Abstract
Clinical research on the deposition of inhaled substances (e.g. inhaled medications, airborne contaminants, fumes) in the lungs necessitates anatomical models of the airways. Current conducting airway models lack three-dimensional (3D) reality as little information is available in the literature on the distribution of the airways in space. This is a limitation to the assessment or predictions of the particle deposition in relation to the subject's anatomy. Detailed information on the full topology and morphology of the airways is thus required to model the airway tree realistically. This paper presents the length, diameter, gravity, coronal and sagittal angles that together describe completely the airways in 3D space. The angle at which the airways branch out from their parent (branching angle) and the rotation angle between successive bifurcation planes are also included. These data are from the study of two sets of airways computed tomography (CT) images. One CT scan was performed on a human tracheobronchial tree cast and the other on a healthy male volunteer. The airways in the first nine generations of the cast and in the first six conducting generations of the volunteer were measured using a computer-based algorithm. The data contribute to the knowledge of the lung anatomy. In particular, the spatial structure of the airways is shown to be strongly defined by the central airways with clear angular lobar patterns. Such patterns tend to disappear with a mean gravity, coronal and sagittal angles of 90° in each generation higher than 13–15. The mean branching angle per generation appears independent of the lobe to which the airways belong. Non-planar geometry at bifurcation is observed with the mean (± SD) bifurcation plane rotation angle of 79 ± 41° (n= 229). This angle appears constant over the generations studied. The data are useful for improving the 3D realism of the conducting airway structure modelling as well as for studying aerosol deposition, flow and biological significance of non-planar airway trees using analytical and computational flow dynamics modelling.
Keywords: bronchial anatomy, image processing, lobar description, modelling, morphometry, topology
Introduction
Little information is available in the literature on human conducting airway morphometry although it is badly needed for various health-related studies where the lung is either a target (inhaled medications) or an unfortunate recipient (e.g. airborne contaminants and fumes). As interest in the fate of inhaled substances in the airways increases, the lack of three-dimensional (3D) information on the bronchial structure is more and more critical.
Particle deposition may be assessed by 3D imaging or predicted using computer modelling of the behaviour of particles in the airway network. Both these techniques use anatomical models of the airways that have been built with the current morphological data available, which essentially come from manual measurements of human airways casts. Lengths and diameters were presented in several studies (e.g. Weibel, 1963; Horsfield & Cumming, 1968; Raabe et al. 1976; Thurlbeck & Horsfield, 1980; Schlesinger & McFadden, 1981; Mortensen et al. 1983). Weibel (1963) plotted the mean diameter per generation and derived the following diameter-generation number relationship:
where d(j) is the mean airway diameter in generation j and the trachea is called generation 1. The coefficient –0.33 in the above equation characterizes the relationship. It was explained by various theories (e.g. minimal resistance production to air flow and minimal volume occupancy: Horsfield & Cumming (1967), minimum entropy production: Wilson (1969), fractal analysis: West et al. (1986)). Relationships between diameters and lengths were also published (Weibel, 1963; Phillips & Kaye, 1995; Kitaoka et al. 1999).
Angular data in the literature are very sparse. This can be explained by two facts. Firstly, manual measurements of 3D angles in casts are very difficult. Secondly, only branching angles (angle formed between the parent airway direction and the studied airway direction) and gravity angles (angle of inclination with respect to gravity) were needed in studies on fluid dynamics or particle deposition in the lungs, using rather simple anatomical models. More refined models are nowadays required, mainly because of increasing interest in targeted pulmonary drug delivery in which the site of deposition of the particles may be a key factor in the success or failure of the treatment (Laube, 1996). A realistic description of the 3D orientation of the airways is thus necessary (Martonen et al. 1995; Finlay et al. 1996; Fleming et al. 1996). Three-dimensional sets of angles are a requirement for modelling and assessing radiolabelled aerosol studies in terms of deposition per airway; they are directly relevant to studies looking at the effect of the subject's breathing position on the distribution of the inhaled aerosol deposition in the lungs (e.g. Baskin et al. 1990). Also, with the increasing use of 3D imaging techniques, lobar and segmental structures are more relevant than the average structure of the whole lung.
