 lymph nodes from 67 patients with gallbladder carcinoma (pN0, n = 40; pN1, n = 27) who underwent curative resection were immunostained with monoclonal antibody against cytokeratins 8 and 18. The results were correlated with clinical and pathologic features and with patient survival.
Results:
Lymph node micrometastases were detected immunohistochemically in 23 (34.3%) of the 67 patients and in 37 (2.5%) of the 1476 nodes examined. Of the 37 nodal micrometastases, 21 (56.8%) were single-cell events, and the remaining 16 were clusters. Five micrometastases were detected in the paraaortic nodes. Clinicopathologic features showed no significant associations with the presence of lymph node micrometastases. Survival was worse in the 27 patients with pN1 disease than in the 40 with pN0 disease (5-year survival; 22.2% vs. 52.6%, P = 0.0038). Similarly, survival was worse in the 23 patients with micrometastasis than in the 44 without micrometastasis (5-year survival; 17.4% vs. 52.7%, P = 0.0027). Twenty-eight patients without any lymph node involvement had the best prognosis, whereas survival for the 11 patients with both types of metastasis was dismal. The grade of micrometastasis (single-cell or cluster) had no effect on survival. The Cox proportional hazard model identified perineural invasion, lymph node micrometastasis, and microscopic venous invasion as significant independent prognostic factors.
Conclusions:
Lymph node micrometastasis has a significant survival impact in patients with pN0 or pN1 gallbladder carcinoma who underwent macroscopically curative resection. Extensive lymph node sectioning with keratin immunostaining is recommended for accurate prognostic evaluation for patients with gallbladder carcinoma.
A total of 1476 lymph nodes from 67 patients with gallbladder carcinoma who underwent curative resection were examined immunohistochemically for micrometastasis; this was detected in 23 patients (34.3%) and in 37 (2.5%) of the nodes examined. Multivariate analysis identified lymph node micrometastasis as a significant independent prognostic factor.
The majority of patients with gallbladder carcinoma are treated at late stages of the disease, resulting in a dismal prognosis overall.1–5 Lymph node spread is the most common form of progression of gallbladder carcinoma, and nodal status is known to be an important predictor of survival after surgery.1,3,6,7 We have demonstrated that perineural invasion and lymph node metastasis are important prognostic factors in gallbladder carcinoma.8 In addition, we documented that paraaortic and regional lymph nodes frequently are involved in advanced gallbladder carcinoma and that extended lymphadenectomy possibly provides a survival benefit in selected patients.9,10
Identification of lymph node involvement represents an integral component of tumor staging. Traditional histologic examination consists of single sectioning of nodes sampled from resected specimens, with hematoxylin and eosin staining. This practice may underestimate the incidence of micrometastasis in lymph nodes, leading to understaging of patients. Immunohistochemical techniques using antibodies against cytokeratin can identify lymph node micrometastasis missed by routine hematoxylin and eosin staining. In recent years, the clinical significance of immunohistochemically detected lymph node micrometastases has been evaluated in various cancers including those of breast,11,12 lung,13,14 esophagus,15–17 stomach,18,19 and colon.20,21 However, only three reports22–24 have addressed this issue in gallbladder carcinoma. Of the three reports, two22,23 were from the same institution, and the remaining one24 did not show survival data.
The purpose of this study was to investigate whether immunohistochemically detected lymph node micrometastasis has prognostic significance in patients with advanced gallbladder carcinoma (pT2–4 tumors). For this purpose, a large number of lymph nodes were examined immunohistochemically, and the impact of lymph node micrometastasis on prognosis was assessed.
PATIENTS AND METHODS
Patients
Between January 1982 and December 2003, 139 patients with gallbladder carcinoma underwent macroscopically curative resection of the primary cancer with systematic extended lymphadenectomy at the First Department of Surgery, Nagoya University Hospital. Fifty-one patients (36.7%) had M1 disease (paraaortic lymph node metastasis, small liver metastasis, and/or local dissemination), while 8 others died of postoperative complications. The remaining 80 patients had no lymph node metastasis (pN0 disease) or regional node metastasis (pN1 disease) detected by routine pathologic examination with hematoxylin and eosin staining. Eight patients were excluded because their archival histologic specimens of dissected lymph nodes could not be located. Five others with pT1 disease were also excluded because pT1 tumors usually spread only locally without nodal involvement and the outcome after resection is excellent.1,6,23 The remaining 67 patients represented the study population, including 23 men and 44 women with a mean age of 63 ± 11 years (range, 33–82 years). Of the 67 study patients, 40 had no lymph node disease (pN0) and the remaining 27 had regional lymph node involvement (pN1). Patient survival was determined from the time of surgery to the time of death or most recent follow-up. No patient was lost to follow-up.
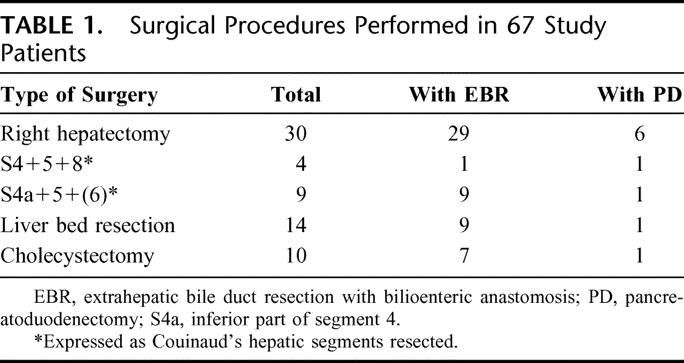
Several types of hepatectomy were performed in 57 (85.1%) of the 67 patients. Combined resection of the extrahepatic bile duct with bilioenteric anastomosis (n = 55, 82.1%), and pancreatoduodenectomy (n = 10, 14.9%) were performed additionally in selected patients (Table 1). Extended lymph node dissection was carried out as follows. After en bloc resection of the primary tumor and nodes of the hepatoduodenal ligament and head of the pancreas with skeletonization of the portal vein andhepatic arteries, the paraaortic connective tissue containing lymph nodes was dissected between the levels of the celiac and inferior mesenteric arteries. The left renal vein and the right renal artery were skeletonized between the aorta and the inferior vena cava.
TABLE 1. Surgical Procedures Performed in 67 Study Patients
Lymph node group and staging were evaluated using the TNM Classification of Malignant Tumors by the International Union Against Cancer.25 The definitions of some regional node groups in the TNM classification are obscure; accordingly, the General Rules for Surgical and Pathologic Studies on Cancer of the Biliary Tract edited by the Japanese Society of Biliary Surgery26 were used to define the topographic relationships of lymph nodes to surrounding structures. Although none of our patients had gross residual tumor, microscopic resection margin status was judged to be positive when cancer cells were apparent on the cut surface of the resected specimen.
Immunohistochemistry
A total of 1476 lymph nodes (22.0 nodes/patient), including 836 regional, 415 paraaortic, and 225 paragastric or paracolic nodes, were retrieved from the 67 surgical specimens. Five serial sections were cut from archival formalin-fixed, paraffin-embedded specimens of lymph nodes. The first and fifth 5-μm sections were stained with hematoxylin and eosin for histologic reexamination for metastatic tumor cells, and the remaining 3 sections were stained with CAM 5.2 monoclonal antibody (Becton Dickinson, San Jose, CA). Immunohistochemical staining was performed with a standard streptavidin-biotin method.27 Briefly, the paraffin sections were dewaxed, hydrated, and treated with 0.1% trypsin (Sigma Chemical, St. Louis, MO) in 0.1% calcium chloride, at pH 7.8, at 37°C for 30 minutes. Endogenous peroxide activity was blocked with 0.3% hydrogen peroxide in absolute methanol. Sections were incubated with the primary monoclonal antibody CAM 5.2 at 25 μg/mL at room temperature for 1 hour. After rinsing, the sections were incubated with secondary antibody, followed by peroxidase-labeled streptavidin (Nichirei, Tokyo, Japan). Reaction products were visualized with diaminobenzidine as the chromogen, and sections were counterstained with hematoxylin.
Both hematoxylin and eosin and immunohistochemically stained sections were examined independently for metastasis by an experienced pathologist. Micrometastasis was recognized when tumor cells were detected only by immunostaining, having not been evident by hematoxylin and eosin staining. Lymph node micrometastases were classified into 3 grades as follows: grade I, single-cell metastasis; grade II, a small cluster of cancer cells; and grade III, a large cluster or multiple clusters of cancer cells (Fig. 1).