Gravity angles were largely recorded only in one report, often called the ‘Lovelace report’ (Raabe et al. 1976). The measurements were performed on a 60-year-old male silicone rubber cast. The individual values of gravity angle ranged from 0° (straight down) to 180° (straight up). The mean gravity angle of all the airways in the same generation and in the same lobe was published in Yeh & Schum (1980), up to generation 12–15. However, Yeh & Schum (1980) converted the individual gravity angles γ greater than 90° to (180 – γ)°, before calculating the mean angle per generation for each lobe. The largest mean gravity angle is 68° (Right Upper lobe, generation 7). Although this data set is relevant to fluid dynamics properties in the airways during a complete breathing cycle, it cannot show the distribution of the airways in space. To our knowledge, the orientation of the airways with respect to the coronal and sagittal directions has not been studied before. Experiments and theory agree on an ‘ideal’ branching angle value of 37° as in the study by Horsfield & Cumming (1967) which used 232 angles from 116 parent airways. One hundred of these were randomly chosen in a population of airways of diameter greater or equal to 0.7 mm; 16 of these were randomly chosen in the central airways down to broncho-pulmonary segmental bronchi. Lastly, the spatial orientation of the airways before and after a bifurcation is of principal interest for modelling the airway tree closely to reality in 3D but no data have been found in the literature. Recent research in arterial branching geometry suggests that non-planarity between two consecutive bifurcation planes is an important factor influencing flow dynamics. Implications such as a swirling type of flow that would improve clearance of material from bends may not be restricted to vascular systems but extended to general piping systems (Caro et al. 1996). Most recently, Caro et al. (2001) have studied the air flow in a physical glass model consisting of a ‘trachea’ followed by two generations of planar branches with orthogonal sequential bifurcation planes. They observed a swirling flow resembling this for non-planar bifurcations. The rotation angle of the bifurcation plane may thus be of considerable interest for modelling aerosol deposition behaviour at the bifurcations.
As the current conducting airway models are simple and unrealistic, a few researchers have made assumptions on the orientation of the airways to develop 3D models. These assumptions are such as a downward tendency (Yeh & Schum, 1980; Koblinger & Hofmann, 1985; Hofmann & Koblinger, 1990), a uniform radial distribution (Fleming et al. 1995) or fixed angular values (e.g. Martonen et al. 1995). The rotation angle between two consecutive bifurcation planes is assumed to be 90° in the models presented in Martonen et al. (1995) and Kitaoka et al. (1999), although the latter allows it to vary depending on the shape of the region to supply with air flow. In the other airway tree models, it does not appear. Experimental data are needed to validate these assumptions. Better knowledge of the precise location of the conducting airways in 3D space will improve the realism of modelling.
In this paper, we present the diameter, length and 3D topology of the first conducting airways as well as their branching angle and rotation angle between successive bifurcations, from two computed tomography (CT) studies. One study was performed on a human tracheobronchial cast; the other study was performed on a male volunteer. The angular variations and patterns are detailed in the pulmonary lobes and airway generations. The data are analysed and discussed in terms of their use for 3D modelling of the conducting airway network and their relevance for predicting aerosol deposition.
Materials and methods
Firstly, a human lung airways plastic cast was CT scanned (1-mm-thick adjacent slices). It contained a large number of conducting airways but a trachea less than 1 cm long. Secondly, a 12-cm-long CT scan containing part of the trachea was performed on a healthy 27-year-old male volunteer at the end of inspiration of a tidal volume breathing cycle. During the scan, the subject held his breath to prevent major motion image artefacts. Very low X-ray power settings (120 kV, 120 mA) were used to minimize the radiation dose to the subject. This study was agreed by an Ethics Committee and performed with the understanding and consent of the volunteer.