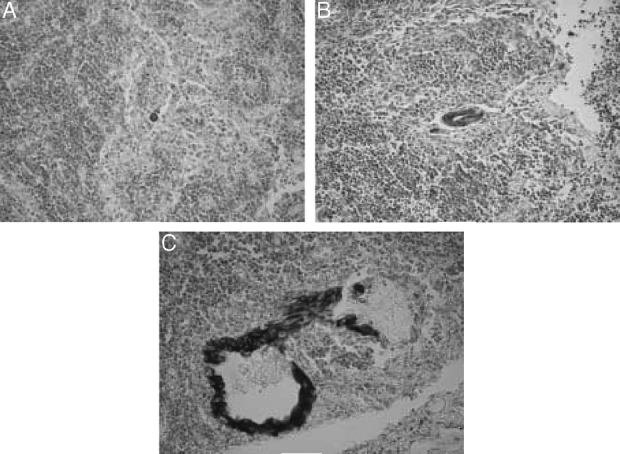
FIGURE 1. Immunohistochemical staining of lymph node micrometastasis by CAM 5.2 monoclonal antibody, showing (A) grade I, (B) grade II, and (C) grade III micrometastasis.
Statistics
Results are expressed as mean ± SD. Statistical analysis was performed using the Fisher exact test probability test and the Mann-Whitney U test, where appropriate. Postoperative survival was calculated using the Kaplan-Meier method. Differences in survival curves were compared using the log-rank test. Cox proportional hazard model was used for multivariate analysis. P < 0.05 was considered statistically significant.
RESULTS
Detection of Nodal Micrometastases
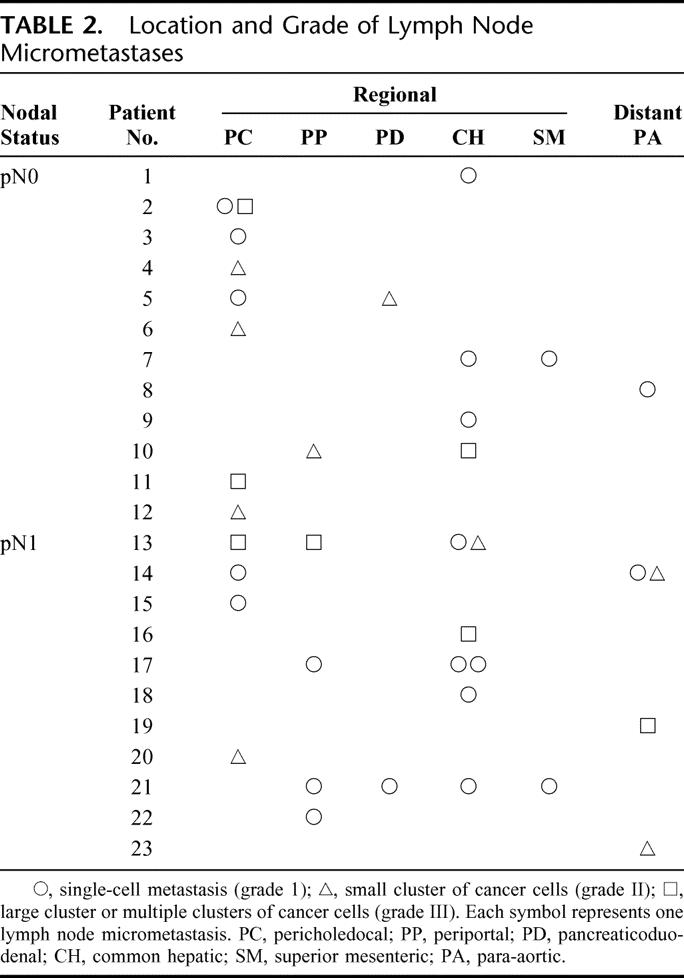
In the 40 patients with pN0 disease, micrometastases were detected in 12 (30.0%) patients and in 16 (1.9%) of 856 lymph nodes examined. In the 27 patients with pN1 disease, micrometastases were found in 11 (40.7%) patients and in 21 (3.4%) of 620 lymph nodes examined. The micrometastases were found more frequently in the latter group; patient-based incidence did not differ significantly (P = 0.365) between pN0 and pN1 groups, and lymph node-based incidence was marginal (P = 0.065). The 37 micrometastases included 21 grade I micrometastases (56.8%), 9 grade II micrometastases (24.3%), and 7 grade III micrometastases (18.9%). Nodal micrometastases were most frequently found in the pericholedochal nodes, followed by the common hepatic nodes. Five micrometastases in the paraaortic nodes were found in 4 patients (Table 2).
TABLE 2. Location and Grade of Lymph Node Micrometastases
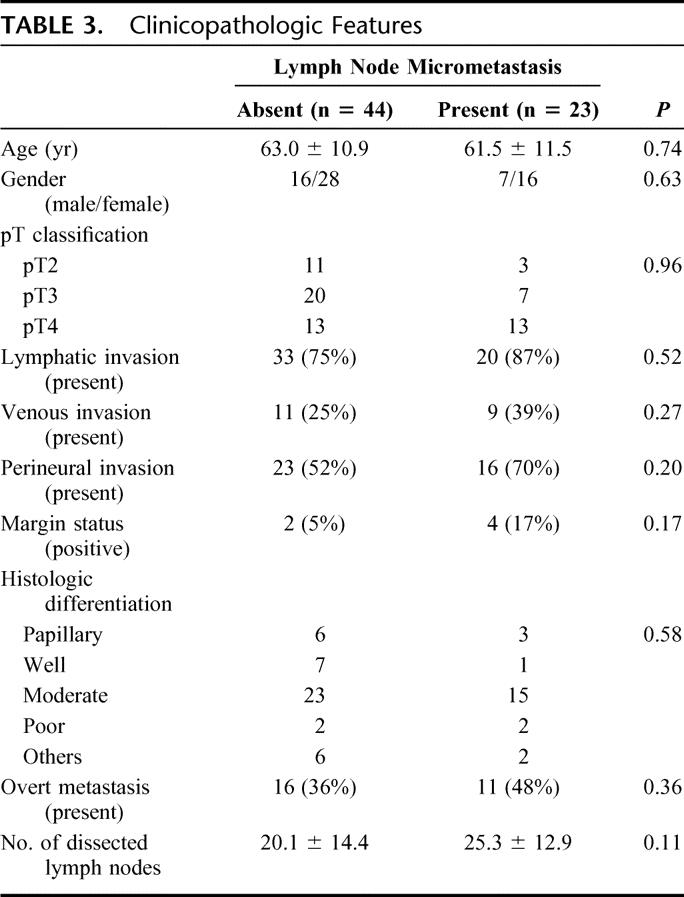
Clinicopathologic details of the 67 study patients with (n = 23) and without lymph node micrometastases (n = 44) are shown in Table 3; no statistically significant associations were found for the presence of lymph node micrometastases.
TABLE 3. Clinicopathologic Features
Impact of Lymph Node Micrometastasis on Prognosis
Survival was worse in the 27 patients with overt lymph node metastasis (pN1 disease) than in the 40 patients without overt lymph node metastasis (pN0 disease) (5-year survival rate; 22.2% vs. 52.6%, P = 0.0038; Fig. 2). Similarly, survival for the 23 patients with lymph node micrometastasis was worse than that for the 44 patients without lymph node micrometastasis (5-year survival rate; 17.4% vs. 52.7%, P = 0.0027; Fig. 3). For the combination of overt metastasis and micrometastasis, the 28 patients without either type of metastasis had the best prognosis, showing a 5-year survival rate of 61.7%. In contrast, survival for the 11 patients with both types of metastasis was dismal; most died within 3 years. Survival curves in patients with either of the 2 types of metastasis were essentially similar, and the difference in survival was not statistically significant (P = 0.932; Fig. 4). Four patients who had micrometastasis in the paraaortic nodes died of recurrence 10, 12, 18, and 32 months after surgery.
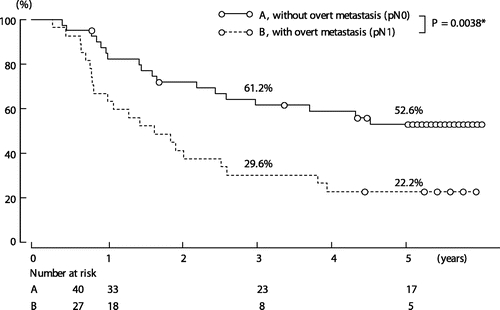
FIGURE 2. Survival according to the presence or absence of overt lymph node metastasis. *By log-rank test.