CT scanning provides a series of transverse digital sections of the volume imaged. By stacking each adjacent slice on top of each other, a 3D digital volume is reconstructed. Quantitative information on the orientation and dimensions of the airways in 3D space can then be obtained using image processing and computed calculations on this volume. A cast airway description is obtained quickly, compared with lengthy and difficult manual measurements. CT also provides insights into in vivo airway networks, at the limit of X-ray dose to the person and image resolution. The method used to obtain geometrical data on the airways requires the airway tree to be segmented out from the CT image background. This background is air for the cast, lung tissue for the volunteer. All the airways in the first nine conducting airway generations were segmented out by thresholding of the images. Such an approach was used for the cast airway tree because the contrast between plastic material (the cast) and air is high. Sauret et al. (1999) pointed out that this was correct in airways large compared with the CT imaging resolution (i.e. no loss of data). In small airways, segmentation by thresholding may not be equivalent to manual segmentation; it is one of the reasons why airways in generations higher than 9 are not studied in this paper. Segmenting out the airways by thresholding in the in vivo CT images is only feasible for the trachea, primary and secondary airways because of the presence of thick airway walls that appear bright (high contrast with background) in CT images. The walls in the smaller airways are too thin to appear on the CT images and the contrast between lung tissue and air inside the airways is low. In clinical situations, manual delimitation of the airway boundary is thus done by an expert eye. In the volunteer study Dr I. W. Brown segmented out all the airways in the first six generations. Higher generations were not complete as branches were smaller than the CT resolution (3-mm-thick adjacent slices), i.e. indistinguishable, or outside the scan field of view. In the preliminary task of segmenting the airways out for the cast and the volunteer CT images, we thus used the most appropriate method for each case to provide a correct definition of the airway contours.
The topology and morphometry of the airways in both digital sets of airways were measured using the semiautomated algorithm described and validated against manual measurements in Sauret et al. (1999). The algorithm finds the middle line of the airways (skeletonization process from Ma & Sonka, 1996) and calculates for each airway defined by a starting point A and end point B its length l, diameter d, gravity angle γ and branching angle β. The algorithm code was extended to the calculation of (i) the airway coronal and sagittal angles, and (ii) the rotation angle between two consecutive bifurcation planes. Together, the gravity angle γ, the coronal angle θ and the sagittal angle ψ completely describe the orientation of the airway AB in space (Fig. 1). The rotation angle between successive bifurcation planes describes the change in orientation of the airways at bifurcation. For clarity, all the angles are now defined. The gravity angle is defined as the angle formed between ABand nγ, the gravity direction when the subject is standing up. It was calculated from the equation:
Fig. 1.
Definition of the length l and diameter d of an airway i and its orientation in space described by the three angles θ, ψ and γ. The middle line of the airway starts in A and ends in B.
The coronal angle is defined as the angle formed between ABand nθ, the gravity direction when the subject is lying on the right side. It was calculated from the equation:
The sagittal angle is defined as the angle formed between ABand nψ, the gravity direction when the subject is lying on his back. It was calculated from the equation:
The branching angle is defined as the angle formed between ABand the parent direction Dand was calculated from the equation:
Each set of two airways in generation j and having the same parent airway i was used to define the bifurcation plane Pj,i characterized by its normal vector nPj, i. The rotation angle Rj, i between two consecutive bifurcation planes of normal vectors nPj, i and nPj+1,i, referred to as the bifurcation plane rotation angle, was then calculated using the equation:
The mean bifurcation plane rotation angle in each generation j≥ 5 was calculated.
Lengths and diameters relationships within each whole data set are presented, as are the mean of lengths and diameters in each generation. The mean of each angular parameter in each lobe and each generation is presented to analyse lobar patterns. Diameters, lengths and angles are compared with previous published data, when available. The cast and the volunteer data sets are compared by studying the significance of the difference of (i) the cast and volunteer mean diameter and length in each airway generation, and (ii) the mean angles in each airway generation and in each lobe (intersubject study). The test statistic used is the difference of the means over standard error (t-test for small independent samples: Bland, 1997). The significance of the difference in the mean angles per generation or lobe within the same data set is also studied (intrasubject study). In this case, an analysis of variance significance test anova(Bland, 1997), which is appropriate to examine the difference between two or more group means of one single variable, is used. As in the statistical study by Koblinger & Hofmann (1985), the relative standard deviation for each graph is given to allow for future use of the data by airway tree geometry modellers.
Results
Figure 2 shows the two sets of airways that were studied (six airway generations in the volunteer, nine airway generations in the cast). The number of airways per generation and per lobe in each set is given in Table 1. The branching patterns are dichotomous, except in the cast where trifurcations were found.
Fig. 2.
Front view of the two sets of conducting airways studied: (a) all the airways in the first six generations (volunteer); (b) all the airways in the first nine generations (cast).
Table 1.
Number of airways per generation and per lobe
| Volunteer | Cast | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole lung | RU | RM | RL | LU | LL | Whole lung | RU | RM | RL | LU | LL | |||||
| 1 | 1 | 1 | ||||||||||||||
| 2 | 2 | 1 | 1 | 2 | 1 | 1 | ||||||||||
| 3 | 4 | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 1 | ||||||
| 4 | 8 | 2 | 1 | 1 | 2 | 2 | 8 | 2 | 1 | 1 | 2 | 2 | ||||
| 5 | 16 | 4 | 2 | 2 | 4 | 4 | 16 | 4 | 2 | 2 | 4 | 4 | ||||
| 6 | 32 | 8 | 4 | 4 | 8 | 8 | 33 | 8 | 5a | 4 | 8 | 8 | ||||
| 7 | 66 | 16 | 10 | 8 | 16 | 16 | ||||||||||
| 8 | 128 | 32 | 16b | 16 | 32 | 32 | ||||||||||
| 9 | 249 | 64 | 33a | 32 | 55a,c | 65a | ||||||||||
One trifurcation;
two airways terminate at generation 7;
five airways terminate at generation 8. Lobes: right upper (RU), right middle (RM), right lower (RL), left upper (LU), left lower (LU).
The mean ratios presented in Table 2 summarize the relationships between (i) daughter diameters and lengths, and (ii) daughter and parent diameters. They may be compared with equivalent Weibel data (Weibel, 1963), obtained from the manual measurements of cast airways. Figure 3 shows the change in diameter and length as a function of the generation number. To allow comparison with Weibel's diameter-generation number relationship, the diameter data were fitted to a curve of similar form: a· 2b(j−1)where j is the generation number and a and b are constants. This curve is a straight line using a logarithmic scale and the coefficient b is related to its slope. The same curve fitting was done using the length data; the trachea length was not included in the fitting as only a part of this airway was imaged, in both the cast and the volunteer. The diameters are shown consistently bigger in the cast than in the volunteer. In generations 2–6, the difference between the mean diameter in the cast and in the volunteer is significant (t-test for small independent samples, P< 0.05). More variation appears in the length patterns than the diameter patterns and the relative standard deviation (SD) for the mean lengths per generation is consistently greater than for the mean diameters per generation. The difference between the cast and volunteer mean length in generations 2–6 is not significant at P= 0.05.
Table 2.
Airway diameters and lengths mean ratios
| Volunteer measurements (six generations) | Cast measurements (nine generations) | Weibel (1963) | |
|---|---|---|---|
| Length/diameter† | 3.09 | 3.14 | Range: 2.8–3.25 |
| Conjugate daughters diameters‡ | 0.82; | 0.74; | 0.86*; |
| Range: 0.3–1.0 | Range: 0.1–1.0 | Range: 0.5–1.0* | |
| Conjugate daughters lengths§ | 0.58 | 0.58 | 0.62* |
| Daughter diameter/parent diameter | 0.83 | 0.78 | 0.79** |
For 1 mm < diameters < 4.6 mm.
Smaller/larger.
Shorter/longer.
Generations 6–8.
Generations 1–10.
Fig. 3.
Mean diameter and length per generation and relative SD. (closed/open diamonds) Cast/volunteer diameter; (closed/open squares) cast/volunteer length. The curve fitting the cast data is a solid line, the curve fitting the volunteer data is a dashed line.
The mean gravity, coronal and sagittal angles for both sets of measured airways are presented in Table 3. They are close to 90° for all the airways in both the volunteer's and the cast data. The most central airways (generations 2–4) have a smaller angular range. Very different lobar patterns are shown for each angle in Figures 4–6. Comparison of the mean lobar angles in generations 4–6 (right upper, left upper and left lower lobes) or 5–6 (right middle and lower lobes) shows the cast and volunteer data not significantly different at P= 0.05, except for the mean coronal angle in generation 6 of the left lower lobe. This could be explained by differences between the cast and volunteer orientation in the thorax, but could also have occurred by chance given the multiple comparisons being performed in this study. One in 20 comparisons in which there was no real difference would be expected to show significance at the 5% confidence level. The angular distribution within a given generation was studied in generation 9 of the cast, which had the largest number of airways (Figure 7). The gravity angles are distributed symmetrically around a peak at 80–100° (mean = 89°, SD = 39.3°). The distribution within the lobes is, respectively, positively and negatively skewed in the left upper and left lower lobes. The coronal angles present two clusters, located between 40° and 80° and between 100° and 140°. The sagittal angles are distributed around 100° (mean = 98°, SD = 37.1°). anovatests considering each lobe as a group showed a significant difference at the 0.05 level in the gravity angle (F= 3.81, d.f. (explained) = 4 and d.f. (residuals) = 244, P= 0.005) as well as the coronal angle (F= 9.76, d.f. (explained) = 4 and d.f. (residuals) = 244, P< 10−3); in the sagittal angle, no significant difference was shown at the 0.05 level (F= 2.17, d.f. (explained) = 4 and d.f. (residuals) = 244, P= 0.07).