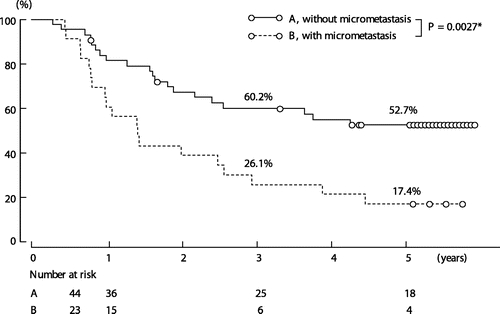
FIGURE 3. Survival according to the presence or absence of lymph node micrometastasis. *By log-rank test.
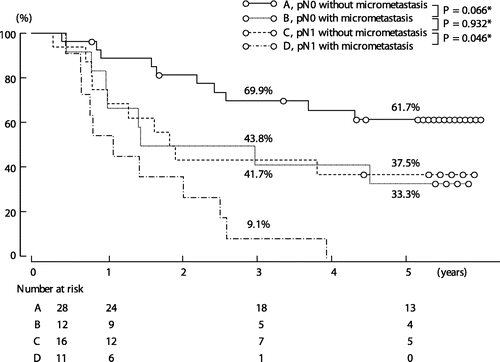
FIGURE 4. Survival according to the presence or absence of overt metastasis and micrometastasis. *By log-rank test.
Outcomes in patients with lymph node micrometastasis also were analyzed according to grade of micrometastasis. Survival rates were similar between the 10 patients with only grade I micrometastasis and the 13 with grade II or III micrometastasis (5-year survival rate; 20.0% versus 15.4%, P = 0.576).
Analysis of Prognostic Factors
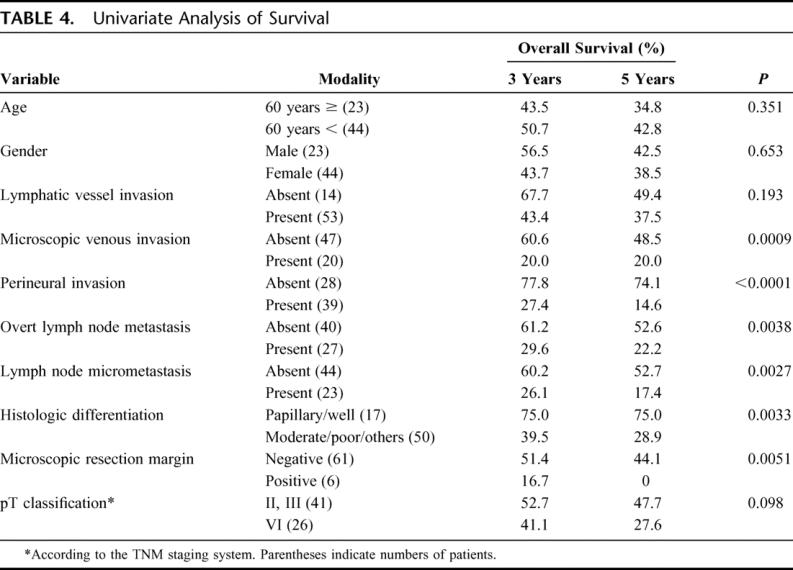
Ten independent clinicopathologic variables were analyzed as possible prognostic factors in pN0 or pN1 gallbladder carcinoma. On univariate analysis, microscopic venous invasion, perineural invasion, overt lymph node metastasis, lymph node micrometastasis, histologic differentiation, and microscopic resection margin status were statistically significant factors. pT classification showed a marginal impact (Table 4).
TABLE 4. Univariate Analysis of Survival
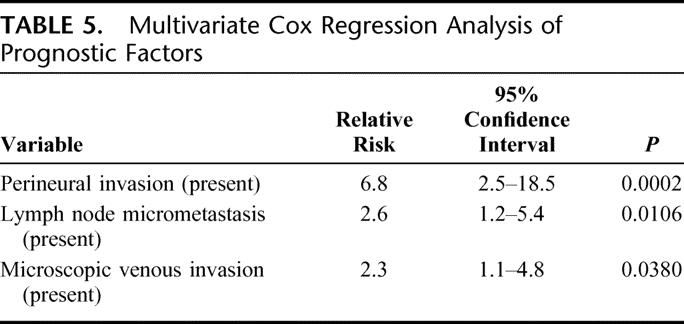
Multivariate analysis by the Cox proportional hazard model was performed using the 6 significant and one marginal variables noted above. On multivariate analysis, perineural invasion, lymph node micrometastasis, and microscopic venous invasion were identified as significant independent prognostic factors in patients with pN0 or pN1 gallbladder carcinoma who underwent macroscopically curative resection (Table 5).