Table 3.
Mean angles (degrees)
| Volunteer measurements (six generations) | Cast measurements (nine generations) | |||||
|---|---|---|---|---|---|---|
| All generations | ||||||
| Gravity angle γ | 84 | 5 | 19–167 | 89 | 2 | 1–170 |
| Coronal angle θ | 84 | 5 | 0–151 | 90 | 2 | 0–180 |
| Sagittal angle ψ | 86 | 5 | 4–169 | 100 | 2 | 0–180 |
| Branching angle β | 39 | 2 | 12–78 | 43 | 1 | 2–121 |
| Generations 2–4 | ||||||
| Gravity angle γ | 77 | 12 | 23–166 | 75 | 10 | 28–143 |
| Coronal angle θ | 86 | 10 | 30–151 | 96 | 12 | 32–158 |
| Sagittal angle ψ | 83 | 9 | 4–135 | 109 | 6 | 68–150 |
Fig. 4.
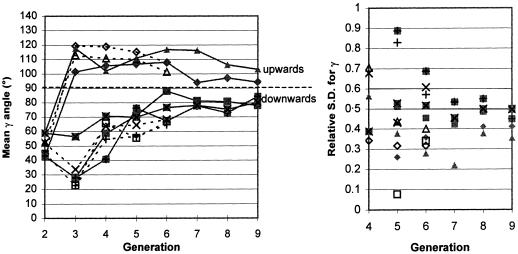
Mean gravity angle per generation for each lobe and relative SD. (closed/open diamonds) Cast/volunteer right upper lobe; (closed/open squares) cast/volunteer right middle lobe; (closed/open crosses) cast/volunteer right lower lobe; (closed/open triangles) cast/volunteer left upper lobe; (closed/open X's) cast/volunteer left lower lobe. The solid lines link the cast data, the dashed lines link the volunteer data.
Fig. 6.
Mean sagittal angle per generation for each lobe and relative SD. Legend as in Figure 4.
Fig. 7.
Frequency distribution of (a) the gravity angle, (b) the coronal angle, (c) the sagittal angle and (d) the branching angle, measured for all the cast airways belonging to generation 9. The width of each class is 20°.
The mean branching angle for each generation and each lobe does not show different lobar patterns and the asymptotic values are around 40° for both the cast and the volunteer's data sets (Figure 8). Comparison of the mean lobar angle in generations 4–6 shows the cast and volunteer data not significantly different at P= 0.05. The mean branching angle averaged for all airways is given in Table 3. The distribution of the branching angles in generation 9 of the cast (Figure 7d) is narrow around its mean (mean = 48°, SD = 24°, n= 249). anovatest (0.05 level) considering each lobe as a group confirmed the hypothesis of no difference between lobes in generation 9 (F= 0.84, d.f. (explained) = 4 and d.f. (residuals) = 244, P= 0.5).
Fig. 8.
Mean branching angle per generation for each lobe and relative SD. Legend as in Figure 4.
The mean value of the bifurcation plane rotation angle in generations 5–8 in the cast is 79° (SD = 41°, n= 229). Considering each generation as a group, anovatest showed no significant difference between each generation at 0.05 level (F= 0.20, d.f. (explained) = 3 and d.f. (residual) = 255, P= 0.89).
Discussion
The algorithm used for this study considers the airways as straight tubes. Sauret et al. (1999) showed that this is a reasonable assumption, except for the first left main bronchus being slightly curved. In the same paper, computed values of airway length, diameter and gravity angle were verified against manual measurements of the cast. They are re-plotted here for the ease of the reader. The cast coronal, sagittal and rotation angles are new data as are all the volunteer measurements. These data are all valuable, as the literature is very poor in quantitative spatial airway measurements. Phillips & Kaye (1995) wrote: ‘there is only one human subject for which comprehensive data is in the public domain (Raabe et al. (1976): manual measurements of a human cast) … we believe there is a pressing need for further measurements before it is clear how great the variations between subjects are, and to what extent the geometrical features identified are universal’. The data published here add new information on the morphometry and topology of the airways. Diameter and length measurements will be briefly discussed first. The angular data are new and will thus be analysed in greater detail.
Length and diameter
The various relationships between length and diameter for both the volunteer and the cast compare well with Weibel's data (Table 2). The diameter-generation number relationship (Figure 3) fits the form of the Weibel description although with slightly different values of coefficient b(eqn 1). This also holds for the mean length, in agreement with West et al. (1986). The ratio length to diameter increases with generation number, suggesting a non-proportional variation between them (Figure 3). This supports the Lovelace data analysis by Phillips & Kaye (1995) in which length increased with diameter but not as fast as proportionality would imply (Weibel, 1963; Phalen et al. 1978). The difference in the cast and volunteer diameters may be explained by a different inflation state between the airways. Large changes in lung volume are found, for instance, between supine and upright positions (Nunn, 1977).
3D orientation
The airways in the central generations have a large influence on overall lung shape, which is primarily inferior to the tracheal bifurcation. Table 3 shows that the gravity angle in generations 2–4 is less than both 90° and the overall mean value. In all generations, the mean gravity angle in the upper lobes for both the volunteer and cast is over 90°, thus clearly upwards (Figure 4). The middle lobe follows the downward pattern of the lower lobes. Extrapolation of gravity angle data suggests that in high generation number (j> 13) the mean gravity angle would be 90° for all lobes. The data concur with the Yeh & Schum analysis of the Lovelace measurements taking into account the angle transformation applied.
Figure 5 shows that right and left lobe airways on average point, respectively, to the right (θ < 90°) and to the left (θ > 90°). This is also shown in the coronal angle histogram for cast generation 9 (Figure 7b). Extrapolation of the Figure 5 data shows the mean coronal angle tending to 90° at high generation number for all lobes.
Fig. 5.
Mean coronal angle per generation for each lobe and relative SD. Legend as in Figure 4.
Figure 6 shows that, unlike the gravity and coronal angle patterns, the sagittal angles for the volunteer and cast data are very different in the first main bronchi (Figure 9). The volunteer's trachea splits towards the back (ψ = 75° and 77° in, respectively, right and left main bronchus) whereas the cast trachea splits towards the front (ψ = 116° and 106° in, respectively, right and left main bronchus). This underlines the intersubject variability that has been seen in several studies (e.g. Raabe et al. 1976; Soong et al. 1979; Koblinger & Hofmann, 1985). In these top views, nψis perpendicular to the subject's back as he lies on the horizontal bed of the CT scanner, but since the cast does not include the tissue around the airways, a disorientation of the cast on the CT bed is possible. We thus estimate a 10° error on the cast absolute angular values. Potential angular shrinkage during the casting procedure may also occur. These points could explain why the sagittal lobar patterns for the cast appear to extrapolate to a value above 90° at higher generations. In generation 9 of the cast, lobar patterns cannot be distinguished (anovatest and Figure 6) and slightly more airways go towards the front of the chest than the back, extreme values around 0° or 180° being rare (Figure 7c).
Fig. 9.
Top views of the airways. It is clear that the trachea bifurcation into the right and left main bronchi points towards the back in the volunteer (a) and towards the front in the cast (b).
The standard deviation for each of the angles (Figures 4–6) also varied with generation number and with lobe but the pattern of variation is similar. There was greater variability in angles in the lower generations, which reduced with generation number, coinciding with all angles converging to 90°.
In view of these results, arbitrary values or hypotheses of a uniform distribution of the gravity, sagittal and coronal angles in the whole lung do not seem valid in the central conducting airways. Angular patterns in the generations studied vary considerably from one lobe to another; only in high generations does extrapolation suggest a distribution centred at 90° for all lobes. The spatial structure of the airways is thus strongly defined by the central airways.
Geometry at branching
Given the number of airways studied, the mean branching angles of 39° in the volunteer and 43° in the cast agree with the theoretically ideal angle of 37° (Table 3). In each generation the cast and volunteer mean angles were not significantly different. The branching angle was not dependent on the lobe (Figures 7d and 8). The rotation angle between consecutive bifurcation planes has been measured for the first time in this study (79°) and confirms visual observations of casts that suggest a value close to 90°. The anovatest did not show significant variation in the generations investigated. Data in higher generations would be valuable to confirm a constant mean value over the whole airway tree.