TABLE 5. Multivariate Cox Regression Analysis of Prognostic Factors
DISCUSSION
Many authors have reported on the survival impact of immunohistochemically detected lymph node micrometastases in various carcinomas.11–23 Some investigators have shown that this finding is a significant prognostic factor,11,14,15,18,23 while others suggest that it is not.13,16,17,20,21 Overall, no consensus on the clinical significance of lymph node micrometastasis has been reached. In the present study, we have demonstrated that nodal micrometastasis is an independent prognostic factor in gallbladder carcinoma, being compatible with findings of a previous study by Nagakura et al.23
Previously, we documented that lymph node micrometastasis has no survival impact in patients with pN0 hilar cholangiocarcinoma.28 The 3- and 5-year survival rates, respectively, were 63.6% and 43.6% in patients with lymph node micrometastasis, and 66.9% and 42.1% in those without micrometastasis. Survival curves in these 2 patient groups were essentially similar (P = 0.983). Microscopic venous invasion, microscopic resection margin status, and histologic differentiation were significant prognostic factors in patients with pN0 hilar cholangiocarcinoma. Therefore, we first postulated that similar findings would be achieved in gallbladder carcinoma because both carcinomas belong to the same disease entity, ie, biliary carcinoma. The incidence of lymph node micrometastasis in pN0 disease was similar between cholangiocarcinoma and gallbladder carcinoma (24.4% vs. 30.0% of study patients, and 1.4% vs. 1.9% of lymph nodes examined, respectively). In addition, clinicopathologic features showed no significant associations with the presence of lymph node micrometastases in both carcinomas. However, results concerning associations in the present study were considerably different from those in our previous study. Although this divergence is difficult to explain, it may involve differences in biologic behavior between these 2 types of biliary carcinoma which we cannot yet clarify.
Nagakura et al23 examined 1136 nodes taken from 63 gallbladder cancer patients who underwent macroscopically curative resection (18.0 nodes examined per patient), finding lymph node micrometastases in 27 nodes (2.3%) from 19 patients (30.2%). We examined 1476 nodes from 67 patients (mean number of nodes examined per patient, 22.0) and found micrometastasis in 37 nodes (2.5%) from 23 patients (34.3%). Numbers of study patients and nodes examined and the incidence of micrometastasis thus were very similar between the 2 studies. They demonstrated that nodal micrometastasis is the strongest independent prognostic factor (relative risk = 11.0) in patients with gallbladder carcinoma, regardless of the overt nodal status. Surprisingly, survival for patients with only micrometastasis was significantly worse than that for patients with only overt metastasis (18% vs. 80% in 5-year survival rate, P = 0.0108). In their series, tumor relapse occurred predominantly at distant sites in patients with lymph node micrometastasis. They speculated that a single cell or a small cluster of cells constituting micrometastasis could migrate more easily within the lymphatic system to ultimately enter the systemic circulation and disseminate. They concluded that nodal micrometastasis in gallbladder carcinoma closely reflects the aggressiveness of a carcinoma and is also an indicator of systemic spread.
In our series, the 3- and 5-year survival rates, respectively, were 41.7% and 33.3% for patients who had pN0 disease with micrometastasis, and 43.8% and 37.5% for patients who had pN1 disease without micrometastasis. Survival curves in these 2 groups were essentially similar (P = 0.932). Survival for the patients who had pN1 disease with micrometastasis as well was the worst. These findings suggest that the survival impact of lymph node micrometastasis is at least equal to that of overt metastasis. Our results were less dramatic than those of the study by Nagakura et al.23 However, multivariate analysis identified lymph node micrometastasis, but not overt metastasis, as a significant prognostic factor. From our results and the Nagakura et al study,23 it is evident that lymph node micrometastasis is a significant prognostic factor in gallbladder carcinoma, unlike cholangiocarcinoma. Unexpectedly, its survival impact may be stronger than that of overt lymph node disease.