Applications
This study provides a complete description of the airways in 3D space, which will allow validation of anatomical airway tree models. The data will help in identifying unrealistic parameters in present models and in correcting them. The study on the volunteer was part of a project assessing radiolabelled aerosol deposition per airway generation and per lobe. The volunteer anatomical data presented here will allow improved analysis of these images.
The 3D description of the airway network image will be of use in airflow studies using computational fluid dynamics (CFD). They are available on demand from the authors. The cast and the volunteer airway data sets are both interesting for clinical use: the cast data contain more generations but their history is unknown, while the volunteer data contain fewer generations but the breathing-state and scanning position are clear. Both allow the study of airflow and aerosol deposition distribution. The image volume sets also show the shape of airway connections, whereas existing models connect crudely two rigid tubes. Studying how this affects CFD simulation results would be useful. To gain a model representative of the variation of pulmonary anatomy, examination of many subjects is necessary.
The computed measurement method used here was shown to be adequate to obtain airway description from CT digital images and the algorithm could be used to analyse more airway casts. The number of airways assessed is limited by the CT resolution that, for in vivo imaging, is related to the X-ray dose to the subject. By demonstrating the use of such a description for anatomy, aerosol deposition and airflow modellers, it is hoped to encourage research into other imaging methods such as 3He-MRI (Johnson et al. 1998) which has no radiation dose. In vivo imaging overcomes uncertainties in cast formation. It could also provide data on the anatomical variation of the airway with patient position and breathing state.
Conclusion
The detailed values and variations of the geometric parameters of the airway structure presented here contribute to the knowledge of the human lung anatomy. They will be useful for improving the 3D realism of the conducting airway structure modelling and for 3D viewing and teaching purposes. Furthermore, visualization of the airway tree in terms of 3D spatial distribution opens the way to new research on aerosol deposition, flow and biological significance of non-planar airway trees, using analytical modelling and computational flow dynamics simulation.
Acknowledgments
We wish to thank Dr M. Sonka for providing the skeletonization algorithm code, and Drs J. Conway and P. C. Jackson for their advice. The project was financed by a University of Southampton, Faculty of Engineering studentship.
References
- Baskin MI, Abd AG, Ilowite JS. Regional deposition of aerosolized pentamidine. Effects of body position and breathing pattern. Ann. Intern. Med. 1990;113:677–683. doi: 10.7326/0003-4819-113-9-677. [DOI] [PubMed] [Google Scholar]
- Bland M. An Introduction to Medical Statistics. 2. Oxford: Oxford Medical Publications; 1997. [Google Scholar]
- Caro CJ, Doorly DJ, Tarnawski M, Scott KT, Long Q, Dumoulin CL. Non-planar and non-planar models of human bronchial airway. Proc. Royal Soc. Series A. 1996;452:185–197. [Google Scholar]
- Caro CG, Schroter RC, Watkins N, Sherwin SJ. Steady inspiratory flow in planar and non-planar models of human bronchial airways. J. Physiol. 2001;531P:98. [Google Scholar]
- Finlay WH, Stapleton KW, Chan HK, Zuberbuhler P, Gonda I. Regional deposition of inhaled hygroscopic aerosols: in vivo SPECT compared with mathematical modeling. J. Appl. Physiol. 1996;81:374–383. doi: 10.1152/jappl.1996.81.1.374. [DOI] [PubMed] [Google Scholar]
- Fleming JS, Nassim M, Hashish AH, Bailey AG, Conway J, Holgate S, et al. Description of pulmonary deposition of radiolabeled aerosol by airway generation using a conceptual three-dimensional model of lung morphology. J. Aerosol. Med. 1995;8:341–356. [Google Scholar]
- Fleming JS, Hashish AH, Conway J, Nassim M, Holgate S, Halson P, et al. Assessment of deposition of inhaled aerosol in the respiratory tract of man using three-dimensional multimodality imaging and mathematical modeling. J. Aerosol. Med. 1996;9:317–327. doi: 10.1089/jam.1996.9.317. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Koblinger L. Monte-Carlo modeling of aerosol deposition in human lungs. Part II: Deposition fractions and their sensitivity to parameter variations. J. Aerosol. Sci. 1990;21:675–688. [Google Scholar]
- Horsfield K, Cumming G. Angles of branching and diameters of branches in the human bronchial tree. Bull. Mathemat. Biophysics. 1967;29:245–259. doi: 10.1007/BF02476898. [DOI] [PubMed] [Google Scholar]
- Horsfield K, Cumming G. Morphology of the bronchial tree in man. J. Appl. Physiol. 1968;24:373–383. doi: 10.1152/jappl.1968.24.3.373. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Hedlund L, MacFall J. A new window into the lung. Physics World. 1998;11:35–38. [Google Scholar]
- Kitaoka H, Takaki R, Suki B. A three-dimensional model of the human airway tree. J. Appl. Physiol. 1999;87:2207–2217. doi: 10.1152/jappl.1999.87.6.2207. [DOI] [PubMed] [Google Scholar]
- Koblinger L, Hofmann W. Analysis of human morphometric data for stochastic aerosol deposition calculations. Physics Med. Biol. 1985;30:541–556. doi: 10.1088/0031-9155/30/6/004. [DOI] [PubMed] [Google Scholar]
- Laube BL. In vivo measurements of aerosol dose and distribution: clinical relevance. J. Aerosol Med. 1996;9(Suppl. 1):S77–S91. doi: 10.1089/jam.1996.9.suppl_1.s-77. [DOI] [PubMed] [Google Scholar]
- Ma CM, Sonka M. A fully parallel 3D thinning algorithm and its applications. Computer Vision Image Understanding. 1996;64:420–433. [Google Scholar]
- Martonen TB, Yang Y, Hwang D, Fleming JS. Computer simulations of human lung structures for medical applications. Computer Biol. Med. 1995;25:431–446. doi: 10.1016/0010-4825(95)00027-2. [DOI] [PubMed] [Google Scholar]
- Mortensen JD, Young JD, Stout L, Stout A, Bagley B, Schaap RN. A numerical identification system for airways in the lung. Anat. Record. 1983;206:103–114. doi: 10.1002/ar.1092060112. [DOI] [PubMed] [Google Scholar]
- Nunn JF. Applied Respiratory Physiology. London: Butterworth and Co (Publishers) Inc; 1977. pp. 66–67. [Google Scholar]
- Phalen RF, Yeh HC, Schum GM, Raabe OG. Application of an idealized model to morphometry of the mammalian tracheobronchial tree. Anat. Record. 1978;190:167–176. doi: 10.1002/ar.1091900202. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Kaye SR. Diameter-based analysis of the branching geometry of four mammalian bronchial trees. Respiration Physiol. 1995;102:303–316. doi: 10.1016/0034-5687(95)00056-9. [DOI] [PubMed] [Google Scholar]
- Raabe OG, Yeh HC, Schum GM, Phalen RF. Tracheobronchial Geometry: Human, Dog, Rat, Hamster (Report LF-53) Albuquerque, NM: Lovelace Foundation Medical Educational Resources; 1976. [Google Scholar]
- Sauret V, Goatman KA, Fleming JS, Bailey AG. Semi-automated tabulation of the 3D topology and morphology of branching networks using CT: application to the airway tree. Physics Med. Biol. 1999;44:1625–1638. doi: 10.1088/0031-9155/44/7/304. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB, McFadden LA. Comparative morphometry of the upper bronchial tree in six mammalian species. Anat. Record. 1981;199:99–108. doi: 10.1002/ar.1091990110. [DOI] [PubMed] [Google Scholar]
- Soong TT, Nicolaides PYuCP, Soong SC. A statistical description of the human tracheobronchial tree geometry. Respiration Physiol. 1979;37:161–172. doi: 10.1016/0034-5687(79)90068-9. [DOI] [PubMed] [Google Scholar]
- Thurlbeck A, Horsfield K. Branching angles in the bronchial tree related to order of branching. Respiration Physiol. 1980;41:173–181. doi: 10.1016/0034-5687(80)90050-x. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Morphometry of the Human Lung. Heidelberg: Springer Verlag; 1963. [Google Scholar]
- West BJ, Bhargava V, Golderberg AL. Beyond the principle of similitude: renormalization in the bronchial tree. J. Appl. Physiol. 1986;60:1089–1097. doi: 10.1152/jappl.1986.60.3.1089. [DOI] [PubMed] [Google Scholar]
- Wilson TA. Design of the bronchial tree. Nature. 1969;18:668–669. doi: 10.1038/213668a0. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Schum GM. Models of human lung airways and their application to inhaled particle deposition. Bull. Mathemat. Biol. 1980;42:461–480. doi: 10.1007/BF02460796. [DOI] [PubMed] [Google Scholar]