A possible reason for contradictory results on the clinical significance of lymph node micrometastasis is different definitions of micrometastasis. Usually, this is defined as tumor cells detectable only by immunostaining.15,18,19 However, some authors set size criteria for micrometastasis, such as deposits less than 2 mm in diameter,16 deposits less than 0.5 mm in diameter,29 and deposits consisting of 5 cancer cells or fewer.23 In the present study, lymph node micrometastases were classified into 3 groups, and all micrometastases were less than 0.5 mm in diameter but included clusters with more than 5 cancer cells. However, no difference in prognosis was noted between grade I and grade II to III micrometastases, which indicated no effect of micrometastasis size in gallbladder carcinoma, irrespective of definition. Micrometastasis has recently been investigated with molecular assay based on the polymerase chain reaction that is more sensitive for detection of micrometastasis. Demeure et al showed that approximately 70% of patients with stage I pancreas cancer harbor mutant K-ras in at least one regional node, indicating the presence of micrometastases not detected by immunohistochemical staining.30 A few authors studied “molecular” metastases from pancreas cancer,30–32 but no reports were found on gallbladder cancer. Further studies using molecular technique is required to assess clinical impact of lymph node micrometastasis from gallbladder cancer.
Greater controversy exists regarding the surgical treatment of gallbladder carcinoma. Recommendations have ranged from simple cholecystectomy without lymphadenectomy to an aggressive resection with extended lymphadenectomy.9,10 Recent evidence, however, suggests that extended lymphadenectomy may prolong survival in selected patients with gallbladder cancer.6,7,9,10 Boniest et al2 reported that regional lymphadenectomy in the hepatoduodenal ligament was effective for improving outcome in gallbladder cancer patients without overt nodal metastasis. The present study showed that lymph node micrometastasis occurred in about one third of patients, and it has a survival impact. Therefore, we recommend dissection of regional nodes including at least the pericholedochal, periportal, common hepatic, and pancreaticoduodenal nodes, even if overt nodal metastasis is absent.
CONCLUSION
Lymph node micrometastasis is an independent significant prognostic factor in patients with pN0 or pN1 gallbladder carcinoma who underwent macroscopically curative resection. Its survival impact is probably stronger than that of overt lymph node involvement. Therefore, we recommend extensive lymph node sectioning with keratin immunostaining foraccurate prognostic evaluation for patients with gallbladder carcinoma.
Footnotes
Reprints: Yuji Nimura, MD, PhD, Department of Surgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan. E-mail: ynimura@med.nagoya-u.ac.jp.
REFERENCES
- 1.Tsukada K, Hatakeyama K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to TNM stage. Surgery. 1996;120:815–822. [DOI] [PubMed] [Google Scholar]
- 2.Boniest S, Panis Y, Fagniez PL, et al. Long-term results after curative resection for carcinoma of the gallbladder. Am J Surg. 1998;175:118–122. [DOI] [PubMed] [Google Scholar]
- 3.Todoroki T, Kawamoto H, Takahashi Y, et al. Treatment of gallbladder cancer by radical resection. Br J Surg. 1999;86:622–627. [DOI] [PubMed] [Google Scholar]
- 4.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito H, Matros E, Brooks DC, et al. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg. 2004;8:183–190. [DOI] [PubMed] [Google Scholar]
- 6.Tsukada K, Kurosaki I, Uchida K, et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997;80:661–667. [PubMed] [Google Scholar]
- 7.Chijiiwa K, Yamaguchi K, Tanaka M. Clinicopathologic differentiation between long-term and short-term postoperative survivors with advanced gallbladder carcinoma. World J Surg. 1997;21:98–102. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi R, Nagino M, Oda K, et al. Perineural invasion has a negative impact on survival of patients with gallbladder carcinoma. Br J Surg. 2002;89:1130–1136. [DOI] [PubMed] [Google Scholar]
- 9.Kondo S, Nimura Y, Hayakawa N, et al. Regional and paraaortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–422. [DOI] [PubMed] [Google Scholar]
- 10.Kondo S, Nimura Y, Kamiya J, et al. Factors influencing postoperative hospital mortality and long-term survival after radical resection for stage IV gallbladder carcinoma. World J Surg. 2003;27:272–277. [DOI] [PubMed] [Google Scholar]
- 11.McGuckin MA, Cummings MC, Walsh MD, et al. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer. 1996;73:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clare SE, Sever SF, Wilkens W, et al. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol. 1997;4:447–451. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein NS, Mani A, Chmielewski G, et al. Immunohistochemically detected micrometastases in peribronchial and mediastinal lymph nodes from patients with T1, N0, M0 pulmonary adenocarcinoma. Am J Surg Pathol. 2000;24:274–279. [DOI] [PubMed] [Google Scholar]
- 14.Ohta Y, Oda M, Wu J, et al. Can tumor size be a guide for limited surgical intervention in patients with peripheral non-small cell lung cancer? Assessment from the point of view of nodal micrometastasis. J Thorac Cardiovasc Surg. 2001;122:900–906. [DOI] [PubMed] [Google Scholar]
- 15.Izbicki JR, Hosch SB, Pichlmeier U, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–1194. [DOI] [PubMed] [Google Scholar]
- 16.Glickman JN, Torres C, Wang HH, et al. The prognostic significance of lymph node micrometastasis in patients with esophageal carcinoma. Cancer. 1999;85:769–778. [PubMed] [Google Scholar]
- 17.Sato F, Shimada Y, Li Z, et al. Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg. 2001;88:426–432. [DOI] [PubMed] [Google Scholar]
- 18.Maehara Y, Oshiro T, Endo K, et al. Clinical significance of occult micrometastasis in lymph nodes from patients with early gastric cancer who died of recurrence. Surgery. 1996;119:397–402. [DOI] [PubMed] [Google Scholar]
- 19.Cai J, Ikeguchi M, Maeta M, et al. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000;127:32–39. [DOI] [PubMed] [Google Scholar]
- 20.Jeffers MD, O'Dowd GM, Mulcahy H, et al. The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. J Pathol. 1994;172:183–187. [DOI] [PubMed] [Google Scholar]
- 21.Oberg A, Stenling R, Tavelin B, et al. Are lymph node micrometastases of any clinical significance in Dukes’ stages A and B colorectal cancer? Dis Colon Rectum. 1998;41:1244–1249. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama N, Shirai Y, Hatakeyama K. Immunohistochemical detection of lymph node micrometastases from gallbladder carcinoma using monoclonal anticytokeratin antibody. Cancer. 1999;85:1465–1469. [DOI] [PubMed] [Google Scholar]
- 23.Nagakura S, Shirai Y, Yokoyama N, et al. Clinical significance of lymph node micrometastasis in gallbladder carcinoma. Surgery. 2001;129:704–713. [DOI] [PubMed] [Google Scholar]
- 24.Tajima Y, Tomioka T, Ikematsu Y, et al. Immunohistochemical demonstration of cytokeratin is useful for detecting micrometastatic foci from gallbladder carcinoma in regional lymph nodes. Jpn J Clin Oncol. 1999;29:425–428. [DOI] [PubMed] [Google Scholar]
- 25.International Union Against Cancer (UICC). TNM Classification of Malignant Tumors, 6th ed. New York: Wiley-Liss, 2002. [Google Scholar]
- 26.Japanese Society of Biliary Surgery. General Rules for Surgical and Pathological Studies on Cancer of the Biliary Tract, 5th ed. Tokyo: Kanehara, 2003. [Google Scholar]
- 27.Sasaki M, Watanabe H, Jass JR, et al. Immunoperoxidase staining for cytokeratins 8 and 18 is very sensitive for detection of occult node metastasis of colorectal cancer: a comparison with genetic analysis of K-ras. Histopathology. 1998;32:199–208. [DOI] [PubMed] [Google Scholar]
- 28.Tojima Y, Nagino M, Ebata T, et al. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with otherwise node-negative hilar cholangiocarcinoma. Ann Surg. 2003;237:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natsugoe S, Mueller J, Stein HJ, et al. Micrometastasis and tumor cell microinvolvement of lymph nodes from esophageal squamous cell carcinoma: frequency, associated tumor characteristics, and impact on prognosis. Cancer. 1998;83:858–866. [PubMed] [Google Scholar]
- 30.Demeure MJ, Doffek KM, Komorowski RA, et al. Adenocarcinoma of the pancreas: detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998;83:1328–1334. [PubMed] [Google Scholar]
- 31.Demeure MJ, Doffek KM, Komorowski RA, et al. Molecular metastases in stage I pancreatic cancer: Improved survival with adjuvant chemotherapy. Surgery. 1998;124:663–669. [DOI] [PubMed] [Google Scholar]
- 32.Niedergethmann M, Rexin M, Hildenbrand R, et al. Prognostic implications of routine, immunohistochemical, and molecular staging in respectable pancreatic adenocarcinoma. Am J Surg Pathol. 2002;26:1578–1587. [DOI] [PubMed] [Google Scholar]


